Abstract
Background
Histamine H1 receptor antagonists are widely used in the treatment of allergic diseases. H1 receptors are expressed on bone cells and histamine takes part in regulation of bone metabolism. Loratadine is often prescribed to children.
Purpose
The aim of the present study was to investigate the effects of loratadine on the skeletal system of young rats.
Material and methods
Loratadine (0.5, 5, and 50 mg/kg p.o. daily) was administered for 4 weeks to male Wistar rats, 6-week-old at the start of the experiment. Bone mass, mass of bone mineral, calcium, and phosphorus content in the bone mineral of the tibia, femur, and L-4 vertebra, histomorphometric parameters of the femur, mechanical properties of the proximal tibial metaphysis, femoral diaphysis and femoral neck, and serum levels of bone turnover markers were examined.
Results
Loratadine at 0.5 and 5 mg/kg did not significantly affect the skeletal system of young rats. At 50 mg/kg, loratadine decreased the femoral length, increased content of calcium and phosphorus in the bone mineral of the vertebra, and tended to improve mechanical properties of the tibial metaphysis.
Conclusion
High-dose loratadine slightly but significantly affected development of the skeletal system in rapidly growing rats.
Keywords: loratadine, histamine H1 receptor antagonists, bone, rats
Introduction
The period of childhood and adolescence plays a key role in the development of the skeletal system. The human skeleton is subjected to intense modeling and remodeling processes. Achieving the optimum bone mass requires proper interaction of life-style, dietetic, and physical activity factors.1 Chronic pediatric diseases and their treatment may strongly affect bone health, leading to both short-term and long-term consequences.2
High prevalence of allergic disorders in children worldwide makes an important public health problem; for example, the calculated worldwide prevalence of allergic rhinitis is 12.66%.3 The International Study for Asthma and Allergies in Childhood (ISAAC) demonstrated an increase in the prevalence of symptoms of some allergic diseases in children in many centers.4 Histamine plays a fundamental role as a mediator of allergic reactions, mainly through H1 receptors. Antagonists of H1 histamine receptors are indicated, among others, in acute allergic reactions in food allergy, allergic rhinitis, and chronic spontaneous urticaria. They belong to the most commonly prescribed drugs in children.5 Nowadays, second-generation antihistamines (like cetirizine, loratadine) are preferable to first-generation drugs (like promethazine, hydroxyzine) in the treatment of most of the allergic symptoms, due to their longer duration of action and better safety profile.5 There are no data on the effects of antihistamines, usually used long term, on the skeletal system in children.
Bone modeling and remodeling are controlled by autocrine, paracrine and endocrine, as well as central nervous system regulation. One of the endogenous factors affecting bone remodeling is histamine. Histamine plays an essential role as a mediator of allergic reactions and in the regulation of gastric acid secretion; it is also a central nervous system neurotransmitter.6,7 Histamine exerts its effects through four histamine receptors (H1–H4). In vitro and in vivo experimental studies demonstrated that histamine is involved in the regulation of bone metabolism.8–13 Expression of histamine H1 and H2 receptors in bone cells (osteoblasts and osteoclasts) is well documented,8–10,14 and histamine is synthesized in osteoclast precursors.8 Histamine increases bone resorption both directly, acting on osteoclast precursors and osteoclasts, and indirectly, by stimulation of the RANKL expression in osteoblasts.8–10 In some, but not all in vivo experimental studies, H1 and H2 receptor antagonists were demonstrated to exert favorable effects on the bone tissue.
The role of stimulation and blockade of histamine receptors in the development of skeletal changes in humans is not clear. The epidemiological and clinical data are extremely scarce. Mastocytosis, a disease characterized by clonal accumulation and/or proliferation of mast cells in various organs, is associated with increased risk of osteoporosis;15 histamine is the most abundant mediator released from mast cells.16 In postmenopausal women suffering from pollen-allergy, increased bone fracture prevalence was observed.17 Despite the very wide use of histamine H1 and H2 receptor antagonists, little is known about their effects on bone metabolism in humans. There is no consensus on the effects of H2 receptor antagonists, used in upper gastrointestinal diseases, on bones.18–23 In experimental animals (rats), H2 antagonists counteracted the development of estrogen-deficiency-induced osteoporosis12,24,25 and did not affect the skeletal system in control rats with normal estrogen levels.12,24–26
As far as H1 receptor antagonists, widely used in the treatment of allergic diseases, are concerned, to our knowledge, only three reports addressed the effect of H1 antagonists on human bones.21,27,28 Promethazine was reported to increase vertebral bone mineral content in postmenopausal women.27 In a population-based study of older adults, the users of H1 receptor antagonists had slightly higher femoral neck bone mineral density than the non-users.21 In the most recent observational case–control study, although users of H1 antagonists were at marginally increased risk of all fractures, they had lower risk of hip fractures than non-users.28
In experimental conditions, an inhibitory effect of H1 receptor antagonists on osteoclastogenesis in vitro was demonstrated for mepyramine and meclozine.8,10,29 In vivo, promethazine, but not another H1 receptor antagonist chlorpheniramine, favorably affected the skeletal system of old mice.30 Promethazine and meclozine counteracted the development of estrogen deficiency-induced bone changes in rats and mice, respectively;29,31 however, the effects of meclozine were mediated partially by upregulation of pregnane X receptor (PXR).29 Inhibition of osteoclast recruitment and differentiation in a rat synchronized model of localized bone resorption due to short-term treatment with mepyramine was demonstrated.8 It has been shown recently that meclozine increased longitudinal skeletal growth in transgenic mice with achondroplasia and in wild type mice.32 However, mepyramine, promethazine, and meclozine are non-selective, sedating antihistamines (first-generation).
The data on the effects of currently used, selective and non-sedating (second-generation) antihistamines are inconsistent. Although cetirizine was reported to decrease, probably due to inhibition of alveolar bone resorption, orthodontic tooth movement in rats,33 its long-term administration did not exert any beneficial effects both in H+/K+-ATPase beta subunit knockout mice, showing osteoporotic phenotype, and wild type mice.14 In our previous study, loratadine (5 mg/kg p.o. daily for 4 weeks) did not counteract estrogen deficiency-induced bone loss in ovariectomized rats.26 However, in non-ovariectomized rats, we demonstrated that loratadine slightly favorably affected the mineralization and mechanical properties of the femur. The effect on mechanical properties of compact bone was probably connected with increased bone formation.26
Taken together, little is known about the effects of second-generation antihistamines on the skeletal system. None of the human or experimental studies concerned the effects of those drugs on the skeletal system in immature organisms. The aim of the present study was to investigate the effect of loratadine administered for 4 weeks on the skeletal system of young, fast-growing male rats. The doses used (0.5, 5, and 50 mg/kg p.o. daily) ranged from a dose similar to that used in children (10 mg in children above 30 kg body mass),5 through a dose 10 times higher, to a dose far beyond the therapeutic range, respectively. Here we report that loratadine at the lowest dose did not affect the skeletal system in rapidly growing rats, whereas high dose loratadine slightly but significantly affected longitudinal bone growth and mineralization.
