Summary
Aims
Five‐α reductase inhibitor (5ARI) therapy has been associated with sexual dysfunction in some patients. This study assessed the impact of a fixed‐dose combination of the 5ARI dutasteride 0.5 mg and the α1‐adrenoceptor antagonist tamsulosin 0.4 mg (DUT‐TAM FDC) on Men's Sexual Health Questionnaire (MSHQ) domain scores in patients with lower urinary tract symptoms secondary to benign prostatic hyperplasia (BPH).
Methods
This was a post hoc analysis of a double‐blind, randomised, placebo‐controlled, parallel‐group, multicentre study in sexually active patients, aged ≥50 years, with a confirmed clinical diagnosis of BPH. Sexual activity, sexual desire, and bother domain scores of the MSHQ were assessed at baseline and at Months 1, 3, 6, 9, and 12. Correlation between MSHQ sexual activity/desire scores and ejaculation, erection, and satisfaction domains at baseline was also evaluated.
Results
In the intent‐to‐treat population (N = 489), 243 and 246 patients were randomised to DUT‐TAM FDC and placebo groups, respectively. Compared with placebo, DUT‐TAM FDC therapy resulted in statistically significant reductions (worsening) from baseline in adjusted mean MSHQ sexual activity and bother domain scores at Months 1, 3, 6, 9, and 12 (all P < 0.05) and in adjusted mean MSHQ sexual desire domain scores at Months 6, 9, and 12 (all P < 0.05). Significant moderate correlations in the expected direction were observed at baseline between the sexual activity/desire domains and the ejaculation, erection, and satisfaction domains (P < 0.0001).
Conclusions
These findings help clarify the degree and impact of libido changes in sexually active men treated with DUT‐TAM FDC and may support clinical decision‐making.
What's known
Association of 5ɑ reductase inhibitors (5ARIs) with decreased libido has been reported in small numbers of patients with benign prostatic hyperplasia, but reports have not assessed baseline sexual function. The Male Sexual Health Questionnaire (MSHQ) was developed to evaluate aspects of male sexual dysfunction. A recent randomised, placebo‐controlled trial reported that the impact of the 5ARI‐α1‐adrenoreceptor (dutasteride‐tamsulosin) combination on sexual health was driven mainly by changes in ejaculation domain scores.
What's new
In this post hoc assessment of a randomised, placebo‐controlled trial, we conducted an evaluation of libido, using the sexual activity and sexual desire domain scores of the MSHQ. We report modest impairments in these domains induced by dutasteride‐tamsulosin fixed‐dose combination therapy, after 1 year of treatment, which are unlikely to be of clinical relevance.
1. INTRODUCTION
The fixed‐dose combination of the 5α‐reductase inhibitor (5ARI), dutasteride 0.5 mg, and the α1‐adrenoceptor antagonist, tamsulosin 0.4 mg (DUT‐TAM FDC), is recommended as a first‐line treatment for the management of moderate‐to‐severe lower urinary tract symptoms (LUTS) because of benign prostatic hyperplasia (BPH), in patients at risk of disease progression.1 However, the potential for sexual dysfunction with 5ARI‐α1‐blocker combination therapies can limit their use in clinical practice, despite patient satisfaction with treatment2 and clinical studies indicating efficacy in reducing symptoms, clinical progression, and the risk of surgery.3, 4, 5, 6 A systematic review and meta‐analysis revealed that erectile dysfunction (ED) and libido alteration were notably more prevalent in patients treated with combination therapy compared with those treated with α1‐blocker monotherapy alone.7 The risk of ED was higher in those treated with combination therapy but the risk of libido alteration was the same for both patients treated with 5ARI monotherapy and those treated with combination therapy.7
Although previous research has reported libido alterations as part of the assessment of sexual adverse events (AEs), the discussions within the literature have focused on the effects of 5ARIs on erectile and ejaculatory function.5, 8 Evidence from epidemiological studies has suggested that it is also important to consider sexual desire and satisfaction when assessing male sexual dysfunction.9, 10 It should be noted that the assessment of sexual function in most studies in this field has been restricted to spontaneous reporting of sexual AEs, including decreased libido, as part of regular clinical trial AE reporting.3, 4, 5, 6, 11 Spontaneous AE reporting presents a disadvantage as it is not quantitative. Furthermore, information regarding the onset, character, and resolution of AEs is subject to patients’ interpretation and the potential but not uncommon misunderstanding of the domains of sexual function.12 Another disadvantage of spontaneous AE reporting is its dependence on the patient's decision to mention it during the study visit (without prompt). Consequently, understanding of the sexual AEs associated with combination therapy is limited.
