Abstract
Aim
To quantify the magnitude and specific contributions of known cardiovascular risk factors leading to higher cardiovascular risk and all‐cause mortality caused by type 2 diabetes (T2D).
Methods
Mediation analysis was performed to assess the relative contributions of known classical risk factors for vascular disease in T2D (insulin resistance, systolic blood pressure, renal function, LDL‐cholesterol, triglycerides and micro‐albuminuria), and what proportion of the effect of T2D on cardiovascular events and all‐cause mortality these factors mediate in the Second Manifestations of ARTerial disease (SMART) cohort consisting of 1910 T2D patients.
Results
Only 35% (95% CI 15‐71%) of the excess cardiovascular risk caused by T2D is mediated by the classical cardiovascular risk factors. The largest mediated effect was through insulin resistance [proportion of mediated effect (PME) 18%, 95% CI 3‐37%], followed by elevated triglycerides (PME 8%, 95% CI 4‐14%) and micro‐albuminuria (PME 7%, 95% CI 3‐17%). Only 42% (95% CI 18‐73%) of the excess mortality risk was mediated by the classical risk factors considered. The largest mediated effect was by micro‐albuminuria (PME 18%, 95% CI 10‐29%) followed by insulin resistance (PME 15%, 95% CI 1‐33%).
Conclusion
A substantial amount of the increased cardiovascular risk and all‐cause mortality caused by T2D cannot be explained by traditional vascular risk factors. Future research should focus on identifying non‐classical pathways that might further explain the increased cardiovascular and mortality risk caused by T2D.
Keywords: cardiovascular risk, mediation analysis, risk factors, type 2 diabetes
1. INTRODUCTION
Despite significant advances in cardiovascular risk management, patients with type 2 diabetes are still at high risk of cardiovascular disease and mortality.1 This higher risk in patients with type 2 diabetes compared with patients without diabetes is in part explained by traditional risk factors for cardiovascular disease including glycaemic control, but the excess risk cannot be solely attributed to the higher prevalence of traditional vascular risk factors in patients with type 2 diabetes (e.g. hypercholesterolaemia, hypertension). Other non‐traditional risk factors, such as insulin resistance, micro‐albuminuria or inflammation, may be important in the pathophysiology of cardiovascular disease and vascular mortality.2 The presence of micro‐albuminuria has been associated with a 2‐fold increased risk of cardiovascular morbidity or mortality in patients with type two diabetes.3 Insulin resistance, as measured by Homeostatic Model Assessment of Insulin Resistance (HOMA‐IR), has been identified as an independent predictor of cardiovascular events in patients with type 2 diabetes. A one unit increase in HOMA‐IR was related to 31% higher risk of cardiovascular events.4 Inflammation, as assessed by elevated high‐sensitivity C‐reactive protein (hs‐CRP) levels (>3 mg/L), has been related to an increased risk of coronary heart disease of 72%.5 To improve cardiovascular risk management in patients with type 2 diabetes, it is of importance to elucidate and understand the causal pathways leading from type 2 diabetes to cardiovascular disease and mortality, as this may direct therapy and quantify the need for future research to find unknown risk factors and treatment targets. While there have been many studies addressing the relation between different risk factors and cardiovascular disease in this specific population, the relative contribution of these risk factors to cardiovascular risk is unknown. In epidemiologic research, understanding causal pathways from an exposure to an outcome can be assessed using mediation analysis techniques. Mediation analysis offers a tool to assess the magnitude of different pathways (mediators) leading to the outcome.6 The aim of this study is to quantify the magnitude and relative contributions of known cardiovascular risk factors in the pathway from type 2 diabetes to increased cardiovascular risk and all‐cause mortality.
2. METHODS
2.1. Study population and baseline measurements
The Second Manifestations of ARTerial disease (SMART) is an ongoing prospective cohort at the University Medical Center Utrecht, The Netherlands. Newly referred patients aged 18 to 79 years with vascular disease or with important risk factors for atherosclerosis (e.g. diabetes, hypertension or hyperlipidaemia) were asked to participate. After inclusion, information on medical history, history of vascular disease (coronary artery disease, cerebrovascular disease, peripheral arterial disease, abdominal aortic aneurysm), medication use and cardiovascular risk factors (e.g. smoking, alcohol consumption, physical activity, hypertension, hyperlipidaemia) was obtained with the use of questionnaires. Additionally, patients underwent physical examination and traditional cardiovascular risk factors were measured (blood pressure, blood sample for plasma lipids, urine sample for albuminuria and creatinine excretion). LDL‐c was calculated using the Friedewald formula up to plasma triglyceride levels of 9 mmol/L to avoid missing LDL‐c.7 A rationale and detailed description of the SMART study has been previously published.8 The study was approved by the Medical Ethics Committee of the University Medical Center Utrecht and informed consent was obtained from all participants.
2.2. Type 2 diabetes diagnosis
Type 2 diabetes was defined as self‐reported type 2 diabetes, a referral diagnosis of type 2 diabetes, a glucose plasma concentration of ≥7.0 mmol/L at baseline with commencement of glucose‐lowering therapy within 1 year after inclusion or the use of glucose‐lowering medication at inclusion.
2.3. Follow‐up
During follow‐up, information on hospitalization and outpatient clinic visits was obtained through biannual questionnaires. All available data on reported events were collected. Death was reported by relatives, the general practitioner or treating specialist. All events were independently evaluated by three members of the SMART study endpoint committee. Primary outcomes for this study were a composite of major events [myocardial infarction (MI), stroke and vascular mortality] and all‐cause mortality. MI was defined as at least two of the following criteria: (a) chest pain for at least 20 minutes, not disappearing after administration of nitrates; (b) elevation of the ST‐segment >1 mm in two following leads on an electrocardiogram or a left bundle branch block; and (c) cardiac enzyme elevation (troponin above clinical cut‐off value or creatinine kinase of at least two times the normal value and a myocardial band fraction >5% of the total creatinine kinase). Sudden cardiac death was also considered as MI. Vascular mortality was defined as death caused by MI, stroke, congestive heart failure, rupture of abdominal aortic aneurysm and vascular death from other causes. The period between patient inclusion and first cardiovascular event, death, loss to follow‐up, or the predefined date of 1 March 2015, was defined as the follow‐up duration. In total, 655 patients (6.0%) in the SMART cohort were lost to follow‐up because of relocation or discontinuation of the study.