Materials and methods
Animals and drugs used
The experiments were performed on 6-week-old male Wistar rats obtained from the Center of Experimental Medicine, Medical University of Silesia, Katowice. The rats were fed a standard laboratory diet (Labofeed B, Wytwórnia Pasz “Morawski”, Kcynia, Poland). Before the rats were divided into experimental groups, they acclimated for a week. The protocol for the experiments on animals was approved by the Local Ethics Commission, Katowice, Poland (the permission number 86/2011). The European Union guidelines (Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes) were followed.
Drugs used: loratadine tablets 10 mg (Flonidan, Sandoz, Kundl, Austria), ketamine (Bioketan, Vetoquinol Biowet, Gorzów Wlkp., Poland), xylazine (Xylapan, Vetoquinol Biowet, Gorzów Wlkp., Poland), and tetracycline hydrochloride (Sigma-Aldrich Co., St. Louis, MO, USA).
Animals were divided into 4 groups (n=10):
I – control rats
II – rats treated with loratadine at a dose of 0.5 mg/kg p.o.
III – rats treated with loratadine at a dose of 5 mg/kg p.o.
IV – rats treated with loratadine at a dose of 50 mg/kg p.o.
Loratadine was administered as tap water suspensions, prepared with the addition of Tween 20 (quantum satis), at a volume of 4 mL/kg. The rats from the control group were given the vehicle at the same volume. The drug or the vehicle were administered to the rats by oral gavage, once daily for 4 weeks. In order to mark the calcification front, tetracycline hydrochloride was administered twice at a dose of 20 mg/kg i.p. (1 day before the start and on the last day of the loratadine or vehicle administration). During the experiment, 4 rats died, probably due to errors made during the administration of the drug or vehicle. Therefore, at the end of the experiment, the number of rats for groups I, II, III, and IV was 9, 10, 9, and 8, respectively.
After 4 weeks of loratadine or vehicle administration, after overnight fasting, the animals were anesthetized with ketamine and xylazine, and sacrificed by cardiac exsanguination. The liver was isolated and weighed. The tibial and femoral bones, and L-4 vertebras, were isolated and cleaned of soft tissue. The left tibias, femurs, and vertebras were weighed, and the length and the diameter of the long bones at half-length were measured. The left tibia, left femur, L-4 vertebra, and the proximal part of the right femur of each rat were wrapped in gauze soaked in 0.9% NaCl solution and stored below −18°C for further studies.34 The right tibias and distal parts of the femurs were used for histomorphometric evaluations. The blood serum was also frozen before the measurements.
Biochemical studies
Serum concentrations of osteocalcin (a marker of bone formation) and C-terminal telopeptide fragments of type I collagen (CTX-I; a marker of bone resorption) were measured by enzyme immunoassays (Rat-MID Osteocalcin EIA and RatLaps EIA, respectively, Immunodiagnostic Systems, Boldon, Tyne and Wear, UK). Serum total calcium and inorganic phosphorus concentrations were measured spectrophotometrically, using kits produced by Pointe Scientific, Canton, MI, USA. The manufacturers’ instructions were followed.
Bone composition and mineralization studies
The left tibia, femur, and L-4 vertebra were lyophilized for 9 days using FreeZone 6 lyophilizer (temperature: −51°C, pressure: 0.03 mBa; Labconco, Kansas City, MO, USA) and weighed. The bones were then ashed at 640°C for 48 hrs in a muffle furnace L9/11/C6 (Nabertherm, Lilienthal, Germany). The contents of bone mineral, bone organic substances, and water in the bones were calculated (as the ratios to the bone mass). The ashed bones were dissolved in 6 M HCl and then diluted in distilled water in order to spectrophotometrically assess the calcium and phosphorus contents in the bone mineral, with the use of Pointe Scientific, Canton, MI, USA, kits.
Bone histomorphometric studies
The histomorphometric measurements of the longitudinal cross-sections of the femoral metaphysis were conducted on the decalcified slides stained with hematoxylin and eosin. The histomorphometric measurements of the transverse cross-sections of the tibial and femoral diaphysis were carried out on the unstained, undecalcified preparations, prepared as previously described.35,36
The measurements were carried out using the OsteoMeasure system with OsteoMeasure XP v1.3.0.1 software (OsteoMetrics, Decatur, GA, USA). The standardized nomenclature developed by the American Society for Bone and Mineral Research (ASBMR) was used for description of the histomorphometric measurements.37 The bone volume to tissue volume ratio (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and trabecular number (Tb.N) were measured in cancellous bone of the distal femoral metaphysis. The width of the reserve, proliferative, and hypertrophic zones of the epiphyseal cartilage (growth plate)38 was also measured in the longitudinal preparations from the distal femoral bone.
In cortical bone (tibial and femoral diaphysis), the following parameters were measured: transverse cross-sectional area of the whole diaphysis (Tt.Ar), transverse cross-sectional area of the marrow cavity (Ma.Ar), and the Ma.Ar/Tt.Ar ratio. Moreover, the mineral apposition rate (MAR) in the tibial and femoral diaphysis was calculated based on the measurements of the distance between the stripes of tetracycline incorporated to the bone, on the periosteal (Ps) and marrow cavity (endosteal; Es) side.
Bone mechanical properties studies
Instron 3342 apparatus (measuring range 0–500 N; Instron, Norwood, MA, USA) was used for measurements of bone mechanical properties. To estimate the mechanical strength of the left femoral diaphysis (cortical bone) and left proximal tibial metaphysis (mostly cancellous bone), 3-point bending tests were used. A compression test was used to determine the strength of the right femoral neck. The data obtained during the measurements were analyzed using the Bluehill 2 version 2.14 software (Instron, Norwood, MA, USA). The frequency of sampling was 100 Hz.
In the bending tests, values of extrinsic parameters (depending on the dimensions of the bones): load, displacement, and the energy, absorbed in the range of load from the start to the given load point, were evaluated at 3 points: the yield point (0.05% offset), the maximum load point, and the fracture point. The intrinsic parameters (independent of the size of the bones): Young’s modulus and stress were also determined.
In order to determine the strength of the left femoral diaphysis, the bone was placed on supporting points, and the load was directed perpendicularly to the long axis of the femur in the half-length of the bone, as previously described.34,36 In order to determine the Young’s modulus and the stress values, it was assumed that the femoral diaphysis was an elliptical pipe. To determine the moment of inertia, necessary for calculations, the transverse cross-sections of the right femoral diaphysis were made in the mid-length, and the internal and external diameters of the diaphysis were measured using OsteoMeasure system (OsteoMetrics, Decatur, GA, USA).