The validated 25‐item Male Sexual Health Questionnaire (MSHQ) was developed for use in a BPH registry to assess specific aspects of male sexual dysfunction.13 The questionnaire contains three core domains (erection, ejaculation, and satisfaction) and includes additional items related to sexual activity and desire, as well as bother, all of which may be affected by the increased severity of LUTS.8, 13 Sexual desire is thought to more accurately reflect libido than sexual activity, though the latter can be considered a surrogate marker for libido; therefore, these domains are complimentary.
We recently reported the results of the first domain‐specific quantitative evaluation of DUT‐TAM FDC therapy on three sexual function domains of the MSHQ.14 Aiming to further understand the impact of DUT‐TAM FDC therapy on libido, this post hoc analysis assessed the change in prospectively collected MSHQ sexual activity and desire domain scores in sexually active men with LUTS secondary to BPH who were treated with 12 months of DUT‐TAM FDC therapy, or placebo. For completeness, we also present the results from MSHQ items related to bother.
2. MATERIALS AND METHODS
2.1. Study design
This post hoc analysis of a double‐blind, randomised, placebo‐controlled, parallel‐group study was conducted at 51 centres across Europe and Australia (GSK116115/NCT01777269) between 18 February 2013 and 5 April 2016. Following a 4‐week run‐in period, patients were randomised (1:1) to receive once‐daily dutasteride 0.5 mg and tamsulosin 0.4 mg (DUT‐TAM FDC) or placebo for 12 months. Lifestyle advice, relevant to maintaining sexual function and improving LUTS, was provided at baseline to subjects in both treatment groups.
The objective of the current analysis was to evaluate the impact of DUT‐TAM FDC treatment on libido using the scores for the sexual activity, sexual desire and bother domains of the MSHQ, which were prospectively collected from patients included in the intent‐to‐treat (ITT) population from the primary analysis.
The primary study was approved by the appropriate regulatory and ethics committees, and performed in accordance with the Declaration of Helsinki 200815 and Good Clinical Practice guidelines.16 Written informed consent was obtained from each patient prior to study participation.
GSK makes available anonymised individual participant data and associated documents from interventional clinical studies, which evaluate medicines upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access original data for studies that have been reanalysed, other types of GSK sponsored research, for study documents without patient‐level data and for clinical studies not listed, please submit an enquiry via the website.
2.2. Study population
Eligibility criteria for enrollment in the study have been reported previously.14 In brief, patients were male, aged ≥50 years, who were sexually active (engaged in sexual activity with a partner during the past 4 weeks and planning to be active during the next 4 weeks), with a confirmed clinical diagnosis of BPH, and International Prostate Symptom Score of ≥12 at screening, a prostate volume of ≥30 cc as assessed by transrectal ultrasonography and a total serum prostate‐specific antigen (PSA) of ≥1.5 ng/mL at screening. Prior use of BPH therapy was permitted, with the exception of 5ARIs.
Patients with a total serum PSA of >10.0 ng/mL at screening were excluded as were those with a history or evidence of prostate cancer and/or those who had used prohibited medications.
2.3. End‐points
The primary study assessed changes in the sexual function domains of the MSHQ over a 12‐month study period; details of the primary and secondary end‐points have been published previously.14 In this post hoc analysis, sexual activity, sexual desire, and bother were assessed at baseline and at Months 1, 3, 6, 9, and 12, using the MSHQ sexual activity, sexual desire, and bother domain scores respectively. Table 1 provides a summary of the MSHQ items included in this analysis and the scoring system applied to each domain. The change in sexual activity, sexual desire, and bother domain scores from baseline were also assessed at Months 1, 3, 6, 9, and 12. Additionally, the correlation between sexual activity/sexual desire domains and the ejaculation, erection, and satisfaction domains, reported in the primary publication,14 was evaluated at baseline.
Table 1.
