Abstract
Nivolumab is the first anti–programmed death‐1 agent approved in China for treatment of locally advanced or metastatic non–small cell lung cancer (NSCLC). Here, we characterize the population pharmacokinetics (PPK) of nivolumab monotherapy in Chinese patients with previously treated advanced/recurrent solid tumors, including NSCLC and nasopharyngeal cancer (NPC), using data from 2 predominantly Chinese (CheckMate 077 and 078), and 5 global (MDX1106‐01, CA209‐003, and CheckMate 017, 057, and 063) studies. The PPK model was developed by reestimating parameters of a prior global population model with Chinese patient data. Model reestimates showed nivolumab pharmacokinetics (PK) to be linear and dose proportional. Race did not have a clinically meaningful effect on nivolumab clearance. Body weight, Asian race, sex, and performance status had significant effects on clearance. Baseline clearance was 9% lower in the Asian versus the global population but not considered clinically relevant. Change in time‐varying clearance and predicted nivolumab exposures with 3 mg/kg every 2 weeks (Q2W) were similar in Chinese, non‐Chinese Asian, and non‐Asian patients. In Chinese patients, the predicted nivolumab exposure with a 240‐mg Q2W regimen was ∼25% higher than with 3 mg/kg Q2W, but ∼62% lower than that of a previously evaluated, well‐tolerated regimen of 10 mg/kg Q2W (global population). Differences in nivolumab baseline clearance and exposures between patients with NPC and NSCLC were not clinically meaningful (<20%). Overall, PPK analysis demonstrated that nivolumab was not sensitive to race when evaluated in Chinese and non‐Asian patients and exhibited similar PK in NSCLC and NPC.
Keywords: Chinese population, fixed dosing, insensitivity to race, nivolumab, non–small cell lung cancer, population pharmacokinetics
Nivolumab, a programmed death‐1 (PD‐1) checkpoint inhibitor, is approved as monotherapy for the treatment of patients with metastatic non–small cell lung cancer (NSCLC) and progression on or after platinum‐based chemotherapy in the United States,1 and adults with locally advanced or metastatic NSCLC after prior chemotherapy in the European Union.2 The initial approval was granted for a body weight–based dosing regimen of 3 mg/kg of nivolumab administered every 2 weeks (Q2W). This initial approval was based on the global CheckMate 017 (NCT01642004) and CheckMate 057 (NCT01673867) studies in squamous cell and nonsquamous cell advanced/metastatic NSCLC, respectively, which demonstrated improved overall survival (OS) compared with docetaxel (75 mg/m2).1, 3, 4 In a pooled 3‐year follow‐up analysis of CheckMate 017 and 057, nivolumab 3 mg/kg demonstrated long‐term OS benefit versus docetaxel, with estimated 3‐year OS rates of 17% versus 8%.5
In China, lung cancer is the leading cause of cancer death.6 The increasing incidence of lung cancer, including NSCLC, highlights the current unmet need for new treatments.6 Nivolumab monotherapy for the treatment of advanced NSCLC has been evaluated in 2 clinical studies in a predominantly Chinese patient population, CheckMate 077 (NCT02593786) and CheckMate 078 (NCT02613507). The phase 1/2 open‐label CheckMate 077 study investigated the use of nivolumab, including 3 mg/kg and 240 mg Q2W, in Chinese patients with previously treated advanced or recurrent solid tumors, comprising NSCLC, hepatocellular carcinoma, and nasopharyngeal carcinoma (NPC).7 In this study, nivolumab was well tolerated, and its pharmacokinetic (PK) profile in Chinese patients was similar to that previously reported in a US population.7, 8 The open‐label, randomized, phase 3 CheckMate 078 study evaluated nivolumab 3 mg/kg Q2W versus docetaxel 75 mg/m2 every 3 weeks in a predominantly Chinese patient population with advanced or metastatic NSCLC (squamous or nonsquamous) and disease progression during or after 1 prior platinum‐based doublet chemotherapy regimen. Results showed an improved OS benefit for nivolumab compared with docetaxel, with a hazard ratio of 0.68 (97.7% confidence interval [CI], 0.52‐0.90; P = .0006), which was consistent with the findings of the global CheckMate 017 and 057 studies.3, 4, 5, 9 In June 2018 nivolumab 3 mg/kg Q2W was approved as the first PD‐1 checkpoint inhibitor in China for the treatment of locally advanced or metastatic NSCLC after prior platinum‐based chemotherapy in adult patients without EGFR or ALK genomic tumor alterations.10
Importantly, ethnic/racial differences may impact risk–benefit profiles and dosing regimens of medications.11 Intrinsic factors, such as genetic and physiologic characteristics, and extrinsic factors, such as culture and environment, may ultimately contribute to differences in drug exposures and therefore efficacy and/or safety in distinct ethnic/racial groups.11, 12, 13 Such differences must be taken into consideration when establishing prescribing information for different regions. Two important factors are race and body weight; race largely relates to intrinsic factors, which can affect drug metabolism, whereas lower body weight can lead to a decreased clearance and therefore greater drug exposure.11, 12, 14 Body weight is particularly relevant for Asian patients, including Chinese patients, who have a lower mean body weight compared with non‐Asian races, especially when using a fixed dose of a therapeutic agent.15, 16 In addition to body weight–based dosing regimens, use of fixed doses is also approved for PD‐1/programmed death ligand‐1 inhibitors including nivolumab (240 mg Q2W).1, 2 As such, characterization and evaluation of drug PK for various races is essential to predict the exposure and ultimately mitigate the potential risks associated with different drug exposures.17
These analyses evaluated the PK of nivolumab in Chinese patients in comparison to other populations via a population pharmacokinetic (PPK) modeling approach. The objectives were (1) to compare nivolumab 3‐mg/kg Q2W clearance and exposures in Chinese, non‐Chinese Asian, and non‐Asian patients; (2) to compare predicted nivolumab 240‐mg Q2W and 3‐mg/kg Q2W exposures in Chinese patients; and (3) to evaluate the exposure measures of nivolumab 240‐mg Q2W in Chinese patients versus a 10‐mg/kg Q2W dosing regimen in a global population. For this analysis, data for Chinese patients were pooled from the CheckMate 077 and CheckMate 078 studies. The global population was pooled from the MDX1106‐01 (NCT00441337), CA209‐003 (NCT00730639), CheckMate 017 (NCT01642004), CheckMate 057 (NCT01673867), and CheckMate 063 (NCT01721759) studies; non‐Chinese patients were also included from the CheckMate 078 study.
