Abstract
Importance
The transscleral XEN Glaucoma Gel Microstent (XEN‐GGM, Allergan Plc., Parsippany, New Jersey) is implanted by a minimally invasive ab interno technique.
Background
The present study aims to assess the long‐term clinical outcomes in patients after XEN‐GGM implantation.
Design
This prospective, non‐randomized, multi‐centred study was conducted in three countries (Austria, Canada and Germany).
Participants
Sixty‐four consecutive eyes of 64 patients with open angle glaucoma received the XEN‐GGM (63 μm) without Mitomycin C. Thirty‐five (55%) were solo procedures, and 29 (45%) were combined with cataract surgery.
Methods
Visits were planned at baseline, 6 months, 1, 2, 3 and 4 years postoperatively.
Main Outcome Measures
The main outcome measures were mean intraocular pressure (IOP), mean number of IOP lowering medication. Secondary outcome parameters were: visual acuity, visual fields and complete surgical failure (defined as presence of a secondary IOP lowering procedure or loss of light perception) at 4 years, postoperatively.
Results
Mean best‐medicated baseline IOP was 22.5 ± 4.2 mmHg and decreased significantly to 13.4 ± 3.1 mmHg 4 years postoperatively (−40%, n = 34, P < 0.001). Mean number of IOP lowering medication decreased significantly from 2.4 ± 1.3 preoperatively to 1.2 ± 1.3 (−50%, n = 34, P < 0.001) postoperatively. Visual field mean deviation showed no significant change between preoperative and postoperative examinations. Complete surgical failure rate per year was 10%.
Conclusions and Relevance
The XEN‐GGM resulted in lower IOP and a reduction in medications from baseline over 4 years of follow‐up. There was no detectable decrease in visual fields over the study. The surgical failure rate is comparable to other filtration surgeries.
Keywords: efficacy, long‐term, MIGS, safety, XEN glaucoma gel microstent
1. INTRODUCTION
Since the introduction of trabeculectomy in 1968, much effort has been spent on improving glaucoma filtration surgery.1 Of the novel devices that have been introduced,2, 3 the XEN Glaucoma Gel Microstent (XEN‐GGM, Allergan Plc., Parsippany, New Jersey) is the first ab interno minimally invasive glaucoma surgery (MIGS) device that leads to subconjunctival outflow. All other ab interno MIGS devices target Schlemm's Canal or the suprachoroidal space.2, 3 The XEN‐GGM is a transscleral gelatin stent placed through a clear‐corneal incision using a preloaded injector. The implant acts as a shunt between the anterior chamber and the subconjunctival space bypassing the outflow resistance in the trabecular meshwork and beyond. The main principle of the intraocular pressure (IOP) reduction is comparable to the principal of the classic trabeculectomy. However, one of the potential advantages of this new minimally invasive glaucoma surgery over trabeculectomy is that it does not require a conjunctival incision.2, 4
This study aimed to assess the long‐term efficacy after XEN‐GGM implantation with an inner diameter of 63 μm. This 63 μm XEN‐GGM differs from the recently FDA approved 45 μm model.
2. METHODS
This prospective, one‐armed, non‐randomized, multi‐centre evaluation was conducted in three countries in three University Eye Hospitals (Salzburg, Austria; Toronto, Canada; and Bochum, Germany). The study and data accumulation were carried out with previous approval from the appropriate Institutional Review Boards. The authors confirm that the study and data accumulation were in conformity with all country, federal, or state laws, informed consent was obtained, and the study was in adherence to the tenets of the Declaration of Helsinki.
Patients with insufficiently controlled IOP or intolerance of topical therapy in open angle glaucoma (OAG: primary open angle glaucoma, pseudoexfoliation glaucoma and pigmentary dispersion glaucoma) were treated with one XEN‐GGM with or without combined phacoemulsification. Exclusion criteria were angle closure glaucoma, congenital glaucoma, neovascular glaucoma, uveitic glaucoma, previous conjunctival surgery, scarring of the conjunctiva, as well as previous intraocular surgery with the exception of selective laser trabeculoplasty or uncomplicated phacoemulsification with intraocular lens implantation. If the patient received the XEN‐GGM in both eyes, only the first eye was included in the study.
