Abstract
Objective
To evaluate the application of contrast‐enhanced ultrasonography (CEUS) for the diagnosis of renal allograft chronic rejection (CR).
Methods
A total of 104 patients who were suspected to have AR or CR were enrolled in this study (derivation group, n = 66; validation group, n = 38). Before biopsy, all patients received an ultrasound examination.
Results
In the CR group, rising time (RT) and time to peak (TTP) of medulla (RTm and TTPm, respectively) were significantly longer compared to those in the AR group. The kidney volume was significantly decreased in the CR group but was increased in the AR group. In the derivation group, age, change in kidney volume, and TTPm were identified as independent predictors by multivariate analysis. Based on the multivariate analysis results and area under receiver operating characteristic (ROC) curves (AUROCs) of individual markers, we constructed a new index as follows: P = −5.424 + 0.074 × age −9.818 × kidney volume change + 0.115 × TTPm; New Index = eP/(1 + eP). The new index discriminates CR from AR and had better AUROCs than any other parameters.
Conclusion
In conclusion, the new index provides a new diagnosis model for CR.
Keywords: contrast media, diagnostic test, graft Rejection, renal transplantation, ultrasonography
Abbreviations
- ABMR
antibody‐mediated rejection
- AR
acute rejection
- ATN
acute tubular necrosis
- AUROC
area under receiver operating characteristic curve
- BMI
body mass index
- CEUS
contrast‐enhanced ultrasonography
- CNI
calcineurin inhibitor
- CR
chronic rejection
- eGFR
estimated glomerular filtration rate
- ISP
immunosuppressive protocol
- +LR
positive likelihood ratio
- ‐LR
negative likelihood ratio
- MDRD
Modification of Diet in Renal Disease
- MMF
mycophenolate mofetil
- NPV
negative predictive value
- PPV
positive predictive value
- PRA
panel reactive antibody
- Pred
prednisone
- QOF
quality of fit
- RI
resistive index
- ROC
receiver operating characteristic
- RT
rising time
- RTc
rising time of cortex
- RTi
rising time of interlobar artery
- RTm
rising time of medulla
- RTs
rising time of segmental artery
- SCr
serum creatinine
- Sen
sensitivity
- Spe
specificity
- SRL
sirolimus
- TCMR
T cell‐mediated rejection
- TICs
time‐intensity curves
- TTP
time to peak
- TTPc
time to peak of cortex
- TTPi
time to peak of interlobar artery
- TTPm
time to peak of medulla
- TTPs
time to peak of segmental artery
- ΔRTm‐c
change in RT between medulla and cortex
- ΔTTPm‐c
change in TTP between medulla and cortex
1. INTRODUCTION
Conventional ultrasound in combination with color Doppler imaging is a common and protocol examination for patients after renal transplantation. However, conventional ultrasound in combination with Doppler imaging can diagnose renal artery stenosis and vein thrombosis, but it is not possible to display subtle microvascular tissue perfusion. Apart from immunological factors, one of the most important factors to ensure a stable allograft function is renal blood supply, which is mainly influenced by parenchymal blood perfusion. Over 90% of renal blood flow in the renal cortex is provided by small renal arterioles and capillaries.1, 2 Therefore, conventional ultrasound is not able to provide accurate and more information for the evaluation of acute and chronic allograft dysfunctions.
Contrast‐enhanced ultrasonography, which employs microbubble contrast agents and complementary harmonic pulse sequences to demonstrate parenchymal perfusion, can be a helpful problem‐solving tool in several clinical scenarios, including kidney diseases.3 The main ultrasound contrast agent approved in Europe is SonoVue (BR1, Bracco, Milan, Italy). Contrast‐enhanced ultrasonography is safe and well‐tolerated in patients without renal toxicity and cross‐allergy anaphylactic reaction.3, 4 The application of CEUS in renal transplants highlights its versatility in immediate problem solving without recourse to other potentially nephrotoxic agents. In addition, CEUS can uniquely provide additional information not available from other modalities about the microcirculation.5
In our previous prospective study, we discriminated AR from ATN in renal allografts using CEUS. After establishing a mathematic model, AR can also be distinguished from non‐AR recipients at any time period after transplantation.6 However, whether CEUS can diagnose renal allograft CR has not been reported. Up to 50% of all rejection episodes are subclinical and occur without changes in standard parameters, such as creatinine or blood urea nitrogen.7, 8 CR, which is mainly mediated by antibodies against donor antigens, has been the major cause of long‐term graft loss. Vasculopathy and disturbances in allograft perfusion occur in CR.9, 10 Most of these vascular insults affect small parenchymal arteries and arterioles, which cannot be assessed by conventional Doppler ultrasound. Thus, it might be helpful to provide CR diagnostic evidence by evaluating renal allograft microperfusion.