Methods
Study Approvals and Informed Consent
All clinical studies referenced as data sources in this manuscript were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Guidelines for Good Clinical Practice, and local regulations. For all clinical studies, an institutional review board or independent ethics committee at each site approved the protocol, consent form, and any other written information provided to patients or their legal representatives. All patients or their legal representatives gave written informed consent prior to study entry and provided consent for additional data analyses following the initial clinical study.
Patient Populations
The Chinese population included in the PPK analyses was from 2 clinical studies, CheckMate 078 and 077. CheckMate 078 is a phase 3, open‐label, randomized multinational study assessing the efficacy and safety of nivolumab (3 mg/kg Q2W) compared with docetaxel (75 mg/m2 every 3 weeks) as second‐line treatment in a predominantly Chinese population (N = 504) with advanced or metastatic NSCLC (squamous and nonsquamous). CheckMate 077 is a phase 1/2 open‐label study, with a secondary objective of characterizing the PK of nivolumab monotherapy (including 3‐mg/kg Q2W and 240‐mg Q2W dosing regimens) in Chinese patients (N = 35) with previously treated advanced or recurrent solid tumors, comprising NSCLC (n = 10), NPC (n = 23), and hepatocellular carcinoma (n = 2). Additional information on the CheckMate 077 and 078 studies is provided in the supporting information in the online version of this paper (section 1). Patient data from MDX‐1106‐01, CA209‐003, CheckMate 017, 057, and 063 global clinical studies were also included in the PPK analysis (for further details, see Tables S1 and S2).
PK Samples and Quantification of Nivolumab Concentrations
The nivolumab PPK analysis used nivolumab concentration values from patients who received nivolumab monotherapy—including patients who had ≥1 prior line of therapy—and from global clinical studies, as well as patients from CheckMate 077 and 078, who were further classified by the nivolumab dosing regimen (240 mg Q2W, 3 mg/kg Q2W, 10 mg/kg Q2W) and race (Chinese, non‐Chinese Asian, non‐Asian). Patients for whom no serum concentration data were available were excluded from the analysis; nivolumab serum concentration values were excluded if they were below the lower limit of quantification or missing the sample date/time. If the data of an associated dose (dose amount, infusion duration) were missing, information was imputed into the analysis data set and not excluded to enable inclusion of PK samples associated with subsequent doses. Missing covariates were imputed using the median (continuous) or mode (categorical) of the covariate variable if <10% of the values were missing. Race, body weight, and baseline estimated glomerular filtration rate (eGFR) were the only covariates that had missing values, but those were <1%. Serum concentrations could be considered outliers and not included in the analysis if they were not considered to be physiologically possible or potentially affected by protocol deviations, such as improper storage conditions. The PK samples collected were analyzed for nivolumab serum concentrations by validated ligand‐binding enzyme‐linked immunosorbent assay (Immunochemistry Department [ICD] 316) or by the validated electrochemiluminescence (ELC) assays ICD 416 and 14BASM122V1. All 3 assays were cross validated. PK samples for the MDX‐1106‐001 study were analyzed using the ICD 316 assay. Some PK samples collected from the CA209‐003 study were analyzed using the ICD 316 assay, the remaining samples were analyzed using the ELC ICD 416 assay. The ELC ICD 416 assay was also used to analyze PK samples collected from other global studies included in this analysis. The nivolumab PK samples collected during the CheckMate 077 and 078 studies were analyzed at WuXi App Tec in China using the validated ELC 14BASM122V1 assay, which was cross validated with the ICD 416 assay. Additional details on all 3 assays are provided in Table S3.
PPK Analysis
PPK Model Development and Evaluation
Nivolumab PK were characterized by a PPK model, developed by reestimating the parameters of a previously reported PPK model for nivolumab monotherapy. This updated model includes data from the Chinese population of the PPK analysis data set shown in Tables S1 and S2.18 As described, the previous final model was developed by backward elimination of covariates one at a time, removing statistically nonsignificant covariates and confounding effects.18
The current PPK model involved a 2‐compartment, zero‐order intravenous infusion and time‐varying clearance (sigmoidal function of the estimate of the maximal change in clearance [Emax]), with a proportional residual error model and random effect on clearance, volume of the central compartment, volume of the peripheral compartment, Emax, and correlation of random effect between clearance and volume of the central compartment. The current model covariates were based on the previously established nivolumab PPK model18 and included the effects of baseline body weight, eGFR, Eastern Cooperative Oncology Group performance status (a scale to determine a patient's health and functional status ranging from 0 [fully active] to 5 [death]),19 sex, race, and tumor type on clearance. The effects of baseline body weight and sex on volume of the central compartment were also included. In this analysis, NPC tumor type was added as a new categorical covariate and tumor types besides NSCLC and NPC were categorized as “other tumor types.” Asian race and NPC were evaluated as categorical covariates in the current PPK model.