Visits were planned at baseline, then 6, 12, 24, 36 and 48 months postoperatively. Additional visits in this pilot study were introduced at the discretion of the surgeon. At baseline, demographic data, prior surgical procedures, type of glaucoma, central corneal thickness (CCT), IOP measurement using a two‐person method, number of glaucoma medication, best corrected visual acuity in Snellen (VA), and visual field mean deviation in Humphrey perimeter (Carl Zeiss AG, Oberkochen, Germany) or visual field mean defect in Octopus perimeter (Haag Streit International, Koeniz, Switzerland) were collected (VF). Goldmann applanation tonometry was used to measure IOP. After the operator of the Goldmann applanation tonometer adjusted the measuring drum, the reader recorded the reading on the dial. IOP readings were repeated until two consecutive or nonconsecutive measurements were obtained differing by 1 mmHg or less, and the average of the two readings served as the IOP measurement for the visit. The 48 month postoperative visit included IOP measurement, number of glaucoma medication, VA, VF, number of needling procedures, and if applicable time to and type of secondary IOP lowering procedure. VF had to be longitudinally analysed separately at each study centre, because of different visual field machines.
The XEN‐GGM, which was applied in the present study, has an inner diameter of 63 μm, an outer diameter of 240 μm and a length of 6 mm. The XEN‐GGM (63 μm) offers 2 to 3 mmHg of outflow resistance at physiologic aqueous humour production rates calculated with the Hagen‐Poiseuille Equation.2 All consecutive XEN‐GGMs were implanted without adjuvant Mitomycin C injection. Needlings were performed by the surgeon's discretion including the decision if an antifibrotic agent was used during needling procedure. The postoperative care regimen included a stop of IOP lowering drops postoperatively. Topical steroid eye drops (TID) were applied for a minimum of 6 weeks postoperatively and slowly tapered out after 6 weeks. Topical antibiotic eye drops (TID) were additionally applied in the first postoperative week.
The primary outcomes of the study were the IOP and medication reductions 4 years after XEN‐GGM implantation compared to baseline. Secondary outcome parameters were major safety measures as defined below.
Derived from the guidelines of the World Glaucoma Association,5 surgical success was defined as a postoperative IOP ≤ 18 mmHg and ≥ −20% IOP reduction at 4 years compared to baseline. Surgical success was further characterized according to whether this had been achieved without ocular hypotensive medication (complete surgical success) or regardless of the number of ocular hypotensive medication (qualified surgical success). In cases of loss of light perception, secondary IOP lowering procedure (with the exception of needling procedures or standard cataract surgery), or a postoperative IOP < 6 mmHg the patient was not classified as surgical success.
Complete surgical failure 5 was defined as the need for a secondary IOP lowering procedure (with the exception of needling procedures or standard cataract surgery) or loss of light perception, while a mismatch of above defined relative or absolute IOP criteria was not defined as complete surgical failure (since a single elevation in IOP might also be treated conservatively). For surgical success and failure analysis patients who were lost to follow‐up were excluded from analysis.
Major postoperative complications and interventions were recorded and analysed. These included: Secondary surgical procedures (including interventions due to hypotony), exposure of the XEN‐GGM, inner obstruction of the implant, cataract operation because of cataract progression and loss of light perception.
2.1. Statistical analysis
Due to unknown variability of the procedure's effect on IOP and medication, a formal calculation of sample size was not performed. Data consistency was checked and data were screened for outliers by using quantile plots. Cross tabulation tables with χ 2 or Pearson's test were used to analyse crosstabs. Beside descriptive statistics paired t‐tests, a linear regression model, Kaplan‐Meyer survival curves and generalized linear models (binominal and normal) were computed. All reported tests were two‐sided, and P values<0.05 were considered as statistically significant. All statistical analyses in this report were performed by use of IBM SPSS Statistics (Ver. 21, New York).
3. RESULTS
Sixty‐four eyes of 64 patients were treated with the XEN‐GGM between 2009 and 2012. About 35/64 (55%) men, 29/64 (45%) women, 29/64 (45%) right eyes and 35/64 (55%) left eyes were included. In 29/64 eyes (45%) the XEN‐GGM implantation was done in combination with cataract surgery, while 35/64 eyes (55%) were treated in a solo XEN‐GGM procedure. The study population consisted mainly of Caucasians (87%), along with 6% Asians, 5% Africans and 2% Caribbean. About 52% had diagnosed primary open angle glaucoma, 41% pseudoexfoliation glaucoma and 7% pigmentary dispersion glaucoma at baseline.