Summary of the items within the sexual activity and sexual desire domains of the MSHQ and the scoring system applied
| MSHQ domain | Options for answer | Scoring rangea |
|---|---|---|
| Sexual activity (items 19 and 20) | ||
| 19. In the last month, how often have you had sexual activity, including masturbating, intercourse, oral sex, or any other type of sex? (Check only one) |
5‐Daily or almost daily 4‐More than 6 times per month 3‐4‐6 times per month 2‐1‐3 times per month 1‐0 times per month |
2‐10 |
| 20. Compared with one month ago, has the number of times you have had sexual activity increased or decreased? |
5‐Increased a lot 4‐Increased moderately 3‐Neither increased nor decreased 2‐Decreased moderately 1‐Decreased a lot |
|
| Sexual desire (questions 22, 23, and 25) | ||
| 22. In the last month, how often have you felt an urge or desire to have sex with your main partner? |
5‐All of the time 4‐Most of the time 3‐About half of the time 2‐Less than half of the time 1‐None of the time |
3‐15 |
| 23. In the last month, how would you rate your urge or desire to have sex with your main partner? |
5‐Very high 4‐High 3‐Moderate 2‐Low 1‐Very low or none at all |
|
| 25. Compared with one month ago, has your urge or desire for sex with your main partner increased or decreased? |
5‐Increased a lot 4‐Increased moderately 3‐Neither increased nor decreased 2‐Decreased moderately 1‐Decreased a lot |
|
| Sexual bother (Q4, 12, 21, and 24) | ||
| 4. In the last month, if you have had difficulty getting hard or staying hard without using drugs like Viagra, have you been bothered by this problem? |
5‐Not at all bothered/Did not have a problem with erection 4‐A little bit bothered 3‐Moderately bothered 2‐Very bothered 1‐Extremely bothered |
4‐20 |
| 12. In the last month, if you have had any ejaculation difficulties or have been unable to ejaculate, have you been bothered by this? |
5‐Not at all bothered 4‐A little bit bothered 3‐Moderately bothered 2‐Very bothered 1‐Extremely bothered |
|
| 21. In the last month, have you been bothered by these changes in the number of times you have had sexual activity? |
5‐Not at all bothered 4‐A little bit bothered 3‐Moderately bothered 2‐Very bothered 1‐Extremely bothered |
|
| 24. In the last month, have you been bothered by your level of sexual desire |
5‐Not at all bothered 4‐A little bit bothered 3‐Moderately bothered 2‐Very bothered 1‐Extremely bothered |
|
MSHQ: Men's Sexual Health Questionnaire.
Higher scores indicate increased sexual activity/desire and less sexual bother.
The incidence of AEs, including serious AEs (SAEs), drug‐related AEs, serious drug‐related AEs, and AEs leading to discontinuation of the study medication or study withdrawal, were assessed. AEs of special interest were also evaluated (including sexual and breast AEs). Abnormal laboratory test results or other safety assessments were recorded as AEs or SAEs. Participants with unresolved sexual AEs at the end of the study (after discontinuation of treatment) were followed up via phone call after 6 months.
2.4. Statistical analysis
The sample size was based on the change in the total MSHQ score. Assuming a 6‐unit treatment difference with a standard deviation (SD) of 18 units, 190 patients per treatment group were required to provide a 90% power at a 0.05 significance level. Assuming a 20% withdrawal rate, 238 patients per treatment group were required for randomisation.
The changes in the MSHQ domain scores from baseline were analysed using a mixed model repeated measures analysis. As for the primary publication, as the end‐points were assessed across multiple time points, a step‐down procedure for interpreting P‐values was adopted. Data for each end‐point were analysed in the ITT population.
Pearson correlations were calculated to measure the strength of a linear association between MSHQ sexual activity/desire domain scores at baseline and MSHQ ejaculation, erection, and satisfaction domain scores at baseline (0 = no association; < 0 = negative association; > 0 = positive association).
3. RESULTS
3.1. Study population
As described previously,14 489 patients were included in the ITT population; 243 in the DUT‐TAM FDC group and 246 in the placebo group. Demographics and baseline characteristics were similar in the two treatment groups, and indicative of a population at increased risk of disease progression.
3.2. MSHQ sexual activity domain
Between baseline and Month 12, mean (SD) MSHQ sexual activity domain scores ranged between 5.1 (1.44) and 5.7 (1.23) in the DUT‐TAM FDC group and between 5.4 (1.18) and 5.7 (1.13) in the placebo group (Figure 1A). DUT‐TAM FDC therapy resulted in a statistically significant (P < 0.05) reduction (worsening) from baseline in adjusted mean MSHQ sexual activity domain scores at Months 1, 3, 6, 9, and 12, compared with placebo (Table 2; Figure 1B).
Figure 1.