In this study, patients who were suspected of allograft rejection with normal serum cyclosporine A and tacrolimus concentrations received renal allograft biopsy. We evaluated the application of CEUS in the assessment of different pathologic renal allograft dysfunction (AR and CR) and further established a novel and simple noninvasive model for predicting CR.
2. MATERIALS AND METHODS
2.1. Patients
A total of 66 renal transplant recipients in the derivation group were enrolled in this prospective study from January 2011 to December 2016. For the validation group, 38 recipients were enrolled from January 2017 to September 2017. The validation group underwent the same studies as the derivation group. All patients received living‐related or deceased donor kidneys. Due to increased SCr either rapidly or slightly, all patients received renal allograft biopsy for pathological diagnosis. Before biopsy, all patients were admitted to our abdominal ultrasound unit for ultrasound examination. Patients with renal allograft artery stenosis, artery or venous thrombus, renal allograft urinary obstruction, perirenal hematoma, ATN, BK virus‐associated nephropathy, tubulointerstitial nephritis, CNI toxicity, and thrombotic microangiopathy were excluded. This study protocol was approved by the Ethics Committee. Procedures in this study were in accordance with the Helsinki Declaration of 2000, with informed consent from the participants.
2.2. Ultrasound examination and principle of contrast‐enhanced ultrasonography
All patients in our study underwent ultrasound‐based measurement of RI and assessment of renal allograft perfusion by CEUS quantification by two experienced ultrasound physicians with at least 10 years of experience in clinical ultrasound examinations. The doctors were blinded to the clinical and laboratory data of patients. To calculate the volume of the transplanted kidney, we used the ellipsoid formula as follows: volume (cm3) = length (cm) × width (cm) × thickness (cm) × π/6. The three dimensions of the transplanted kidney were measured by B‐mode ultrasound examination.
All patients were examined at 8‐10 am on an empty stomach without fluid infusion or caffeine intake. Patients were examined in the supine position. CEUS examination was performed using a Philips iU‐22 ultrasonic apparatus with a C5‐1 probe (Philips, Amsterdam, the Netherlands) with an intravenous bolus injection of 0.6 mL SonoVue. After routine B‐mode ultrasound and color Doppler examination, we chose the longitudinal section of the transplant kidney as the fixed section for CEUS, which showed the renal hilus and the maximum area of the transplant kidney. Image acquisition began at the start of the SonoVue injection, and a capture of 120 s was recorded continuously onto the local hard drive as a DICOM file. The main gain, focus position, TGC, and other presets remained constant when CEUS was being performed. The mechanical index (MI) was set at 0.07.
The proprietary software SonoLiver (TomTec Imaging Systems Gmbh, Munich, Germany) was employed for quantitative data analysis. A 50 mm2 ROI was placed over the area, including the cortex, medulla, interlobar artery, and segmental artery in the mid pole of the transplanted kidneys; consequently, 4 TICs were generated for each patient (Figure 1). In this study, the segmental artery was chosen as the reference region, and the QOF ≥ 75% between the reference region and the analysis region was regarded as the permission of enrolling. We used two quantitative parameters, RT and TTP, for further analysis. The repeatability was good, as previously described.6
Figure 1.
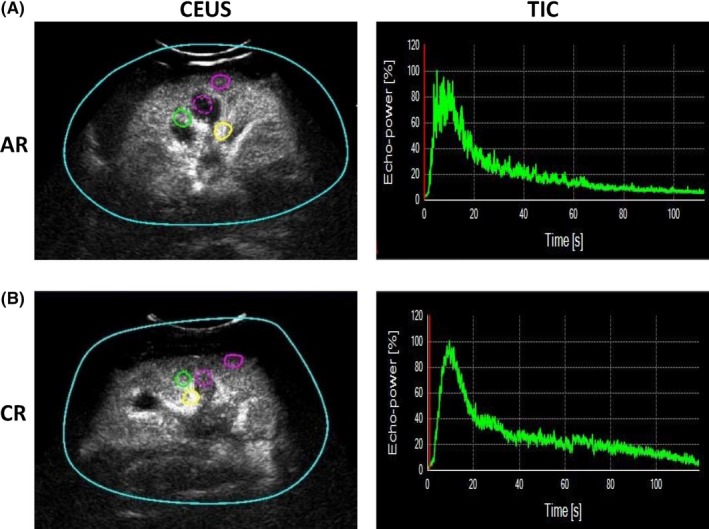
CEUS quantification measurement and TIC. A, The 4 regions of interest were demonstrated. Yellow circle: segmental artery; green circle: interlobar artery; purple dotted circle: medulla; purple solid circle: cortex. B, In AR and CR kidneys, the TIC was coarse, especially in the AR kidney, with apparent ups and downs. In addition, the peak of the TIC for CR was sharper than AR
2.3. Blood and pathological examinations
Blood samples were taken on the same day. All clinical blood tests were performed in the Department of Clinical Laboratory, including routine blood and renal function tests. The eGFR was calculated with the simplified MDRD Study equation. Renal allograft biopsy and histologic examination based on the Banff 2013 classification were performed.