2.4. Data analyses
Mediation analysis using marginal structural models was performed, which is based on the counterfactual framework.9 In brief, this type of analysis provides a tool to deconstruct the total effect of a given exposure on an outcome into a natural direct effect and an indirect effect. The total effect estimates the risk of cardiovascular events and all‐cause mortality in type 2 diabetes patients compared with that in patients without type 2 diabetes. The natural direct effect compares the risk of cardiovascular events and all‐cause mortality in type 2 diabetes patients with that of patients without type 2 diabetes, if the risk factor levels for type 2 diabetes patients were set to the levels that would have been observed if they had been patients without type 2 diabetes. This is achieved by constructing counterfactual values for the risk factor variables. To illustrate, imagine three versions of the same patient (i.e. except for the difference described hereafter, these three versions of the patient are exactly the same). In the first version, the patient has type 2 diabetes and the risk factor levels (mediators) have the natural levels of a patient with type 2 diabetes. In the second version, the patient has type 2 diabetes, but the risk factor levels are set to the value of a patient without type 2 diabetes. In the third version, the patient does not have type 2 diabetes and the risk factor levels have the natural levels of a patient without type 2 diabetes. The total effect will be the difference in outcome between the patient in versions 1 and 3. The natural direct effect will be the difference in outcome between the patient in versions 2 and 3. The indirect effect will be the difference in outcome between the patient in versions 1 and 2. The interpretation of these effects is as follows: the indirect effect is the (relative) difference in cardiovascular events that can be attributed to mediation through the risk factor, whereas the natural direct effect is the (relative) difference in cardiovascular events that can be attributed to a direct path from type 2 diabetes to cardiovascular events. This path will include mediation through other risk factors that are not included in the analysis. Finally, the total effect is the (relative) difference in cardiovascular events between those who have type 2 diabetes compared with those without type 2 diabetes, which equals the sum of the natural direct effect and indirect effect. A summary of this approach can be found in Figure S1. The risk factors used as mediators in this model are insulin resistance assessed by triglyceride‐glucose index (TyG index), systolic blood pressure, kidney function estimated by the Modification of Diet in Renal Disease (MDRD) formula, LDL‐c, triglycerides and presence of micro‐albuminuria. To ensure stability of the models, the mediators were dichotomized using target levels described in the European Society of Cardiology (ESC) guidelines: systolic blood pressure <140 mmHg, estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2, LDL‐c < 2.6 mmol/L, triglycerides <1.7 mmol/L.10 The mediator insulin resistance as measured by the TyG index was dichotomized using the lower bound of the fourth quartile as a cut‐off point (TyG index <9.3).
Figure 1 shows the underlying model of this analysis. It assumes a causal relationship between the selected well‐established risk factors and cardiovascular events and all‐cause mortality. Age and sex were included in the models to adjust for confounding of the type 2 diabetes mediator, type 2 diabetes endpoint of interest, and mediator endpoint of interest associations. Estimates of the direct and indirect effects were obtained from weighted Cox proportional hazards models for the outcome cardiovascular events and all‐cause mortality. The proportional hazards assumption was visually checked by use of a hazard function plot and showed no signs of violation. The linearity assumption was visually checked by plotting martingale residuals. The plots showed no violation of the linearity assumption.
Figure 1.
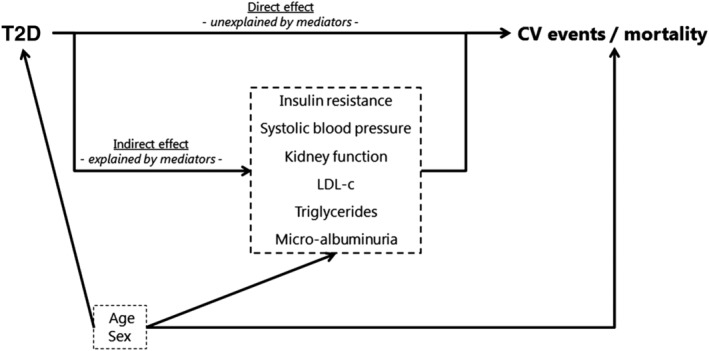
Path diagram of the relation between type 2 diabetes (exposure), cardiovascular risk factors (mediators) and cardiovascular events/all‐cause mortality (outcome) in the presence of measured confounders: age and sex. CV, cardiovascular; T2D, type 2 diabetes
For determining the weights needed in the Cox model, inverse probability weighting was used by regressing the binary mediators TyG index, systolic blood pressure, eGFR, LDL‐c, triglycerides and micro‐albuminuria on type 2 diabetes, age and sex with separate binomial logistic regression models. The effect of all mediators was assessed simultaneously by multiplying the weights derived from the regression models. The total, direct and indirect effect are estimated as hazard ratios with 95% confidence intervals and proportion mediated effect (PME) with 95% confidence intervals. The PME is a measure relative to the total effect which is defined as 100%. The contribution of direct and indirect effects to the total type 2 diabetes effect was estimated on the ln(hazard ratio) scale as the effects are additive on this scale. To obtain 95% confidence intervals for the total effect and (combined) indirect effect, bootstrapping was used with 1000 bootstrap samples. The bounds of the 95% confidence interval were based on the 2.5 and 97.5 percentiles of the different distributions of effects. Single imputation methods were used to reduce missing covariate data for glucose (n = 74; 0.7%), triglycerides (n = 64; 0.6%), systolic blood pressure (n = 19; 0.2%), LDL‐c (n = 162; 1.5%), micro‐albuminuria (n = 512; 4.7%) and eGFR (n = 60; 0.5%). Given the very low percentages of missing data, multiple imputation was deemed unnecessary. To assess whether the relative contributions of the mediators were different for patients with and without previous cardiovascular disease, subgroup analyses were performed in strata of presence of cardiovascular disease. All analyses were performed using statistical package R 3.2.2. For all analyses, P < 0.05 was considered significant.
