Abstract
We evaluated the relationship between benralizumab (30 mg every 4 and 8 weeks (Q4W, Q8W)) pharmacokinetic (PK) exposure and end points of asthma exacerbation rates (AERs) and change from baseline in prebronchodilator forced expiratory volume in 1 second (FEV 1) for patients with severe, uncontrolled eosinophilic asthma in the SIROCCO/CALIMA phase III trials. In empirical assessment, AER ratios in SIROCCO were similar across PK quartiles. However, the lowest PK quartile in CALIMA had reduced efficacy; low CALIMA placebo AER possibly confounded this result. In population modeling, estimated benralizumab 90% effective concentration for AER reduction was 927 ng/mL, below the Q8W dosage steady‐state average PK concentration (1,066 ng/mL). Benralizumab treatment resulted in more rapid FEV 1 improvement vs. placebo (estimated half‐maximum time: 7.6 vs. 18 days); this response was greater for patients with greater baseline eosinophil counts. These results confirmed 30 mg Q8W is the optimal benralizumab dosage for patients with severe eosinophilic asthma.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ In the phase III SIROCCO and CALIMA trials, benralizumab 30 mg every 4 weeks (Q4W) and every 8 weeks (Q8W; first three doses Q4W) significantly improved asthma exacerbation rates (AERs), forced expiratory volume in 1 second (FEV1), and symptoms for patients with severe, uncontrolled eosinophilic asthma.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ We aimed to evaluate the relationship between benralizumab pharmacokinetic (PK) exposure and efficacy end points of AER and change from baseline in prebronchodilator FEV1 for patients in the SIROCCO/CALIMA trials.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ We report that empirical and population analyses of AER and prebronchodilator FEV1 confirm that benralizumab 30 mg Q8W, with an additional dose at week 4, is the optimal dosage for the treatment of patients with severe asthma.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ Although we found that patients with greater baseline blood eosinophil counts may have slightly better response, benralizumab still provides benefit for patients with baseline blood eosinophil counts < 300 cells/μL, and no dosage adjustment by anti‐drug antibody (ADA) status or weight is necessary.
Benralizumab is an interleukin‐5 receptor alpha–directed cytolytic monoclonal antibody1 indicated for the add‐on maintenance treatment of patients with severe asthma aged 12 years and older and with an eosinophilic phenotype.2 Benralizumab elicits rapid and near‐complete depletion of eosinophils in the lung tissue, sputum, blood, and bone marrow via enhanced antibody‐dependent, cell‐mediated cytotoxicity.1, 3, 4
In a 52‐week, phase IIb dosage‐ranging trial, based on the exposure–response analysis of three clinical end points (asthma exacerbation rate (AER), Asthma Control Questionnaire, and forced expiratory volume in 1 second (FEV1)),5 investigators projected benralizumab 30 mg every 8 weeks (first 3 doses every 4 weeks (Q4W); Q8W) as the 90% effective dosage (ED90) and a candidate regimen to be studied in phase III trials.6 In the phase III SIROCCO and CALIMA trials, the sponsor‐selected regimens of benralizumab 30 mg Q4W and Q8W to evaluate benralizumab efficacy and safety for patients with severe, uncontrolled, eosinophilic asthma.7, 8 Both regimens significantly reduced AERs by up to 51%, decreased asthma symptoms, and increased lung function for patients receiving high‐dosage inhaled corticosteroids plus long‐acting β2‐agonists (ICS/LABA) with baseline blood eosinophil counts ≥ 300 cells/μL.7, 8 A subsequent population modeling analysis of nine phase II−III clinical trials found that the pharmacokinetic (PK) profile of benralizumab was dose‐proportional across a wide dosage range.9
The objective of this analysis was to evaluate the relationship between benralizumab PK exposure and the end points of AER (primary end point) and FEV1 (secondary end point) for patients who participated in the SIROCCO and CALIMA trials and, thereby, to verify the optimal dosing regimen of benralizumab for patients with severe, uncontrolled eosinophilic asthma.
Results
Patients
In total, 1,204 and 1,306 patients with severe, uncontrolled asthma were randomized and received treatment in the SIROCCO and CALIMA trials, respectively. All patients in SIROCCO and 1,091 patients in CALIMA were receiving high‐dosage ICS/LABA. For this analysis, we excluded 215 patients from CALIMA who received medium‐dosage ICS/LABA. Five patients with extreme PK concentration outliers were excluded from the analysis, leaving 1,461 patients from the SIROCCO (n = 747) and CALIMA (n = 714) trials who had adequate benralizumab PK data for inclusion in the empirical assessment. For the AER modeling analysis, 2,268 patients (placebo, n = 777; Q4W, n = 756; Q8W, n = 735) in both trials had adequate benralizumab PK data to include in this analysis. For the FEV1 modeling analysis, 24 patients had missing baseline prebronchodilator FEV1 values, resulting in the inclusion of 2,244 patients (placebo, n = 764; Q4W, n = 747; Q8W, n = 733).