Eleven out of 64 (17%) eyes were lost to follow‐up after 4 years. Nineteen out of 64 eyes were classified to secondary surgical procedures (see below in the complete surgical failure section). Therefore, 34/64 eyes (53%) were analysed for IOP change, change in number of IOP lowering medication, VA change and VF change.
Additional baseline characteristics and data on needling procedures are presented in Table 1.
Table 1.
Patient baseline characteristics and needlings after XEN glaucoma gel microstent implantation
| n | Minimum | Maximum | Mean | SD | |
|---|---|---|---|---|---|
| Age (y) | 34 | 18.0 | 85.2 | 66.4 | 12.4 |
| Central corneal thickness (μm) | 34 | 490 | 621 | 535.0 | 29.5 |
| Days of follow‐up (d) | 34 | 1279 | 2243 | 1419 | 290 |
| Number of needlings (#) | 34 | 0 | 6 | 1.3 | 1.7 |
| Days to first needling (d) | 18 | 14 | 1061 | 271.0 | 335.0 |
| First needling in 1st year | 13 (72%) | ||||
| First needling in 2nd year | 3 (17%) | ||||
| First needling in 3rd year | 2 (11%) | ||||
| First needling in 4th year | 0 (0%) | ||||
Patients with complete data at 4 years follow‐up and the lack of secondary IOP lowering procedures were included in this analysis. After a mean of 3.9 years an average number of 1.3 needlings was performed. Eighteen out of 34 (53%) of the patients had one or more needlings.
3.1. Intraocular pressure
IOP decreased significantly from 22.5 ± 4.2 mmHg (mean ± SD) preoperatively to 13.4 ± 3.1 mmHg after 4 years (n = 34, P < 0.001, Figure 1). The overall mean IOP at baseline (22.7 ± 4.1 mmHg, n = 64) was not statistically different compared to mean IOP of patients reaching the 4 years endpoint (P = 0.76). No patient demonstrated ocular hypotony at 4 years (IOP < 6 mmHg), but 3/64 (5%) patients required interventions due to low IOP between surgery and 4 years follow‐up. Among these three patients, one had an explantation of the stent due to persistent hypotony postoperatively and was therefore considered as having a secondary IOP lowering procedure. The preoperative IOP (r = 0.64, P < 0.001) as well as a higher number of needlings (r = 0.45, P = 0.008) correlated directly with the relative IOP reduction. There was no difference in mean IOPs between solo vs combined cataract surgery with XEN‐GGM after 4 years (solo: 13.2 ± 2.7, combined: 13.7 ± 3.7, P = 0.65).
Figure 1.
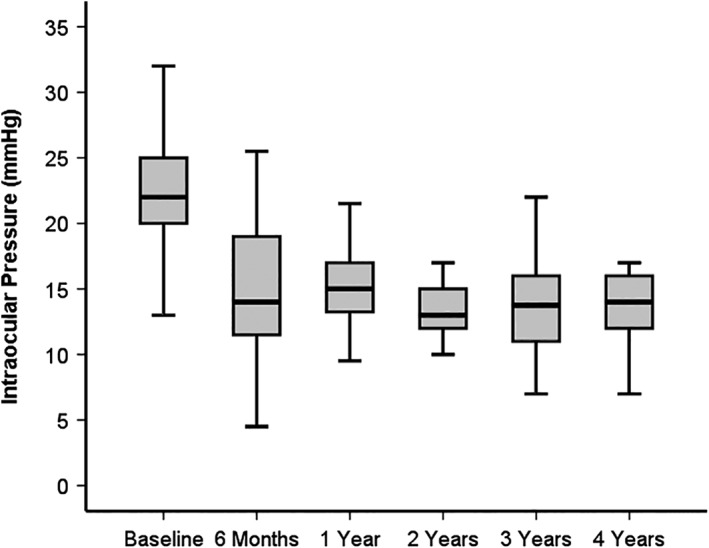
Boxplot of intraocular pressure (IOP) over 4 years after XEN glaucoma gel microstent implantation
A statistically significant reduction of mean IOP of −40% was detected 4 years postoperatively (P < 0.001, n = 34).