Overview of (A) mean (±SE) sexual activity domain score (range 2‐10; observed cases), and (B) adjusted mean (±SE) change in sexual activity domain score (ITT population) from baseline through to Month 12. DUT‐TAM FDC, fixed‐dose combination of dutasteride (0.5 mg) and tamsulosin (0.4 mg); ITT, intent‐to‐treat; MSHQ, Men's Sexual Health Questionnaire; SE, standard error
Table 2.
Summary of MMRM analysis for change from baseline in sexual activity and sexual desire domain scores (ITT population)
| Visit | Change from baseline (DUT‐TAM FDC therapy‐placebo) | |||||
|---|---|---|---|---|---|---|
| Sexual activity domain score | Sexual desire domain score | |||||
| Estimatea | 95% CI | P | Estimatea | 95% CI | P | |
| Month 1 | –0.28 | –0.49, –0.08 | 0.007 | –0.53 | –0.83, –0.24 | < 0.001b |
| Month 3 | –0.25 | –0.48, –0.02 | 0.030 | –0.33 | –0.66, 0.01 | 0.057 |
| Month 6 | –0.33 | –0.56, –0.10 | 0.006 | –0.89 | –1.27, –0.51 | < 0.001 |
| Month 9 | –0.29 | –0.53, –0.06 | 0.015 | –0.65 | –1.00, –0.30 | < 0.001 |
| Month 12 | –0.31 | –0.53, –0.09 | 0.006 | –0.35 | –0.70, –0.01 | 0.045 |
CI: confidence interval; DUT‐TAM FDC: fixed‐dose combination of dutasteride (0.5 mg) and tamsulosin (0.4 mg); ITT: intent‐to‐treat; MMRM: mixed model repeated measures.
A negative estimate indicates a worsening with DUT‐TAM FDC therapy compared with placebo.
The P‐value for Month 1 could not be interpreted as statistically significant because of the stepdown multiplicity criteria.
3.3. MSHQ sexual desire domain
In the DUT‐TAM FDC therapy group, mean (SD) MSHQ sexual desire domain scores ranged from 9.0 (2.35) to 10.0 (1.77) between baseline and Month 12. Values in the placebo group ranged from 9.7 (1.64) to 9.9 (1.72) (Figure 2A).
Figure 2.
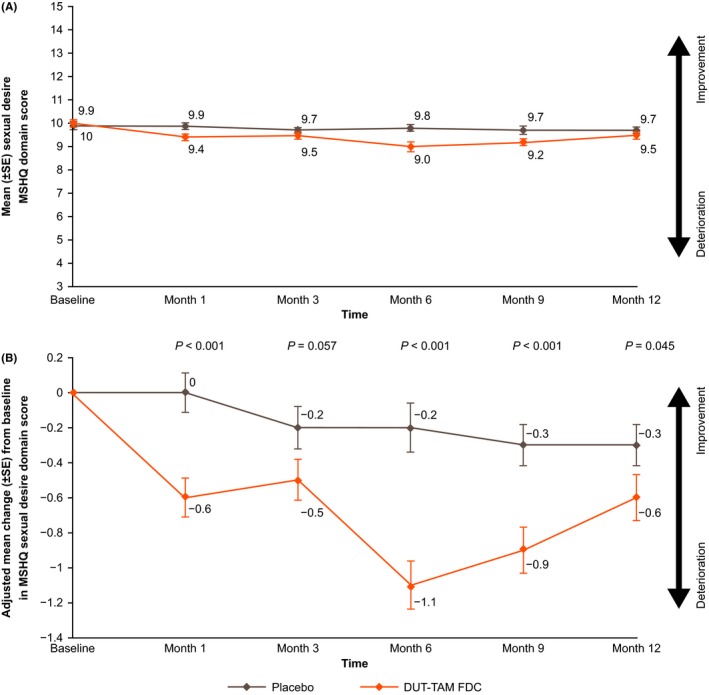
Overview of (A) mean (±SE) sexual desire domain score (range 3‐15; observed cases) and (B) adjusted mean (±SE) change in sexual desire domain score (ITT population), from baseline through to Month 12. DUT‐TAM FDC, fixed‐dose combination of dutasteride (0.5 mg) and tamsulosin (0.4 mg); ITT, intent‐to‐treat; MSHQ, Men's Sexual Health Questionnaire; SE, standard error
Patients receiving DUT‐TAM FDC had a statistically significant (P < 0.05) reduction (worsening) from baseline in adjusted mean MSHQ sexual desire domain scores compared with placebo at Months 6, 9, and 12 (Table 2; Figure 2B). No statistically significant between treatment group differences were seen at Month 3. The treatment difference observed at Month 1 (P < 0.001) was nominally significant because of the multiplicity step‐down procedure employed.
