Abstract
Objectives
Laboratory monitoring of patients using lithium is important to prevent harm and to increase effectiveness. The aim of this study is to determine compliance with the guidelines for laboratory monitoring of patients treated with lithium overall and within subgroups.
Methods
Patients having at least one lithium dispensing for 6 months or longer between January 2010 and December 2015 were identified retrospectively using data from the Dutch PHARMO Database Network. Laboratory monitoring was defined as being compliant with the Dutch Multidisciplinary Clinical Guideline Bipolar Disorders when lithium serum levels, creatinine and thyroid‐stimulating hormone (TSH) had been measured at least every 6 months during lithium use.
Results
Data were analyzed from 1583 patients with a median duration of 7‐ to 6‐months period of lithium use. Results indicated that patients had been monitored over 6‐month period for lithium serum levels 65% of the time, for creatinine 73% of the time and for TSH 54% of the time. Just over one seventh (16%) of patients had been monitored in compliance with the guidelines for all three parameters during total follow‐up. Especially males, patients aged below 65 years, patients receiving prescriptions solely from general practitioners, prevalent users of lithium, patients without interacting co‐medication, and patients without other days with laboratory measurements had been monitored less frequently in compliance with the guidelines.
Conclusions
A considerable proportion of patients had not been monitored in accordance with the guidelines. Further research is needed to understand the reasons for noncompliance and to implement strategies with the ultimate goal of optimizing safety and effectiveness for patients treated with lithium.
Keywords: compliance, guideline, guideline compliance, lithium, monitoring
1. INTRODUCTION
Mood stabilizers aim to prevent manic and depressive relapses in patients with bipolar disorder during maintenance therapy.1 The balance between benefit and harm of pharmacotherapy needs to be monitored and evaluated for each patient before and periodically during treatment.2 Clinical practice guidelines (CPGs) provide instructions regarding both clinical and biomarker monitoring.2, 3, 4 Clinical monitoring includes monitoring of both signs and symptoms, while biomarker monitoring can be divided into monitoring of physical parameters and of laboratory parameters.2 Although the need for monitoring of patients treated with psychotropic drugs is widely recognized, adherence to monitoring guidelines is often found to be suboptimal.5, 6, 7, 8 For example, monitoring rates of metabolic parameters such as weight, glucose, and lipids in patients using antipsychotic drugs have been found not to correspond with advice given in CPGs, posing unnecessary risks of morbidity to patients.9, 10
Lithium is approved for the acute treatment of mania and prophylaxis of bipolar disorder and is additionally used to augment antidepressant therapy in unipolar depression.11, 12 Monitoring of lithium serum levels is important to prevent harm and to improve effectiveness among its users. The effectiveness of the treatment may decrease during subtherapeutic lithium serum levels, with a risk of relapse or recurrence, while higher lithium serum levels may be associated with a higher risk of adverse effects.13 Adverse effects with regard to renal and thyroid function frequently occur.14 A significant decline in renal function has been reported in patients treated with lithium,15, 16, 17, 18 in rare cases resulting in renal failure.19, 20 Thyroid function may decline, with a risk of developing goiter. Based on these risks, monitoring of lithium serum levels, renal and thyroid function is important.12
In a previous survey by our group, prescribers of lithium reported to monitor at least one parameter within the first 6 months and during maintenance treatment in patients using lithium.21 Especially lithium serum levels, creatinine, and TSH were reported to be monitored by almost all prescribers of lithium.21 Although prescribers report to monitor patients using lithium, it is unknown whether in clinical practice patients are actually monitored for lithium serum levels, creatinine, and TSH. The objective of this study is to determine compliance with the guidelines for laboratory monitoring of patients treated with lithium among ambulatory patients in the Netherlands overall and within subgroups.
2. METHODS
2.1. Setting and source population
For this retrospective follow‐up study, monitoring of ambulatory patients treated with lithium was assessed in multiple regions in the Netherlands. Data were obtained from the PHARMO Database Network, a population‐based network of electronic databases from primary and secondary health care settings in the Netherlands. The PHARMO Database Network is a large patient‐centered data network, including multiple linked observational databases designed for use in pharmacoepidemiology and outcomes studies of drugs, which collates patient records in geographically defined areas throughout the Netherlands. Mandatory health insurance requires patients to register with a general practitioner and most patients are registered with a single pharmacy in the Netherlands. To address the objectives of the current study, data from the Out‐patient Pharmacy Database and the Clinical Laboratory Database were used.