3. RESULTS
3.1. Baseline characteristics
In total, 10 915 patients were included, of which 1910 (17%) were identified as type 2 diabetes patients. The patients with type 2 diabetes were on average aged 60 ± 10 years and were predominantly male (70%) (Table 1). Patients with type 2 diabetes had an average body mass index (BMI) of 29.0 ± 5.0 kg/m2. Current smoking and alcohol use were 50% and 14%, respectively (Table 1).
Table 1.
Baseline characteristics of patients without type 2 diabetes (T2D) versus patients with type 2 diabetes
| N | No T2D | T2D |
|---|---|---|
| n = 9005 | n = 1910 | |
| Men, n (%) | 5912 (66) | 1329 (70) |
| Age, years; mean (SD) | 56 (12) | 60 (10) |
| BMI, kg/m2; mean (SD) | 26.5 (4.1) | 29.0 (5.0) |
| Smoking current, n (%) | 3885 (43) | 946 (50) |
| Pack‐years, median (IQR) | 11 (0‐28) | 13 (0‐31) |
| Alcohol use, n (%) | 809 (9) | 272 (14) |
| Systolic blood pressure, mmHg; mean (SD) | 141 (22) | 145 (21) |
| Diastolic blood pressure, mmHg; mean (SD) | 83 (13) | 83 (12) |
| Duration of diabetes, years; median (IQR) | ‐ | 4 (1‐10) |
| Medication | ||
| Glucose‐lowering, n (%) | ‐ | 1262 (66) |
| Insulin, n (%) | ‐ | 455 (24) |
| Lipid‐lowering, n (%) | 4906 (55) | 1218 (64) |
| Blood pressure‐lowering, n (%) | 5927 (66) | 1473 (77) |
| Type of vascular disease | ||
| Coronary artery disease, n (%) | 3684 (41) | 842 (44) |
| Cerebrovascular disease, n (%) | 1857 (21) | 364 (19) |
| Peripheral artery disease, n (%) | 1114 (12) | 269 (14) |
| Abdominal aortic aneurysm, n (%) | 552 (6) | 92 (5) |
| Laboratory measurements | ||
| Glucose, mmol/L; mean (SD) | 5.7 (0.7) | 8.7 (2.9) |
| HbA1c, %; mean (SD) | 5.6 (0.4) | 7.1 (1.3) |
| Triglyceride glucose index; mean (SD) | 8.8 (0.6) | 9.4 (0.7) |
| eGFR, ml/min/1.73 m2; mean (SD) | 78.5 (18.1) | 78.5 (22.1) |
| Micro‐albuminuria, n (%) | 463 (9) | 417 (23) |
| Total cholesterol, mmol/L; mean (SD) | 5.2 (1.4) | 4.8 (1.4) |
| LDL‐c, mmol/L; mean (SD) | 3.2 (1.2) | 2.7 (1.1) |
| HDL‐c, mmol/L; mean (SD) | 1.3 (0.4) | 1.1 (0.3) |
| Non‐HDL‐c, mmol/L; mean (SD) | 3.9 (1.4) | 3.7 (1.4) |
| Triglycerides, mmol/L; median (IQR) | 1.4 (1.0‐2.0) | 1.7 (1.2‐2.5) |
Note: Continuous variables are depicted as mean ± SD, count variables as n (%) and non‐normally distributed variables as median (interquartile range).
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate.
3.2. Mediation analysis of type 2 diabetes and cardiovascular events
During follow‐up, the event rate for patients without diabetes was 17.1 per 1000 person‐years, while patients with type 2 diabetes had an event rate of 27.5 per 1000 person‐years. The total effect of type 2 diabetes on cardiovascular events was an increased risk with an adjusted hazard ratio of 1.46 (95% CI 1.29‐1.70). The direct effect and indirect effect contributions were 65% (95% CI 29‐85%) and 35% (95% CI 15‐71%), respectively (Table 2). The largest mediated effect contributing to the indirect effect was insulin resistance (PME 18%, 95% CI 3%‐37%). The second largest mediated effect was through elevated triglycerides (PME 8%, 95% CI 4‐14%), followed closely by presence of micro‐albuminuria (PME 7%, 95% CI 3‐17%) and reduced kidney function (PME 6%, 95% CI 2‐12%) (Table 2). The excess risk was not mediated through elevated systolic blood pressure or high LDL‐c (PME 1%, 95% CI ‐1‐4% and PME ‐5%, 95% CI ‐10‐0%) (Table 2). Subgroup analyses showed that LDL‐c > 2.5 mmol/L appears to be a mediator for patients without previous cardiovascular disease (PME 17%, 95% CI ‐3 to −57%) and not for patients with previous cardiovascular disease (PME ‐8%, 95% CI ‐14 to −3%).
Table 2.