Empirical assessment of asthma exacerbation rate
Median steady‐state trough benralizumab concentrations within each dosage were consistent across studies. Steady‐state trough concentrations for the Q4W dosage were approximately five times that for the Q8W dosage because of the shorter dosing interval. Estimates of AER ratios in the SIROCCO trial were relatively flat across the trough concentration quartiles, confirming that the benralizumab 30 mg Q8W dosage reached the efficacy plateau for AER. In CALIMA, the lowest trough PK quartile had a smaller AER response compared with other quartiles. However, low efficacy in the lowest trough concentration quartiles was observed in both Q4W and Q8W dosages (Figure 1 and Table S1 ).
Figure 1.
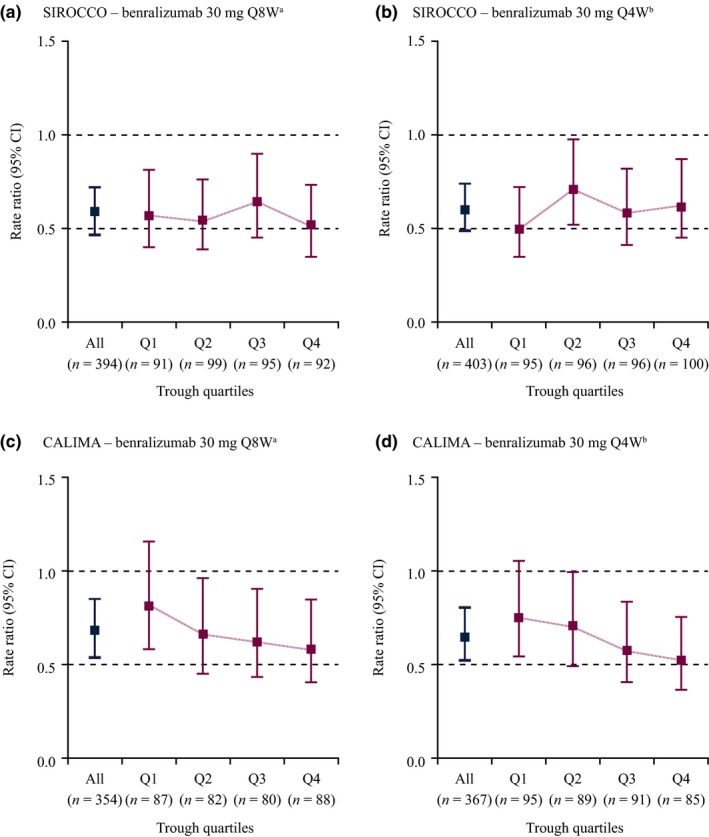
Relative risk of asthma exacerbation by treatment regimens and steady‐state trough concentration quartiles for patients with severe asthma requiring high‐dosage inhaled corticosteroids/long‐acting β2‐agonists in the SIROCCO and CALIMA trials. CI, confidence interval; Q, quartile; Q4W, every 4 weeks; Q8W, every 8 weeks (first three doses Q4W). aQ1: < 140 ng/mL; Q2: 140–256 ng/mL; Q3: 256–442 ng/mL; Q4: ≥ 442 ng/mL. b Q1: < 797 ng/mL; Q2: 797–1,220 ng/mL; Q3: 1,220–1,690 ng/mL; and Q4: ≥ 1,690 ng/mL. [Colour figure can be viewed at wileyonlinelibrary.com]
Population exposure–response modeling for asthma exacerbation rate
Asthma exacerbation rate modeling
There was negligible difference in the parameter estimates of the population Poisson‐normal and negative binomial–normal count models, and, hence, the Poisson‐normal model was selected to move forward. AER modeling identified the number of prior exacerbations in the previous year as the most significant covariate, for which the greater the number of prior exacerbations, the greater the exacerbation risk was. Patients without oral corticosteroid (OCS) usage and from Central/Eastern Europe were found to have a lesser exacerbation risk.
Baseline blood eosinophil count did not have a significant effect on exacerbation risk. The estimated benralizumab half‐maximum effective concentration (EC50) for AER was 103 ng/mL, corresponding to 90% of the maximal effective concentration (EC90) of 927 ng/mL for patients with severe asthma. The typical average steady‐state concentrations of benralizumab within a dosing interval were 1,066 and 2,132 ng/mL for the 30 mg Q8W and 30 mg Q4W dosage, respectively. There was a positive trend toward improved benralizumab efficacy for patients with greater baseline blood eosinophil counts; however, the effect was not significant. The difference in treatment effects for adolescents and adults also was evaluated but was not statistically significant because of the limited sample size of adolescent patients.
Trial simulation for effect on asthma exacerbation rate
A trial simulation of 5,000 virtual patients with asthma across all spectrums of baseline eosinophil counts with a typical weight of 72 kg and antidrug antibody (ADA) incidence, as observed in the SIROCCO and CALIMA trials, showed that the projected median AER ratios were comparable for the benralizumab Q4W and Q8W regimens (0.61 vs. 0.63, respectively; Figure 2 a), which were consistent with the observations. Although the presence of ADAs in some patients in the SIROCCO and CALIMA trials was associated with an increase in benralizumab clearance,9 the presence of ADAs had no significant effect on benralizumab efficacy in this model. In the rate ratios computed under the extreme scenarios, no ADA incidence and 100% ADA incidence were close to that of the base scenario under the observed ADA incidence in SIROCCO and CALIMA (Figure 2 b). We did not identify adolescence as a significant covariate for AER; the large uncertainty in the prediction interval in the trial simulations indicated that the limited sample size precluded conclusive results (Figure 2 c).