The Out‐patient Pharmacy Database comprises all general practitioner‐ and specialist‐ prescribed health care products dispensed by out‐patient pharmacies and covers a catchment area representing 4.2 million residents. The dispensing records include information on type of product, strength, dosage regimen, dispensing date, quantity dispensed, and prescriber specialty. Dispensed drugs are coded according to the World Health Organization (WHO) Anatomical Therapeutic Chemical (ATC) Classification System. The Clinical Laboratory Database comprises results of tests performed on clinical specimens. These laboratory tests are requested by general practitioners and medical specialists in order to get information concerning diagnosis, treatment, and prevention of disease. The electronic records include information on date and time of testing, test result, unit of measurement, and type of clinical specimen. Laboratory tests are coded according to the Dutch WCIA coding system.22 Clinical laboratory data cover a catchment area representing 1.2 million residents. Patient data included sex and date of birth and a unique patient identification code to follow patients’ medication and laboratory data over time. In the period 2010 to 2015 approximately 170 000 patients had data available from both the Out‐patient Pharmacy Database and the Clinical Laboratory Database and these data were used as the source population.
The data from the PHARMO Database Network do not include any information allowing identification of individual persons; data were collected anonymously. In terms of current Dutch law, no ethical approval for this study was required.
2.2. Study population
All patients who had been dispensed lithium (ATC code N05AN01) during the period from 1 January 2010 to 31 December 2015 were identified from the source population. The date of the first lithium dispensing in the study period was defined as the index date. Patients were included if they were aged 18 years or older at the index date, had at least 1 year of medication history, had at least 1 year of follow‐up, and had used lithium for at least 6 months. The theoretical duration of lithium use was determined by dividing the amount dispensed by the prescribed dosage regimen. Because dosing of lithium varies, lithium episodes were considered to be continuous if gaps between subsequent dispensed prescriptions were less than 30 days, based on the start date of dispensing and the theoretical end date of the previous lithium dispensing for the same patient. All patients having at least one episode of lithium treatment for a duration of 6 months were identified. Individual follow‐up for all patients was divided into fixed time periods of 6 months (182 days) (Figure 1A).
Figure 1.
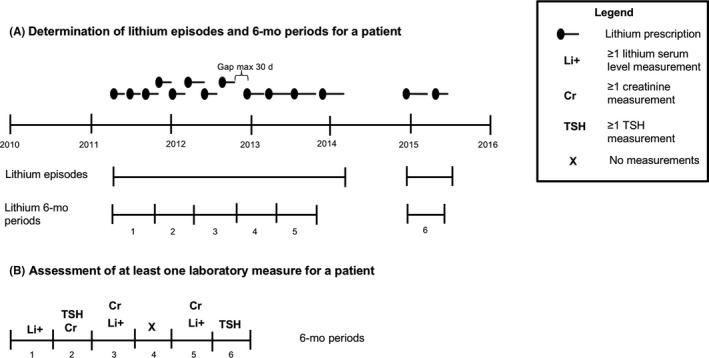
Schematic representation of assessment of laboratory measurements
2.3. Patient characteristics
For all patients in the study population, sex, age at start of periods, and number of 6‐month period of lithium use were determined. To provide further insight into the patient population, dispensed psychotropic drugs (based on ATC codes, Appendix 1) were determined within the 6‐month period before lithium dispensing. Furthermore, the type of prescriber within the first 6‐month period, treatment status, and number of dispensed medications in addition to lithium were determined.
2.4. Period characteristics
Age (as determined by year of birth) was assessed in a time‐dependent manner at the start of each period. The characteristics of prescribers during lithium use were analyzed; the type of prescriber for lithium was assessed within every period from the pharmacy records and categorized into “medical specialist,” “general practitioner,” or “both.” A medical specialist could, for example, be a psychiatrist or geriatrist.
A distinction was made between patients based on duration of lithium use. Patients were divided into (re‐)initiators and prevalent users at the start of each 6‐month period. An (re‐)initiator of lithium was defined as having a dispensed lithium prescription without having a lithium dispensing within the 6 months before the prescription, while being noninstitutionalized (defined as having at least one drug dispensing event for any other drug within 6 months before dispensing). A patient was defined as a prevalent user (maintenance) during consecutive lithium periods of 6 months after the index date. Patients may have had more than one lithium treatment episode.
The number of different dispensed medications (based on ATC codes) was determined within each period. Patients were divided into patients with polypharmacy (≥5 medications including lithium) or patients with <5 medications and it was assessed whether patients with polypharmacy were monitored more often in accordance with the guidelines than patients with <5 medications.
Co‐medications interacting with lithium requiring monitoring of lithium according to the G‐standard, an evidenced‐based professional guideline for the management of drug‐drug interactions, were assessed within periods.23
The number of days with laboratory measurements other than lithium serum levels, creatinine and TSH were determined in each period as a measure of laboratory monitoring intensity in general.