In order to determine the strength of the proximal metaphysis of the left tibia, the proximal epiphysis was removed and the load was applied perpendicularly to the long axis of the tibia (3 mm from the proximal edge of the bone), as previously described.36,39 In order to determine the Young’s modulus and the stress values, it was assumed that the cross-section of the bone at the fracture site had the shape of a circle.
To determine the femoral neck strength, the diaphysis, cut at the mid-length of the femur, was fixed in a methacrylate plate and the load was applied to the head of the femur.36 The maximum load was measured.
Statistical analysis
Since results for many parameters failed to meet ANOVA assumptions, Kruskal–Wallis test followed by Mann–Whitney U test (Statistica 13.1; StatSoft Polska, Kraków, Poland) were used for evaluation of the significance of the results. P-values <0.05 were considered significant and marked in the tables and figures. Moreover, those results, which differed from the results of the control rats at P<0.1 in Mann–Whitney U test (if Kruskal–Wallis test P<0.1) were described as tendencies in the text. The results are presented as median (min–max).
Results
Body mass gain and serum biochemical parameters
The rats on which the experiments were carried out were in the phase of the most rapid growth, since the body mass of the control rats increased by 80% during the 4-week period of observation (Table 1). Administration of loratadine at doses of 0.5 and 5 mg/kg p.o. daily did not affect the body mass gain, whereas the body mass gain tended to decrease in the rats to which loratadine was administered at a dose of 50 mg/kg daily in comparison with the control rats. In rats administered the highest loratadine dose, mass of the liver tended to increase (not shown).
Table 1.
Effects of loratadine (0.5–50 mg/kg p.o.) on the body mass gain, serum levels of total calcium, inorganic phosphorus, and bone turnover markers in young male rats
| Parameter/group | C | L0.5 | L5 | L50 |
|---|---|---|---|---|
| Initial body mass (g) | 150.0 (135.0–168.0) | 155.5 (140.0–174.0) | 154.0 (140.0–179.0) | 159.0 (152.0–182.0) |
| Body mass gain after four weeks (g) | 122.0 (110.0–143.0) | 124.5 (109.0–131.0) | 118.0 (108.0–141.0) | 116.0 (87.0–119.0) |
| Total calcium (mg/100 mL) | 10.18 (9.67–11.15) | 10.45 (9.36–11.15) | 10.61 (9.91–11.39) | 10.76 (9.91–11.70) |
| Inorganic phosphorus (mg/100 mL) | 8.16 (7.39–10.42) | 8.69 (7.36–9.71) | 8.33 (6.83–9.37) | 8.89 (7.49–9.78) |
| Osteocalcin (ng/mL) | 327.4 (279.7–366.0) | 329.8 (251.2–366.6) | 301.2 (272.9–369.3) | 292.6 (218.3–373.2) |
| CTX-I (ng/mL) | 44.0 (37.7–63.3) | 48.4 (40.4–57.3) | 50.7 (40.2–73.5) | 50.8 (40.3–63.3) |
Notes: Results are presented as median (min–max); n=8–10. Loratadine at doses of 0.5 mg/kg, 5 mg/kg, or 50 mg/kg was administered orally, once daily for 4 weeks. The final measurements of the body mass were made before the last loratadine or vehicle administration. Kruskal–Wallis test followed by Mann–Whitney U test were used for statistical evaluation of loratadine effect in comparison with the control rats.
Abbreviations: C, control rats; CTX-I, C-terminal type I collagen fragments; L0.5, rats treated with loratadine at a dose of 0.5 mg/kg p.o.; L5, rats treated with loratadine at a dose of 5 mg/kg p.o.; L50, rats treated with loratadine at a dose of 50 mg/kg p.o.
Administration of loratadine did not affect the serum concentrations of total calcium, inorganic phosphorus and the markers of bone turnover: bone formation (osteocalcin) and bone resorption (C-terminal telopeptides of type I collagen – CTX-I) in the blood serum in comparison to the control rats (Table 1).
Bone length, composition, and mineralization
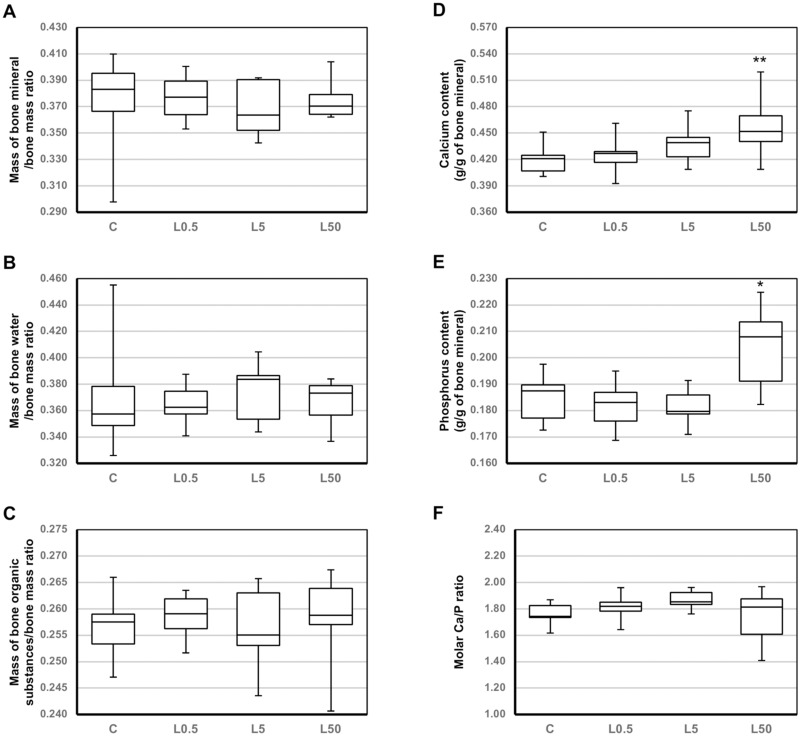
The use of loratadine at lower doses (0.5 and 5 mg/kg p.o.) did not significantly affect the macrometric parameters (length, diameter) of the long bones (tibia and femur), as well as bone mass, mineral mass, composition (the ratios of the mass of bone mineral, bone organic substances and bone water to the bone mass), and calcium and phosphorus content in the bone mineral in the long bones and the L-4 vertebra (Table 2, Figure 1, data for the tibia not shown). However, after administration of loratadine at 5 mg/kg p.o., bone mineralization in the femur (the bone mineral mass/bone mass ratio; Table 2) insignificantly decreased, and concomitantly, in the vertebra mineral, calcium content tended to increase and molar calcium/phosphorus ratio insignificantly increased (Figure 1). Also, the femoral length tended to decrease in rats administered the medium loratadine dose.