Results of mediation analysis: total, direct and indirect effects of type 2 diabetes on cardiovascular events; adjusted for age, sex and mediators
| Effects T2D vs. no T2D | Three mediators | Four mediators | Five mediators | Six mediators | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | PME (95% CI) | HR (95% CI) | PME (95% CI) | HR (95% CI) | PME (95% CI) | HR (95% CI) | PME (95% CI) | |
| Total effect | 1.41 (1.25‐1.58) | 100 | 1.44 (1.29‐1.61) | 100% | 1.44 (1.29‐1.62) | 100% | 1.46 (1.29‐1.70) | 100% |
| Direct effecta | 1.26 (1.11‐1.43) | 66.7% (45.4‐82.6) | 1.31 (1.16‐1.48) | 73.2% (54.5‐87.8) | 1.25 (1.09‐1.42) | 59.7% (32.8‐78.4) | 1.28 (1.08‐1.52) | 64.5% (29.3‐85.0) |
| Indirect effect, combinedb | 1.12 (1.06‐1.17) | 33.3% (17.4‐54.6) | 1.10 (1.05‐1.16) | 26.8% (12.2‐45.5) | 1.16 (1.09‐1.23) | 40.3% (21.6‐67.2) | 1.14 (1.06‐1.23) | 35.5% (15.0‐70.7) |
| Indirect effect through:b | ||||||||
| Insulin resistance | 1.08 (1.04‐1.13) | 23.9% (9.6‐41.5) | 1.08 (1.04‐1.14) | 23.5% (11.039.0) | 1.10 (1.05‐1.15) | 26,1% (13.5‐43.0) | 1.07 (1.01‐1.12) | 17.9% (3.0‐37.2) |
| Systolic blood pressure | 1.01 (1.00‐1.02) | 1.9% (−0.7‐5.2) | 1.01 (1.00‐1.02) | 2.0% (−0.4‐4.8) | 1.01 (1.00‐1.02) | 2.0% (−0.3‐5.2) | 1.00 (0.99‐1.01) | 0.8% (−1.4‐4.2) |
| Kidney function | 1.03 (1.01‐1.04) | 7.5% (3.2‐13.5) | 1.02 (1.01‐1.04) | 6.8% (3.0‐12.1) | 1.03 (1.01‐1.04) | 6.9% (2.8‐12.9) | 1.02 (1.01‐1.05) | 6.0% (2.4‐12.2) |
| LDL‐c | ‐ | ‐ | 0.98 (0.96‐0.99) | −5.6% (−11.0‐1.7) | 0.98 (0.97‐1.00) | −4.4% (−9.8 to −0.4) | 0.98 (0.97‐1.00) | −5.3% (−10.1‐0.3) |
| Triglycerides | ‐ | ‐ | ‐ | ‐ | 1.04 (1.02‐1.05) | 9.8% (5.1‐16.3) | 1.03 (1.01‐1.05) | 8.0% (3.6‐14.0) |
| Micro‐albuminuria | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 1.03 (1.01‐1.05) | 7.3% (2.6‐16.7) |
Abbreviations: CI, confidence interval; HR, hazard ratio; PME, proportion mediated effect; T2D, type 2 diabetes.
Effect of type 2 diabetes not explained by mediators.
Effect of type 2 diabetes through mediator(s).
3.3. Mediation analysis of type 2 diabetes and all‐cause mortality
During follow‐up, the event rate for patients without diabetes was 18.5 per 1000 person‐years, while patients with type 2 diabetes had an event rate of 29.6 per 1000 person‐years. The total effect of type 2 diabetes on all‐cause mortality was an increased risk with an adjusted hazard ratio of 1.37 (95% CI 1.20‐1.53). The direct effect and indirect effect contributions were 58% (95% CI 27‐82%) and 42% (95% CI 18‐73%), respectively (Table 3). The largest mediated effect contributing to the indirect effect was by presence of micro‐albuminuria (PME 18%, 95% CI 10‐29%), followed closely by insulin resistance (PME 15%, 95% CI 1‐33%), and then by elevated triglycerides (PME 7%, 95% CI 2‐14%) and decreased kidney function (PME 7%, 95% CI 3‐13%) (Table 3). The excess risk was not mediated through the other mediators; elevated systolic blood pressure (PME 3%, 95% CI 0‐7%) or high LDL‐c (PME ‐7%, 95% CI ‐15 to −2%). Subgroup analyses showed that LDL‐c > 2.5 mmol/L appears to be a mediator for patients without previous cardiovascular disease (PME 20%, 95% CI 3‐54%) and not for patients with previous cardiovascular disease (PME ‐13%, 95% CI ‐25 to −5%) (Table 4).
Table 3.
Results of mediation analysis: total, direct and indirect effects of type 2 diabetes on all‐cause mortality; adjusted for age, sex and mediators
| Effects T2D vs. no T2D | Three mediators | Four mediators | Five mediators | Six mediators | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | PME (95% CI) | HR (95% CI) | PME (95% CI) | HR (95% CI) | PME (95% CI) | HR (95% CI) | PME (95% CI) | |
| Total effect | 1.35 (1.21‐1.51) | 100% | 1.37 (1.50‐2.07) | 100% | 1.37 (1.22‐1.54) | 100% | 1.37 (1.52‐2.13) | 100% |
| Direct effecta | 1.24 (1.11‐1.41) | 71.0% (48.7‐86.5) | 1.28 (1.22‐1.52) | 78.6% (59.7‐93.2) | 1.25 (1.11‐1.41) | 70.8% (46.8‐89.5) | 1.21 (1.56‐1.62) | 57.8% (27.4‐81.7) |
| Indirect effect, combinedb | 1.09 (1.04‐1.14) | 29.0% (13.5‐51.3) | 1.07 (1.02‐1.12) | 21.4% (6.8‐40.3) | 1.09 (1.03‐1.16) | 29.2% (10.5‐53.2) | 1.13 (1.00‐1.30) | 42.2% (18.3‐72.6) |
| Indirect effect through:b | ||||||||
| Insulin resistance | 1.05 (1.02‐1.10) | 17,8% (5.2‐35.0) | 1.06 (1.02‐1.10) | 18,5% (5.0‐34.4) | 1.06 (1.02‐1.10) | 18,3% (5.9‐35.1) | 1.05 (1.02‐1.06) | 15,4% (0.8‐32.7) |
| Systolic blood pressure | 1.01 (1.00‐1.02) | 3.7% (0.8‐7.9) | 1.01 (1.00‐1.02) | 3.3% (0.6‐7.0) | 1.01 (1.00‐1.02) | 3.4% (0.4‐7.1) | 1.01 (0.98‐1.02) | 2.7% (−0.1‐6.9%) |
| Kidney function | 1.02 (1.01‐1.04) | 7.5% (3.1‐14.4) | 1.02 (1.01‐1.04) | 7.3% (3.1‐13.5) | 1.02 (1.01‐1.04) | 7.3% (2.9‐13.1) | 1.02 (1.02‐1.05) | 6.7% (2.5‐13.0) |
| LDL‐c | ‐ | ‐ | 0.98 (0.96‐0.99) | −7.6% (−14.5 to −3.0) | 0.98 (0.97‐0.99) | −7.1% (−13.6 to −2.3) | 0.98 (0.98‐1.01) | −7.3% (−15.0 to −2.3) |
| Triglycerides | ‐ | ‐ | ‐ | ‐ | 1.02 (1.01‐1.04) | 7.3% (2.4‐14.0%) | 1.02 (0.99‐1.03) | 7.0% (1.9‐13.9) |
| Micro‐albuminuria | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 1.05 (1.03‐1.07) | 17.6% (9.9‐29.0) |
Abbreviations: CI, confidence interval; HR, hazard ratio; PME, proportion mediated effect; T2D, type 2 diabetes.