2.5. Assessment of laboratory measurements
Within each 6‐month period of lithium use and during total follow‐up of lithium use of patients, it was determined whether lithium serum levels, creatinine and TSH had been measured in compliance with the guidelines (Figure 1B). The 2008 published Dutch Multidisciplinary Clinical Guideline Bipolar Disorders recommended to monitor lithium serum levels, creatinine and TSH at least every 6 months for patients using lithium.24 Monitoring was defined as compliant with the guidelines if lithium serum levels, creatinine and TSH had been determined at least every 6 months during lithium use. For every 6‐month period, it was determined whether a laboratory measurement of lithium serum level, creatinine and TSH had been performed.
2.6. Data analysis
Data were analyzed using the Statistical Package for Social Sciences Version 24.0 for Windows (SPSS 24.0; SPSS Inc, Chicago, IL). Descriptive statistics were used to determine the percentage of patients who had been monitored in compliance with the guidelines for lithium serum levels, creatinine and TSH in 6‐month period. Thereby, the percentages of patients being monitored during total follow‐up of lithium use were determined for all three parameters. A sensitivity analysis was performed to determine the proportion of patients being monitored in 9‐month period for lithium serum levels, creatinine and TSH.
The strength of the association between the patient and period characteristics and guideline monitoring adherence was assessed with logistic regression analysis and expressed as odds ratios (ORs) with corresponding 95% confidence intervals (95%CI). ORs for being monitored according to the guidelines compared to not being monitored according to the guidelines were calculated for the covariates sex, age, type of prescriber, treatment status (initiation or maintenance), number of prescribed medications (<5 or ≥5 medications), interacting co‐medication, and days with laboratory measurements other than lithium, creatinine, or TSH. All ORs were adjusted for all patient and period characteristics.
3. RESULTS
3.1. Study population
From the source population, a total of 2279 different patients were identified with at least one lithium dispensing in the period between 1 January 2010 and 31 December 2015 (Figure 2). After applying the exclusion criteria, 1583 patients were identified who had used lithium for at least one 6‐month period, generating a total of 10 202 patient periods of 6 months.
Figure 2.
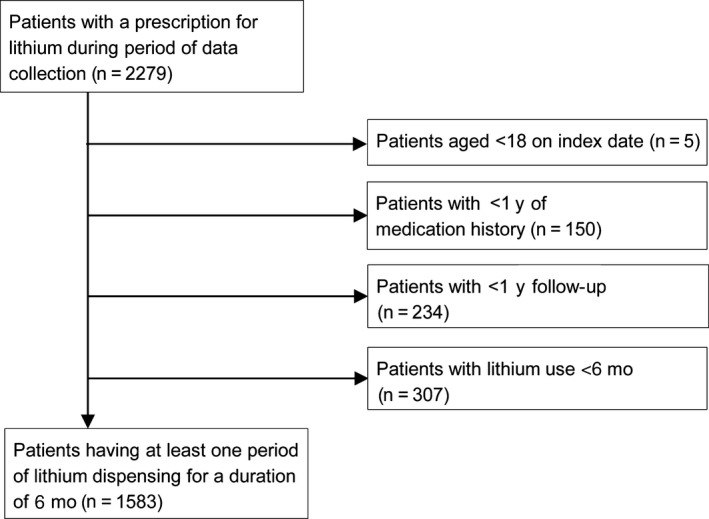
Flowchart for patient inclusion
The study population thus included 1583 patients (mean age 57 (SD 14) years, 60% female), with a median number of seven 6‐month period (interquartile range (IQR) 3‐10) (Table 1). Most patients had been prescribed lithium in the first 6‐month period by a medical specialist (59%), about one fifth had been prescribed lithium by a general practitioner (21%), and about one fifth had been prescribed lithium by both a medical specialist and a general practitioner (20%). Most patients had been maintenance users within the first 6‐month period (95%). In half of the first 6‐month period, patients had used five or more other medications (50%) with a median number of four (IQR 2‐7) other medications per 6‐month period.
Table 1.