Table 2.
Effects of loratadine (0.5–50 mg/kg p.o.) on the length, mass, composition, and mineralization of the femur in young male rats
| Parameter/Group | C | L0.5 | L5 | L50 |
|---|---|---|---|---|
| Bone length (mm) | 33.84 (33.02–34.92) | 33.81 (32.81–34.70) | 33.30 (29.72–34.33) | 33.26 (32.30–33.67)** |
| Bone mass (g) | 0.678 (0.622–0.718) | 0.692 (0.635–0.739) | 0.677 (0.593–0.722) | 0.688 (0.580–0.729) |
| Bone mineral mass (g) | 0.258 (0.244–0.276) | 0.257 (0.245–0.289) | 0.249 (0.211–0.273) | 0.259 (0.206–0.286) |
| Mass of bone mineral /bone mass ratio | 0.387 (0.359–0.402) | 0.379 (0.361–0.400) | 0.368 (0.355–0.396) | 0.377 (0.355–0.414) |
| Mass of bone water /bone mass ratio | 0.373 (0.341–0.407) | 0.378 (0.355–0.396) | 0.385 (0.362–0.399) | 0.381 (0.333–0.404) |
| Mass of bone organic substances/bone mass ratio | 0.241 (0.234–0.257) | 0.244 (0.240–0.254) | 0.246 (0.236–0.253) | 0.241 (0.239–0.253) |
| Calcium content (g/g of bone mineral) | 0.379 (0.352–0.391) | 0.377 (0.358–0.404) | 0.375 (0.352–0.387) | 0.382 (0.358–0.404) |
| Phosphorus content (g/g of bone mineral) | 0.163 (0.156–0.176) | 0.160 (0.141–0.180) | 0.161 (0.157–0.182) | 0.162 (0.154–0.184) |
| Molar Ca/P ratio | 1.79 (1.59–1.91) | 1.86 (1.60–2.04) | 1.80 (1.60–1.88) | 1.82 (1.61–1.97) |
Notes: Results are presented as median (min–max); n=8–10. Loratadine at doses of 0.5 mg/kg, 5 mg/kg, or 50 mg/kg was administered orally, once daily for 4 weeks. Kruskal–Wallis test followed by Mann–Whitney U test were used for statistical evaluation of loratadine effect in comparison with the control rats. ** P<0.01 in comparison with the control rats.
Abbreviations: C, control rats; Ca, calcium; L0.5, rats treated with loratadine at a dose of 0.5 mg/kg p.o.; L5, rats treated with loratadine at a dose of 5 mg/kg p.o.; L50, rats treated with loratadine at a dose of 50 mg/kg p.o.; P, phosphorus.
Figure 1.
Effects of loratadine (0.5–50 mg/kg p.o.) on the composition and mineralization of the L-4 vertebra in young male rats. Results are presented as median (line), interquartile range (box), and minimum and maximum values (whiskers); n=8–10. Loratadine at doses of 0.5 mg/kg, 5 mg/kg, or 50 mg/kg was administered orally, once daily for 4 weeks. Kruskal–Wallis test followed by Mann–Whitney U test were used for statistical evaluation of loratadine effect in comparison with the control rats. *P<0.05, **P<0.01 – in comparison with the control rats.
Abbreviations: C, control rats; L0.5, rats treated with loratadine at a dose of 0.5 mg/kg p.o.; L5, rats treated with loratadine at a dose of 5 mg/kg p.o.; L50, rats treated with loratadine at a dose of 50 mg/kg p.o.
Similarly, after administration of loratadine at the highest dose (50 mg/kg p.o.), most of those parameters remained unaffected. However, a significant decrease in the length of the femur was observed (Table 2). Moreover, administration of loratadine at the highest dose induced a significant increase in the content of calcium and phosphorus in the bone mineral of the L-4 vertebra, with no effect on their molar ratio (Figure 1).
Bone histomorphometry
Loratadine at all used doses did not significantly affect the investigated histomorphometric parameters of the femur (Table 3). The width of the proliferative and hypertrophic zones of the femoral epiphyseal cartilage insignificantly decreased in rats treated with loratadine at 50 mg/kg p.o., in comparison with the control rats.
Table 3.
Effects of loratadine (0.5–50 mg/kg p.o.) on histomorphometric parameters of the distal metaphysis, diaphysis, and distal epiphyseal cartilage in the femur in young male rats
| Parameter/group | C | L0.5 | L5 | L50 | |
|---|---|---|---|---|---|
| Metaphysis | BV/TV (%) | 37.4 (31.7–48.6) | 40.9 (32.1–47.2) | 38.0 (31.2–49.7) | 40.0 (28.5–54.7) |
| Tb.Th (μm) | 55.5 (52.1–78.8) | 62.9 (49.4–67.4) | 64.2 (54.5–72.0) | 59.1 (51.1–74.5) | |
| Tb.Sp (μm) | 108.4 (66.1–112.1) | 94.1 (74.8–104.9) | 112.9 (72.0–128.5) | 90.6 (54.4–128.1) | |
| Tb.N (mm−1) | 6.10 (5.37–7.78) | 6.68 (6.00–7.13) | 5.60 (5.19–7.70) | 6.72 (5.58–8.33) | |
| Diaphysis | Ct.Ar (mm2) | 4.30 (4.01–4.74) | 4.40 (3.97–4.77) | 4.22 (3.89–4.67) | 4.08 (3.62–4.76) |
| Ma.Ar (mm2) | 3.90 (3.19–4.73) | 3.97 (3.46–5.13) | 3.91 (3.59–5.19) | 3.95 (3.61–4.53) | |
| Ma.Ar/Tt.Ar | 0.476 (0.443–0.503) | 0.485 (0.442–0.553) | 0.485 (0.446–0.552) | 0.492 (0.441–0.509) | |
| Ps.MAR (µm/day) | 6.37 (3.17–8.02) | 5.11 (3.96–8.36) | 5.79 (5.15–6.60) | 6.02 (4.94–8.03) | |
| Es.MAR (µm/day) | 4.45 (3.15–5.69) | 3.73 (2.82–7.19) | 5.37 (3.14–6.20) | 4.56 (3.71–6.24) | |
| Epiphyseal cartilage | Reserve zone width (μm) | 38.1 (28.7–40.5) | 40.1 (34.0–41.5) | 39.3 (31.9–48.5) | 36.6 (30.5–41.6) |
| Proliferative zone width (μm) | 50.1 (45.1–53.2) | 48.8 (38.8–57.9) | 52.8 (41.7–55.5) | 44.8 (42.0–52.2) | |
| Hypertrophic zone width (μm) | 39.6 (33.9–45.0) | 42.0 (33.3–43.9) | 41.6 (37.4–47.3) | 37.0 (34.0–39.1) | |
Notes: Results are presented as median (min–max); n=8–10. Loratadine at doses of 0.5 mg/kg, 5 mg/kg, or 50 mg/kg was administered orally, once daily for 4 weeks. Kruskal–Wallis test followed by Mann–Whitney U test were used for statistical evaluation of loratadine effect in comparison with the control rats.