2.4. Data management and statistical analysis
The results were expressed as the mean values ± SD or median (interquartile range). In the derivation group, one‐way ANOVA tests or independent sample t tests were used to compare the markers among the groups. Significant variables from the univariate analysis (P < 0.05) were then subjected to multivariate analysis by forwarding logistic regression to identify independent factors associated with either end point. CR was considered as a positive result and AR as a negative result. A predictive index was constructed by modeling the values of the independent variables. The diagnostic values of parameters were assessed by calculating the AUROCs. The best cutoff points were selected from the ROC curve to identify the presence or absence of CR. For that purpose, we selected cutoff points with a 90% certainty for the presence and absence of CR, thus regarding 10% false‐negative or false‐positive results as clinically acceptable; we also selected an optimal cutoff point according to the best Youden index. The diagnostic accuracy was calculated using Sen, specificity, NPV and PPV, and likelihood ratios. Then, the index derived in the derivation group was tested in the validation group. All data were analyzed using SPSS 19.0 (SPSS Inc., Chicago, USA).
3. RESULTS
3.1. Demographics and baseline characteristics
In the derivation group, the mean age of all 66 enrolled patients (53 males, 13 females) was 39.1 ± 12.1 years. All patients were administered triple immunosuppressants, including MMF, cyclosporine A or tacrolimus, SRL, and Pred. The mean time from renal transplantation was 51 months (duration of 1 week to 180 months). In the validation group, the mean age of all patients (28 males, 10 females) was 41.4 ± 9.9 years. Patients in the validation group also received a standard triple ISP. The mean time from renal transplantation was 61 months (duration of 3 weeks to 228 months). In the derivation group, 41 patients presented AR, and 25 patients presented CR with a biopsy‐proven histologic examination. In the validation group, there were 22 patients with AR and 16 patients with CR. There were no significant differences between the AR and CR groups in terms of sex, weight, BMI, or ISP in either the derivation or validation groups. The demographics and baseline characteristics of each group are shown in Table 1. For the derivation group, the baseline SCr and eGFR in the AR and CR groups were 115.4 ± 30.2 vs. 105.6 ± 25.4 μmol/L and 75.2 ± 37.6 vs. 80.7 ± 44.4 ml/min/1.73 m2, respectively. The baseline SCr and eGFR were not significantly different between the AR and CR groups. The renal function data from the biopsy and ultrasound examination are presented in Table 2.
Table 1.
Demographic characteristics and ultrasound indexes of patients
| Derivation group (n = 66) | Validation group (n = 38) | |||||
|---|---|---|---|---|---|---|
| AR (n = 41) | CR (n = 25) | P value | AR (n = 22) | CR (n = 16) | P value | |
| Sex (Male/Female) | 33/8 | 20/5 | >0.05 | 18/4 | 10/6 | >0.05 |
| Age (Years) | 36.29 ± 12.21 | 43.76 ± 10.59 | 0.014 | 40.14 ± 8.54 | 43.13 ± 11.34 | >0.05 |
| Weight (kg) | 63.87 ± 4.87 | 62.76 ± 5.34 | >0.05 | 65.01 ± 5.23 | 64.91 ± 6.08 | >0.05 |
| BMI | 23.82 ± 1.87 | 23.39 ± 2.12 | >0.05 | 23.75 ± 2.01 | 23.12 ± 2.32 | >0.05 |
| Post‐transplant time at US examination (month) | 27.61 ± 32.04 | 90.76 ± 53.64 | <0.001 | 35.98 ± 57.12 | 96.94 ± 53.96 | <0.001 |
| Pretransplant PRA (%) | ||||||
| Class I | 3.59 ± 12.92 | 4.92 ± 12.35 | 0.087 | 0.59 ± 2.06 | 4.00 ± 8.21 | >0.05 |
| Class II | 1.32 ± 5.96 | 12.20 ± 25.24 | 0.008 | 3.00 ± 10.19 | 2.00 ± 5.57 | >0.05 |
| ISP | NA | NA | ||||
| CNI + MMF + Pred | 41 | 25 | 20 | 14 | ||
| SRL + MMF + Pred | 0 | 0 | 2 | 2 | ||
| RI | 0.60 ± 0.07 | 0.64 ± 0.10 | >0.05 | 0.65 ± 0.12 | 0.62 ± 0.09 | >0.05 |
| Kidney volume change (%) | 11.56 ± 15.92 | ‐10.90 ± 17.54 | <0.001 | 20.44 ± 26.38 | ‐15.30 ± 17.58 | <0.001 |
| RTm | 12.97 ± 6.13 | 15.85 ± 3.82 | 0.040 | 10.21 ± 3.05 | 13.82 ± 3.87 | 0.003 |
| TTPm | 14.25 ± 6.08 | 17.39 ± 4.61 | 0.030 | 12.45 ± 4.33 | 16.25 ± 5.29 | 0.024 |
Data are presented as the mean values with the standard error of the mean.