3.4. MSHQ bother domain
Baseline MSHQ bother scores were similar between treatment groups, with the mean (SD) scores of 16.7 (3.36) and 17.1 (3.18) in the DUT‐TAM FDC therapy group and in the placebo group, respectively. Mean MSHQ bother scores decreased in both treatment groups at each postbaseline assessment (adjusted mean MSHQ bother score changes from baseline ranging from –0.9 to –1.6 in the DUT‐TAM FDC therapy group compared with 0 to ‐0.5 in the placebo group). Statistically significant treatment differences (P < 0.05) were observed between treatment groups at each postassessment visit (Months 1, 3, 6, 9, and 12) in the change from baseline MSHQ bother score. Adjusted mean (95% confidence interval) treatment differences (DUT‐TAM FDC‐placebo) were –0.63 (–1.16, –0.10) at Month 1 (P = 0.021), –0.67 (–1.24, –0.09) at Month 3 (P = 0.023), –1.57 (–2.20, –0.95) at Month 6 (P < 0.001), –1.18 (–1.79, –0.57) at Month 9 (P < 0.001), and –1.20 (–1.81, –0.60) at Month 12 (P < 0.001).
Figure 3 highlights the changes from baseline in the sexual activity, sexual desire, and bother domains at Months 1, 3, 6, 9, and 12 in relation to the changes in ejaculation, erection, and satisfaction domains.
Figure 3.
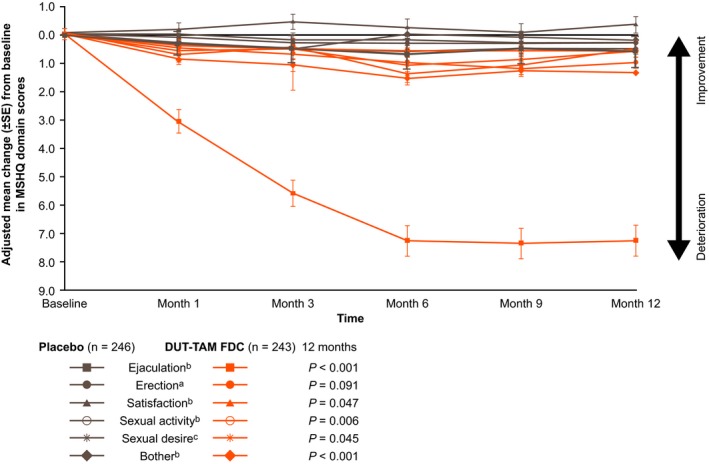
Adjusted mean change (±SE), from baseline to Month 12, in the ejaculation, erection, satisfaction, sexual activity, sexual desire, and bother domain scores (observed cases) of the MSHQ (ITT population). DUT‐TAM FDC, fixed‐dose combination of dutasteride (0.5 mg) and tamsulosin (0.4 mg); ITT, intent‐to‐treat; MSHQ, Men's Sexual Health Questionnaire; SE, standard error
aNo statistically significant difference at any studied time point post baseline
bStatistically significant difference at any studied time point post baseline
cStatistically significant difference at Months 6, 9, and 12 post baseline
3.5. Domain correlations at baseline
Moderate correlations were observed at baseline between the sexual activity domain scores and the scores for the ejaculation (correlation coefficient [r] 0.43), erection (r = 0.41), and satisfaction (r = 0.35) domains assessed in the primary analysis (Table 3). Each of these correlations were found to be significant (P < 0.0001).
Table 3.
Pearson correlation coefficients for domain scores at baseline (ITT population)
| Baseline values | Ejaculation domain | Erection domain | Satisfaction domain |
|---|---|---|---|
| Sexual activity domain score | |||
| Correlation coefficient | 0.43a | 0.41a | 0.35a |
| Number of observations | 447 | 456 | 432 |
| Sexual desire domain score | |||
| Correlation coefficient | 0.40a | 0.46a | 0.51a |
| Number of observations | 444 | 447 | 431 |
ITT: intent‐to‐treat.
All of the pairwise correlations are significantly different from 0, with P‐value < 0.0001.
Similar correlations were seen between the sexual desire domain scores and those for the ejaculation (r = 0.40), erection (r = 0.46), and satisfaction domains (r = 0.51) (Table 3). Each of these correlations were also found to be significant (P < 0.0001).