Figure 2.
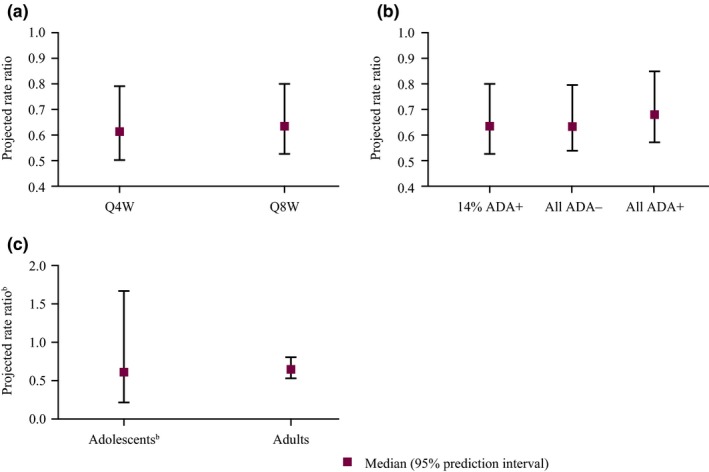
Projected asthma exacerbation risk by treatment regimen, antidrug antibody (ADA) status, and age group from clinical trial simulations.a Q4W, every 4 weeks; Q8W, every 8 weeks. aOne hundred trial simulations with 500 patients for each scenario. bThe prediction interval for adolescents was wider than that for adults because of the smaller numbers enrolled in the SIROCCO and CALIMA trials compared with adult patients. [Colour figure can be viewed at wileyonlinelibrary.com]
Exposure–response assessment for forced expiratory volume
The correlation plot of steady‐state average serum concentration (Cavg,ss) data vs. observed prebronchodilator FEV1 change from baseline at end of treatment (EOT) data was greatly scattered (Figure S1 ) and did not reveal any trend of FEV1 response with increasing benralizumab steady‐state concentrations. The presence of ADAs had no effect on FEV1 response. Subsequent population exposure–response modeling confirmed a similar finding: the estimated EC50 (1.36 ng/mL) was smaller than the assay's lower limit of quantitation (i.e., 3.86 ng/mL). This suggested a potentially flat exposure–response curve for FEV1 at 30 mg efficacy plateau dosage (Q8W and Q4W); therefore, accurate estimation of EC50 is not possible.
Longitudinal modeling for forced expiratory volume response
Given the flat exposure–response relationship, the prebronchodilator FEV1 data were modeled longitudinally. Placebo effect modeling identified standing height and age to have significant effects on individual model‐predicted maximum placebo effect (Pmax; Figure S2 ). Taller patients tended to have a greater placebo effect than shorter patients, and adolescents had the strongest placebo effect compared with other age groups (with the same standing height). No placebo effect was observed for patients aged > 65 years.
For the treatment effect, only baseline blood eosinophil count was identified as a relevant covariate (Figure 3). Patients with greater baseline eosinophil counts were associated with superior FEV1 response. In addition to the height and age effects on Pmax and the influence of baseline blood eosinophil count on maximum benralizumab effect (Emax), standing height, age, sex, and concomitant use of theophylline or aminophylline at baseline were found to have significant effects on baseline prebronchodilator FEV1 (Figure S3 ). A typical female patient had a lower baseline FEV1 than a male patient of the same age and height. FEV1 at baseline decreased with age, and taller patients tended to have greater baseline FEV1 values than shorter patients. Concomitant use of theophylline at baseline was associated with an ~10% decrease in baseline FEV1. However, only ~12% of patients in the SIROCCO and CALIMA trials were receiving theophylline or aminophylline, and this concomitant medication had no impact on placebo or drug effect. Race had no effect on FEV1 values.
Figure 3.
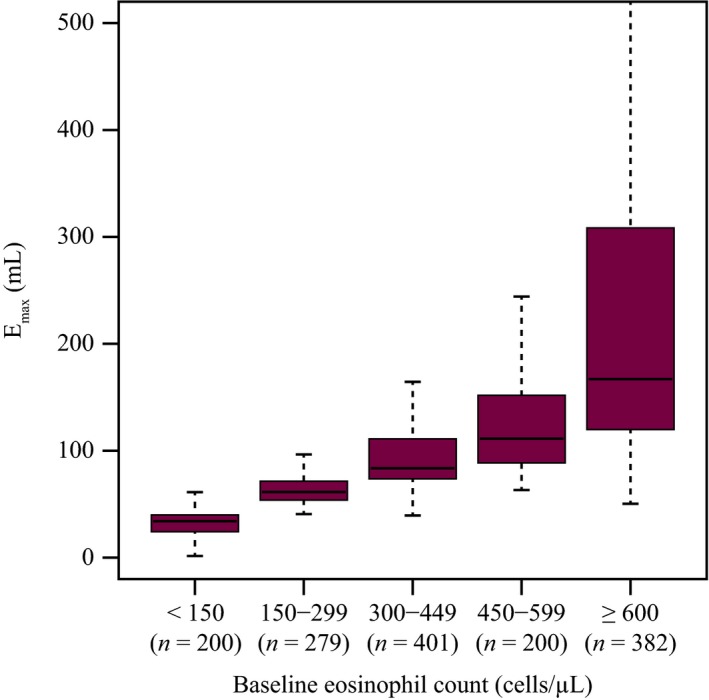
Effect of baseline blood eosinophil count on individual model‐predicted benralizumab efficacy. Emax, maximum forced expiratory volume in 1 second (efficacy). Boxplot: horizontal black line: median; box: spans the interquartile range (25th percentile–75th percentile); whiskers: span up to 1.5‐times the interquartile range. [Colour figure can be viewed at wileyonlinelibrary.com]
The estimated typical placebo and drug effects were 184.0 mL and 95.8 mL, respectively (i.e., the observed apparent effect in benralizumab‐treated patients was 279.8 mL). Benralizumab treatment was associated with a rapid improvement of FEV1 over placebo; time associated with half‐maximum response to benralizumab treatment (T50) was 7.64 days for benralizumab compared with an estimated half‐life of 18 days associated with the emergence of placebo effect.