Table 2.
Renal function
| Derivation group (n = 66) | Validation group (n = 38) | |||||
|---|---|---|---|---|---|---|
| AR (n = 41) | CR (n = 25) | P value | AR (n = 22) | CR (n = 16) | P value | |
| SCr (μmol/L) | 230.32 ± 134.44 | 340.52 ± 205.09 | 0.012 | 236.50 ± 171.70 | 226.00 ± 164.49 | >0.05 |
| eGFR (mL/min/1.73 m2) | 39.45 ± 17.83 | 26.29 ± 14.62 | 0.003 | 39.32 ± 18.87 | 39.06 ± 19.86 | >0.05 |
Data are presented as the mean values with standard error of the mean.
3.2. Comparison of renal function and CEUS parameters
The patients with CR demonstrated significantly higher SCr and lower eGFR compared to the AR group (Figure 2A,B). No difference was observed in RI between these two groups (Figure 2C). The kidney volume change was calculated as (volume 2‐volume 1)/volume 1. The volume 2 was tested by B‐mode ultrasound examination before CEUS was performed. The volume 1 was tested within 24 hours post‐transplantation. In the CR group, the kidney volume change was significantly decreased compared to that in the AR group (Figure 2D). The RT and TTP of the medulla (RTm and TTPm) were significantly longer in the CR group than those in the AR group (Figure 2E,F).
Figure 2.
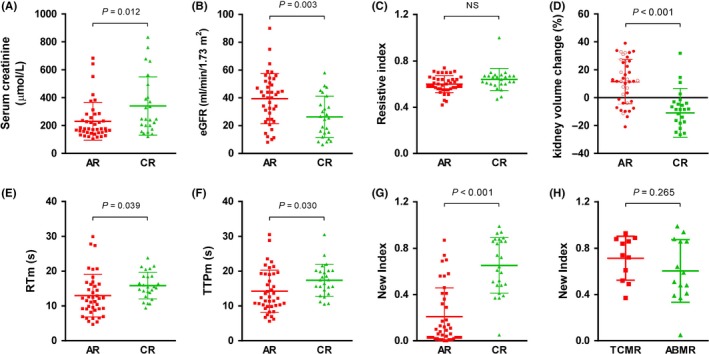
Comparison of renal function and ultrasound features between AR and CR patients in the derivation group. A, SCr; (B) eGFR; (C) RI; (D) kidney volume change; (E) RTm; (F) TTPm; (G) New index between the AR and CR groups; (H) New index between the TCMR and ABMR groups in CR patients. Data are expressed as the mean ± SD
3.3. Predictors of CR and establishment of the diagnostic model
All patients enrolled were divided into the CR group and AR group. In the univariate analysis, variables including age, SCr, eGFR, kidney volume change, RTm, and TTPm were identified as predictors of CR. Among them, three variables were identified as independent predictors by multivariate analysis as follows: age (OR = 1.077, 95% CI 1.012‐1.146, P = 0.019), kidney volume change (OR = 5.45E‐5, 95% CI 2.28E‐7‐0.013, P < 0.001), and TTPm (OR = 1.122, 95% CI 1.001‐1.258, P = 0.048). ROC curves were estimated for individual markers in all patients. The AUROCs for age, kidney volume change, and TTPm in diagnosing renal allograft CR were 0.696, 0.831, and 0.687, respectively (Table 3 and Figure 3A).
Table 3.
ROC analysis in the derivation group to evaluate discrimination ability
| Parameters | AUROC | 95% CI | P value |
|---|---|---|---|
| RTs | 0.650 | 0.519‐0.781 | 0.042 |
| RTi | 0.681 | 0.551‐0.812 | 0.014 |
| RTc | 0.672 | 0.542‐0.802 | 0.020 |
| RTm | 0.701 | 0.577‐0.826 | 0.006 |
| ΔRTm‐c | 0.663 | 0.528‐0.799 | 0.027 |
| TTPs | 0.579 | 0.439‐0.718 | 0.287 |
| TTPi | 0.668 | 0.540‐0.796 | 0.023 |
| TTPc | 0.682 | 0.554‐0.810 | 0.014 |
| TTPm | 0.687 | 0.561‐0.814 | 0.011 |
| ΔTTPm‐c | 0.645 | 0.507‐0.782 | 0.050 |
| eGFR | 0.709 | 0.582‐0.836 | 0.005 |
| Age | 0.696 | 0.566‐0.828 | 0.008 |
| Kidney volume change | 0.831 | 0.729‐0.933 | <0.001 |
| RI | 0.640 | 0.502‐0.778 | 0.058 |
| New index | 0.886 | 0.807‐0.965 | <0.001 |
Figure 3.