Patient characteristics (N = 1583)
| Patient characteristics | Number of patients (%) |
|---|---|
| Sex | |
| Male | 634 (40%) |
| Female | 949 (60%) |
| Mean age at index date | 57 (SD: 14) |
| Age range in y | 18 ‐ 95 |
| Age bands, y | |
| 18‐45 | 358 (23%) |
| 46‐55 | 387 (24%) |
| 56‐65 | 406 (26%) |
| >65 | 432 (27%) |
| Number of 6 month periods of lithium use | |
| 1‐4 | 555 (35%) |
| 5‐8 | 443 (28%) |
| 9‐12 | 585 (37%) |
| Psychotropic drugs use within the 6‐month period before the first lithium dispensing | |
| Antidepressants | 731 (46%) |
| Antipsychotics | 644 (41%) |
| Anxiolytics, hypnotics and sedatives | 681 (43%) |
| Psychostimulants | 9 (1%) |
| Mood stabilizers | 162 (10%) |
| Prescriber within the first 6‐month period | |
| General practitioner | 336 (21%) |
| Medical specialist | 938 (59%) |
| Both | 309 (20%) |
| Treatment status within the first 6‐month period | |
| (Re‐)Initiation | 82 (5%) |
| Maintenance | 1501 (95%) |
| Number of dispensed medications in addition to lithium within the first 6‐month period | |
| <5 medications | 794 (50%) |
| ≥5 medications | 789 (50%) |
| Median number of medications (IQR) | 4 (2‐7) |
Psychotropic drugs had commonly been dispensed within the 6‐month period before the first dispensing of lithium. Antidepressants had been dispensed to 46% of patients, antipsychotics to 41%, anxiolytics, hypnotics, and sedatives to 43%, mood stabilizers to 10% and psychostimulants had been almost extremely rare (1%).
3.2. Outcomes
Lithium serum levels were monitored in 65% of periods of lithium use, creatinine in 73%, and TSH in 54% of periods of lithium use (Table 2).
Table 2.
Laboratory tests conducted compliant with the guidelines
| Laboratory tests | Percentage monitored compliant with the guidelines for patients during total follow‐up of lithium use | Percentage monitoring compliant with the guidelines within periods |
|---|---|---|
| Lithium serum level | 724/1583 (46%) | 6584/10 202 (65%) |
| Creatinine | 747/1583 (47%) | 7441/10 202 (73%) |
| Thyroid stimulating hormone (TSH) | 338/1583 (21%) | 5545/10 202 (54%) |
About half of all patients were monitored according to the guidelines during total follow‐up of lithium use for lithium serum levels (46%) and creatinine (47%) and about one fifth for TSH (21%). About one seventh (16%) of patients were monitored according to the guidelines for all three parameters during the total period of lithium use.
Sensitivity analysis results indicate that the percentages of monitoring compliant with the guidelines in periods were increased to 69%, 79%, and 68%, respectively, for lithium serum levels, creatinine and TSH when measured over 9‐month period. During total follow‐up, the percentage of monitoring in compliance with the guidelines increased to 56%, 63%, and 42% for lithium serum levels, creatinine and TSH, respectively.
3.2.1. Characteristics of periods monitored in compliance with the guidelines
Monitoring of lithium serum levels, creatinine and TSH seemed to be correlated with the different characteristics (Figure 3). Between subgroups, the odds for being monitored differed for some characteristics. Males were less often monitored for TSH according to the guidelines compared to females (52% vs 56%; OR 0.83; 0.77‐0.90).
Figure 3.
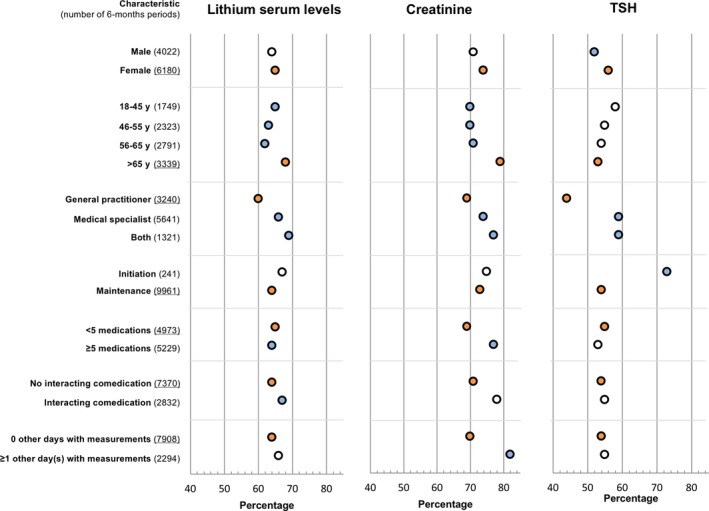
Subgroup analysis of characteristics on percentage of monitoring compliant with the guidelines in 6‐month period (adjusted for all patient and period characteristics). Reference categories are underlined at the Y‐axis and presented as orange dots. Blue dots represent significant differences compared to the reference group (P < 0.05) [Colour figure can be viewed at wileyonlinelibrary.com]
Within age categories, the odds of being monitored was significantly lower for patients aged between 18 and 65 years compared to patients >65 years old for lithium and creatinine.