3.6. Safety
As previously reported, the proportion of patients with any AEs, SAEs, and drug‐related AEs was significantly higher in the DUT‐TAM FDC therapy group than in the placebo group.14 The proportion of patients with altered (decreased) libido AEs (including decreased libido, loss of libido, and sexual dysfunction) was numerically higher in the DUT‐TAM FDC group than in the placebo group (10% [n = 24] vs 5% [n = 12], respectively); however, none were serious. Four subjects in the DUT‐TAM FDC therapy group and two in the placebo group withdrew from the study because of altered (decreased) libido AEs.
4. DISCUSSION
This was the first study to prospectively assess the impact of DUT‐TAM FDC therapy on sexual activity, sexual desire, and bother in sexually active men with LUTS secondary to BPH, using the MSHQ instrument to evaluate specific aspects of male sexual dysfunction. The findings from this post hoc analysis of a double‐blind, randomised, placebo‐controlled, parallel‐group study revealed a statistically significant reduction (i.e. worsening) in MSHQ domain scores for sexual activity (Months 1, 3, 6, 9, and 12), sexual desire (Months 6, 9, and 12), and bother (Months 1, 3, 6, 9, and 12), in patients treated with DUT‐TAM FDC, compared with placebo. At baseline, a moderate correlation was seen between the sexual activity and sexual desire domains and the ejaculation, erection, and satisfaction domains presented in the primary analysis.14
Although the observed reductions in sexual activity and sexual desire domain scores were significantly greater with DUT‐TAM FDC than with placebo, the treatment differences in the mean domain scores across the time points were numerically small for sexual desire (–0.33 to –0.89) and sexual activity (–0.25 to –0.33) and unlikely to be clinically relevant, although a minimum clinically meaningful change in MSHQ score has yet to be determined. These results are in line with those observed in the primary analysis in that the absolute changes from baseline and the differences at 12 months between placebo and the DUT‐TAM FDC therapy group, in terms of the erection and overall satisfaction domains, were numerically very small and unlikely to be of clinical relevance.14 Changes in the total MSHQ score are driven largely by changes in the scores for the ejaculation domain, which in the primary analysis, reduced by an average of 7.5 points from baseline to Month 12 in the DUT‐TAM FDC therapy group compared with 0.6 points in the placebo group (P < 0.001).14 However, as for MSHQ total score, a minimum clinically meaningful change in ejaculation domain scores is yet to be determined. Previous studies have shown sensitivity of the total MSHQ scores to both diagnostic status and treatment conditions.17, 18, 19 The post hoc correlation analyses between sexual activity/desire and ejaculation, erection, and satisfaction domains were all in the expected direction, and in line with previously described correlations between sexual desire, erectile dysfunction, and sexual satisfaction.20
Currently, the potential mechanisms of loss or alteration of libido are unknown. A complex combination of biological, psychological, and social factors contributes to sexual desire.21 Based on the major influence androgens have on sexual desire, a reduction in libido may indicate androgen deficiency arising from either pituitary or testicular dysfunction, which can be evaluated by measurement of serum testosterone.22 If testosterone concentrations fall within the normal range, it is unlikely that endocrine factors are responsible for loss of libido.22 Interestingly, 5ARIs have been shown to elevate serum testosterone concentrations during treatment,23 which may suggest that endocrine factors are unlikely to be responsible for the loss of libido seen in this study. Serum concentrations of dihydrotestosterone (DHT), a more potent androgen than testosterone,24 decrease during treatment with 5ARIs because of inhibition of conversion from testosterone.25 Whether this contributes to loss of libido remains to be elucidated; however, it has been suggested that male sexual function is primarily related to testosterone as opposed to DHT.26 Evidence to support this comes from male pseudohermaphrodites, who are deficient in DHT and the 5α‐reductase type 2 enzyme, but have been reported to have an intact sex‐drive.26, 27
A limitation of the findings presented in this paper is that the clinical relevance of the observed changes in MSHQ domain scores remains uncertain. In addition, there was no in‐depth assessment of quality of life to accompany domain score changes. The 12‐month study duration did not allow for evaluation of the long‐term effects of DUT‐TAM FDC on sexual activity and desire. Furthermore, MSHQ domain scores for sexual activity, sexual desire, and bother have not been independently validated, and therefore should be interpreted with caution. Finally, this post hoc analysis assessed the effect of DUT‐TAM FDC therapy on the MSHQ sexual activity and desire domain scores, as compared with placebo, but it was not powered to compare the impact of either 5ARI or adrenoreceptor antagonist monotherapies with placebo. Therefore, we cannot infer what impact these therapies might have on male libido when used in isolation.
