Abstract
Reports on patients who lack ownership over their entire body are extremely rare. Here, we present patient SA who suffered from complete body disownership after a tumour resection in the right temporoparietal cortex. Neuropsychological assessment disclosed selective bilateral ownership problems, despite intact primary visual and somatosensory senses. SA's disownership seems to stem from a suboptimal multimodal integration, as shown by the rubber hand illusion and the beneficial effect during and after simple exercises aiming at multisensory recalibration.
Keywords: body ownership, body representation, bilateral ownership problems, rubber hand illusion, affective touch
Background
Following brain damage patients can experience that parts of their body do not belong to themselves. It usually concerns the affected limb contralateral to the affected hemisphere, typically the (hemiparetic) left limb after right hemispheric damage (Baier & Karnath, 2008; Gandola et al., 2012; Nightingale, 1982; Vallar & Ronchi, 2009). Vallar and Ronchi (2009) suggested that disownership of limbs in patients with somatoparaphrenia, a misidentification and confabulation of limbs, could be the result of a ‘defective’ integration of visual, tactile and proprioceptive information in concurrence with problems in the spatial representation of the body. The integration of this information has been associated with the inferior posterior parietal cortex (Dijkerman & de Haan, 2007; Kammers, de Vignemont, Verhagen, & Dijkerman, 2009) and the ventral premotor cortex (Ehrsson, Spence, & Passingham, 2004; Zeller, Gross, Bartsch, Johansen‐Berg, & Classen, 2011). While disownership over one body part has been reported regularly, patients who lack ownership over their entire body are extremely rare. Here, we present a 46‐year‐old man, patient SA, who suffered from a diminished sense of ownership over his complete body. The main aim of this study was to examine the (cognitive) mechanism underlying his subjective reports of body disownership.
Patient characteristics
Patient SA was a 46‐year‐old man who was diagnosed 5 years before the current consultation with an intraventricular brain tumour, located in the right posterior part of the lateral ventricle (Figure 1). The tumour was an incidental finding on a cranial CT scan, performed after an unrelated head trauma. In retrospect, SA reported that problems in body representation developed gradually before the first consultation (>5 years ago). On neurological examination, no focal deficits were present. Within 2 months after the diagnosis, SA underwent an elective transcortical resection of the tumour through the right parietal lobe. Neuropsychological examination 5 months after resection stated that ‘despite problems in orientation in space (i.e., navigation) and time, the neuropsychological assessment shows overall a strong analytic and beyond average cognitive profile’ (Table S1 in Appendix S2). Importantly, performance on several other spatial tests including left‐right orientation, judgement of line orientation and line bisection was unimpaired suggesting that the spatial problem was specific for navigation and that there was no general spatial perception deficit. Although he did also report problems in the feeling of body ownership, this was not examined at that time. At the time of this study, 5 years after his initial diagnosis and surgery, SA reported that his problems in body ownership (see Subjective reports below) had become more pronounced. No new focal neurological deficits were identified at the time of the study compared with previous examination.
Figure 1.
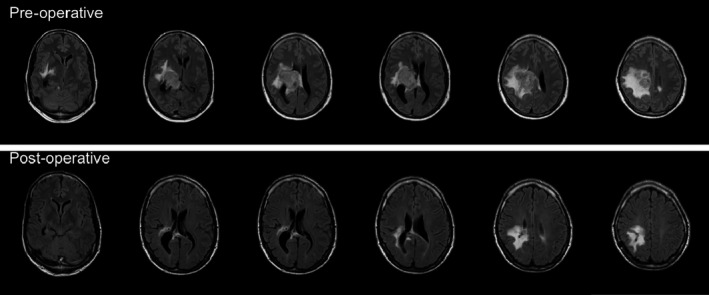
MRI scans with T2‐weighted images with fluid‐attenuated inversion recovery (FLAIR) in axial plane. The upper row represents pre‐operative images showing a circumscript right intraventricular lesion with dimensions 5 × 4 × 4 cm (with T1 enhancement after gadolinium administration; not shown). The lesion is accompanied by a large area of subcortical oedema extending cranially from the tumour as well as some mesiotemporal oedema. The bottom row represents post‐operative images (6 months prior to the current study). These images show residual right parietal hyper intensities (gliosis) and a small area of post‐operative cortical changes located posteriorly in the parietal lobe. Some residual T1 gadolinium enhancement of the lateral ventricle wall was seen (unchanged compared to previous post‐operative MRI scans; images not shown). Note that images are in radiological orientation (left side of the image is the right side of the brain).