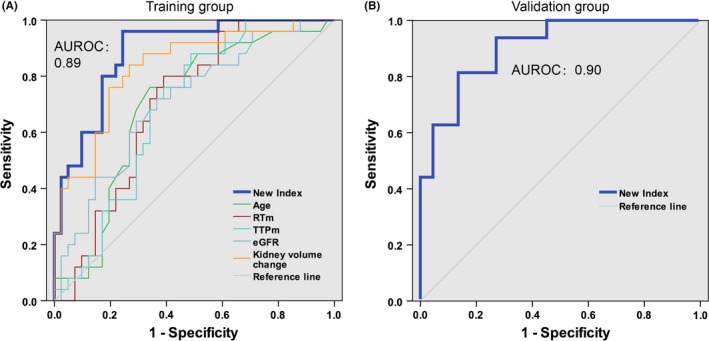
Area under ROC curves. AUROCs estimated the diagnostic performance of the new index, age, RTm, TTPm, eGFR and kidney volume change in the derivation group (A) and validation group (B)
Based on the multivariate analysis results and AUROCs of individual markers, we constructed a new index expressed by the following formula:
We found that this new index was significantly elevated in CR patients compared with AR patients (median (interquartile range), 0.64 (0.48, 0.87) vs 0.09 (0.03, 0.39), P = 1.71E‐7) (Figure 2G). No significant differences were observed between the TCMR and ABMR subgroups in CR patients (Figure 2H). Moreover, the highest AUROC was observed for this index (AUROC = 0.89, 95% CI 0.81‐0.97). Two cutoff values were chosen to identify the absence (less than 0.36) and presence (greater than 0.70) of renal allograft CR (Table 4). By applying the lower cutoff value (a score below 0.36), 30 of the 31 patients with AR were correctly identified, and the Sen and specificity were 96% and 73%, respectively. At the cutoff value of 0.70, the Sen and specificity were 48% and 93%, respectively, and 12 of 15 patients with CR were correctly identified. Using these two cutoff values, approximately 70% of patients could be correctly diagnosed, with over 90% accuracy.
Table 4.
Sensitivity, specificity, predictive values, and likelihood ratios of new index according to different cutoffs for the diagnosis of CR
| Cutoff | CR predicted by New index |
All patients n (%) |
Renal allograft biopsy | Sen | Spe | NPV | PPV | ‐LR | +LR | |
|---|---|---|---|---|---|---|---|---|---|---|
|
AR n (%) |
CR n (%) |
|||||||||
| Derivation group (n = 66) | ||||||||||
| 0.36 | AR | 31 (47%) | 30 (73%) | 1 (4%) | 96% | 73% | 97% | 69% | 0.05 | 3.6 |
| CR | 35 (53%) | 11 (27%) | 24 (96%) | |||||||
| 0.70 | AR | 51 (77%) | 38 (93%) | 13 (52%) | 48% | 93% | 75% | 80% | 0.56 | 6.6 |
| CR | 15 (23%) | 3 (7%) | 12 (48%) | |||||||
| Validation group (n = 38) | ||||||||||
| 0.36 | AR | 21 (55%) | 18 (82%) | 3 (19%) | 81% | 82% | 86% | 76% | 0.23 | 4.5 |
| CR | 17 (45%) | 4 (18%) | 13 (81%) | |||||||
| 0.70 | AR | 28 (74%) | 21 (95%) | 7 (44%) | 56% | 95% | 75% | 90% | 0.46 | 12 |
| CR | 10 (26%) | 1 (5%) | 9 (56%) | |||||||
3.4. Comparison of the new index with individual markers for predicting CR
Area under ROC curves were used to evaluate the overall diagnostic performance of this new index and individual markers (Figure 3A). We found that this new index had a better AUROC than that of kidney volume change, eGFR, and individual CEUS markers. Additionally, this new index was superior to individual markers when optimal cutoff values were applied (Table 5).
Table 5.
Sensitivity, specificity, predictive values, and likelihood ratios of models according to optimal cutoff for the diagnosis of CR in the derivation group
| Optimal cutoff point | ||||||
|---|---|---|---|---|---|---|
| Sen | Spe | PPV | NPV | +LR | −LR | |
| New index | 96% | 76% | 71% | 97% | 3.9 | 0.05 |
| Kidney volume change | 72% | 80% | 69% | 83% | 3.7 | 0.35 |
| RTm | 80% | 61% | 56% | 83% | 2.1 | 0.33 |
| eGFR | 64% | 71% | 57% | 76% | 2.2 | 0.51 |
The optimal cutoff points of the new index, kidney volume change (rate), RTm and eGFR are 0.37, 4.0%, 12.7 and 30.0, respectively, based on the best Youden index in our study.