3.2. Number of IOP lowering medication
Mean number of medication decreased from 2.4 ± 1.3 (mean ± SD) preoperatively to 1.2 ± 1.3 at the 4 years visit (P < 0.001, n = 34, Figure 2). Regarding the mean number of medications of all 64 patients included at baseline (2.6 ± 1.3, n = 64) compared to the mean number of medications of patients reaching the 4 years control (n = 34), no statistical difference could be detected (P = 0.10). There was no difference in mean number of medications between solo vs combined cataract surgery with XEN‐GGM after 4 years (solo: 1.1 ± 1.3, combined: 1.4 ± 1.4, P = 0.40).
Figure 2.
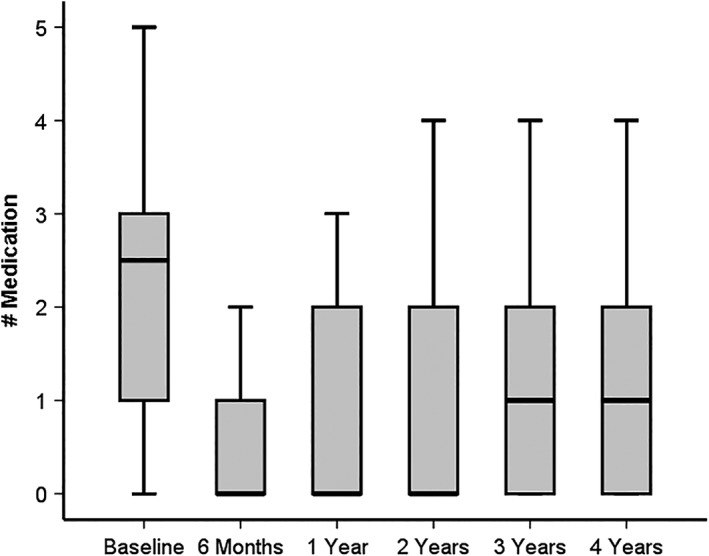
Boxplot of number of intraocular pressure (IOP) lowering medication over 4 years after XEN glaucoma gel microstent implantation
A statistically significant reduction of mean IOP lowering medication of −50% was detected 4 years postoperatively (P < 0.001, n = 34).
3.3. Visual acuity
Combined as well as solo procedures revealed no deterioration of VA during the observed period in mean. Solo procedures had a mean preoperative VA of 0.79 and mean postoperative VA of 0.80 (P = 0.73) after 4 years. When combined with cataract surgery, patients had a mean preoperative VA of 0.53 and a mean postoperative VA of 0.61 (P = 0.398). Three out of 34 patients had a loss of BCVA of two lines or more after 4 years (one patient −2 lines in solo procedures; one patient −3 lines and one patient −4 lines in combined procedures), while eight out of 34 patients showed a gain of BCVA of two lines or more at 4 years, postoperatively compared to baseline (three patients +2 lines in solo procedures; two patients +2 lines, two patients +3 lines and one patient +5 lines in combined procedures). Seven out of 34 patients were excluded from VA analysis because of a secondary standard cataract procedure between baseline and 4 year follow‐up. No patient showed a loss of light perception.
3.4. Visual field
Due to different perimeter models in the study sites, the mean visual field data is displayed separately. Baseline mean deviations (Humphrey perimeter) were −10.2 ± 7.0 dB and −12.2 ± 7.5 dB, and baseline mean defect (Octopus perimeter) was 3.4 ± 3.9 dB. The 4 years postoperative mean deviations were −11.9 ± 10.0 dB (P = 0.506) and −14.0 ± 8.9 dB (P = 0.248), and mean defect was 3.7 ± 4.9 dB (P = 0.710). There was no statistically significant change in the visual fields at each study centre over 4 years.
3.5. Surgical success
Twenty‐eight out of 53 (53%) patients achieved qualified surgical success. Qualified surgical success correlated with gender: female patients (68%) had higher qualified surgical success rates than male patients (39%, P = 0.037). Twelve out of 53 (25%) patients achieved complete surgical success after 4 years. There was no difference among gender in complete surgical success: 28% (female) vs 18% (male), P = 0.38.