Patients who had received lithium prescriptions from general practitioners were monitored less often in compliance with the guidelines regarding lithium serum levels (60% vs 66%; OR 0.72; 0.67‐0.80), creatinine (69% vs 74%; OR 0.63; 0.57‐0.69), and TSH (44% vs 59%; OR 0.54; 0.49‐0.59) compared to patients who had received prescriptions from medicals specialists. In addition, patients who had received lithium prescriptions from a general practitioner only were additionally monitored less often in compliance with the guidelines for all three parameters compared to patients who had received lithium prescriptions from both a general practitioner and medical specialist.
Patients defined as initiators were more often monitored in compliance with the guidelines for TSH compared to prevalent users (73% vs 54%; OR 2.21; 1.65‐2.95).
Patients were dispensed on average five different other medications (IQR2‐8) per 6‐month period in addition to lithium. The proportion of patients monitored in compliance with the guidelines was slightly higher for patients on fewer than five medications compared to the proportion of patients with polypharmacy (≥5 medications) for lithium serum levels (65% vs 64% OR 1.11; 1.02‐1.22), lower for creatinine (69% vs 77%; OR 0.82; 0.74‐0.90), but not significantly different for TSH.
Patients not receiving interacting co‐medication were monitored less often in accordance with the guidelines for lithium serum levels (64% vs 67%; OR 0.87; 0.78‐0.96) compared to patients receiving interacting co‐medication, but not for creatinine and TSH.
Patients with zero 'other days with laboratory measurements' were monitored less often for creatinine (70% vs 82%; OR 0.60; 0.53‐0.68) compared to patients with one or more 'other days with laboratory measurements', but not significantly less often for lithium serum levels or TSH.
4. DISCUSSION
A considerable number of patients using lithium were not monitored according to the Dutch guidelines. Compliance was relatively higher for laboratory monitoring of creatinine compared to monitoring of lithium serum levels and TSH. The higher proportion of patients being monitored for creatinine might be explained by the fact that creatinine is more often included in laboratory protocols than lithium serum levels, for example, during admission laboratory measurements or when needed for other medications. In elderly patients, renal function will gradually decline and this may be the reason why this parameter is more frequently monitored in this group.25 The most recent version of the Dutch Guideline for Bipolar Disorders (2015) states that TSH needs to be monitored at least once a year instead of every 6 months.4 It is possible that some health care professionals may have questioned the advice in the previous guidelines to monitor TSH every 6 months and for this reason have been less compliant with the guidelines before 2015. Some health care professionals may have implemented guidelines of 2015 directly and have monitored at least TSH once a year in the period starting from February, when the guideline was issued.
In general, males were monitored less often in compliance with the guidelines for TSH, but not for creatinine and lithium serum levels. Compliance was especially higher in older patients (>65 years) compared to patients aged <65 years. Patients using <5 medications were monitored less often in compliance with the guidelines for creatinine, but not for lithium serum levels and TSH. Patients defined as initiators were more often monitored in compliance with the guidelines for TSH compared to maintenance users.
Compliance with laboratory monitoring in patients with prescriptions from general practitioners was less compared to patients with prescriptions from medical specialists or from both a medical specialist and a general practitioner. An earlier small‐scale Dutch study reported that general practitioners monitored lithium serum levels significantly less often than psychiatrists. Furthermore, the study indicated that creatinine and TSH were monitored less often than the guidelines prescribed.26
If co‐medication interacting with lithium had been prescribed within periods, patients were monitored more often compliant with the guidelines for lithium serum levels. This may be caused by alerts from clinical decision support systems, which remind the prescriber to monitor lithium serum levels.27
Patients without other days with laboratory measurements were less often monitored in accordance with the guidelines for creatinine. The reasons for this difference can be related to the willingness of patients to have blood tests, comorbidities, or medications requiring additional monitoring.
Many guidelines advise to monitor lithium serum levels at least twice a year.3 Therefore, our findings could be compared to monitoring compliance in other countries. In agreement with other monitoring studies on adherence to guidelines, improvement of laboratory monitoring of patients using lithium in clinical practice is needed.6, 7, 8 The study by Collins et al, reported that 68% of patients had undergone two or more tests for lithium serum levels per year, 55% had undergone two or more tests for creatinine, and 82% had undergone at least one TSH test per year after at least 1 year of lithium use. The time periods in which laboratory measurements had been assessed were different (one‐year periods compared to six‐month periods for lithium, creatinine and TSH), but their results indicate a similar trend. Paton et al described in a later study that monitoring of lithium serum levels, creatinine and TSH had increased to 80%, 70%, and 92%, respectively.7 Monitoring rates increased after a quality improvement program consisting of a patient safety alert mandating health care professionals to put in place systems to inform patients on lithium safety, to monitor lithium in line with National Institute for Health and Care Excellence (NICE) guidance and to communicate monitoring tests to involved laboratories and clinicians.7 Eagles at al reported that monitoring of patients using lithium did improve after distribution of guidelines, but still exhibited several inadequacies.8 Ooba et al reported that the prevalence of monitoring increased after a regulatory warning requiring compliance with the measurement of blood levels during lithium treatment, but remained low.28 Our results further illustrate that there are inadequacies in monitoring, highlighting that patients are less often monitored than prescribed by the guidelines and may be in need of additional care.
