Figure 2.
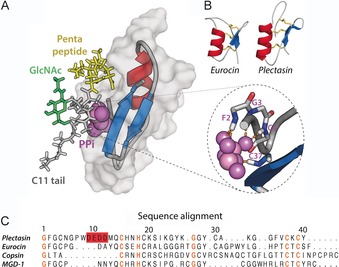
A) Docking model of the plectasin⋅lipid II complex, based on NMR data acquired in DPC micelles. Plectasin coordinates the pyrophosphate group (PPi) through backbone amide protons of the N terminus and the C‐terminal β‐strand (see zoom, right). Moreover, in this model the pentapeptide (in yellow) is involved in plectasin binding. Plectasin is shown in surface representation (in gray) and in cartoon representation. B) CSαβ defensins such as eurocin exhibit a fold similar to that of plectasin yet low sequence homology. C) Sequence alignment of several CSαβ defensins. Conserved residues are highlighted in orange whereas the anionic patch in plectasin is in red. The first ten residues of the copsin N terminus were not included in the alignment.