3.6. Complete surgical failure
19/53 patients (36%) had a secondary IOP lowering procedure between baseline and 48 months follow‐up and were for this reason classified as complete surgical failure (Figure 3). One eye had an exposure of the implant and therefore needed a bleb revision and also underwent a trabeculectomy. No patient suffered from loss of light perception. Based on a computed 50% percentile of surgical failure in the Kaplan‐Meyer curve we calculated a mean surgical failure rate of 10% per year for the first 5 years postoperatively. Males (50%) had more complete surgical failures than females (20%; 95% CI for effect: 4.3%‐56%, P = 0.023). Between gender no significant differences in type of procedure (XEN solo male: 63%, female: 37%, P = 0.154), age (male: 68.7 ± 10.6, female: 68.2 ± 11.7, P = 0.84), preoperative number of medication (male: 2.8 ± 1.3, female: 2.4 ± 1.4, P = 0.21), preoperative IOP (male: 23.3 ± 4.5 mmHg, female: 22.3 ± 3.6 mmHg, P = 0.35), as well as mean number of needlings (male: 1.3 ± 1.6, female: 1.4 ± 1.8, P = 0.92) could be detected. Postoperative interventions and major complications are listed in Table 2.
Figure 3.
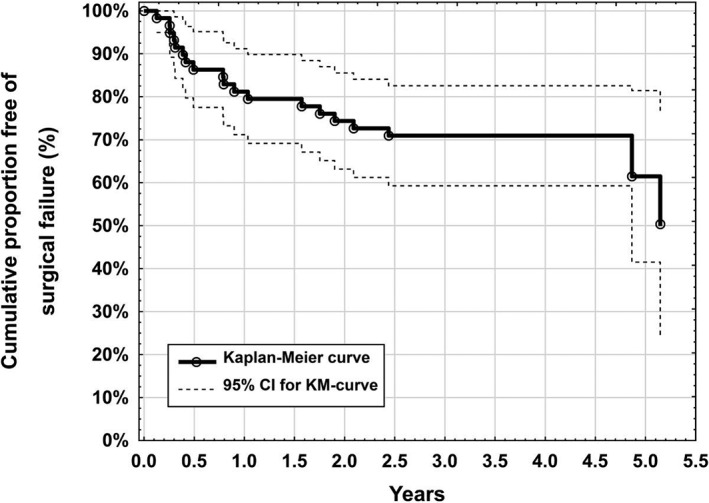
Kaplan‐Meyer survival function for the need of a secondary intraocular pressure lowering surgery after XEN glaucoma gel microstent implantation
Table 2.
Complications after XEN glaucoma gel microstent implantation in a postoperative period of 4 years
| Postoperative complications | N = 64 | |
|---|---|---|
| n | % | |
| Secondary surgical procedure (unrelated; ex: Nd:YAG capsulotomy due to secondary cataract) | 2 | 3 |
| Secondary surgical procedure (related to XEN) due to high IOP | ||
| Selective laser trabeculoplasty | 1 | 2 |
| Filtering glaucoma surgeries (trabeculectomy n = 10, Bearveldt glaucoma implant n = 1, Ahmed valve n = 1) | 12 | 19 |
| Cyclodestructive procedures | 3 | 5 |
| Secondary surgical procedure (related to XEN) due to hypotony (defined as: Choroidal effusion, shallow anterior chamber, hypotony maculopathy) | ||
| Anterior chamber refill† | 3 | 5 |
| Exposure of the XEN implant‡ (and additional filtering glaucoma surgery) | 1 | 2 |
| Inner obstruction of the implant—Resolved with Nd:YAG laser | 2 | 3 |
| Cataract operation due to cataract progression | 7 | 11 |
| Loss of light perception | 0 | 0 |
| Lost of follow‐up | 11 | 17 |
In one patient an explantation of the XEN implant was necessary because of persistent hypotony.
In one patient bleb revision was required due to exposure of the XEN implant and further filtering glaucoma surgery was necessary; intraocular pressure (IOP).
About 50% percentile is estimated to 1880 days and 75% percentile survival to 640 days, respectively.
4. DISCUSSION
While many studies report 12 months results,6, 7, 8 this study reports a much longer follow‐up period. Long‐term efficacy and safety data on MIGS devices and techniques are sparse. We analysed long‐term data on our early consecutive XEN‐GGM implantations. This analysis is primarily intended to show long‐term efficacy and secondarily major safety parameters, since the power of a more detailed safety analysis is limited because of the relatively small number of patients and lack of control group.