Diagnostic plots of the current model are presented as observed versus predicted population or individual concentration (Figure S1). The prediction‐corrected visual predictive checks were performed with 500 simulated data sets obtained using parameter values from the current model for the patients dosed with 3 mg/kg or 240 mg Q2W for all concentrations after previous dose (Figure S2A), and for trough concentrations after first dose (Figure S2B).
The PPK analysis was performed using the NONMEM computer program (Version 7.2, ICON Development Solutions, Hanover, Maryland), compiled using Intel Fortran, and diagnostic plots were prepared using R software. Visual predictive checks and bootstrap analysis were carried out through Perl Speaks NONMEM. The bootstrap analysis included resampling of the PK data set for 1000 iterations to estimate the 95%CI of parameters.
PPK Model Application
Individual baseline clearance and time‐dependent clearance were assessed and compared across Chinese, non‐Chinese Asian, and non‐Asian patients. The steady‐state clearance, as a percentage of baseline, was calculated per the following formula: Steady‐state clearance , where Emax was the estimate of the maximal change in clearance. The maximal decrease as a percentage of baseline was calculated as: , where Emax was the estimate of the maximal change in clearance. Covariate effects of <20% were not considered clinically meaningful.
Nivolumab clearances expressed as a percentage of typical values in Chinese, non‐Chinese Asian, and non‐Asian groups were also compared to confirm that the covariate effects included in the model adequately described the clearance across these racially distinct populations.
To compare nivolumab exposures in Chinese, non‐Chinese Asian, and non‐Asian groups, measures of individual exposure with the nivolumab 3‐mg/kg Q2W body weight–based dosing regimen were obtained and summarized from the current model for each patient with available estimates of PK parameters of minimum, maximum, and time‐average of nivolumab concentrations after the first dose (Cmin1, Cmax1, Cavg1) and at steady state (Cminss, Cmaxss, Cavgss). The comparison of the above‐mentioned measures of predicted exposure with nivolumab 3 mg/kg Q2W and clearance by race was also evaluated. In Chinese patients from the CheckMate 077 and 078 studies, the terminal half‐life at the start of treatment (THALFβ) and steady state (THALFβss), as well as the Cavgss range within weight distribution according to treatment with 240‐mg Q2W fixed‐dose regimen and 3‐mg/kg Q2W body weight–based dosing regimen, were also assessed.
In Chinese patients, predicted exposures for the nivolumab 3‐mg/kg Q2W body weight–based dosing regimen and the 240‐mg Q2W fixed‐dose regimen were compared. The predicted exposures for the 240‐mg Q2W fixed‐dose regimen in Chinese patients were also compared with those from the previously evaluated and well‐tolerated 10‐mg/kg Q2W dosing regimen in patients from the MDX‐1106‐01 and CA209‐003 global phase 1 clinical studies.
Results
Patient Population
Exposure with nivolumab monotherapy was evaluated based on a total data set of 1200 patients, of whom 959 had previously treated advanced or metastatic NSCLC (nonsquamous, n = 544; squamous, n = 415) and 23 had NPC. Patients were categorized by nivolumab monotherapy treatment regimen and race into 3 patient groups: Chinese, non‐Chinese Asian, and non‐Asian patients (Table 1). The Chinese group included 289 patients with NSCLC, all 23 cases of NPC, and the only 2 patients with hepatocellular carcinoma who were part of the data set. A total of 6945 concentration records were available. A summary of the concentration records by study and dosing regimen is provided in Tables S4A and S4B. Select baseline characteristics of Chinese patients from Checkmate 077 and 078 are presented in Table S5.
Table 1.
Patients Included in the PPK Analysis Data Set by Treatment and Race/Ethnicity Category
| Nivolumab Treatment Regimen | Chinese (n = 314) | Non‐Chinese Asian (n = 21) | Non‐Asian (n = 865) |
|---|---|---|---|
| 0.1 mg/kg | 0 | 0 | 17 |
| 0.3 mg/kg | 0 | 0 | 24 |
| 1 mg/kg | 0 | 0 | 92 |
| 3 mg/kg | 294 | 18 | 584 |
| 10 mg/kg | 0 | 3 | 148 |
| 240 mg Q2W | 20 | 0 | 0 |
PPK indicates population pharmacokinetic; Q2W, every 2 weeks.
Chinese patients are those with race/ethnicity specified as Chinese in studies CheckMate 077 and 078, non‐Chinese Asian patients are whose race are Asian but ethnicity not specified as Chinese, non‐Asian patients are those with neither Asian nor Chinese race. The global population is the sum of all non‐Chinese Asian and non‐Asian patients (n = 886).
Current PPK Model Development and Evaluation
The previously developed final PPK model employed in this analysis was updated with data from Chinese patients and had the tumor type covariates revised from the previously reported final PPK model for nivolumab monotherapy to include both NSCLC and NPC tumor types.18 The current model was as follows:
where i refers to the values of an individual subject, was the typical value of clearance, CLNPC was the effect of NPC tumor type on the change of clearance relative to NSCLC (the reference tumor type), and CLOTH was the change of clearance for the other tumor types, including renal cancer carcinoma, melanoma, colorectal cancer, prostate cancer, and hepatocellular carcinoma, relative to NSCLC. Clearance values are also indicated for Black (RAAA) and Asian races (RAAS), as well as body weight (BW) and performance status (PS). Additional information on covariates in the PPK analysis data set, including numbers of patients with each type of tumor, is provided in Table S6.