Effect of type 2 diabetes not explained by mediators.
Effect of type 2 diabetes through mediator(s).
Table 4.
Subgroup analyses; mediation analysis: total, direct and indirect effects of type 2 diabetes on cardiovascular events and all‐cause mortality; adjusted for age, sex and mediators
| Effects T2D vs. no T2D | Cardiovascular events | All‐cause mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| Subgroup | Presence of cardiovascular disease (n = 7469) | No cardiovascular disease (n = 2745) | Presence of cardiovascular disease (n = 7469) | No cardiovascular disease (n = 2745) | ||||
| HR (95% CI) | PME (95% CI) | HR (95% CI) | PME (95% CI) | HR (95% CI) | PME (95% CI) | HR (95% CI) | PME (95% CI) | |
| Total effect | 1.49 (1.29‐1.71) | 100% | 1.71 (1.21‐2.41) | 100% | 1.35 (1.16‐1.53) | 100% | 1.85 (1.33‐2.57) | 100% |
| Direct effecta | 1.32 (1.14‐1.55) | 69.2% (43.1‐49.5) | 1.49 (0.98‐2.24) | 69.2% (−11.0‐100) | 1.20 (1.03‐1.37) | 59.1% (19.8‐84.5) | 1.45 (0.97‐2.18) | 59.7% (0‐99.3) |
| Indirect effect, combinedb | 1.13 (1.04‐1.22) | 30.8% (10.5‐56.9%) | 1.15 (0.93‐1.40) | 30.8% (−16.1‐100) | 1.12 (1.05‐1.20) | 40.9% (15.5‐80.2) | 1.28 (1.01‐1.58) | 40.3% (0.7‐100) |
| Indirect effect through:b | ||||||||
| Insulin resistance | 1.06 (1.01‐1.12) | 16,0% (3.1‐31.3) | 1.06 (0.93‐1.19) | 12.1% (−14.5‐50.3) | 1.05 (1.00‐1.09) | 16.7% (1.7‐38.6) | 1.04 (0.92‐1.17) | 6.3% (−14.5‐29.3) |
| Systolic blood pressure | 1.00 (0.99‐1.02) | 1.1% (−2.4‐4.7) | 0.97 (0.93‐1.00) | −6.3% (−19.9‐0.5) | 1.01 (1.00‐1.03) | 4.7% (0.2‐10.9) | 0.99 (0.96‐1.01) | −2.2% (−8.7‐1.9) |
| Kidney function | 1.02 (1.01‐1.04) | 6.3% (2.0‐11.6) | 1.00 (0.97‐1.03) | 0.2% (−8.0‐8.1) | 1.02 (1.01‐1.04) | 7.3% (2.95‐15.7) | 1.00 (0.97‐1.03) | 0.2% (−5.0‐5.9) |
| LDL‐c | 0.97 (0.95‐0.99) | −7.8% (−13.8 to −3.0) | 1.07 (0.98‐1.18) | 16.6% (−2.8‐55.7) | 0.97 (0.95‐0.98) | −12.7% (−25.0 to −5.4) | 1.12 (1.02‐1.23) | 20.2% (3.3‐53.9) |
| Triglycerides | 1.03 (1.01‐1.04) | 6.4% (2.0‐11.7) | 1.02 (0.98‐1.05) | 3.5% (−4.7‐15.9) | 1.02 (1.00‐1.03) | 5.9% (0.2‐14.2) | 1.02 (0.98‐1.06) | 3.5% (−3.4‐12.5) |
| Micro‐albuminuria | 1.03 (1.01‐1.06) | 8.8% (2.9‐16.6) | 1.02 (0.98‐1.07) | 4.7% (−4.0‐19.9) | 1.05 (1.03‐1.08) | 19.1% (9.6‐36.8) | 1.08 (1.02‐1.15) | 12.3% (3.7‐27.1) |
Abbreviations: CI, confidence interval; HR, hazard ratio; PME, proportion mediated effect; T2D, type 2 diabetes.
Effect of type 2 diabetes not explained by mediators.
Effect of type 2 diabetes through mediator(s).