The observation that improvement is needed in terms of compliance with monitoring guidelines is not only seen in patients treated with lithium. The prevalence of lipid and glucose monitoring in patients treated with second‐generation antipsychotic agents was found by earlier studies to be low as well.29, 30
The PHARMO Database Network covers a broad area in the Netherlands. Therefore, one can assume that our findings are generalizable to other patients using lithium in the Netherlands. With this rich database, the odds of being monitored for diverse patient characteristics could be assessed.
Because patients may go to other laboratories not included in the PHARMO Database Network, there is a possible risk of bias due to incomplete laboratory data. In addition, the possibility of hospitalization cannot be ruled out for some patients during the study period or within the 6‐month period before initiation of lithium. Some patients may have been admitted to an institution or hospital for a short duration and lithium treatment may have been initiated there. To lower bias due to missing data, only patients with at least one dispensing in the 6‐month period before lithium treatment were included. The data provided no information regarding clinical indications for lithium use or comorbidities.
Ideally, the type of health care professional requesting laboratory parameters should be evaluated, to have insight into which health care professional is most involved in patient monitoring. Only information on the prescriber of lithium was available; the health care professional requesting monitoring parameters was unknown. Responsibility for patient monitoring could be transferred to a health care professional other than the prescriber. Data on the type of health care professional requesting laboratory parameters could give insight into how ultimate medical responsibility is organized, as patients may be monitored by a different health care professional than the prescriber. For example, a medical specialist may prescribe lithium, while a general practitioner monitors the patient. Our data indicate that if both a medical specialist and general practitioner prescribed lithium, compliance with the guidelines for laboratory monitoring of patients using lithium was higher compared to a general practitioner solely. Based on a previous study by our research group, prescribers most often reported to effectuate monitoring themselves.31
The number of patients using interacting comedication could have been underestimated. Information on use of over‐the‐counter medication, such as certain nonsteroidal anti‐inflammatory drugs (NSAIDs) was not available in the database. NSAIDs can increase lithium serum levels, but can be dispensed in the Netherlands without a prescription.
Our results raise questions as to why patients are not monitored in compliance with the guidelines. To ensure the safe and effective treatment of patients using lithium, the reasons behind these results need to be assessed. First, it should be assessed whether monitoring parameters are requested by health care professionals, to determine whether health care professionals do not request lithium serum levels, creatinine and TSH, or whether patients are hesitant to comply with monitoring parameters that are requested. Previous studies have identified patient factors that influence monitoring rates, which included the willingness of patients to have blood tests.6, 32, 33 On the other hand, prescribers may think the guidelines are too burdensome and recommend testing too frequently. It is not clear if the recommended monitoring scheme in the guidelines is the optimal frequency, because most recommendations have not been examined in controlled studies. Some prescribers could have also used different guidelines that may include other recommendations for monitoring of patients using lithium.34 Qualitative studies may provide in‐depth insight into the personal rationale for choices.
Klann et al suggested an algorithm for automating drug monitoring by health care professionals. This information technology system automatically invites patients for laboratory testing when medication is dispensed. When a physician prescribes medication, laboratory tests for lithium serum levels, creatinine and TSH will be scheduled for the patient at the same time. This system is known as a “corollary order.”35
Methods to improve patient compliance with monitoring can include methods to reduce time consumption by patients, such as the implementation of point‐of‐care tests (POCTs) in pharmacies for lithium and creatinine.36, 37 Testing patients in pharmacies when medication is dispensed would save time for patients and could lead to improved monitoring compliance.
5. CONCLUSION
A considerable proportion of patients had not been monitored in accordance with the Dutch guidelines. In order to ensure patient safety and the effectiveness of lithium treatment, it is crucial to understand why patients are not being monitored according to the guidelines. Future research is needed to provide insight into the causes of missing values of patient monitoring. Based on those findings, methods to improve the monitoring of patients using lithium can be developed and evaluated in clinical practice.
DISCLOSURES
There are no conflicts of interest for any of the authors.