Although the number of study centres was small, no single study centre statistically influenced any of the parameters analysed. It should be noted that this study represents the first consecutive patients, which received the XEN‐GGM with an inner lumen size of 63 μm. Since this study was performed in an early period of this novel technique, a learning curve effect may have negatively influenced the results in terms of efficacy, complications or success rates. Furthermore, none of the patients received a Mitomycin C pre‐treatment or other antifibrotic metabolites during or prior XEN‐GGM surgery, which might have decreased fibrosis rates of this filtering procedure. On the other hand, a study population of 88% Caucasians potentially have a higher rate of success than an African‐derived population for example. For biological and statistical reasons we decided only to analyse the first operated eye in patients who were operated in both eyes.
4.1. Efficacy parameters
Glaucoma filtration surgery is effective in terms of lowering IOP and decreasing medication burden.9, 10 Lochhead et al reported a −45% relative IOP reduction after trabeculectomy.11 Ahmed and Baerveldt tube shunts reduce IOP by −50% and −56%, respectively. IOP reductions were reported by Christakis et al at 4 years after surgery.12 We found a −40% IOP reduction at 4 years after XEN‐GGM. Baseline IOPs in our study population were lower than in these studies.11, 12 Therefore, the differences in relative IOP reduction might be explained by differences in study populations between these studies.13 Absolute mean IOPs after trabeculectomy are between 11.7 and 15.5 mmHg in long‐term literature data.9, 11 The mean postoperative IOPs after XEN‐GGM are therefore comparable to trabeculectomy results reported in the literature (recent XEN‐GGM study: 13.4 ± 3.1 mmHg).11, 13, 14, 15 The reduction of IOP lowering medication of 50% with the XEN‐GGM is also comparable to reported rates of reduction after tube shunt and trabeculectomy (ie, TVT Study: Tube group—61%, Trabeculectomy group—59%).13, 16
Pseudoexfoliation and pigmentary glaucomas are risk factors for rapid glaucoma disease progression.17 In this study, there was no difference in efficacy with the presence of pseudoexfoliation or pigmentary glaucoma. It is also notable that the results were comparable whether surgery was performed as a stand‐alone procedure or in combination with cataract surgery. Prior reports indicate that phacotrabeculectomy is not as effective as trabeculectomy in reducing IOP.11 A reduced extent of conjunctival trauma during XEN‐GGM surgery may explain the differences in our study compared to priors.
The long‐term rates of failure after trabeculectomy have been reported to be as high as 50% at 5 years.13, 18, 19 In this study, we calculated a complete surgical failure rate of 10% per year within the first 5 years, which seems to be fairly equal to prior trabeculectomy studies.13, 20 An additional application of adjuvant Mitomycin C during XEN‐GGM implantation might reduce the surgical failure rate. On the other hand, the surgical failure rate as assessed in this study might be underestimated compared to other studies,13, 18, 19 because of the exclusion of absolute IOP or relative IOP reduction criteria from the definition of surgical failure. However, surgical success rates reported in the literature for trabeculectomy or tubes shunt surgery were comparable to this study, although our study applied strict definitions for surgical success (IOP ≤ 18 mmHg).13, 18, 19 Secondary IOP lowering procedures have been recorded accurately in our patients, although a rate of 17% loss to follow‐up should be taken into consideration. The rates for lost to follow‐up are comparable to other studies (ie, TVT study: Tube group: 15% and Trabeculectomy group: 7% at 4 years).13 The reason for a significantly higher success rate in women and higher failure rate in males may be explained by slightly higher preoperative IOP and higher preoperative number of medication in males compared to females. Our results contradict a retrospective study on risk factors for failure after XEN‐GGM, in which men were not associated with higher failure.21 Further prospective randomized trials are therefore needed to investigate surgical success and failure between trabeculectomy and XEN‐GGM, as well as outcome differences in gender after XEN‐GGM.
Bleb needlings are performed frequently after trabeculectomy.22, 23 In this study, the highest risk for receiving an initial needling was in the first year and decreased over time. Because of the lack of a defined postoperative regimen or a needling protocol in this study, the decisions for needlings were made by the surgeons' discretion and are inherently variable. This and the fact that no antifibrotic metabolite was part of the XEN‐GGM procedure might explain our high needling rates.