In a post hoc analysis of the relationship between the number of exacerbations in the previous year with Emax, there was an apparent trend of improving FEV1 for patients with ≥ 3 exacerbations in the previous year. There was no impact of body weight on prebronchodilator FEV1 change from baseline for patients receiving high‐dosage ICS/LABA with baseline blood eosinophil counts ≥ 300 cells/μL (Figure 4).
Figure 4.
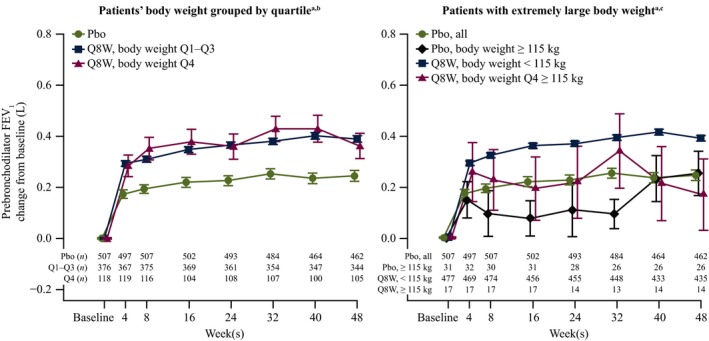
Impact of body weight on prebronchodilator forced expiratory volume in 1 second (FEV 1) change from baseline for patients with baseline blood eosinophil counts ≥ 300 cells/μL receiving high‐dosage inhaled corticosteroids/long‐acting β2‐agonists. Pbo, placebo; Q, quartile; Q8W, every 8 weeks. aSymbols represent the mean, and error bars are standard error of the mean. bQ1–Q3: 40–89 kg; Q4: > 89 kg. cBody weight cutoff = 115 kg (top 5%). [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
High serum concentrations of benralizumab are required for rapid airway eosinophil depletion, possibly because of the low partitioning of serum immunoglobulin G to lung epithelial fluid lining and sputum. An additional dose at week 4 for the Q8W regimen may have facilitated benralizumab distribution and depletion of eosinophils in tissues early in the course of treatment. The greater PK exposure and sustained blood eosinophil depletion for a greater percentage of patients associated with the additional week 4 dose were expected to have expedited the onset of benralizumab treatment benefit.
Both empirical and population analyses of AER and FEV1 confirmed benralizumab 30 mg Q8W, with an additional dose at week 4, as the optimal dosing regimen for patients with severe asthma receiving high‐dosage ICS/LABA. This is the recommended dosage of benralizumab for the add‐on maintenance treatment of patients with severe asthma aged 12 years and older and with an eosinophilic phenotype.2 In the current analysis, no additional efficacy was observed with increased benralizumab dosing frequency or exposure, which is in agreement with the results reported in the SIROCCO and CALIMA trials.7, 8
In the empirical assessment of AERs, similar rate ratios were observed across all PK trough concentrations regardless of baseline blood eosinophil counts in SIROCCO and agreed with a priori projection, confirming 30 mg Q8W at the efficacy plateau for AER reduction (Figures 2 and 5). In CALIMA, on the other hand, efficacy was less pronounced in the lowest PK quartile groups for patients receiving both 30 mg Q4W and 30 mg Q8W. This result emerged despite a greater PK exposure for patients who received the Q4W regimen than for patients in the greater trough concentration quartiles who received the Q8W regimen. The marginal exacerbation rate for patients receiving placebo was much lower in the CALIMA trial compared with the SIROCCO trial (1.01 vs. 1.52), and this may have confounded the interpretation of the empirical trough PK–AER assessment of this study. However, overall efficacy was comparable between the two treatment groups, confirming benralizumab 30 mg Q8W as the optimal dosing regimen for patients with severe, eosinophilic asthma. Therefore, a greater benralizumab dose or more frequent dosing would not provide additional benefit, and a dosage lower than 30 mg Q8W could potentially risk reducing efficacy. Furthermore, the subsequent AER modeling found the estimated benralizumab EC90 for AER to be 927 ng/mL, which is lower than the typical steady‐state average PK concentration (1,066 ng/mL) for the Q8W dosage. The optimal dosage of 30 mg Q8W was also confirmed by the analysis of prebronchodilator FEV1, as no exposure‐relationship was identified, indicating the efficacy plateau was attained.
Figure 5.