4. DISCUSSION
The aim of the current study was to assess and quantify the contribution of known risk factor pathways in the relation between type 2 diabetes and cardiovascular disease and all‐cause mortality. The main findings are that 65% of the cardiovascular risk and 58% of the mortality risk could not be explained by traditional risk factors. Only 35% of the excess cardiovascular risk and 42% of the mortality can be attributed to the classical pathways leading to cardiovascular disease and mortality. The pathway contributing the most to cardiovascular disease was the presence of insulin resistance (18%), followed by elevated triglycerides (8%), presence of micro‐albuminura (7%) and reduced kidney function (6%), whereas the pathways contributing the most to mortality were presence of micro‐albuminuria (18%), insulin resistance (15%), elevated triglycerides (7%) and reduced kidney function (6%).
Even although there have been significant advances in cardiovascular risk management and treatment, there is still an excess risk of cardiovascular disease and mortality in patients with type 2 diabetes.1, 11 The results of the current study show that a substantial amount of the type 2 diabetes‐related cardiovascular and mortality risk is unexplained by classical pathways. Many factors may contribute to the increased cardiovascular and mortality risk that is observed in patients with type 2 diabetes. The present study assessed mediators in the relation between type 2 diabetes and cardiovascular events or all‐cause mortality, therefore known cardiovascular risk factors such as lifestyle (e.g. smoking, alcohol use, physical exercise, diet), which are not a causal consequence of type 2 diabetes, are not evaluated in this analysis and will contribute to the unexplained type 2 diabetes‐related cardiovascular and mortality risk.
Non‐traditional risk factors are potentially of importance and may contribute to unexplained type 2 diabetes‐related cardiovascular and mortality risk, as only a quarter of the excess risk was explained by the traditional risk factors. These non‐traditional risk factors were not evaluated in the mediation analysis because this analytical approach assumes non‐intertwining pathways and most of the non‐traditional risk factors have a common soil.
One of the non‐traditional risk factors is adipose tissue dysfunction contributing to low‐grade inflammation. Adipose tissue is an active endocrine organ secreting hormones and cytokines. When excess adipose tissue becomes dysfunctional, it produces tumor necrosis factor (TNF‐α) and interleukin‐6 (IL‐6), resulting in a state of systemic low‐grade inflammation and insulin resistance.12 Inflammation has been related to cardiovascular disease in an apparently healthy population, although the study population included a small proportion of patients with type 2 diabetes.13, 14 Part of the unexplained type 2 diabetes cardiovascular risk may be caused by adipose tissue dysfunction and low‐grade inflammation. Impaired fibrinolysis and thrombosis may also be the result of adipose tissue dysfunction.12 Insulin resistance has been linked to increased expression of plasminogen activator inhibitor‐1 (PAI‐1), leading to decreased fibrinolytic activity.15, 16, 17 In addition, markers of thrombosis (fibrinogen, von Willebrand factor, factor VIII) were found to be risk factors for coronary heart disease in patients with type 2 diabetes.18 Impaired fibrinolysis and thrombosis may therefore also contribute to the unexplained type 2 diabetes‐related cardiovascular risk.
Patients with type 2 diabetes have high levels of advanced glycation end products (AGEs). These are a group of molecules produced by non‐enzymatic glycation and oxidation of lipids, proteins and nucleic acids.19 Glycation can alter the structure of low‐density lipoprotein and thereby prevent its clearance from the circulation resulting in subsequent higher uptake in monocytes causing formation of foam cells. Serum AGE levels are higher in patients with type 2 diabetes compared with patients without type 2 diabetes and, in addition, serum AGE levels are in turn higher in patients with type 2 diabetes and coronary heart disease compared with patients with type 2 diabetes without coronary heart disease.20 Also, increased serum AGE levels have been related to more severe coronary atherosclerosis.21 A different route is through crosslinking of extracellular matrix proteins in the arterial wall (e.g. elastin, collagen) leading to arterial stiffness.19 Indeed, increased serum AGE levels are related to increased arterial stiffness, which is thought to explain part of the increased cardiovascular risk in patients with type 2 diabetes.22, 23 The formation of AGEs and presence of arterial stiffness may also have contributed to the unexplained type 2 diabetes‐related cardiovascular risk.
Glucose‐lowering treatment might not be only beneficial but may also induce cardiovascular harm. As such, the size of the remaining unexplained risk related to type 2 diabetes may be in part caused by detrimental or beneficial (side) effects of glucose‐lowering pharmacotherapy. A recent meta‐analysis consisting of 14 trials showed that glucose‐lowering drugs (especially peroxisome proliferator‐activated receptor agonists and dipeptidyl peptidase‐4 inhibitors) increased the risk of heart failure in patients with type 2 diabetes.24 Another meta‐analysis showed that severe hypoglycaemia, which is a common adverse effect of glucose‐lowering treatment, is also related to a higher risk of cardiovascular events in patients with type 2 diabetes.25
Besides non‐traditional risk factors, a different explanation might be that the increased risk of cardiovascular disease and all‐cause mortality has presumably already accumulated during the metabolic syndrome phase, preceding type 2 diabetes.26, 27, 28 This would imply that cardiovascular risk management should be focused on preventing metabolic syndrome and the progression of patients with metabolic syndrome into type 2 diabetes. In cases of patients with already established type 2 diabetes, a multifactorial approach should be considered where, in addition to the traditional modifiable risk factors, non‐traditional risk factors are also targeted. An example of such an approach is implemented in the Steno‐2 trial, which resulted in a significant decrease in cardiovascular events.29
Even though there is a substantial residual cardiovascular and mortality risk because of type 2 diabetes, it is important to emphasize that about a quarter of the risk is explained by traditional modifiable risk factors. Inherent to the mediation analysis technique, the present study would imply that if the optimal levels of the classical risk factors were achieved, an additional 25% relative risk reduction could be achieved.