Diagnostic plots for the current PPK model (Figure S1) showed that nivolumab PK were linear and dose proportional, and were well characterized with a 2‐compartment model with zero‐order infusion in Chinese, non‐Chinese Asian and non‐Asian patients. The prediction‐corrected visual predictive check plots (Figures S2A and S2B) show that the model adequately characterized the trough data from the 5th to the 95th percentiles: the solid and dashed lines representing the 5th, 50th, and 95th percentiles of the observed data pass through the center of the respective 90% prediction interval (shaded area) of the PK data. Thus, the predictions of the model were considered appropriate for its intended purpose. The model provided a good fit of the data for the Chinese patient population in both the 3‐mg/kg and 240‐mg Q2W dosing regimens. Parameter estimates for the current model are presented in Table S7.
Predicted Nivolumab Clearance in Chinese, Non‐Chinese Asian, and Non‐Asian Patients
The magnitude of the effects of baseline covariates on nivolumab clearance and volume of the central compartment, assessed using the current PPK model, was within ±20% of the reference values as assessed by 95%CI, with exception of body weight (Figure 1). Importantly, neither race nor tumor type (NPC) had a clinically relevant effect on nivolumab clearance (<20%). For race, a 9% lower baseline clearance for Asian versus non‐Asian patients was observed as statistically significant, but not clinically meaningful. For NPC tumor type, a 9% higher baseline clearance for patients with NPC versus NSCLC was observed; however, the effect was not statistically significant (95%CI included 1). It is notable that uncertainty is observed due to the small sample size (n = 23) of patients with NPC. Female patients appeared to have a lower clearance and volume of the central compartment versus male patients, whereas patients with Eastern Cooperative Oncology Group PS > 0 versus PS = 0 exhibited a 15% increase in nivolumab clearance; however, these differences were unlikely to be clinically relevant.20 Additionally, the PPK analyses showed that the change of time dependence of clearance in Chinese patients was similar to that of non‐Asian patients. Specifically, a decrease of nivolumab clearance with time was seen in the Chinese patient cohort, reaching a maximal decrease of approximately 32% (Figure 2A). Changes in nivolumab clearance occurred soon after treatment initiation. The THALFβ and THALFβss in Chinese patients was 605.5 hours (25.2 days) and 861.8 hours (35.9 days), respectively. The half‐maximal change was reached at approximately 2 months (time of half‐maximal change [t50] of 1380 hours). Predicted baseline clearance for 3 mg/kg Q2W nivolumab was 12% (10.2 mL/h) and 20% (9.2 mL/h) lower in Chinese and non‐Chinese Asian patients, respectively, than in non‐Asian patients (11.6 mL/h; Figure 2B). Furthermore, the predicted baseline clearance of nivolumab was similar between patients with NPC and previously treated NSCLC, with a <3% difference (Figure 3).
Figure 1.
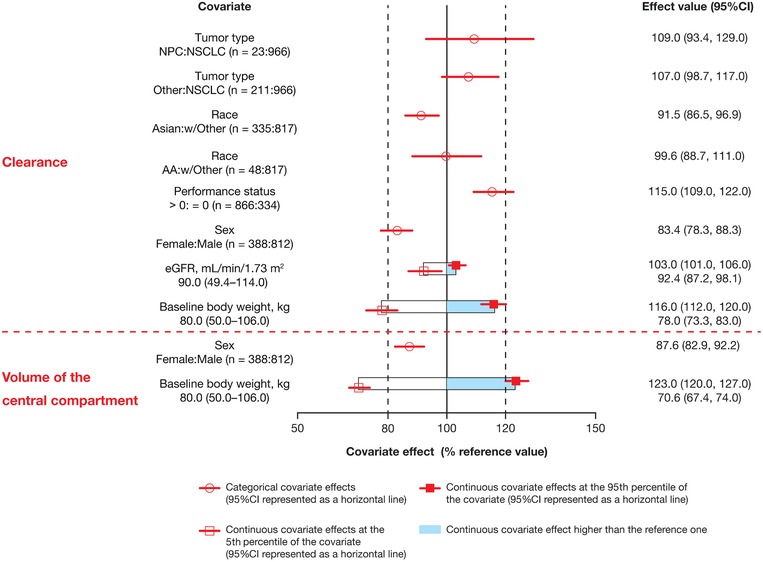
Effect of covariates on nivolumab clearance using the current PPK model. The effect of categorical covariates is presented as comparator to reference; the effect of continuous covariates is presented as reference value (5th percentile, 95th percentile). The reference patient is a white/other male weighing 80 kg with a baseline performance status of 0, eGFR of 90 mL/min/1.73 m2, and 2L+ NSCLC. Parameter estimate in reference patient is considered 100% (vertical solid line), and dashed vertical lines are at 80% and 120% of this value. 2L+ indicates second line or later; AA, Black; CI, confidence interval; eGFR, estimated glomerular filtration rate; NPC, nasopharyngeal carcinoma; NSCLC, non–small cell lung cancer; PPK, population pharmacokinetics.
Figure 2.