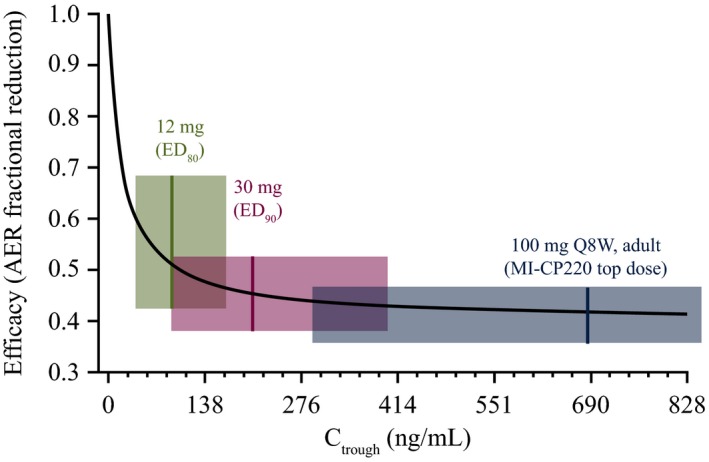
Simulated exposure–response for benralizumab effect on asthma exacerbation rate.5 Symbol: median exposure; shaded area: interquartile range; Stochastic simulation: n = 3,000 virtual patients/dosage. AER, asthma exacerbation rate; Ctrough, observed trough concentration; ED 80/90, estimated effective dosage that gave 80%/90% inhibition; Q8W, every 8 weeks. [Colour figure can be viewed at wileyonlinelibrary.com]
At the benralizumab ED90 (30 mg Q8W), the potential impact of PK variability on efficacy was reduced. Although body weight and the presence of ADAs were identified as significant PK covariates, based on observed data and population PK/pharmacodynamic modeling,10 the presence of ADAs and large body weight (> 115 kg) had no impact on blood eosinophil depletion.
Patients with ≥ 3 prior exacerbations in the past 12 months tended to have greater efficacy for FEV1 than those with two prior exacerbations. The population modeling was unable to confirm the impact of the number of prior exacerbations on benralizumab efficacy for AER, as this covariate was already included in the baseline exacerbation rate and the absence of observed individual profiles precluded more thorough covariate effect assessment.
Being a woman and using theophylline concomitantly with benralizumab each corresponded to an ~10% reduction in typical FEV1 value at baseline. The effects were slightly greater than the typical day‐to‐day variation (≤ 9.5% in amplitude) of FEV1 in patients with asthma11; however, neither was found relevant for placebo effect or benralizumab treatment effect in the population modeling. Race effect was not confirmed using this model. However, previous studies have identified African‐Americans as having reduced FEV1. 12
In the analysis of exacerbation events, there was a trend for greater efficacy for AER for patients with greater baseline eosinophil counts, but this was not statistically significant. Patients with baseline eosinophil counts < 300/μL still benefited from benralizumab treatment but to a lesser extent. The strongest effect of baseline eosinophil count was on benralizumab efficacy for FEV1. The greater the baseline eosinophil count was, the greater the FEV1 improvement over placebo was. However, the differences for Emax for patients with baseline blood eosinophil counts < 150/μL and 150–299/μL (a substantial number of the primary population) and those with counts 300–449/μL were negligible (Figure 3) when compared with the estimated maximum placebo effect, 196 mL.
Benralizumab treatment was associated with rapid improvement of FEV1, with a half‐maximum time of 7.6 days. For the SIROCCO and CALIMA trials, week 4 was the first FEV1 measurement after first dose. The speed of onset of treatment effect (T50) will be further evaluated in a phase IIIb study, SOLANA, with earlier and more frequent FEV1 measurement (days 3, 7, and 14) after first dose (NCT02869438). The development of a placebo effect was considerably slower, with a half‐life estimate of ~18 days.
Limitations
Certain limitations are associated with this analysis. There was a strong placebo effect for reduced exacerbations and improved FEV1 observed in the SIROCCO and CALIMA trials,7, 8 which is a common observation in clinical trials for patients with asthma.13, 14 The placebo effect on FEV1 is usually a result of better patient adherence to maintenance therapy because of the active monitoring that accompanies controlled clinical trial enrollment. However, we did not observe a placebo effect for patients aged > 65 years, and we saw the strongest placebo effect in the adolescent group. Adolescents may have benefited more from compliance to maintenance therapy relative to other age groups during the treatment phase. The effect of growth and corresponding increase in lung volume over the 1‐year study period also may have contributed to the placebo effect in adolescents in SIROCCO and CALIMA, as standing height had a significant impact on the placebo effect and baseline FEV1. However, our modeling analysis found age was not a significant covariate of drug effect, and there was no difference in drug effect between adolescents and adults, supporting the treatment of this subpopulation with benralizumab. Despite a strong placebo effect, the large sample size allowed full covariate assessments for baseline FEV1 as well as the placebo effect. The estimation of the onset of placebo effect and treatment effect on FEV1 improvement is limited by lack of measurements prior to week 4 of the study, but the impending SOLANA study should provide a more accurate characterization of these effects. We did not identify age as a relevant covariate (modeled using a nonlinear power function) for benralizumab efficacy for AER or FEV1, and the model‐projected exacerbation ratio for adolescents was similar to that for adults but with a much wider 95% confidence interval. It is possible that the small sample size for the adolescent population in these trials contributed to age not being identified as a relevant covariate.