Abbreviations: BV/TV, bone volume/tissue volume ratio; C, control rats; Ct.Ar, transverse cross-sectional area of the cortical bone; Es.MAR, endosteal mineral apposition rate; L0.5, rats treated with loratadine at a dose of 0.5 mg/kg p.o.; L5, rats treated with loratadine at a dose of 5 mg/kg p.o.; L50, rats treated with loratadine at a dose of 50 mg/kg p.o.; Ma.Ar, transverse cross-sectional area of the marrow cavity; Ma.Ar/Tt.Ar, transverse cross-section of the marrow cavity/total diaphysis area ratio; Ps.MAR, periosteal mineral apposition rate; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness.
Only in the transverse cross-sections of the tibial diaphysis, loratadine at a dose of 5 mg/kg p.o. induced an increase in the endosteal mineral apposition rate (P<0.01; not shown); after the highest loratadine dose, this parameter only tended to increase.
Bone mechanical properties
Administration of loratadine at the lower doses did not affect the mechanical properties of the proximal tibial metaphysis, built mostly of cancellous bone (Table 4, data for the yield point not shown), femoral diaphysis built of compact bone (not shown), and femoral neck (compact and cancellous bone; not shown). After administration of loratadine at the highest dose (50 mg/kg p.o.), there was a strong tendency to increase Young’s modulus of the tibial metaphysis in relation to the control rats. Other parameters of cancellous bone of the tibial metaphysis: the load, stress, and energy for maximum load and fracture points insignificantly increased.
Table 4.
Effects of loratadine (0.5–50 mg/kg p.o.) on mechanical properties of the tibial metaphysis in young male rats
| Parameter/group | C | L0.5 | L5 | L50 |
|---|---|---|---|---|
| Young’s modulus (MPa) | 577 (428–862) | 560 (447–915) | 662 (559–961) | 861 (452–1010) |
| Maximum load (N) | 36.1 (29.2–38.5) | 34.4 (28.4–38.2) | 32.4 (27.6–44.1) | 43.0 (30.6–62.2) |
| Displacement for maximum load (mm) | 0.922 (0.642–1.971) | 1.081 (0.713–2.007) | 1.211 (0.700–1.735) | 1.319 (0.591–2.001) |
| Energy for maximum load (mJ) | 21.0 (13.0–50.0) | 24.5 (14.0–57.0) | 26.0 (13.0–49.0) | 37.0 (13.0–61.0) |
| Stress for maximum load (MPa) | 19.0 (13.5–23.8) | 18.2 (15.2–22.1) | 19.1 (13.8–25.7) | 23.1 (16.0–33.8) |
| Fracture load (N) | 27.3 (23.2–35.5) | 31.0 (20.2–36.4) | 29.0 (26.8–34.6) | 34.4 (27.0–42.3) |
| Displacement for fracture load (mm) | 2.001 (1.476–2.006) | 2.002 (1.585–2.007) | 2.001 (1.498–2.004) | 2.001 (1.713–2.002) |
| Energy for fracture load (mJ) | 51.0 (42.0–56.0) | 52.0 (37.0–61.0) | 51.0 (46.0–63.0) | 63.0 (47.0–84.0) |
| Stress for fracture load (MPa) | 15.1 (11.5–23.4) | 16.3 (10.7–22.1) | 15.9 (13.7–19.8) | 17.5 (15.8–22.9) |
Notes: Results are presented as median (min–max); n=8–10. Loratadine at doses of 0.5 mg/kg, 5 mg/kg, or 50 mg/kg was administered orally, once daily for 4 weeks. Kruskal–Wallis test followed by Mann–Whitney U test were used for statistical evaluation of loratadine effect in comparison with the control rats.
Abbreviations: C, control rats; L0.5, rats treated with loratadine at a dose of 0.5 mg/kg p.o.; L5, rats treated with loratadine at a dose of 5 mg/kg p.o.; L50, rats treated with loratadine at a dose of 50 mg/kg p.o.
Discussion
Although experimental data suggest that histamine plays a role in regulating bone metabolism via, among others, H1 receptors, there are no data on the effects of antihistamines on the skeletal system of young organisms. The problem of the effects of widely used drugs on the bone system in adolescents is important because bone fractures often occur in this age group; the incidence of fractures in children is much bigger than in adults.40 The fracture rate in children is reported to be in the range of 12.0–36.1/1000 per year, with the occurrence peaking approximately at age 14 years for boys and 11 years for girls.41 Boys are more prone to fractures than girls.41 In the present study, the effects of 4-week administration of loratadine were investigated in immature male rats (6-week-old at the start of the experiment). The rats were at their 11. week of age at the end of the experiment. Most of the male rats reach the sexual maturity by this time,42 so it may be assumed that the rats in our study were adolescent.43 Taking into account the rat life-span, the 4-week period of drug administration in rats corresponded to about 2.5 years in humans.43
During 4 weeks of observation, the body mass of control rats almost doubled, reaching a mean value of about 275 g; however, bone strength was much lower than in adult rats. A comparison of the results regarding bone strength of young rats in this study with the results of our previous study in which the measurements were conducted in 28-week-old male rats (weighing about 380 g) indicated that the young control animals had much lower bone strength. In the femoral diaphysis (built of compact bone), the mean maximum load was lower by 39% in young rats than in adult rats, and in femoral neck (compact and cancellous bone) by 40%. The biggest differences were observed in the proximal tibial metaphysis (built mostly of cancellous bone): the maximum load in young rats was lower by 65%.44
In the present study, loratadine was administered to rats at 0.5, 5, and 50 mg/kg p.o. daily for 4 weeks. We used the dose of 5 mg/kg, because that dose induced some slight skeletal effects in our previous study carried out on mature female rats26 and was in the range of doses which had been earlier used in rats.45 It should be stated, however, that there is very small number of preclinical studies on loratadine administered orally in rats. For example, a dose of similar range (8 mg/kg p.o.) was used to investigate loratadine metabolism in different species.46 The dose of 0.5 mg/kg corresponds to the doses used in children (10 mg daily in children above 30 kg body mass, and 5 mg in children below 30 kg of body mass).5 Doses usually used in experiments on rats are higher than in humans because of faster rat metabolism. The rat doses equivalent to human doses may be calculated using a factor of 6.2 based on body surface area.47 However, comparison of the metabolism and pharmacokinetic parameters of loratadine in rats and monkeys46 indicates that such an approach may be not justified in the case of loratadine. Therefore, it seems that only the dose of 0.5 mg/kg corresponded to the doses used in humans and that both higher doses were above the therapeutic range.