The current study also showed that in patients with type 2 diabetes the remaining cardiovascular and mortality risk was not mediated through LDL‐c < 2.5 mmol/L or systolic blood pressure > 140 mmHg. It is important to underline that the current study does not imply that LDL‐c and hypertension are not important risk factors in cardiovascular disease and mortality, but they do not explain the increased risk in the relation between type 2 diabetes and cardiovascular events. This is most probably because of the heterogeneity of the population, as the subgroup analyses showed that LDL‐c does explain part of the cardiovascular and mortality risk.
The strength of this prospective study is the large real‐world study population with a considerable number of type 2 diabetes patients with a standardized data collection. Another strength is that this analytical approach allows classification of the relation between type 2 diabetes and cardiovascular events or all‐cause mortality, and is able to quantify the specific contributions of the mediators. Also, the combined indirect effect for cardiovascular events was higher than that for all‐cause mortality, which is expected, and therefore adds to the validity of the results. A study limitation may be the assumption that all confounders in the relation between type 2 diabetes and the outcomes, and all the confounders in the relation between mediator and the outcomes, were adjusted for in this analysis. Furthermore, only baseline data of the mediators were available; they may have changed and fluctuated over the course of follow‐up. This may have amplified or diminished the mediator effect. Also, as only baseline data were available for the diagnosis of type 2 diabetes, it could not be assessed as a time‐varying variable, which may dilute the reference group and attenuate the results. Future research incorporating time‐varying analysis is therefore needed. In addition, with mediation analysis it is assumed that pathways are non‐intertwining and that the contributions of the specific mediators are additive. However, cardiovascular risk factors tend to cluster, which can lead to some uncertainty around the precise estimates in the mediation analysis. Also, the subgroup of patients without previous cardiovascular disease consisted only of 2745 patients, which leads to wide confidence intervals, making interpretation more difficult. In addition, the subgroup analyses may have been confounded by indication as the group of patients with cardiovascular disease will be treated to lower LDL‐c levels. This may explain the reversed mediation by LDL‐c in the subgroup analyses. To stress further upon subgroups, it is known that cardiovascular risk factors may vary by ethnicity. As patients were included in the central part of The Netherlands and it was necessary to understand Dutch, almost all of the patients were of Caucasian descent. The potential problem with generalizability to other ethnic groups is presumably limited as differences in risk factors by ethnicity mostly have an impact on the prevalence of the risk factor in different populations, and not on the role of the risk factor in the pathogenesis of atherosclerosis. Finally, there may be risk of immortal time bias, as the definition of type 2 diabetes needs survival of 1 year in order to establish whether the patients with glucose plasma concentration of ≥7.0 mmol/L at baseline commenced with glucose‐lowering medication. However, this would result in dilution of the reference group and would have attenuated the current results.
In conclusion, a substantial amount of the increased cardiovascular risk and all‐cause mortality because of type 2 diabetes cannot be explained by traditional vascular risk factors. Future research should focus on identifying non‐classical pathways that might further explain the increased cardiovascular and mortality risk caused by type 2 diabetes.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
S.S. contributed to the conception and design of the work, analyzed the data, contributed to the interpretation of the data and wrote the manuscript; R.H.H.G. contributed to the interpretation of the data and critically revised the manuscript; G.F.N.B. contributed to the analyses of the data and critically revised the manuscript; Y.v.d.G. and M.J.C. contributed to the acquisition of the data and critically revised the manuscript; F.L.J.V. contributed to the acquisition and interpretation of the data and critically revised the manuscript; J.W. contributed to the conception and design of the work, acquisition and interpretation of the data, and critically revised the manuscript. All authors approved the final version of the manuscript for publication and they are in agreement to be accountable for all aspects of the work.
J.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supporting information
Figure S1. Mediation analysis based on counterfactual variables. Three versions of a patient are created. In the first version the patient has type 2 diabetes and the risk factor levels (mediators) have the natural levels of a patient with type 2 diabetes (Real T2DM). In the second version, the patient has type 2 diabetes, but the risk factor levels are set to the value of a patient without type 2 diabetes (Counterfactual T2DM). In the third version, the patient does not have type 2 diabetes and the risk factor levels have the natural levels of a patient without type 2 diabetes (Real no T2DM).
ACKNOWLEDGMENTS
We gratefully acknowledge the contribution of the SMART research nurses; R. van Petersen (data‐manager); B.G.F. Dinther (vascular manager) and the participants of the SMART Study Group: A. Algra MD, PhD; Y. van der Graaf, MD, PhD; D.E. Grobbee, MD, PhD; G.E.H.M. Rutten, MD, PhD, Julius Center for Health Sciences and Primary care; F.L.J. Visseren, MD, PhD, Department of Internal Medicine; G.J. de Borst, MD, PhD, Department of Vascular Surgery; L.J. Kappelle, MD, PhD, Department of Neurology; T. Leiner, MD, PhD, Department of Radiology; H.M. Nathoe, MD, PhD, Department of Cardiology.
Sharif S, Groenwold RHH, van der Graaf Y, et al. Mediation analysis of the relationship between type 2 diabetes and cardiovascular events and all‐cause mortality: Findings from the SMART cohort. Diabetes Obes Metab. 2019;21:1935–1943. 10.1111/dom.13759
Contributor Information
Jan Westerink, Email: j.westerink-3@umcutrecht.nl.