Conclusions
The results we report, on both empirical and population analyses of AER and prebronchodilator FEV1, confirm that 30 mg Q8W with an additional dose at week 4 is the optimal dosage of benralizumab for the treatment of patients with severe asthma. Although patients with greater baseline blood eosinophil counts may have slightly better response, benralizumab still provides benefit for patients with baseline blood eosinophil counts < 300 cells/μL. No dosage adjustment by ADA status or weight is necessary.
Methods
This analysis was a population efficacy modeling study with exposure–response assessment and longitudinal modeling followed by covariate analysis.
Patients and trial designs
The pooled data from the SIROCCO (NCT01928771) and CALIMA (NCT01914757) phase III randomized controlled trials were used for this analysis (Figure 6).7, 8 These trials included male and female patients aged 12–75 years who had physician‐diagnosed asthma that required medium‐dosage (CALIMA only) to high‐dosage ICS/LABA for ≥ 12 months and had experienced ≥ 2 asthma exacerbations in the previous 12 months. Patients with baseline blood eosinophil counts ≥ 300 cells/μL and < 300 cells/μL were recruited to the trials in a 2:1 ratio. Patients were randomized 1:1:1 to receive 48 weeks (SIROCCO) or 56 weeks (CALIMA) of treatment with subcutaneous injections of benralizumab 30 mg Q4W, benralizumab 30 mg Q8W (placebo was administered at the 4‐week interim visits to maintain blinding), or placebo Q4W. Patients continued their prescribed maintenance ICS/LABA during the trials. Full details and results of these trials have been published.7, 8
Figure 6.
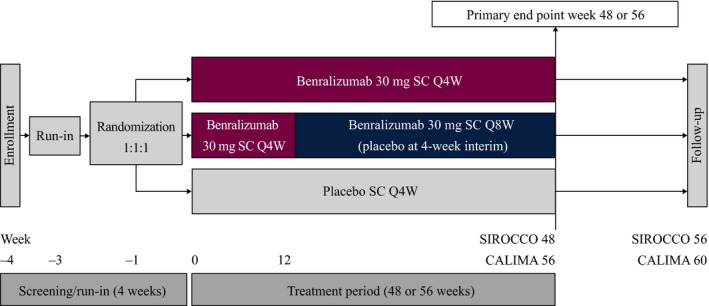
SIROCCO and CALIMA trial designs. Q4W, every 4 weeks; Q8W, every 8 weeks (first three doses Q4W). [Colour figure can be viewed at wileyonlinelibrary.com]
For both SIROCCO and CALIMA, an independent ethics committee or institutional review board at each study center approved the clinical study protocol, and the national regulatory authority either approved the clinical study protocol or received a notification according to local regulations. All patients provided written informed consent at enrollment. The study was conducted in accordance with the principles of the Declaration of Helsinki and was consistent with the International Council for Harmonisation/Good Clinical Practice and applicable regulatory requirements and AstraZeneca company policy on bioethics.
The primary efficacy end point of the SIROCCO and CALIMA trials was the AER for patients receiving high‐dosage ICS/LABA with baseline blood eosinophil counts ≥ 300 cells/μL.7, 8 Serum samples were collected prior to dosing and throughout the treatment period for determination of benralizumab concentrations and PK analyses. A key secondary end point of the SIROCCO and CALIMA trials was lung function, as assessed by the change of prebronchodilator FEV1.
Data analyses and prior knowledge
The analysis population consisted of patients from the placebo arms of both trials who received high‐dosage ICS and was limited to 2,268 patients from the treatment arms that were included in the PK analysis population. In the prebronchodilator FEV1 analysis, the patients were further excluded if the baseline FEV1 values were missing. Prior population efficacy end point modeling of benralizumab in a 52‐week phase IIb study (MI‐CP220) was conducted in 606 adults with uncontrolled eosinophilic asthma. Patients received subcutaneous injections of placebo or benralizumab (2, 20, or 100 mg) on weeks 1, 4, 8, 16, 24, 32, and 40.6 The dose‐response analysis of asthma exacerbation events projected that 30 mg, the ED90, was the optimal dose to be further evaluated in the primary registration studies, as illustrated by Figure 5.5 The EC90 dose was expected to maximize therapeutic efficacy while reducing the impact of steady‐state PK variability on efficacy outcome. The selection of the 30 mg Q8W regimen was further supported by the exposure–response analyses of FEV1 and Asthma Control Questionnaire responses, two key secondary efficacy end points in the SIROCCO and CALIMA trials. In addition, the baseline eosinophil count was identified as a significant covariate for benralizumab efficacy evaluated by FEV1 response.
Exposure–response analysis of asthma exacerbation rate
The exposure–response analysis of AER was conducted using an empirical PK trough concentration quartile–AER correlation method and a population‐modeling approach.
Empirical assessment of asthma exacerbation rate
For the empirical assessment, observed AERs were correlated with benralizumab steady‐state trough concentration quartiles to assess the effect of benralizumab exposure on exacerbation risk. Asthma exacerbation relative risk values for trough concentration quartiles vs. placebo for the Q8W and Q4W regimens were estimated using negative binomial regression. Trough concentration quartiles were computed based on pooled data for each regimen.