4.2. Safety parameters
VA and VF's were stable at 4 years postoperatively. Although we detected a loss of BCVA of ≥2 lines in 3/34 (9%) of the patients, this did not significantly affect the mean BCVA change postoperatively. Conversely, VA was shown to deteriorate (≥2 lines in Snellen) in 34% of the patients 3 years after trabeculectomy and mean BCVA decreases further with time after trabeculectomy.24, 25 VF stability in this study was analysed independently at each site since different perimeters were used. However, no statistically significant progression in visual fields were detected at 4 years after XEN‐GGM implantation. Depending on the study, ocular hypotony is a relatively common complication after trabeculectomy and occurs in 1% to 18% of the cases.26, 27, 28 Although 3/64 (5%) patients had persistent clinically significant hypotony after XEN‐GGM implantation, no patient had hypotony at 4 years. Ocular hypotony rates may be higher in the 63 μm version of the stent, due to the wider inner diameter compared to the recent model (45 μm) now available commercially.
The XEN‐GGM lowered IOP and reduced medications at 4 years. The reduction in IOP and medications was comparable to trabeculectomy results reported in prospective trials.13, 16 In this study, patients did not lose vision and maintained visual field stability. Rates for complete surgical failure were comparable to data for invasive procedures like trabeculectomy and tube shunts.13 The XEN‐GGM is a promising option in the surgical treatment of glaucoma. Prospective randomized trials should be conducted to compare this device to trabeculectomy and tube shunt surgery.
CONFLICTS OF INTEREST
Dr. Ahmed is a consultant to and investigator for Allergan Plc., Glaukos Corp., Ivantis Inc., Transcend Medical Inc. and AqueSys Inc., and has received speaker honoraria from Neomedix Inc. Dr. Reitsamer and Dr. Dick are consultants to and investigators for AqueSys Inc., Allergan Plc. and investigator for Transcend Medical Inc. Dr. Sheybani is a consultant for Allergan Plc., Others: nothing to declare.
Lenzhofer M, Kersten‐Gomez I, Sheybani A, et al. Four‐year results of a minimally invasive transscleral glaucoma gel stent implantation in a prospective multi‐centre study. Clin. Experiment. Ophthalmol. 2019;47:581–587. 10.1111/ceo.13463
Dr. Lenzhofer and Dr. Kersten‐Gomez contributed equally to this work.
REFERENCES
- 1. Cairns JE. Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol. 1968;66(4):673‐679. [PubMed] [Google Scholar]
- 2. Sheybani A, Lenzhofer M, Hohensinn M, Reitsamer H, Ahmed II. Phacoemulsification combined with a new ab interno gel stent to treat open‐angle glaucoma: pilot study. J Cataract Refract Surg. 2015;41:1905‐1909. [DOI] [PubMed] [Google Scholar]
- 3. Fea A, Cannizzo PM, Consolandi G, Lavia CA, Pignata G, Grignolo FM. Managing drawbacks in unconventional successful glaucoma surgery: a case report of stent exposure. Case Rep Ophthalmol Med. 2015;2015:847439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lewis RA. Ab interno approach to the subconjunctival space using a collagen glaucoma stent. J Cataract Refract Surg. 2014;40(8):1301‐1306. [DOI] [PubMed] [Google Scholar]
- 5. Grehn Shaarawy S.. Guidelines on design and reporting of glaucoma surgical trials. 2009.