On behalf of the SMART study group:
R. van Petersen, B.G.F. Dinther, A. Algra, Y. van der Graaf, D.E. Grobbee, G.E.H.M. Rutten, F.L.J. Visseren, G.J. de Borst, L.J. Kappelle, T. Leiner, and H.M. Nathoe
REFERENCES
- 1. Tancredi M, Rosengren A, Svensson AM, et al. Excess Mortality among Persons with Type 2 Diabetes. N Engl J Med. 2015;373:1720‐1732. [DOI] [PubMed] [Google Scholar]
- 2. Fonseca V, Desouza C, Asnani S, et al. Nontraditional risk factors for cardiovascular disease in diabetes. Endocr Rev. 2004;25:153‐175. [DOI] [PubMed] [Google Scholar]
- 3. Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non‐insulin‐dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med. 1997;157:1413‐1418. [PubMed] [Google Scholar]
- 4. Bonora E, Formentini G, Calcaterra F, et al. HOMA‐estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care. 2002;25:1135‐1141. [DOI] [PubMed] [Google Scholar]
- 5. Soinio M, Marniemi J, Laakso M, et al. High‐sensitivity C‐reactive protein and coronary heart disease mortality in patients with type 2 diabetes: a 7‐year follow‐up study. Diabetes Care. 2006;29:329‐333. [DOI] [PubMed] [Google Scholar]
- 6. Lange T, Vansteelandt S, Bekaert M. A simple unified approach for estimating natural direct and indirect effects. Am J Epidemiol. 2012;176:190‐195. [DOI] [PubMed] [Google Scholar]
- 7. Tremblay AJ, Morrissette H, Gagne, JM, et al. Validation of the Friedewald formula for the determination of low‐density lipoprotein cholesterol compared with beta‐quantification in a large population. Clin Biochem. 2004;37:785‐790. [DOI] [PubMed] [Google Scholar]
- 8. Simons PC, Algra A, van de Laak MF, et al. Second manifestations of ARTerial disease (SMART) study: rationale and design. Eur J Epidemiol. 1999;15:773‐781. [DOI] [PubMed] [Google Scholar]
- 9. Lange T, Rasmussen M, Thygesen LC. Assessing natural direct and indirect effects through multiple pathways. Am J Epidemiol. 2014;179:513‐518. [DOI] [PubMed] [Google Scholar]
- 10. Piepoli MF, Hoes AW, Agewall S, et al. European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention and Rehabilitation (EACPR). Eur Heart J. 2016, 2016;37:2315‐2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Sande NG, Dorresteijn JA, Visseren, FL, et al. Individualized prediction of the effect of angiotensin receptor blockade on renal and cardiovascular outcomes in patients with diabetic nephropathy. Diabetes Obes Metab. 2016;18:1120‐1127. [DOI] [PubMed] [Google Scholar]
- 12. Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959‐2971. [DOI] [PubMed] [Google Scholar]
- 13. Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973‐979. [DOI] [PubMed] [Google Scholar]
- 14. Ridker PM, Buring JE, Shih J, et al. Prospective study of C‐reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731‐733. [DOI] [PubMed] [Google Scholar]
- 15. Haffner SM, D'Agostino RJr, Mykkanen L, et al. Insulin sensitivity in subjects with type 2 diabetes. Relationship to cardiovascular risk factors: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 1999;22:562‐568. [DOI] [PubMed] [Google Scholar]
- 16. Sobel BE. Insulin resistance and thrombosis: a cardiologist's view. Am J Cardiol. 1999;84:37 J‐41 J. [DOI] [PubMed] [Google Scholar]
- 17. Calles‐Escandon J, Mirza SA, Sobel BE, et al. Induction of hyperinsulinemia combined with hyperglycemia and hypertriglyceridemia increases plasminogen activator inhibitor 1 in blood in normal human subjects. Diabetes. 1998;47:290‐293. [DOI] [PubMed] [Google Scholar]
- 18. Saito I, Folsom AR, Brancati FL, et al. Nontraditional risk factors for coronary heart disease incidence among persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Ann Intern Med. 2000;133:81‐91. [DOI] [PubMed] [Google Scholar]
- 19. Hegab Z, Gibbons S, Neyses L, et al. Role of advanced glycation end products in cardiovascular disease. World J Cardiol. 2012;4:90‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kilhovd BK, Berg TJ, Birkeland KI, et al. Serum levels of advanced glycation end products are increased in patients with type 2 diabetes and coronary heart disease. Diabetes Care. 1999;22:1543‐1548. [DOI] [PubMed] [Google Scholar]
- 21. Kiuchi K, Nejima J, Takano T, et al. Increased serum concentrations of advanced glycation end products: a marker of coronary artery disease activity in type 2 diabetic patients. Heart. 2001;85:87‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51:527‐539. [DOI] [PubMed] [Google Scholar]
- 23. Yoshida N, Okumura K, Aso Y. High serum pentosidine concentrations are associated with increased arterial stiffness and thickness in patients with type 2 diabetes. Metabolism. 2005;54:345‐350. [DOI] [PubMed] [Google Scholar]
- 24. Udell JA, Cavender MA, Bhatt DL, et al. Glucose‐lowering drugs or strategies and cardiovascular outcomes in patients with or at risk for type 2 diabetes: a meta‐analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2015;3:356‐366. [DOI] [PubMed] [Google Scholar]
- 25. Goto A, Arah OA, Goto M, et al. Severe hypoglycaemia and cardiovascular disease: systematic review and meta‐analysis with bias analysis. BMJ. 2013;347:f4533. [DOI] [PubMed] [Google Scholar]
- 26. Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta‐analysis. Am J Med. 2006;119:812‐819. [DOI] [PubMed] [Google Scholar]
- 27. Ballantyne CM, Hoogeveen RC, McNeill AM, et al. Metabolic syndrome risk for cardiovascular disease and diabetes in the ARIC study. Int J Obes (Lond). 2008;32:S21‐S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilson PW, D'Agostino RB, Parise H, et al. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066‐3072. [DOI] [PubMed] [Google Scholar]
- 29. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383‐393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Mediation analysis based on counterfactual variables. Three versions of a patient are created. In the first version the patient has type 2 diabetes and the risk factor levels (mediators) have the natural levels of a patient with type 2 diabetes (Real T2DM). In the second version, the patient has type 2 diabetes, but the risk factor levels are set to the value of a patient without type 2 diabetes (Counterfactual T2DM). In the third version, the patient does not have type 2 diabetes and the risk factor levels have the natural levels of a patient without type 2 diabetes (Real no T2DM).