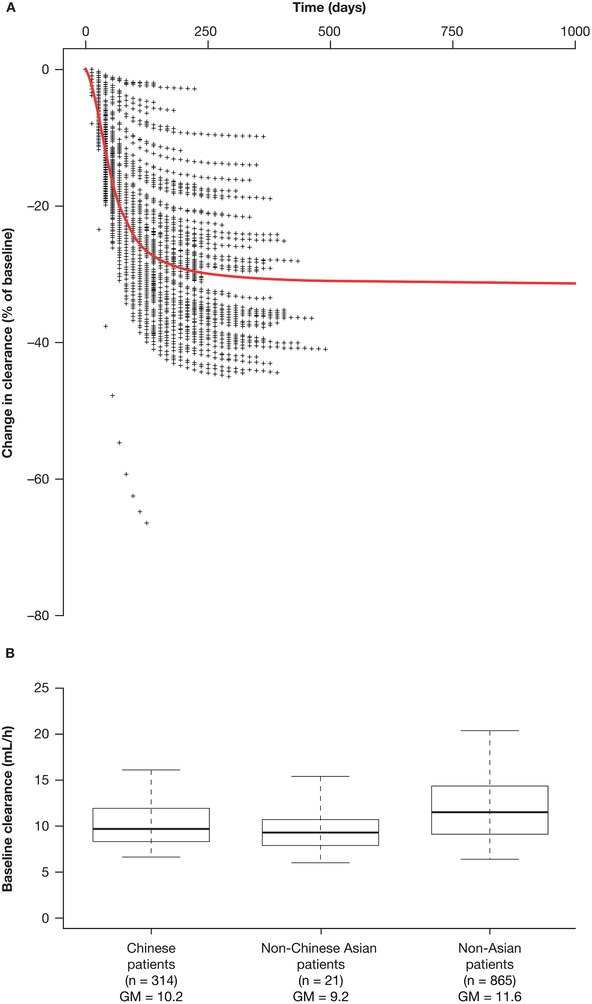
Predicted change in nivolumab clearance over time in (A) Chinese patients and predicted baseline clearance by (B) race/ethnicity. The red line represents the clearance–time profile for a typical patient. Percentage difference in clearance was calculated using the following equation: . The box plots represent the median (bold line) and 25th to 75th percentiles of the distribution. Whiskers represent the 5th and 95th percentiles of the distribution. CL indicates clearance; CLt, clearance at time t; CLt=0, baseline clearance; GM, geometric mean.
Figure 3.
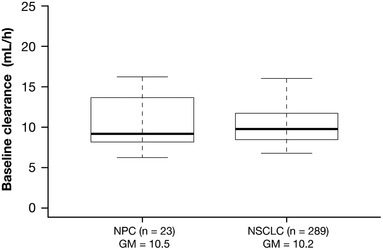
Comparison of baseline clearance in Chinese patients with NPC and previously treated NSCLC. The box plots represent the median (bold line), and 25th to 75th percentiles of the distribution. Whiskers represent the 5th and 95th percentiles of the distribution. GM indicates geometric mean; NPC, nasopharyngeal cancer; NSCLC, non–small cell lung cancer.
Predicted Nivolumab 3‐mg/kg Q2W Exposures in Chinese, Non‐Chinese Asian, and Non‐Asian Patients
The overall differences in geometric means for all exposure measures were <10% lower for Chinese compared with non‐Asian patients (eg, geometric mean Cavg1 was 25.6 µg/mL and 27.5 µg/mL in Chinese and non‐Asian patients, respectively; Table 2).
Table 2.
Predicted Exposures With Nivolumab 3 mg/kg Q2W by Race/Ethnicity
| Chinese (n = 294)a | Non‐Asian (n = 584) | ||||
|---|---|---|---|---|---|
| Predicted Exposure (µg/mL) | Geometric Mean | CV (%) | Geometric Mean | CV (%) | Difference in Geometric Means (%) |
| Cmin1 | 16.3 | 23.4 | 17.2 | 29.3 | −5.2 |
| Cmax1 | 56.2 | 13.8 | 61.3 | 59.6 | −8.3 |
| Cavg1 | 25.6 | 17.5 | 27.5 | 23.1 | −6.9 |
| Cminss | 62.0 | 73.5 | 65.9 | 79.3 | −5.9 |
| Cmaxss | 120.0 | 44.8 | 129.0 | 56.1 | −7.0 |
| Cavgss | 80.2 | 61.5 | 85.6 | 65.0 | −6.3 |
Cavg1 indicates postdose 1 time‐averaged serum concentration; Cavgss, time‐averaged serum concentration at steady state; Cmax1, postdose 1 peak serum concentration; Cmaxss, peak serum concentration at steady state; Cmin1, postdose 1 trough serum concentration; Cminss, trough serum concentration at steady state; CV, coefficient of variation; NPC, nasopharyngeal carcinoma; NSCLC, non–small cell lung cancer; Q2W, every 2 weeks.
Represents Chinese patients receiving 3 mg/kg only; includes 6 patients with NPC and 288 patients with NSCLC.
Predicted Nivolumab 240‐mg Q2W and 3‐mg/kg Q2W Exposures in Chinese Patients
Simulation‐based predicted nivolumab exposure in Chinese patients showed the geometric mean estimates of exposure to be approximately 25% higher with the 240‐mg Q2W fixed dose than with 3 mg/kg Q2W for all 6 measures of individual nivolumab exposure (Table 3). For example, the geometric mean Cavg1 was 32.4 µg/mL versus 25.6 µg/mL for the 240‐mg Q2W fixed dose versus 3 mg/kg Q2W in Chinese patients (Table 3). The median and 90% prediction intervals (5th to 95th percentile) for the simulated nivolumab Cavg1 by dosing regimen in Chinese patients (240 mg Q2W and 3 mg/kg Q2W) are presented in Figure 4. Figure S3 presents the Cavgss of both dosing regimens versus the baseline body weight of Chinese patients. There was a trend for higher Cavgss with 240 mg dosing versus 3 mg/kg for baseline body weights up to approximately 80 kg.
Table 3.