3.5. Validation of the new index
The characteristics of patients in the validation group are summarized in Table 1. No differences were observed in SCr, eGFR, and RI values between the AR and CR groups (Figure 4A‐C). Similar to the derivation group, the kidney volume change was significantly lower in the CR group than that in the AR group (Figure 4D). The RTm and TTPm were increased in the CR group compared to the AR group (Figure 4D,E). The new index was significantly elevated in CR patients compared with AR patients (median (interquartile range), 0.72 (0.51, 0.94) vs 0.05 (0.13, 0.32), P = 7.68E‐7) (Figure 4G). The AUROC is shown in Figure 3B (AUROC = 0.90, 95% CI 0.81‐0.99, P < 0.001). Upon applying a high cutoff value (New Index = 0.70), CR was predicted in 26% of patients with PPV 90%, and upon applying a low cutoff value (New Index = 0.36), CR was excluded with 86% certainty in 55% of patients (Table 4). Finally, the new index showed no significant differences between the TCMR and ABMR subgroups in CR patients (Figure 4H).
Figure 4.
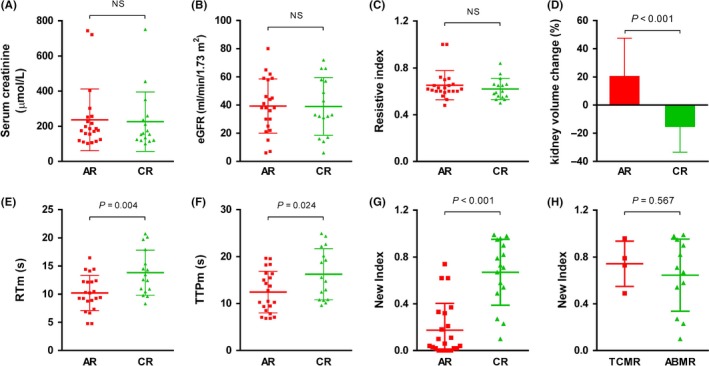
Comparison of renal function and ultrasound features between AR and CR patients in the validation group. A, SCr; (B) eGFR; (C) RI; (D) Kidney volume change; (E) RTm; (F) TTPm; (G) New index between the AR and CR groups; (H) New index between the TCMR and ABMR groups in CR patients. Data are expressed as the mean ± SD
4. DISCUSSION
Although the CEUS demands special analysis software and a more expensive contrast agent, the general advantages of CEUS versus conventional ultrasound in combination with color Doppler imaging in kidney transplantation are notable. Contrast‐enhanced ultrasonography examination is not contraindication for impaired kidney function. Contrast‐enhanced ultrasonography displays microvascular tissue perfusion and allows renal blood flow to be quantified, and this assessment demands no special experience of the investigator.11 There are two substantial arguments for the application of CEUS in the evaluation of renal allograft function. Renal allograft underlies progressive vascular remodeling after transplantation. Most of these vascular insults affect small parenchymal arteries and arterioles, which cannot be assessed by Doppler ultrasonography. Therefore, the major advantage of CEUS is evaluating microperfusion to augment diagnostic evidence and to permit early administration of the appropriate therapy. Second, the superficial position of the transplanted kidney and minimum organ movements due to respiration facilitates examination with a contrast agent largely, while the kidney should be kept in a stable position for the assessment of renal blood flow.12
Based on the results, we found that the essential difference between AR and CR is the microperfusion speed. CR demonstrated a lower microperfusion speed, which means a higher resistance of renal artery and arteriole. Allograft rejection is a complex process that involves the interplay of different cellular and molecular pathways that cause a broad range of allograft injuries. Allograft rejection can be hyperacute (occurring within minutes after the vascular anastomosis), acute (occurring days to weeks after transplantation), late acute (occurring 3 months after transplantation), or chronic (occurring months to years after transplantation). The major pathological changes of AR were lymphocytic infiltration, interstitial edema, intimal arteritis, tubulitis and arteritis.13, 14 However, CR is much more complex than AR. Currently, the main pathology of CR is ABMR. Despite the major advances in molecular biology and gene rearrangement, the diagnosis of ABMR is still dependent on histologic findings.15 The typical pathology of chronic ABMR is vascular endothelium injury.16 Therefore, CEUS, a quantitative method for evaluating microperfusion, is able to discriminate CR from AR. In addition, RT and TTP parameters in CR are significantly increased compared to those in the AR group.