- 6. Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reitsamer HA, Lenzhofer M, Hohensinn M, Hoeh HR, eds. Ab Interno Approach to the Subconjunctival Space: First 852 Eyes Treated with New Minimally Invasive Gel Stent for Treating Glaucoma. San Diego: American Society of Cataract and Refractive Surgery; 2015. [Google Scholar]
- 8. Grover DS, Flynn WJ, Bashford KP, et al. Performance and safety of a new ab interno gelatin stent in refractory glaucoma at 12 months. Am J Ophthalmol. 2017;183:25‐36. [DOI] [PubMed] [Google Scholar]
- 9. Al‐Haddad CE, Abdulaal M, Al‐Moujahed A, Ervin AM, Ismail K. Fornix‐based versus Limbal‐based Conjunctival Trabeculectomy flaps for glaucoma: findings from a Cochrane systematic review. Am J Ophthalmol. 2017;174:33‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Riva I, Roberti G, Katsanos A, Oddone F, Quaranta L. A review of the Ahmed glaucoma valve implant and comparison with other surgical operations. Adv Ther. 2017;34(4):834‐847. [DOI] [PubMed] [Google Scholar]
- 11. Lochhead J, Casson RJ, Salmon JF. Long term effect on intraocular pressure of phacotrabeculectomy compared to trabeculectomy. Br J Ophthalmol. 2003;87(7):850‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christakis PG, Zhang D, Budenz DL, Barton K, Tsai JC, Ahmed IIK. Five‐year pooled data analysis of the Ahmed Baerveldt comparison study and the Ahmed versus Baerveldt study. Am J Ophthalmol. 2017;176:118‐126. [DOI] [PubMed] [Google Scholar]
- 13. Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL. Treatment outcomes in the tube versus trabeculectomy (TVT) study after five years of follow‐up. Am J Ophthalmol. 2012;153(5):789‐803. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qing GP, Wang NL, Wang T, Chen H, Mou DP. Long‐term efficacy of trabeculectomy on Chinese patients with pigmentary glaucoma: a prospective case series observational study. Chin Med J. 2016;129(11):1268‐1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beckers HJ, Kinders KC, Webers CA. Five‐year results of trabeculectomy with mitomycin C. Graefe's Arch Clin Experiment Ophthalmol. 2003;241(2):106‐110. [DOI] [PubMed] [Google Scholar]
- 16. Al Habash A, Aljasim LA, Owaidhah O, Edward DP. A review of the efficacy of mitomycin C in glaucoma filtration surgery. Clin Ophthalmol. 2015;9:1945‐1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan TCW, Bala C, Siu A, Wan F, White A. Risk factors for rapid glaucoma disease progression. Am J Ophthalmol. 2017;180:151‐157. [DOI] [PubMed] [Google Scholar]
- 18. Kirwan JF, Lockwood AJ, Shah P, et al. Trabeculectomy in the 21st century: a multicenter analysis. Ophthalmology. 2013;120(12):2532‐2539. [DOI] [PubMed] [Google Scholar]
- 19. Landers J, Martin K, Sarkies N, Bourne R, Watson P. A twenty‐year follow‐up study of trabeculectomy: risk factors and outcomes. Ophthalmology. 2012;119(4):694‐702. [DOI] [PubMed] [Google Scholar]
- 20. Mietz H, Raschka B, Krieglstein GK. Risk factors for failures of trabeculectomies performed without antimetabolites. Br J Ophthalmol. 1999;83(7):814‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schlenker MB, Gulamhusein H, Conrad‐Hengerer I, et al. Efficacy, safety, and risk factors for failure of standalone ab interno gelatin microstent implantation versus standalone Trabeculectomy. Ophthalmology. 2017;124:1579‐1588. [DOI] [PubMed] [Google Scholar]
- 22. Ung CT, Von Lany H, Claridge KG. Late bleb needling. Br J Ophthalmol. 2003;87(11):1430‐1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feldman RM, Tabet RR. Needle revision of filtering blebs. J Glaucoma. 2008;17(7):594‐600. [DOI] [PubMed] [Google Scholar]
- 24. Kashiwagi K, Kogure S, Mabuchi F, et al. Change in visual acuity and associated risk factors after trabeculectomy with adjunctive mitomycin C. Acta Ophthalmol. 2016;94(7):e561‐e570. [DOI] [PubMed] [Google Scholar]
- 25. Gedde SJ, Heuer DK, Parrish RK. Review of results from the tube versus trabeculectomy study. Curr Opin Ophthalmol. 2010;21(2):123‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schubert HD. Postsurgical hypotony: relationship to fistulization, inflammation, chorioretinal lesions, and the vitreous. Surv Ophthalmol. 1996;41(2):97‐125. [DOI] [PubMed] [Google Scholar]
- 27. Costa VP, Smith M, Spaeth GL, Gandham S, Markovitz B. Loss of visual acuity after trabeculectomy. Ophthalmology. 1993;100(5):599‐612. [DOI] [PubMed] [Google Scholar]
- 28. Suner IJ, Greenfield DS, Miller MP, Nicolela MT, Palmberg PF. Hypotony maculopathy after filtering surgery with mitomycin C. Incidence and treatment. Ophthalmology. 1997;104(2):207‐214. discussion 14‐5. [DOI] [PubMed] [Google Scholar]