Asthma exacerbation rate modeling
A longitudinal dynamic Poisson‐normal count model was previously developed to characterize the AER in MI‐CP220. Both longitudinal dynamic Poisson‐normal and negative binomial–normal count models were explored to characterize the relationship between the benralizumab dosing regimen and the reduction of asthma exacerbation risk. For each patient, the numbers of exacerbation events were computed in an 8‐week interval until the end of follow‐up, except for the last interval, where the number of events were added up from the end of the previous 8‐week interval until the end of the follow‐up. In addition to a dose–response analysis, an efficacy (Emax) model was explored to describe the impact of benralizumab concentration on exacerbation events. In this model, the expected number of exacerbation events in an interval (a, b) could be described by the following equation:
where β0 was an intercept parameter corresponding to the log of asthma exacerbation rate over the interval; β was the parameter associated with effect of covariates X; Emax was the maximal reduction of risk of having exacerbations and was a function of typical βEmax and parameter estimates; βEmax,z, associated with treatment covariates, Z, C(t) was the model‐predicted benralizumab serum concentration at time t; EC50 was 50% of the maximal effective concentration; and η was the normally distributed interindividual variability. We assumed the AER was influenced by both intrinsic asthma exacerbation risk factors (base exacerbation rate) and via treatment effect. The analysis of the base AER covariates began with covariates such as region, number of prior exacerbations in the previous year, the use of maintenance OCS, and baseline eosinophil count. This was followed by the evaluation of covariates on treatment effects, such as baseline blood eosinophil counts. The effect of covariates on efficacy was assessed with the likelihood ratio test, using an acceptance criterion of P < 0.001.
Population modeling of prebronchodilator forced expiratory volume response
The analysis of a key secondary end point in the SIROCCO and CALIMA trials, change from baseline in prebronchodilator FEV1, was composed of three components: the empirical assessment that correlated the steady‐state average concentration of benralizumab within a dosing interval, Cavg,ss, with prebronchodilator FEV1 changes from baseline at the EOT (week 48 and week 56 for SIROCCO and CALIMA, respectively); the exposure–response analysis; and a population longitudinal modeling of FEV1 response.
Empirical exposure–response assessment for forced expiratory volume
The individual steady‐state Cavg,ss at the given dosing intervals (28 days for the Q4W dosage and 56 days for the Q8W dosage), derived based on final population PK model–predicted individual clearance,9 was correlated with the observed prebronchodilator FEV1 changes from baseline at EOT for benralizumab‐treated patients.
Exposure–response analysis for forced expiratory volume
Prebronchodilator FEV1 data from SIROCCO and CALIMA were pooled and simultaneously modeled using a direct Emax model, and placebo effect was characterized using an exponential function:
where Pmax is the maximum placebo effect of FEV1 improvement over baseline as time approaches infinity, and rate‐constant kpbo corresponds to the onset speed of the placebo effect. Emax is the maximum response to benralizumab as time approaches infinity, and T50 is the time associated with half‐maximum response to benralizumab treatment.
Population longitudinal modeling of forced expiratory volume response
The observed FEV1 response (change from baseline) consists of placebo effect and drug (benralizumab) effect (Figure 7). Population analysis was first limited to placebo patients to characterize the placebo effect:
Figure 7.
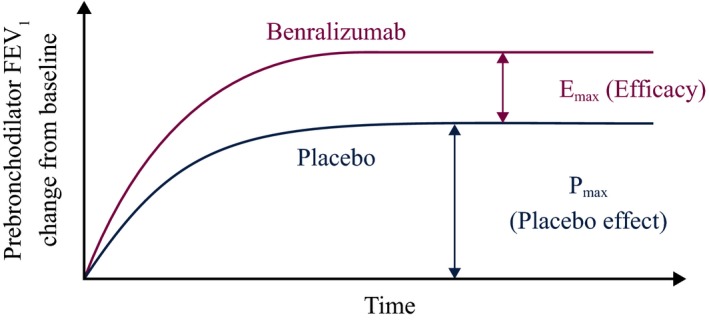
Schematic of the longitudinal model for forced expiratory volume in 1 second (FEV 1) response. Emax, maximum FEV 1 response to benralizumab treatment (efficacy); Pmax, maximum placebo effect. [Colour figure can be viewed at wileyonlinelibrary.com]
Covariates evaluated on placebo effect were age, baseline FEV1 (observed), standing height, creatinine clearance, geographic region, body mass index (BMI), race, baseline eosinophil count, concomitant medication use, and sex.
When modeling the drug effect (Emax), only FEV1 data from benralizumab‐treated patients were analyzed:
where T50 is the time associated with half‐maximum response to benralizumab treatment.
Covariates evaluated on Emax were baseline FEV1, baseline eosinophil count, age, body weight, standing height, BMI, geographic region, race, OCS use, concomitant medication use, ≥ 3 exacerbations in the previous 12 months, sex, ADAs, adolescence, and systemic clearance (a surrogate for steady‐state PK exposure).
In addition to the covariates evaluated for placebo and treatment effects, age, body weight, standing height, BMI, creatinine clearance, systemic clearance, baseline eosinophil count, sex, oral corticosteroid use, geographic region, race, and concomitant medication use were evaluated for their impact on baseline FEV1 using pooled data from both the placebo group and the benralizumab‐treated patients.