Allograft transplantation, not only kidney transplantation but also other solid organ transplantation, needs consecutive monitoring of allograft status. Some doctors suggest protocol biopsies at fixed time points after transplantation to diagnose any subclinical allograft injury or rejection to improve transplant outcomes. However, core needle biopsies are invasive, and frequent biopsies may be associated with severe complications. Moreover, sampling errors and variability in biopsy analysis confound conclusions about graft health. CEUS is an emerging noninvasive method but has not been reported for CR diagnosis thus far. Interestingly, Fischer et al recently tested the efficacy of CEUS in detecting allograft AR and CR using a murine heart transplantation model.17 Compared to the syngeneic groups, a progressive decline in microperfusion was demonstrated in the allografts undergoing acute transplant rejection (40%, 64%, and 92% on days 4, 6, and 8 post‐transplantation, respectively) and CR (33%, 33%, and 92% on days 5, 14, and 30 post‐transplantation, respectively). The data suggest that early endothelial cell injury and platelet aggregation contributed to the early microperfusion decline. Although a 33% decrease in microperfusion in the CR group seems less than 40% or 64% in the AR group, the detection time points are not the same. Animal experiments using CEUS in renal transplants are still lacking.
In addition to the CEUS parameter TTPm, kidney volume change is also an important parameter for the new index formula. In the AR and CR groups, the kidney volume presented absolutely different variation tendencies. Mechanically, the swelling of the renal pyramids that occurs in AR is caused by the intense reaction initiated by the immune response. Although CR is also mediated by immune rejection similar to AR, the immune response is weaker and slower. Han et al analyzed 351 living‐donor kidney transplantation patients. They also found that the low‐graft‐volume group conferred a greater risk of rejection, chronic change, and graft loss than that in the high‐graft‐volume group.18
In addition to CR, interstitial fibrosis and tubular atrophy (IF/TA) is another confounding factor in the late stage of renal transplantation. Therefore, IF/TA is indeed a very important factor that is worth considering. This factor is also a limitation of the current study and will be investigated in the future. Although the precise CEUS parameters that may best predict disease still warrant systematic evaluations, animal models, and limited clinical trials in humans, our study raises hopes that CEUS could outcompete other modalities as a first‐line tool for assessing renal perfusion noninvasively.19 However, due to the limited number of kidney transplantation cases, the validation group was rather small, and a power analysis was not performed. Our results should be validated in further studies with large sample sizes, and the cutoff values may need to be adjusted or modified based on the results from further larger studies.
In our study, the new index cannot discriminate sub‐phenotypes of CR, such as TCMR and ABMR. Although the major cause of CR is ABMR, the therapies for TCMR and ABMR are different. ABMR is mediated by donor‐specific antibodies generated by plasma cells. The common therapeutic strategy for ABMR is IVIG, plasmapheresis, antithymocyte globulin, or rituximab. However, steroid pulse therapy and increased immunosuppressants are usually applied for TCMR. Mechanically, TCMR is mediated by T cells, rather than B cells. Therefore, if CEUS could differentiate TCMR and ABMR, it will be helpful for doctors to makes decisions for treatments. It is inspiring that there are some preliminary animal studies using targeted CEUS. For instance, in a rat kidney transplant study, microbubble contrast agents were coupled with anti‐CD3, anti‐CD4, and anti‐CD8 antibodies. Strikingly, CD3‐mediated ultrasound, which suggests T‐cell infiltration, allows the detection of AR as early as postoperative day 2.20 Similarly, Sun et al detected C4d deposition in vivo in rat kidney and heart transplant models of ABMR using C4d‐targeted microbubbles with a streptavidin‐biotin conjugation.21, 22 These studies further enhanced the specificity of CEUS in detecting allograft rejection and provide the potential for discriminating between ABMR and TCMR in the future.
In conclusion, CEUS is a reliable and useful tool for the diagnosis and follow‐up after kidney transplantation, allows for the visualization of kidney allograft microperfusion in different circumstances and is an accurate, specific, and sensitive method for the assessment of CR. Moreover, the new index established in our study provides assistance in the diagnosis of CR with a high degree of accuracy and convenience.
PERSPECTIVE
Contrast‐enhanced ultrasonography (CEUS) is a noninvasive imaging tool for collecting quantitative measurements of regional renal perfusion and microvascular function. Here we established a model to diagnose renal allograft chronic rejection using CEUS. We believe that antibody‐coupled CEUS which named as molecular ultrasound image will be more specific and attractive in the future.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by the National Key R&D Program of China (2018YFA0107502 to CY), the Medical and Health Talents Training Plan for the Excellent Youth of Shanghai Municipal (2018YQ50 to CY), Shanghai Rising‐Star Program (19QA1406300 to Cheng Yang), the National Natural Science Foundation of China (81270833, 81570674, 81400752, 81770746, and 81500457), and the Science and Technology Commission of Shanghai Municipality (15411964900).