Subjective reports
SA reported absence of ownership over his whole body, specifically, he was unable to recognize, feel or experience his body as its own, despite the fact that he knew it was his own. Although disownership affected his whole body, problems were most pronounced in both hands, left more than right, and while driving a car: ‘As if I am in a shell, a passenger whilst driving myself’. There were no problems in motor planning or executing, nor were there any reports of somatosensory problems. He was able to do all daily activities and played tennis. SA was able to recognize his body as his own when looking at this body. Without vision, he reported that his body became ‘lost’; unable to mentally build an image of the configuration of his body parts (e.g., where his arm was attached to his body) and was missing the sense that he owned a body. In a mirror, this sense of ‘disintegration’ diminished; however, the sense of disownership remained. Other reported problems included problems in left/right discrimination and problems in space perception in general (e.g., estimating the width of a quay). Of these aforementioned complaints, the sense of disownership had the greatest impact on his daily activities and reduced quality of life. SA did report mood problems and was familiar with depressive episodes for which he received therapy at the time of testing. He further stated that these episodes did not modulate the feelings of body disownership, and thus seem unrelated.
Basic sensory and neuropsychological testing
Following his subjective reports, we examined SA twice; the first and second examinations were separated by 2 weeks. In both examinations, we prepared an individually tailored test battery covering the aspects of patient's complaints (i.e., body representation and space perception), see Table 2.
Table 2.
Subjective experience of hand (i.e., left, right) ownership (in %) indicated on a visual analogue scale for patient SA and controls for both session (s) 1 and 2
| Patient SA | Controlsb | |||
|---|---|---|---|---|
| s1 | s2 | s1 | s2 | |
| Left hand | 5b | 40b | 100 | 100 |
| Right hand | 32b | 43b | 100 | 100 |
| Left hand exercisea | n/a | 77b | n/a | 100 |
| Right hand exercisea | n/a | 78b | n/a | 100 |
All controls scored 100 (SD = 0), in order to be able to compute Crawford's statistics, SD was set at 1 for all tests.
Feeling of ownership during the exercises. n/a = not applicable, since these measures were not administered during the first session.
Significantly different from controls (p < .0001).
Primary somatosensory function
Tests for relative position sense and two‐point discrimination were administered for both hands (according to Winward, Halligan, & Wade, 2002), which showed no indication of impairments in proprioception and tactile acuity.
Spatial and structural body representation tasks
Results on the tactile pointing, body localization and implicit relative position sense (see Appendix S1 for detailed information and test instructions) suggested no problems in most aspects of his body representation; hence the spatial, configural and metric aspects as well as conscious perceptions, attitudes, and beliefs concerning a human body seemed intact and therefore did not contribute to his feelings of body disownership. These findings are in line with the ‘Draw‐a‐person task’ in which he was able to configure what a healthy person should look like. However, when he had to draw how he experienced his own body, he only drew the body parts (i.e., the hands) that were visible to him (Figure 2A, and B respectively).
Figure 2.
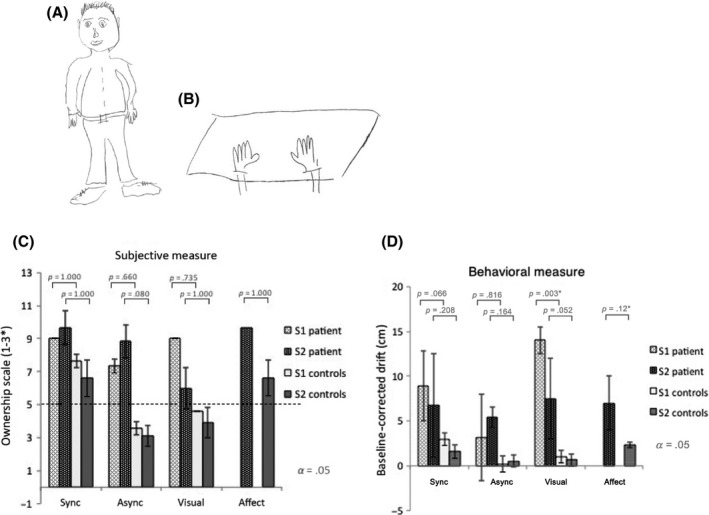
(A) Left panel: drawing of a physical human body and (right panel) drawing of own experienced body. When instructed to draw a person, he drew an appropriately sized body with correctly attached limbs, indicating intact semantic knowledge of the configural layout of a human body. However, when instructed to draw the experienced own physical body, he initially hesitated and needed further encouragement. SA reported that he was not able to feel the presence of his entire body (including his hands) when he did not look at his body. Looking at a part of his body increased a feeling of ownership over this part of the body. He then drew a pair of detached arms and hands, as that is the only body part he saw when looking at the table surface (B). What is particularly striking is that this drawing is indicative of the reports of the patient; it seemed as if he could only draw what he saw, suggesting reliance on vision without incorporating the other senses which are necessary for ownership (i.e., proprioception, touch) (Vallar & Ronchi, 2009). All controls drew two similar persons. (C) Average subjective feeling of ownership for SA and controls (‘ownership scale’ (average Q1–3) of the Embodiment questionnaire), for the stimulated left hand in the synchronous, asynchronous, visual and, affective (only session 2) condition for both test sessions (i.e., S1 and S2). For (C) and (D) Error bars represent within‐subject error in SA, and between‐subject error in controls. *In the visual‐only condition, only one (of three) questions could be answered, the other two questions required tactile input. (D) Average baseline‐corrected (post‐session–pre‐session) proprioceptive drift (in cm) for all conditions for the left hand for SA and controls.