Predicted Exposures With Nivolumab 240 mg Q2W or 3 mg/kg Q2W in Chinese Patients
| Chinese 240 mg Q2W (n = 314)a | Chinese 3 mg/kg Q2W (n = 294)b | ||||
|---|---|---|---|---|---|
| Predicted Exposure (µg/mL) | Geometric Mean | CV (%) | Geometric Mean | CV (%) | Difference in Geometric Means (%) |
| Cmin1 | 20.7 | 24.1 | 16.3 | 23.4 | 27.0 |
| Cmax1 | 71.2 | 18.9 | 56.2 | 13.8 | 27.0 |
| Cavg1 | 32.4 | 18.3 | 25.6 | 17.5 | 27.0 |
| Cminss | 78.3 | 71.8 | 62.0 | 73.5 | 26.0 |
| Cmaxss | 152.0 | 44.7 | 120.0 | 44.8 | 27.0 |
| Cavgss | 101.0 | 58.1 | 80.2 | 61.5 | 26.0 |
Cavg1 indicates postdose 1 time‐averaged serum concentration; Cavgss, time‐averaged serum concentration at steady state; Cmax1, postdose 1 peak serum concentration; Cmaxss, peak serum concentration at steady state; Cmin1, postdose 1 trough serum concentration; Cminss, trough serum concentration at steady state; CV, coefficient of variation; Q2W, every 2 weeks.
Predicted data for all Chinese patients in CheckMate 077 and 078 receiving nivolumab 3 mg/kg (n = 294) or 240 mg Q2W (n = 20) doses.
Predicted data for Chinese patients in CheckMate 077 and 078 receiving nivolumab 3‐mg/kg Q2W (n = 294) dose only.
Figure 4.
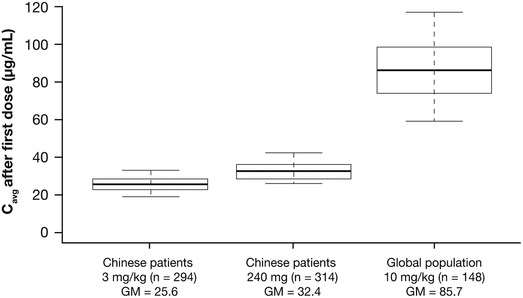
Distribution of predicted nivolumab exposures (Cavg1) with 240 mg Q2W and 3 mg/kg Q2W in Chinese patients and 10 mg/kg Q2W in the global population. The box plots represent the median (bold line), and 25th to 75th percentiles of the distribution. Whiskers represent the 5th and 95th percentiles of the distribution. Cavg indicates time‐averaged nivolumab concentration; Cavg1, first postdose time‐averaged serum nivolumab concentration; GM, geometric mean; Q2W, every 2 weeks.
Predicted Exposures of Nivolumab 240‐mg Q2W in Chinese Patients Versus 10‐mg/kg Q2W Dosing Regimen in the Global Population
The predicted exposure with nivolumab 240‐mg Q2W fixed dosing in Chinese patients was approximately 60% to 62% lower than with the nivolumab 10‐mg/kg Q2W regimen in the global population for all 6 measures of nivolumab exposure assessed (Table 4). For instance, the geometric mean Cavg1 was 32.4 µg/mL for the 240‐mg Q2W fixed dose in Chinese patients, compared with 85.7 µg/mL for the 10‐mg/kg body weight–based dose in the global population (Table 4). The median and 90% prediction intervals (5th to 95th percentile) for the simulated nivolumab Cavg1 by dosing regimen in Chinese patients (240 mg Q2W and 3 mg/kg Q2W) and in a global population (10 mg/kg) is presented in Figure 4.
Table 4.
Predicted Exposures With Nivolumab 240 mg Q2W in Chinese Patients and 10 mg/kg Q2W in the Global Population
| Chinese Patients 240 mg Q2W (n = 314)a | Global Population 10 mg/kg Q2W (n = 148)b | ||||
|---|---|---|---|---|---|
| Predicted Exposure (µg/mL) | Geometric Mean | CV (%) | Geometric Mean | CV (%) | Difference in Geometric Means (%) |
| Cmin1 | 20.7 | 24.1 | 53.6 | 27.5 | −61.0 |
| Cmax1 | 71.2 | 18.9 | 178.0 | 25.2 | −60.0 |
| Cavg1 | 32.4 | 18.3 | 85.7 | 22.0 | −62.0 |
| Cminss | 78.3 | 71.8 | 206.0 | 64.0 | −62.0 |
| Cmaxss | 152.0 | 44.7 | 393.0 | 39.6 | −61.0 |
| Cavgss | 101.0 | 58.1 | 268.0 | 52.3 | −62.0 |
Cavg1 indicates postdose 1 time‐averaged serum concentration; Cavgss, time‐averaged serum concentration at steady‐state; Cmax1, postdose 1 peak serum concentration; Cmaxss, peak serum concentration at steady state; Cmin1, postdose 1 trough serum concentration; Cminss, trough serum concentration at steady state; CV, coefficient of variation; Q2W, every 2 weeks.
Predicted data for all Chinese patients in CheckMate 077 and 078 receiving nivolumab 3‐mg/kg (n = 294) or 240‐mg Q2W (n = 20) doses.
Predicted data for global patients receiving 10 mg/kg Q2W from global studies MDX‐1106‐01 and CA209‐003.