Yang C, Wu S, Yang P, et al. Prediction of renal allograft chronic rejection using a model based on contrast‐enhanced ultrasonography. Microcirculation. 2019;26:e12544 10.1111/micc.12544
Cheng Yang and Shengdi Wu contributed equally to this article.
REFERENCES
- 1. Wei K, Le E, Bin JP, Coggins M, Thorpe J, Kaul S. Quantification of renal blood flow with contrast‐enhanced ultrasound. J Am Coll Cardiol. 2001;37:1135‐1140. [DOI] [PubMed] [Google Scholar]
- 2. Young LS, Regan MC, Barry MK, Geraghty JG, Fitzpatrick JM. Methods of renal blood flow measurement. Urol Res. 1996;24:149‐160. [DOI] [PubMed] [Google Scholar]
- 3. Correas JM, Anglicheau D, Joly D, Gennisson JL, Tanter M, Helenon O. Ultrasound‐based imaging methods of the kidney‐recent developments. Kidney Int. 2016;90:1199‐1210. [DOI] [PubMed] [Google Scholar]
- 4. Piscaglia F, Bolondi L. Italian Society for Ultrasound in M, Biology Study Group on Ultrasound Contrast A: the safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32:1369‐1375. [DOI] [PubMed] [Google Scholar]
- 5. Harvey CJ, Sidhu PS, Bachmann Nielsen M. Contrast‐enhanced ultrasound in renal transplants: applications and future directions. Ultraschall Med. 2013;34:319‐321. [DOI] [PubMed] [Google Scholar]
- 6. Jin Y, Yang C, Wu S, et al. A novel simple noninvasive index to predict renal transplant acute rejection by contrast‐enhanced ultrasonography. Transplantation. 2015;99:636‐641. [DOI] [PubMed] [Google Scholar]
- 7. Rush D, Nickerson P, Gough J, et al. Beneficial effects of treatment of early subclinical rejection: a randomized study. J Am Soc Nephrol. 1998;9:2129‐2134. [DOI] [PubMed] [Google Scholar]
- 8. Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326‐2333. [DOI] [PubMed] [Google Scholar]
- 9. Kreis HA, Ponticelli C. Causes of late renal allograft loss: chronic allograft dysfunction, death, and other factors. Transplantation. 2001;71:SS5‐SS9. [PubMed] [Google Scholar]
- 10. Howard RJ, Patton PR, Reed AI, et al. The changing causes of graft loss and death after kidney transplantation. Transplantation. 2002;73:1923‐1928. [DOI] [PubMed] [Google Scholar]
- 11. Yang C, Hu MS, Zhu TY, He WY. Evaluation of kidney allograft status using novel ultrasonic technologies. Asian J urol. 2015;2:140‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zeisbrich M, Kihm LP, Druschler F, Zeier M, Schwenger V. When is contrast‐enhanced sonography preferable over conventional ultrasound combined with Doppler imaging in renal transplantation? Clin Kidney J. 2015;8:606‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753‐760. [DOI] [PubMed] [Google Scholar]
- 14. Ingulli E. Mechanism of cellular rejection in transplantation. Pediatr Nephrol. 2010;25:61‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gosset C, Lefaucheur C, Glotz D. New insights in antibody‐mediated rejection. Curr Opin Nephrol Hypertens. 2014;23:597‐604. [DOI] [PubMed] [Google Scholar]
- 16. Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d‐negative antibody‐mediated rejection and antibody‐associated arterial lesions. Am J Transplant. 2014;14:272‐283. [DOI] [PubMed] [Google Scholar]
- 17. Fischer K, Ohori S, Meral FC, et al. Testing the efficacy of contrast‐enhanced ultrasound in detecting transplant rejection using a murine model of heart transplantation. Am J Transplant. 2016;17:1791‐1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han SS, Yang SH, Oh YJ, et al. Graft volume as the surrogate marker for nephron number affects the outcomes of living‐donor kidney transplantation. Clin Transplant. 2011;25:E327‐E335. [DOI] [PubMed] [Google Scholar]
- 19. Wang L, Mohan C. Contrast‐enhanced ultrasound: a promising method for renal microvascular perfusion evaluation. J Transl Int Med. 2016;4:104‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grabner A, Kentrup D, Pawelski H, et al. Renal contrast‐enhanced sonography findings in a model of acute cellular allograft rejection. Am J Transplant. 2016;16:1612‐1619. [DOI] [PubMed] [Google Scholar]
- 21. Liao T, Zhang Y, Ren J, et al. Noninvasive quantification of intrarenal allograft C4d deposition with targeted ultrasound imaging. Am J Transplant. 2018;19:259‐268. [DOI] [PubMed] [Google Scholar]
- 22. Liao T, Liu X, Ren J, et al. Noninvasive and quantitative measurement of C4d deposition for the diagnosis of antibody‐mediated cardiac allograft rejection. EBioMedicine. 2018;37:236‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]