Results of the present study indicate that loratadine administered at doses of 0.5 mg/kg and 5 mg/kg p.o. daily for 4 weeks did not significantly affect the skeletal system of young male rats. The results concerning the lower loratadine doses are consistent with those of Aasarød et al who did not demonstrate any significant difference after administration of cetirizine (3 mg/kg p.o. daily for 6 months) to adult female wild type mice. Moreover, no effect of cetirizine was observed in H+/K+-ATPase beta subunit knockout mice, showing osteoporotic phenotype.14 Also in our previous study on adult female rats, no effects of loratadine (5 mg/kg p.o. daily for 4 weeks) on bone mechanical properties and mineralization in estrogen-deficient rats were observed.26
Only after administration of loratadine at a dose of 50 mg/kg p.o. daily, slight skeletal effects were demonstrated in the present study. The body mass gain in those rats tended to decrease, and longitudinal bone growth significantly decreased in relation to the control rats. The decrease in the femoral length and decreases (albeit statistically insignificant) in the width of proliferative and hypertrophic zones of the epiphyseal cartilage (growth plate) were demonstrated in those rats. Those results are completely at variance with the data reported for meclozine. Meclozine, a first-generation antihistamine, increased longitudinal skeletal growth in transgenic mice with achondroplasia, and also in wild type mice.32 Meclozine facilitated proliferation and differentiation of chondrocytes, attenuating abnormally activated FGFR3 signaling in achondroplasia. Since meclozine was chosen to those experiments by screening 1186 drugs, and other antihistamines were found ineffective, it was concluded that effects of meclozine on chondrogenesis were unlikely to be relevant to its antihistamine activity.48 In young children, long-term safety profiles of some second-generation antihistamines were similar to placebo (studies involved, among others, body mass and height measurements).49
Administration of loratadine induced some changes in mineralization of cancellous bone (L-4 vertebra). The calcium content in the bone mineral increased in a dose–dependent manner, reaching significance at the highest dose. Also, after administration of that loratadine dose, an increase in the phosphorus content in the bone mineral of the L-4 vertebra was observed, with no effect on the molar calcium/phosphorus (Ca/P) ratio. On the other hand, after administration of loratadine at 5 mg/kg p.o., bone mineralization (the bone mineral mass/bone mass ratio) in the femur insignificantly decreased and at the same time a tendency to increase the calcium content and an insignificant increase in the molar calcium/phosphorus ratio in the vertebra mineral were demonstrated. No significant changes in the calcium and phosphorus content in bone mineral were observed for the long bones, where the share of compact bone is much bigger than in the vertebra.
The changes in the calcium and phosphorus content in the vertebra bone mineral, although significant, are difficult to interpret. Calcium phosphates, the most important inorganic constituents of biological hard tissues, are present in bone in the form of carbonated hydroxyapatite.50 Alterations from normal crystal size, composition, and structure disorder mechanical integrity of the bone.51 The molar Ca/P ratio for the vertebra of control rats in the present study (mean value: 1.76) was similar to those reported for human bones (1.71) and hydroxyapatite (1.67).50 Increases in the molar ratio may indicate some disorders of the mineral structure, since, for example, the ratio for amorphous calcium phosphate is 1.2–2.2 and for tetracalcium phosphate – 2.0.50 Since on aging the Ca/P ratio and the carbonate content increase,52 it may be speculated that loratadine might affect the maturation of bone hydroxyapatite crystals. Further studies on the effects of antihistamines on bone mineralization should be performed.
In the present study, loratadine did not affect the mechanical properties of compact bone (femoral diaphysis). However, after administration of loratadine at 50 mg/kg p.o. daily, there was a strong tendency to increase Young’s modulus in the proximal tibial metaphysis, indicating improvement of material properties of cancellous bone. This could have resulted from changes in bone mineralization. On the other hand, in our previous study,26 loratadine (5 mg/kg p.o. daily) did not affect mechanical properties of cancellous bone of the proximal tibial metaphysis and slightly increased the strength of the femoral neck and diaphysis (compact bone) in mature female rats. The differences between the results of this study and our previous study might have resulted not only from the different age of rats but also from sex differences. For example, such differences concerning loratadine metabolism were demonstrated for male and female rats.46
It should be stated that although majority of previous studies suggested that histamine H1 receptor antagonists inhibit bone resorption,8,10,29,31,33 they, with the exception of one study,33 concerned first-generation antihistamines, in which effects on other than H1 receptors might play a role. Administration of loratadine at any dose did not affect serum markers of bone resorption (CTX-I) and bone formation (osteocalcin), as well as histomorphometric parameters of cancellous bone (femoral metaphysis) and most of histomorphometric parameters of compact bone. The only significant change was an increase in the endosteal mineral apposition rate in the tibia after administration of loratadine at 5 mg/kg p.o. daily. This increase in bone formation is to some extent consistent with an increase in the transverse growth (periosteal) observed in our previous study on mature female rats.26
In summary, administration of loratadine at the lowest dose (0.5 mg/kg p.o. daily) did not affect the skeletal system in rapidly growing male rats, whereas at the highest dose (50 mg/kg p.o. daily), it significantly affected the longitudinal bone growth and cancellous bone mineralization. Since loratadine at a dose of 50 mg/kg p.o. daily in the present study not only affected the skeletal system but also tended to decrease body mass gain and increase the liver mass, it is possible that the dose was too high to be relevant to the effects of antihistamines in children. In fact, the possibility that the skeletal changes induced by the highest loratadine dose might have been the effect of the toxicity, not only the H1 receptor blockade, cannot be excluded. However, also after administration of loratadine at a dose of 5 mg/kg p.o. daily, some tendencies indicating changes in bone mineralization were demonstrated. The results of this study suggest that long-term use of antihistamine drugs during the growth period in children may affect the skeletal system, which may influence the risk of osteoporosis in the future.