Discussion
The previous final PPK model used to develop the current model used in this analysis had been established for nivolumab treatment of solid tumors using data from a global population of 1895 patients who received 0.3 to 10.0 mg/kg nivolumab across 11 clinical studies.18 Estimates from this model indicated that nivolumab exhibited linear PK (0.1–20.0 mg/kg) with time‐varying clearance. As patients with cancer often exhibit higher rates of protein turnover,21 it is hypothesized that the decrease in nivolumab clearance over time may reflect improved disease status.17, 18 In fact, the magnitude of the change in drug clearance has been correlated to Response Evaluation Criteria in Solid Tumors (v1.1) objective response status: patients who exhibited a complete/partial response had the largest decrease in clearance over time.17 Results from this model also showed that covariate effects on nivolumab PK, including renal and mild hepatic impairment, were not clinically relevant (<20% cutoff value), whereas nivolumab clearance and volume of distribution in the central compartment increased with body weight.18
In the current analysis, the parameters of the previously developed model18 were reestimated using data from 2 clinical studies in predominantly Chinese populations and 5 global clinical studies. Overall, the parameters evaluated here were consistent with previous estimates.18 Additionally, the current PPK model showed that nivolumab PK was linear and dose proportional in Chinese patients and non‐Asian patients, and that the effect of covariates was aligned with that previously observed in a global population and in a time‐varying model.18 Body weight was found to have a statistically significant effect on clearance and volume of the second compartment. The mechanism of nivolumab elimination and the significant relationship between body weight and nivolumab clearance in the current PPK model is consistent with evaluations from the previous PPK analysis.18 Importantly, race did not have a clinically meaningful impact on clearance in this study, indicating that nivolumab PK is not sensitive to race. Similarly, NPC tumor type had no impact on clearance, although this result should be interpreted with caution due to the small NPC sample size. However, these findings are in agreement with our prior population model study, which also found that tumor type (including advanced NSCLC, renal cell carcinoma, and melanoma) had no clinical effect on nivolumab clearance.18 Moreover, the nivolumab baseline clearance in Chinese patients was only approximately 12% lower than that in non‐Asian patients and not considered clinically meaningful. The time‐varying clearance of nivolumab was evident in the Chinese population, namely, for the maximal decrease of approximately 32%, which was similar to that of non‐Asian patients.
Additionally, nivolumab exposure measures both after the first dose (Cmin1, Cmax1, Cavg1), and at steady state (Cminss, Cmaxss, Cavgss) with the approved 3‐mg/kg Q2W dosing regimen were similar among Chinese, non‐Chinese Asian, and non‐Asian patients, confirming that nivolumab PK is not sensitive to race.
Given the use of a fixed‐dosing regimen across various cancer immunotherapeutic antibodies22, 23, 24, 25 and its recognized advantages (improved administration and reduced prescription errors),26 this analysis also compared exposure of a fixed dose with the approved body weight–based nivolumab regimen of 3 mg/kg. Despite the fixed dose of 240 mg Q2W showing approximately 25% higher nivolumab exposure versus the 3‐mg/kg Q2W regimen in Chinese patients, the predicted exposure of this same fixed regimen in Chinese patients was approximately 62% lower than that of the well‐tolerated nivolumab 10‐mg/kg Q2W regimen in the global population.27 Based on the predicted exposures and the finding that race/ethnicity was not a clinically meaningful covariate of nivolumab PPK, it is anticipated that nivolumab fixed dosing would be a suitable approach for Chinese patients.
Limitations of this analysis include the small number of patients in CheckMate 077, which may impact data interpretation; in particular, the low number of patients with NPC (n = 23) makes the interpretation of tumor type effect on clearance difficult. In addition, it is noted that the coefficient of variation across exposure measures was generally higher at steady state than after the first dosing interval. Variability in exposure is a result of variability in clearance, Emax, volume of the central compartment, and volume of distribution of peripheral compartment. Factors contributing to the difference include higher variability in steady‐state clearance than baseline clearance and the higher impact of clearance on steady‐state exposure than on the first dosing interval, due to accumulation in exposure.
Conclusions
In summary, the findings from these analyses demonstrate that, similar to non‐Asian patients, nivolumab PK was linear and dose proportional with time‐dependent clearance in Chinese patients. Race and tumor type did not have a clinically meaningful impact on nivolumab clearance, in the presence of other covariates. The predicted exposures with the approved 3‐mg/kg Q2W regimen were similar among Chinese, non‐Chinese Asian, and non‐Asian patients. Although the predicted exposure with a 240‐mg Q2W fixed dose was approximately 25% higher than that with the 3‐mg/kg Q2W body weight–based regimen in Chinese patients, it was still markedly (60%‐62%) lower than that observed with the previously evaluated and well‐tolerated nivolumab 10‐mg/kg Q2W regimen in a global population.27 Overall, these results indicate that nivolumab is not sensitive to race differences and exhibits similar exposures in Chinese, non‐Chinese Asian, and non‐Asian patients.
Funding
This study was funded by Bristol‐Myers Squibb.
Declaration of Conflicting Interest
JZ is an employee of Bristol‐Myers Squibb. JC, AB, AR, and JS are employees and shareholders of Bristol‐Myers Squibb.
Data Sharing Statement
Bristol‐Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/clinical-trials-and-research/disclosure-commitment.html.
Author Contributions
All authors contributed substantially to the scientific process leading up to the writing of the paper, including the conception and design of the study. JZ and JS contributed to the acquisition and analysis of data, and drafted the manuscript and figures. All authors approved the final version of the article for submission and agree to be accountable for all aspects of the work.
Supporting information
Supporting Information
Figure S1
Figure S2
Figure S3
Acknowledgments
We thank the patients and their families, as well as the participating clinical study teams, for making this analysis possible. This study was sponsored by Bristol‐Myers Squibb and Ono Pharmaceuticals. Medical writing assistance was provided by Amy Horne, MSc, and Katerina Kumpan, PhD, of Caudex, and was funded by Bristol‐Myers Squibb.