APPENDIX 1.
| Drug class | ATC codes |
|---|---|
| Psychotropic drugs | |
| Antidepressants | N06A |
| Antipsychotics | N05A excl. N05AN |
| Anxiolytics, hypnotics, and sedatives | N05B + N05C |
| Psychostimulants | N06B |
| Mood stabilisers | N03AF1, N03AG01, N03AX09 |
Nederlof M, Egberts TCG, van Londen L, et al. Compliance with the guidelines for laboratory monitoring of patients treated with lithium: A retrospective follow‐up study among ambulatory patients in the Netherlands. Bipolar Disord. 2019;21:419–427. 10.1111/bdi.12730
REFERENCES
- 1. Peselow ED, Clevenger S, IsHak WW. Prophylactic efficacy of lithium, valproic acid, and carbamazepine in the maintenance phase of bipolar disorder: a naturalistic study. Int Clin Psychopharmacol. 2016;31:218‐223. [DOI] [PubMed] [Google Scholar]
- 2. Nederlof M, Stoker LJ, Egberts T, Heerdink ER. Instructions for clinical and biomarker monitoring in the summary of product characteristics (SmPC) for psychotropic drugs: Overview and applicability in clinical practice. J Psychopharmacol Oxf Engl. 2015;29:1248‐1254. [DOI] [PubMed] [Google Scholar]
- 3. Malhi GS, Gessler D, Outhred T. The use of lithium for the treatment of bipolar disorder: Recommendations from clinical practice guidelines. J Affect Disord. 2017;27:266‐280. [DOI] [PubMed] [Google Scholar]
- 4. Kupka R, Goossens P, vanBendegem M, et al. Multidisciplinary guideline bipolar disorder [Internet]. Nederlandse Vereniging voor Psychiatrie (NVvP), 2015. http://www.nvvp.net/stream/richtlijn-bipolaire-stoornissen-2015
- 5. Kilbourne AM, Post EP, Bauer MS, et al. Therapeutic drug and cardiovascular disease risk monitoring in patients with bipolar disorder. J Affect Disord. 2007;102:145‐151. [DOI] [PubMed] [Google Scholar]
- 6. Collins N, Barnes TR, Shingleton‐Smith A, Gerrett D, Paton C. Standards of lithium monitoring in mental health Ttrusts in the UK. BMC Psychiatry. 2010;12:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paton C, Adroer R, Barnes T. Monitoring lithium therapy: the impact of a quality improvement programme in the UK. Bipolar Disord. 2013;15:865‐875. [DOI] [PubMed] [Google Scholar]
- 8. Eagles JM, McCann I, MacLeod TN, Paterson N. Lithium monitoring before and after the distribution of clinical practice guidelines. Acta Psychiatr Scand. 2000;101:349‐353. [DOI] [PubMed] [Google Scholar]
- 9. Keller WR, Fischer BA, McMahon R, Meyer W, Blake M, Buchanan RW. Community adherence to schizophrenia treatment and safety monitoring guidelines. J Nerv Ment Dis. 2014;202:6‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coughlin M, Goldie CL, Tregunno D, Tranmer J, Kanellos‐Sutton M, Khalid‐Khan S. Enhancing metabolic monitoring for children and adolescents using second‐generation antipsychotics. Int J Ment Health Nurs. 2018;27:1188‐1198. [DOI] [PubMed] [Google Scholar]
- 11. Bauer M, Adli M, Ricken R, Severus E, Pilhatsch M. Role of lithium augmentation in the management of major depressive disorder. CNS Drugs. 2014;28:331‐342. [DOI] [PubMed] [Google Scholar]
- 12. Sani G, Perugi G, Tondo L. Treatment of bipolar disorder in a lifetime perspective: is lithium still the best choice? Clin Drug Investig. 2017;37:713‐727. [DOI] [PubMed] [Google Scholar]
- 13. Malhi GS, Gershon S, Outhred T. Lithiumeter: Version 2.0. Bipolar Disord. 2016;18:631‐641. [DOI] [PubMed] [Google Scholar]
- 14. McKnight RF, Adida M, Budge K, Stockton S, Goodwin GM, Geddes JR. Lithium toxicity profile: a systematic review and meta‐analysis. Lancet Lond Engl. 2012;379:721‐728. [DOI] [PubMed] [Google Scholar]
- 15. Kirkham E, Skinner J, Anderson T, Bazire S, Twigg MJ, Desborough JA. One lithium level >1.0 mmol/L causes an acute decline in eGFR: findings from a retrospective analysis of a monitoring database. BMJ Open. 2014;4:e006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Szpunar J, Maeshima H, Grossberg GT. Renal concerns relative to the use of lithium in geriatric bipolar disorder patients. Ann Clin Psychiatry Off J Am Acad Clin Psychiatr. 2017;29:283‐290. [PubMed] [Google Scholar]
- 17. Tondo L, Abramowicz M, Alda M, et al. Long‐term lithium treatment in bipolar disorder: effects on glomerular filtration rate and other metabolic parameters. Int J Bipolar Disord. 2017;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aiff H, Attman P‐O, Aurell M, et al. Effects of 10 to 30 years of lithium treatment on kidney function. J Psychopharmacol Oxf Engl. 2015;29:608‐614. [DOI] [PubMed] [Google Scholar]
- 19. Alsady M, Baumgarten R, Deen P, de Groot T. Lithium in the kidney: friend and foe? J Am Soc Nephrol JASN. 2016;27:1587‐1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Azab AN, Shnaider A, Osher Y, Wang D, Bersudsky Y, Belmaker RH. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nederlof M, Heerdink ER, Egberts A, et al. Monitoring of patients treated with lithium for bipolar disorder: an international survey. Int J Bipolar Disord. 2018;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nederlands Huisartsen Genootschap . HIS‐Tabel Diagnostische Bepalingen. [Internet]. 2016.