Conclusion
High dose loratadine slightly but significantly affected the development of the skeletal system in rapidly growing rats. Taking into account wide and long-term use of antihistamines, it seems that the results of the present study should encourage clinical observations regarding the influence of chronic use of antihistamines on the skeletal system in children and adolescents.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Preliminary results of this paper were presented at the 18th International Congress of the Polish Pharmacological Society as a poster presentation. The poster’s abstract was published in “Conference Abstracts” in Pharmacol. Rep. 2013;65(Suppl.1):44. https://doi.org/10.1016/S1734-1140(13)71323-9.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115(12):3318–3325. doi: 10.1172/JCI27071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams KM. Update on bone health in pediatric chronic disease. Endocrinol Metab Clin North Am. 2016;45(2):433–441. doi: 10.1016/j.ecl.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pols DHJ, Wartna JB, van Alphen EI, et al. Interrelationships between atopic disorders in children: a meta-analysis based on ISAAC questionnaires. PLoS One. 2015;10(7):e0131869. doi: 10.1371/journal.pone.0131869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asher MI, Montefort S, Björkstén B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet. 2006;368(9537):733–743. doi: 10.1016/S0140-6736(06)69283-0 [DOI] [PubMed] [Google Scholar]
- 5.Fitzsimons R, van der Poel LA, Thornhill W, du Toit G, Shah N, Brough HA. Antihistamine use in children. Arch Dis Child Educ Pract Ed. 2015;100(3):122–131. doi: 10.1136/archdischild-2013-304446 [DOI] [PubMed] [Google Scholar]
- 6.Lieberman P. Histamine, antihistamines, and the central nervous system. Allergy Asthma Proc. 2009;30(5):482–486. doi: 10.2500/aap.2009.30.3264 [DOI] [PubMed] [Google Scholar]
- 7.Lieberman P. The basics of histamine biology. Ann Allergy Asthma Immunol. 2011;106(2 Suppl):S2–S5. doi: 10.1016/j.anai.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 8.Biosse-Duplan M, Baroukh B, Dy M, de Vernejoul MC, Saffar JL. Histamine promotes osteoclastogenesis through the differential expression of histamine receptors on osteoclasts and osteoblasts. Am J Pathol. 2009;174(4):1426–1434. doi: 10.2353/ajpath.2009.080871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deyama Y, Kikuiri T, Ohnishi GI, et al. Histamine stimulates production of osteoclast differentiation factor/receptor activator of nuclear factor-κB ligand by osteoblasts. Biochem Biophys Res Commun. 2002;298(2):240–246. doi: 10.1016/s0006-291x(02)02440-3 [DOI] [PubMed] [Google Scholar]
- 10.Ikawa Y, Yonekawa T, Ohkuni Y, Kuribayashi M, Fukino K, Ueno K. A comparative study of histamine activities on differentiation of osteoblasts and osteoclasts. J Toxicol Sci. 2007;32(5):555–564. doi: 10.2131/jts.32.555 [DOI] [PubMed] [Google Scholar]
- 11.Fouilloux I, Duplan MB, Baroukh B, Cherruau M, Saffar JL, Lesclous P. Mast cell activation and degranulation occur early during induction of periosteal bone resorption. Bone. 2006;38(1):59–66. doi: 10.1016/j.bone.2005.07.026 [DOI] [PubMed] [Google Scholar]
- 12.Lesclous P, Schramm F, Gallina S, Baroukh B, Guez D, Saffar JL. Histamine mediates osteoclastic resorption only during the acute phase of bone loss in ovariectomized rats. Exp Physiol. 2006;91(3):561–570. doi: 10.1113/expphysiol.2006.033217 [DOI] [PubMed] [Google Scholar]
- 13.Dobigny C, Saffar JL. H1 and H2 histamine receptors modulate osteoclastic resorption by different pathways: evidence obtained by using receptor antagonists in a rat synchronized resorption model. J Cell Physiol. 1997;173(1):10–18. doi: [DOI] [PubMed] [Google Scholar]
- 14.Aasarød KM, Stunes AK, Mosti MP, et al. Effects of the histamine 1 receptor antagonist cetirizine on the osteoporotic phenotype in H+/K+ATPase beta subunit KO mice. J Cell Biochem. 2016;117(9):2089–2096. doi: 10.1002/jcb.25514 [DOI] [PubMed] [Google Scholar]
- 15.Degboé Y, Eischen M, Nigon D, et al. Prevalence and risk factors for fragility fracture in systemic mastocytosis. Bone. 2017;105:219–225. doi: 10.1016/j.bone.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 16.Rossini M, Zanotti R, Orsolini G, et al. Prevalence, pathogenesis, and treatment options for mastocytosis-related osteoporosis. Osteoporos Int. 2016;27(8):2411–2421. doi: 10.1007/s00198-016-3539-1 [DOI] [PubMed] [Google Scholar]
- 17.Ferencz V, Meszaros S, Csupor E, et al. Increased bone fracture prevalence in postmenopausal women suffering from pollen-allergy. Osteoporos Int. 2006;17(3):484–491. doi: 10.1007/s00198-005-0011-z [DOI] [PubMed] [Google Scholar]
- 18.Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology. 2010;139(1):93–101. doi: 10.1053/j.gastro.2010.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Vries F, Cooper AL, Cockle SM, van Staa TP, Cooper C. Fracture risk in patients receiving acid-suppressant medication alone and in combination with bisphosphonates. Osteoporos Int. 2009;20(12):1989–1998. doi: 10.1007/s00198-009-0891-4 [DOI] [PubMed] [Google Scholar]
- 20.Eom CS, Park SM, Myung SK, Yun JM, Ahn JS. Use of acid-suppressive drugs and risk of fracture: a meta-analysis of observational studies. Ann Fam Med. 2011;9(3):257–267. doi: 10.1370/afm.1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinjo M, Setoguchi S, Solomon DH. Antihistamine therapy and bone mineral density: analysis in a population-based US sample. Am J Med. 2008;121(12):1085–1091. doi: 10.1016/j.amjmed.2008.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwok CS, Yeong JKY, Loke YK. Meta-analysis: risk of fractures with acid-suppressing medication. Bone. 2011;48(4):768–776. doi: 10.1016/j.bone.2010.12.015 [DOI] [PubMed] [Google Scholar]
- 23.Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int. 2006;79(2):76–83. doi: 10.1007/s00223-006-0021-7 [DOI] [PubMed] [Google Scholar]
- 24.Lesclous P, Guez D, Saffar JL. Short-term prevention of osteoclastic resorption and osteopenia in ovariectomized rats treated with the H2 receptor antagonist cimetidine. Bone. 2002;30(1):131–136. [DOI] [PubMed] [Google Scholar]