In conclusion, although significantly different from those seen with placebo, changes in sexual activity and sexual desire domain scores over 12 months of DUT‐TAM FDC treatment were numerically small and of questionable clinical relevance. As anticipated, sexual activity and sexual desire domain scores were positively correlated with ejaculation, erection, and satisfaction domain scores in the expected direction. These findings help clarify the impact of combined therapy with DUT‐TAM FDC on libido in sexually active men with BPH and can help inform clinical decision‐making.
DISCLOSURES
Raymond C. Rosen received research support from Boehringer Ingelheim, Bayer Healthcare, Eli Lilly, Shionogi, and Pfizer. Claus G. Roehrborn is a consultant for GSK, Lilly, Procept, NxThera, Neotract, and Sophiris and has previously received grants or research support form NxThera, Neotract, Procept, and Astellas. Michael J. Manyak is a current employee of GSK and owns stocks/shares in GSK. Juan Manuel Palacios‐Moreno is a current employee of GSK and owns stocks/shares in GSK. Timothy H. Wilson is an employee of PARAXEL International (Durham, NC, USA) and owns stocks/shares in GSK. Zrinka Lulic is a current employee of GSK and owns stocks/shares in GSK. Francois Giuliano is a consultant for Pfizer, Sanofi, Menarini, Recordati, and Ipsen.
AUTHOR CONTRIBUTIONS
RCR*, CGR*, MJM, JMP‐M, THW, ZL, and FG* were involved in concept/design of the study, analysed/interpreted the data and were involved in drafting the article or revising it critically for important intellectual content.
*Equal contribution
ACKNOWLEDGEMENTS
This study was funded by GlaxoSmithKline (GSK; study number: GSK116115/NCT01777269). Lisa Auker, PhD, of Fishawack Indicia Ltd, UK, provided medical writing support, which was funded by GSK, but did not contribute to the study design, or acquisition, analysis or interpretation of data.
Rosen RC, Roehrborn CG, Manyak MJ, et al. Evaluation of the impact of dutasteride/tamsulosin combination therapy on libido in sexually active men with lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH): A post hoc analysis of a prospective randomised placebo‐controlled study. Int J Clin Pract. 2019;73:e13282 10.1111/ijcp.13282
RCR, CGR, and FG contributed equally to this work.
REFERENCES
- 1. Gravas S, Bach T, Bachmann A, et al. EAU guidelines on management of non‐neurogenic male lower urinary tract symptoms (LUTS), incl. Benign Prostatic Obstruction (BPO). 2016; https://uroweb.org/wp-content/uploads/Non-Neurogenic-Male-LUTS_2705.pdf. Accessed 02 April 2018.
- 2. Ertel P, Adalig B, Demircan I, et al. Understanding patient and physician perceptions of benign prostatic hyperplasia in Asia Pacific, Latin America and the Commonwealth of Independent States: the Prostate Research on Behaviour and Education (PROBE) II survey. Int J Clin Pract. 2016;70:870‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Debruyne F, Barkin J, van Erps P, et al. Efficacy and safety of long‐term treatment with the dual 5 alpha‐reductase inhibitor dutasteride in men with symptomatic benign prostatic hyperplasia. Eur Urol. 2004;46:488‐494; discussion 95. [DOI] [PubMed] [Google Scholar]
- 4. Roehrborn CG, Boyle P, Nickel JC, et al. Efficacy and safety of a dual inhibitor of 5‐alpha‐reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002;60:434‐441. [DOI] [PubMed] [Google Scholar]
- 5. Kirby RS, Roehrborn C, Boyle P, et al. Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the Prospective European Doxazosin and Combination Therapy (PREDICT) trial. Urology. 2003;61:119‐126. [DOI] [PubMed] [Google Scholar]
- 6. McConnell JD, Roehrborn CG, Bautista OM, et al. The long‐term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387‐2398. [DOI] [PubMed] [Google Scholar]