- 23. van Roon EN, Flikweert S, le Comte M, et al. Clinical relevance of drug‐drug interactions : a structured assessment procedure. Drug Saf. 2005;28:1131‐1139. [DOI] [PubMed] [Google Scholar]
- 24. Nolen W, Kupka R, Schulte P, et al. Guideline Bipolar disorder. [Internet]. Nederlandse Vereniging voor Psychiatrie (NVvP). 2008. www.med-info.nl/Richtlijnen/Geestelijk%20-%20Gedragsstoornissen/Bipolaire%20stoornissen.pdf
- 25. Kurtal H, Schwenger V, Azzaro M, et al. Clinical value of automatic reporting of estimated glomerular filtration rate in geriatrics. Gerontology. 2009;55:288‐295. [DOI] [PubMed] [Google Scholar]
- 26. van de Beek LM, Ouwens MA, de Vries PL, Hummelen JW. Lithium levels need to be monitored: discrepancies between guidelines and practice. Tijdschr Voor Psychiatr. 2010;52:367‐373. [PubMed] [Google Scholar]
- 27. Wilting I, Movig KL, Moolenaar M, et al. Drug‐drug interactions as a determinant of elevated lithium serum levels in daily clinical practice. Bipolar Disord. 2005;7:274‐280. [DOI] [PubMed] [Google Scholar]
- 28. Ooba N, Tsutsumi D, Kobayashi N, et al. Prevalence of therapeutic drug monitoring for lithium and the impact of regulatory warnings: analysis using Japanese claims database. Ther Drug Monit. 2018;40:252‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pereira L, Budovich A, Claudio‐Saez M. Monitoring of metabolic adverse effects associated with atypical antipsychotics use in an outpatient psychiatric clinic. J Pharm Pract. 2018;1:897190017752712. [DOI] [PubMed] [Google Scholar]
- 30. Haupt DW, Rosenblatt LC, Kim E, Baker RA, Whitehead R, Newcomer JW. Prevalence and predictors of lipid and glucose monitoring in commercially insured patients treated with second‐generation antipsychotic agents. Am J Psychiatry. 2009;166:345‐353. [DOI] [PubMed] [Google Scholar]
- 31. Nederlof M, Heerdink ER, Egberts ACG, Wilting I, Stoker LJ, Hoekstra R. Monitoring of patients treated with lithium for bipolar disorder: an international survey. Int J Bipolar Disord. 2018;6(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Padalecki J, Xu KT, Smith C, Carrasco L, Hensley J, Richman PB. How much risk are emergency department patients willing to accept to avoid diagnostic testing. Turk J Emerg Med. 2017;17(1):16‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Glover KJ, Lawley D. How safe is lithium prescribing? audit of a local prescribing framework and patient survey. The Psychiatrist. 2005;29:98‐100. [Google Scholar]
- 34. Nederlof M, Kupka RW, Braam AM, Egberts A, Heerdink ER. Evaluation of clarity of presentation and applicability of monitoring instructions for patients using lithium in clinical practice guidelines for treatment of bipolar disorder. Bipolar Disord. 2018. 10.1111/bdi.12681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klann J, Schadow G, McCoy JM. A recommendation algorithm for automating corollary order generation. AMIA Annu Symp Proc AMIA Symp. 2009;14:333‐337. [PMC free article] [PubMed] [Google Scholar]
- 36. Vrouwe EX, Luttge R, Vermes I, van den Berg A. Microchip capillary electrophoresis for point‐of‐care analysis of lithium. Clin Chem. 2007;53:117‐123. [DOI] [PubMed] [Google Scholar]
- 37. Geerts A, DeKoning F, DeVooght K, Egberts A, DeSmet PA, vanSolinge WW Feasibility of point‐of‐care creatinine testing in community pharmacy to monitor drug therapy in ambulatory elderly patients. J Clin Pharm Ther. 2013;38:416–422. [DOI] [PubMed] [Google Scholar]