- 25.Lesclous P, Guez D, Baroukh B, Vignery A, Saffar JL. Histamine participates in the early phase of trabecular bone loss in ovariectomized rats. Bone. 2004;34(1):91–99. doi: 10.1016/j.bone.2003.08.007 [DOI] [PubMed] [Google Scholar]
- 26.Folwarczna J, Janas A, Pytlik M, Śliwiński L, Wiercigroch M, Brzęczek A. Modifications of histamine receptor signaling affect bone mechanical properties in rats. Pharmacol Rep. 2014;66(1):93–99. doi: 10.1016/j.pharep.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 27.Tyan ML. Effect of promethazine on lumbar vertebral bone mass in postmenopausal women. J Intern Med. 1993;234(2):143–148. [DOI] [PubMed] [Google Scholar]
- 28.Abrahamsen B, Vestergaard P. Proton pump inhibitor use and fracture risk - effect modification by histamine H1 receptor blockade. Observational case-control study using national prescription data. Bone. 2013;57(1):269–271. doi: 10.1016/j.bone.2013.08.013 [DOI] [PubMed] [Google Scholar]
- 29.Guo J, Li W, Wu Y, et al. Meclizine prevents ovariectomy-induced bone loss and inhibits osteoclastogenesis partially by upregulating PXR. Front Pharmacol. 2017;8:693. doi: 10.3389/fphar.2017.00693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tyan ML, Blahd WH. Effects of H1 blocking agents on age-related osteopenia in the mouse. Mech Ageing Dev. 1987;38(3):287–293. [DOI] [PubMed] [Google Scholar]
- 31.Rico H, Gómez M, Revilla M, et al. Effects of promethazine on bone mass and on bone remodeling in ovariectomized rats: a morphometric, densitometric, and histomorphometric experimental study. Calcif Tissue Int. 1999;65(4):272–275. doi: 10.1007/s002239900697 [DOI] [PubMed] [Google Scholar]
- 32.Matsushita M, Hasegawa S, Kitoh H, et al. Meclozine promotes longitudinal skeletal growth in transgenic mice with achondroplasia carrying a gain-of-function mutation in the FGFR3 gene. Endocrinology. 2015;156(2):548–554. doi: 10.1210/en.2014-1914 [DOI] [PubMed] [Google Scholar]
- 33.Meh A, Sprogar Š, Vaupotic T, et al. Effect of cetirizine, a histamine (H1) receptor antagonist, on bone modeling during orthodontic tooth movement in rats. Am J Orthod Dentofac Orthop. 2011;139(4):e323–e329. doi: 10.1016/j.ajodo.2009.11.013 [DOI] [PubMed] [Google Scholar]
- 34.Turner CH, Burr DB. Basic biochemical measurements of bone: a turtorial. Bone. 1993;14(4):595–608. doi: 10.1016/8756-3282(93)90081-K [DOI] [PubMed] [Google Scholar]
- 35.Folwarczna J, Pytlik M, Janiec W. Effects of doxycycline on development of changes in histomorphometric parameters of bones induced by bilateral ovariectomy in rats. Pol J Pharmacol. 2003;55(3):433–441. [PubMed] [Google Scholar]
- 36.Folwarczna J, Pytlik M, Zych M, et al. Favorable effect of moderate dose caffeine on the skeletal system in ovariectomized rats. Mol Nutr Food Res. 2013;57(10):1772–1784. doi: 10.1002/mnfr.201300123 [DOI] [PubMed] [Google Scholar]
- 37.Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res. 2013;28(1):2–17. doi: 10.1002/jbmr.1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villemure I, Stokes IAF. Growth plate mechanics and mechanobiology. A survey of present understanding. J Biomech. 2009;42(12):1793–1803. doi: 10.1016/j.jbiomech.2009.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stürmer EK, Seidlová-Wuttke D, Sehmisch S, et al. Standardized bending and breaking test for the normal and osteoporotic metaphyseal tibias of the rat: effect of estradiol, testosterone, and raloxifene. J Bone Miner Res. 2006;21(1):89–96. doi: 10.1359/JBMR.050913 [DOI] [PubMed] [Google Scholar]
- 40.Rennie L, Court-Brown CM, Mok JYQ, Beattie TF. The epidemiology of fractures in children. Injury. 2007;38(8):913–922. doi: 10.1016/j.injury.2007.01.036 [DOI] [PubMed] [Google Scholar]
- 41.Naranje SM, Erali RA, Warner WC, Sawyer JR, Kelly DM. Epidemiology of pediatric fractures presenting to emergency departments in the United States. J Pediatr Orthop. 2016;36(4):e45–e48. doi: 10.1097/BPO.0000000000000595 [DOI] [PubMed] [Google Scholar]
- 42.Campion SN, Carvallo FR, Chapin RE, et al. Comparative assessment of the timing of sexual maturation in male Wistar Han and Sprague-Dawley rats. Reprod Toxicol. 2013;38:16–24. doi: 10.1016/j.reprotox.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 43.Sengupta P. The laboratory rat: relating its age with human’s. Int J Prev Med. 2013;4(6):624–630. [PMC free article] [PubMed] [Google Scholar]
- 44.Folwarczna J, Pytlik M, Śliwiński L, Cegieła U, Nowińska B, Rajda M. Effects of propranolol on the development of glucocorticoid-induced osteoporosis in male rats. Pharmacol Rep. 2011;63(4):1040–1049. [DOI] [PubMed] [Google Scholar]
- 45.Lieber G, Jimenez J, Hunter JC, McLeod RL, Jia Y. Concomitant activity of histamine and cysteinyl leukotrienes on porcine nasal mucosal vessels and nasal inflammation in the rat. Pharmacology. 2010;85(5):311–318. doi: 10.1159/000299792 [DOI] [PubMed] [Google Scholar]
- 46.Ramanathan R, Alvarez N, Su AD, et al. Metabolism and excretion of loratadine in male and female mice, rats and monkeys. Xenobiotica. 2005;35(2):155–189. doi: 10.1080/00498250500038906 [DOI] [PubMed] [Google Scholar]
- 47.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. doi: 10.4103/0976-0105.177703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsushita M, Kitoh H, Ohkawara B, et al. Meclozine facilitates proliferation and differentiation of chondrocytes by attenuating abnormally activated FGFR3 signaling in achondroplasia. PLoS One. 2013;8(12):e81569. doi: 10.1371/journal.pone.0081569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simons FER, Simons KJ. H1 antihistamines: current status and future directions. World Allergy Organ J. 2008;1(September):145–155. doi: 10.1097/WOX.0b013e318186fb3a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dorozhkin SV, Epple M. Biological and medical significance of calcium phosphates. Angew Chem Int Ed. 2002;41(17):3130–3146. doi: [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez-Florez N, Garcia-Tunon E, Mukadam Q, et al. An investigation of the mineral in ductile and brittle cortical mouse bone. J Bone Miner Res. 2015;30(5):786–795. doi: 10.1002/jbmr.2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Combes C, Cazalbou S, Rey C. Apatite biominerals. Minerals. 2016;6(2):34. doi: 10.3390/min6020034 [DOI] [Google Scholar]