We assessed statistical tests of covariate–parameter relationships using the likelihood ratio test, by forward inclusion (P < 0.05 with 1 degree of freedom) followed by backward elimination (P < 0.001 with 1 degree of freedom). Only those covariate effects with strong correlation with the model parameters were retained in the final model.
Funding
The SIROCCO and CALIMA trials were funded by AstraZeneca and Kyowa Hakko Kirin. This analysis was funded by AstraZeneca and MedImmune LLC.
Conflicts of Interest
L.R., Y.L.C., L.Y., and B.Y. are employees of MedImmune LLC. B.W. was an employee of MedImmune LLC at the time of the analysis. P.B. and M.G. are employees of AstraZeneca.
Author Contributions
Y.L.C., L.Y., B.Y., B.W., P.B., M.G., and L.R. wrote the manuscript. Y.L.C., L.Y., B.Y., B.W., P.B., M.G., and L.R. designed the research. Y.L.C., L.Y., B.Y., B.W., P.B., M.G., and L.R. interpreted the data. Y.L.C., L.Y., B.Y., and B.W. analyzed the data.
Supporting information
Table S1. Relative risk (risk ratio) of exacerbation by treatment groups and PK quartiles for the SIROCCO and CALIMA trials.
Figure S1. Empirical correlation of prebronchodilator FEV1 change from baseline at end of treatment with steady‐state benralizumab concentration for the benralizumab Q8W regimen using data from the SIROCCO and CALIMA trials.
Figure S2. Effects of standing height and age on individual model‐predicted Pmax.
Figure S3. Effects of standing height, age, and sex on individual model‐predicted baseline FEV1.
Acknowledgments
Editorial support was provided by Debra Scates, PhD, of JK Associates, Inc., and Michael A. Nissen, ELS, of AstraZeneca. This support was funded by AstraZeneca.
References
- 1. Kolbeck, R. et al MEDI‐563, a humanized anti‐IL‐5 receptor alpha mAb with enhanced antibody‐dependent cell‐mediated cytotoxicity function. J. Allergy Clin. Immunol. 125, 1344–1353 (2010). [DOI] [PubMed] [Google Scholar]
- 2. FASENRA™ (benralizumab) prescribing information, November 2017 <www.fda.gov>. Accessed December 12, 2017.
- 3. Busse, W.W. et al Safety profile, pharmacokinetics, and biologic activity of MEDI‐563, an anti‐IL‐5 receptor alpha antibody, in a phase I study of subjects with mild asthma. J. Allergy Clin. Immunol. 125, 1237–1244 (2010). [DOI] [PubMed] [Google Scholar]
- 4. Laviolette, M. et al Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J. Allergy Clin. Immunol. 132, 1086–1096 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang, B. et al Exposure‐response analysis for determination of benralizumab optimal dosing regimen in adults with asthma. Am. J. Respir. Crit. Care Med. 189, A1324 (2014). [Google Scholar]
- 6. Castro, M. et al Benralizumab, an anti‐interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose‐ranging study. Lancet Respir. Med. 2, 879–890 (2014). [DOI] [PubMed] [Google Scholar]
- 7. Bleecker, E.R. et al Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high‐dosage inhaled corticosteroids and long‐acting beta2‐agonists (SIROCCO): a randomised, multicentre, placebo‐controlled phase 3 trial. Lancet 388, 2115–2127 (2016). [DOI] [PubMed] [Google Scholar]
- 8. FitzGerald, J.M. et al Benralizumab, an anti‐interleukin‐5 receptor alpha monoclonal antibody, as add‐on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet 388, 2128–2141 (2016). [DOI] [PubMed] [Google Scholar]
- 9. Yan, L. , Wang, B. , Chia, Y.L. & Roskos, L.R. Population pharmacokinetic modeling of benralizumab in adult and adolescent patients with asthma. CPT Pharmacometrics Syst Pharmacol. 6, 249‐257 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang, B. , Yan, L. , Yao, Z. & Roskos, L.K. Population pharmacokinetics and pharmacodynamics of benralizumab in healthy volunteers and patients with asthma. CPT Pharmacometrics Syst. Pharmacol. 6, 249–257 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Troyanov, S. , Ghezzo, H. , Cartier, A. & Malo, J.L. Comparison of circadian variations using FEV1 and peak expiratory flow rates among normal and asthmatic subjects. Thorax 49, 775–780 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hankinson, J.L. , Odencrantz, J.R. & Fedan, K.B. Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med. 159, 179–187 (1999). [DOI] [PubMed] [Google Scholar]
- 13. Dutile, S. , Kaptchuk, T.J. & Wechsler, M.E. The placebo effect in asthma. Curr. Allergy Asthma Rep. 14, 456 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Castro, M. et al Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double‐blind, randomised, placebo‐controlled, phase 3 trials. Lancet Respir. Med. 3, 355–366 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Relative risk (risk ratio) of exacerbation by treatment groups and PK quartiles for the SIROCCO and CALIMA trials.
Figure S1. Empirical correlation of prebronchodilator FEV1 change from baseline at end of treatment with steady‐state benralizumab concentration for the benralizumab Q8W regimen using data from the SIROCCO and CALIMA trials.
Figure S2. Effects of standing height and age on individual model‐predicted Pmax.
Figure S3. Effects of standing height, age, and sex on individual model‐predicted baseline FEV1.