- 7. Favilla V, Russo GI, Privitera S, et al. Impact of combination therapy 5‐alpha reductase inhibitors (5‐ARI) plus alpha‐blockers (AB) on erectile dysfunction and decrease of libido in patients with LUTS/BPH: a systematic review with meta‐analysis. Aging Male. 2016;19:175‐181. [DOI] [PubMed] [Google Scholar]
- 8. Rosen RC. Assessment of sexual dysfunction in patients with benign prostatic hyperplasia. BJU Int. 2006;97:29‐33; discussion 44‐5. [DOI] [PubMed] [Google Scholar]
- 9. Li MK, Garcia LA, Rosen R. Lower urinary tract symptoms and male sexual dysfunction in Asia: a survey of ageing men from five Asian countries. BJU Int. 2005;96:1339‐1354. [DOI] [PubMed] [Google Scholar]
- 10. Vallancien G, Emberton M, Harving N, van Moorselaar RJ. Sexual dysfunction in 1,274 European men suffering from lower urinary tract symptoms. J Urol. 2003;169:2257‐2261. [DOI] [PubMed] [Google Scholar]
- 11. Roehrborn CG, Siami P, Barkin J, et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4‐year results from the CombAT study. Eur Urol. 2010;57:123‐131. [DOI] [PubMed] [Google Scholar]
- 12. La Torre A, Giupponi G, Duffy D, et al. Sexual dysfunction related to drugs: a critical review. part v: alpha‐blocker and 5‐ARI drugs. Pharmacopsychiatry. 2016;49:3‐13. [DOI] [PubMed] [Google Scholar]
- 13. Rosen RC, Catania J, Pollack L, et al. Male Sexual Health Questionnaire (MSHQ): scale development and psychometric validation. Urology. 2004;64:777‐782. [DOI] [PubMed] [Google Scholar]
- 14. Roehrborn CG, Manyak MJ, Palacios‐Moreno JM, et al. A prospective randomised placebo‐controlled study of the impact of dutasteride/tamsulosin combination therapy on sexual function domains in sexually active men with lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH). BJU Int. 2018;121:647‐658. [DOI] [PubMed] [Google Scholar]
- 15. World Medical Association . WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. 2008. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed 02 April 2018.
- 16. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use . ICH Harmonised Tripartite Guideline—Guideline for Good Clinical Practice E6(R1). 1996. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed 02 April 2018.
- 17. Rosen RC, Fitzpatrick JM. Ejaculatory dysfunction in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. BJU Int. 2009;104:974‐983. [DOI] [PubMed] [Google Scholar]
- 18. Song SH, Son H, Kim KT, et al. Effect of tamsulosin on ejaculatory function in BPH/LUTS. Asian J Androl. 2011;13:846‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maggi M, Heiselman D, Knorr J, et al. Impact of Testosterone solution 2% on ejaculatory dysfunction in hypogonadal men. J Sex Med. 2016;13:1220‐1226. [DOI] [PubMed] [Google Scholar]
- 20. Burger B, Weidner W, Altwein JE. Prostate and sexuality: an overview. Eur Urol. 1999;35:177‐184. [DOI] [PubMed] [Google Scholar]
- 21. DeLamater JD, Sill M. Sexual desire in later life. J Sex Res. 2005;42:138‐149. [DOI] [PubMed] [Google Scholar]
- 22. Wein AJ, Kavoussi LR, Partin AW, Peters CA. Campbell‐Walsh Urology. Philadelphia, USA: Elsevier; 2016. [Google Scholar]
- 23. Clark RV, Hermann DJ, Cunningham GR, et al. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha‐reductase inhibitor. J Clin Endocrinol Metab. 2004;89:2179‐2184. [DOI] [PubMed] [Google Scholar]
- 24. Deslypere JP, Young M, Wilson JD, McPhaul MJ. Testosterone and 5 alpha‐dihydrotestosterone interact differently with the androgen receptor to enhance transcription of the MMTV‐CAT reporter gene. Mol Cell Endocrinol. 1992;88:15‐22. [DOI] [PubMed] [Google Scholar]
- 25. Drobnis EZ, Nangia AK. 5α‐reductase inhibitors (5ARIs) and male reproduction. Adv Exp Med Biol. 2017;1034:59‐61. [DOI] [PubMed] [Google Scholar]
- 26. Zhu Y‐S, Sun G‐H. 5α‐reductase isozymes in the prostate. J Med Sci. 2005;25:1‐12. [PMC free article] [PubMed] [Google Scholar]
- 27. Imperato‐McGinley J, Guerrero L, Gautier T, Peterson RE. Steroid 5alpha‐reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science. 1974;186:1213‐1215. [DOI] [PubMed] [Google Scholar]