References
- 1. Bristol‐Myers Squibb . Opdivo® (nivolumab) prescribing information, March 2019. http://packageinserts.bms.com/pi/pi_opdivo.pdf. Accessed March 6, 2019.
- 2. European Medicines Agency . Opdivo™ (nivolumab) summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003985/WC500189765.pdf. Accessed August 14, 2018.
- 3. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med. 2015;373(2):123‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borghaei H, Paz‐Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med. 2015;373(17):1627‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non‐small‐cell lung cancer (CheckMate 017 and CheckMate 057): 3‐year update and outcomes in patients with liver metastases. Ann Oncol. 2018;29(4):959‐965. [DOI] [PubMed] [Google Scholar]
- 6. Cheng TY, Cramb SM, Baade PD, Youlden DR, Nwogu C, Reid ME. The international epidemiology of lung cancer: latest trends, disparities, and tumor characteristics. J Thorac Oncol. 2016;11(10):1653‐1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang L, Fang W, Zhang Y, et al. Preliminary safety and pharmacokinetic results from a phase 1/2 study of nivolumab in previously treated Chinese patients with advanced or recurrent solid tumors (CheckMate 077 cohort A). Presented at the 20th Annual Meeting of the Chinese Society of Clinical Oncology (CSCO); September 26‐30, 2017, Xiamen, China.
- 8. Gettinger S, Horn L, Jackman D, et al. Five‐year follow‐up of nivolumab in previously treated advanced non‐small‐cell lung cancer: results from the CA209‐003 study. J Clin Oncol. 2018;36(17):1675‐1684. [DOI] [PubMed] [Google Scholar]
- 9. Wu YL, Lu S, Cheng Y, et al. Nivolumab versus docetaxel in a predominantly chinese patient population with previously treated advanced non‐small cell lung cancer: results of the phase 3 CheckMate 078 study. American Association for Cancer Research Annual Meeting. Vol Oral CT114. Chicago, IL; 2018. [Google Scholar]
- 10. Bristol‐Myers Squibb . China National Drug Administration approves country's first immuno‐oncology agent, Opdivo (nivolumab injection), for previously treated non‐small cell lung cancer (NSCLC). June 15, 2018. https://news.bms.com/press-release/corporatefinancial-news/china-national-drug-administration-approves-countrys-first-imm. Accessed January 17, 2019.
- 11. Ramamoorthy A, Pacanowski MA, Bull J, Zhang L. Racial/ethnic differences in drug disposition and response: review of recently approved drugs. Clin Pharmacol Ther. 2015;97(3):263‐273. [DOI] [PubMed] [Google Scholar]
- 12. Yasuda SU, Zhang L, Huang SM. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther. 2008;84(3):417‐423. [DOI] [PubMed] [Google Scholar]
- 13. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) . ICH harmonised tripartite guideline: ethnic factors in the acceptability of foreign clinical data E5 (R1). https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E5_R1/Step4/E5_R1__Guideline.pdf. Accessed January 10, 2019.
- 14. Syn NL, Yong WP, Lee SC, Goh BC. Genetic factors affecting drug disposition in Asian cancer patients. Expert Opin Drug Metab Toxicol. 2015;11(12):1879‐1892. [DOI] [PubMed] [Google Scholar]
- 15. Costa RLB, Gradishar WJ. Differences are important: breast cancer therapy in different ethnic groups. J Glob Oncol. 2017;3(4):281‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walpole SC, Prieto‐Merino D, Edwards P, Cleland J, Stevens G, Roberts I. The weight of nations: an estimation of adult human biomass. BMC Public Health. 2012;12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Y, Booth B, Rahman A, Kim G, Huang SM, Zineh I. Toward greater insights on pharmacokinetics and exposure‐response relationships for therapeutic biologics in oncology drug development. Clin Pharmacol Ther. 2017;101(5):582‐584. [DOI] [PubMed] [Google Scholar]
- 18. Bajaj G, Wang X, Agrawal S, Gupta M, Roy A, Feng Y. Model‐based population pharmacokinetic analysis of nivolumab in patients with solid tumors. CPT Pharmacometrics Syst Pharmacol. 2017;6(1):58‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649‐655. [PubMed] [Google Scholar]
- 20. Goncalves J, Araujo F, Cutolo M, Fonseca JE. Biosimilar monoclonal antibodies: preclinical and clinical development aspects. Clin Exp Rheumatol. 2016;34(4):698‐705. [PubMed] [Google Scholar]
- 21. Fearon KC, Hansell DT, Preston T, et al. Influence of whole body protein turnover rate on resting energy expenditure in patients with cancer. Cancer Res. 1988;48(9):2590‐2595. [PubMed] [Google Scholar]
- 22. Genentech . Gazvya® (obinutuzumab) prescribing information, November 2017. https://www.gene.com/download/pdf/gazyva_prescribing.pdf. Accessed August 14, 2018.
- 23. Genentech . Perjeta® (pertuzumab) prescribing information, June 2012. https://www.gene.com/download/pdf/perjeta_prescribing.pdf. Accessed August 14, 2018.
- 24. Merck . Keytruda® (pembrolizumab) prescribing information, November 2017. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf. Accessed August 14, 2018.
- 25. Genentech . Tecentriq® (atezolizumab) prescribing information, April 2018. August 14, 2018. https://www.gene.com/download/pdf/tecentriq_prescribing.pdf.
- 26. Zhao X, Suryawanshi S, Hruska M, et al. Assessment of nivolumab benefit‐risk profile of a 240‐mg flat dose relative to a 3‐mg/kg dosing regimen in patients with advanced tumors. Ann Oncol. 2017;28(8):2002‐2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med. 2012;366(26):2443‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Figure S1
Figure S2
Figure S3