Body ownership
The overall subjective experience of ownership was measured with a visual analogue scale (VAS) and the rubber hand illusion (RHI). These were compared to the data of six1 healthy (gender‐ and age‐matched (average age 46.5 [SD = 7.7]) controls. Design and set‐up for the VAS and RHI in controls were identical to that of SA. Statistical analysis was performed on the VAS and on both outcome measures of the RHI by frequentist statistics with single case–control analyses (Crawford & Howell, 1998) and with Bayesian Single Case Method analyses (Crawford & Garthwaite, 2007).
The VAS showed that SA reported – when asked to what extent his hands felt as his own – hardly any feelings of ownership over both hands, as opposed to controls (see Table 2009 for statistical comparison with healthy controls).
In addition, we presented the classic RHI (see Botvinick & Cohen, 1998 for a detailed procedure); set‐up adopted from Kammers et al. (2009) plus an extra condition where he only had to look at the rubber hand (visual only). On each trial, except for the visual only, the rubber hand and the invisible own hand were stroked for 90 s, either synchronously or asynchronously. Commonly, the synchronous visuotactile stimulation causes the highest feelings of ownership Botvinick and Cohen (1998). Mere visual exposure to a rubber hand is thought to lead to some embodiment, but insufficient to reach full‐blown embodiment over the rubber hand in healthy participants (Ferri, Chiarelli, Merla, Gallese, & Constantini, 2013; Longo, Cardozo, & Haggard, 2008). Asynchronous stimulation would result in the least illusion and usually serves as a control condition. Indeed, this is exactly what we found in our control group for session 1 (Figure 2C,D for statistics). Relative to the control group SA experienced an increased sense of ownership over the rubber hand, as reflected on both outcome measures (e.g., [behavioural] proprioception and [subjective] embodiment questionnaire). Moreover, unlike the controls, there was no difference between synchronous stroking and visual input only on both measures, suggesting that SA did not benefit from multisensory integration (Figure 2C,D). In session one (S1, light pattern), SA scored far above the illusion threshold (5) for the asynchronous and visual condition in the subjective measure. Controls (S1), however, did not score above 5 in these conditions, which is the usual pattern of results (Kammers et al., 2009). In fact, SA reported that the stroking in the synchronous condition interfered with the illusion. Even more so, he reported that visually focusing for 90 s created the feeling ‘that this rubber hand is attached to my body, which I don't experience with my real hand’. Statistical analyses confirmed this pattern of results, especially for the proprioceptive drift measure. Analyses revealed, as expected, a significant difference between controls and the patient for the visual condition (Bonferroni‐corrected p‐values in Figure 2C,D), and a near significant result for the synchronous condition. This was, however, not the case for the subjective measure, probably due to small sample size. Additional Bayesian Single Case Method analyses revealed that the estimated percentage of the control population that would obtain a score lower than SA ranges from 67% to 89% for the conditions in the subjective measures and from 86% to 100% for the behavioural measures. Thus, taking the frequentist approach and the Bayesian together, these results indicate that SA overall has a heightened susceptibility for the RHI in session 1, which is most pronounced in the visual condition.
For the following weeks, we recommended (as well for the controls) some simple exercises to SA consisting of touching and simultaneously looking at his body through a mirror 3 × 5 min a day for 2 weeks. The rationale behind this was taking advantage of his reliance on vision by making use of a mirror and simultaneously stimulates the intact afferent input by touching his limbs. Additionally, viewing the self from third person perspective might reinstate ownership by incorporating other (less affected) body parts, such as face and trunk (Fotopoulou et al., 2011).
Two weeks later we applied a subset of the tests again (Table 1). SA's feelings of subjective ownership had changed profoundly, which is outlined Table 2 (s2). Remarkably, when asked how much ownership he experienced during these exercises, he reported almost complete ownership. This leads to the compelling idea that just 2 weeks of simple multisensory stimulation improved feelings of ownership. We presented the RHI again, and surprisingly the experience of ownership dropped in the visual condition (as opposed to the first test session and other conditions) for both measurements; the pattern now resembled that of a healthy control participant. Statistically, results resembled the first session (Figure 2C,D), except that the visual condition was near significant between the controls and the patient. Additionally, we added a slow stroking (range of 1–10 cm/s) affective touch condition, which has been previously correlated with pleasant emotion and may facilitate the brain's ability to construct a sense of body ownership (Crucianelli, Metcalf, Fotopoulou, & Jenkinson, 2013; Van Stralen, Van Zandvoort, Hoppenbrouwers, & Dijkerman, 2014). During this condition, both measurements (Figure 2D,E), but particularly his verbal reports indicate that he embodied the rubber hand the most, ‘… this seems more like my own hand than the experience in the other conditions. Really 100%! I had no idea where my own hand was’. This was confirmed by statistical analyses (Figure 2C,D).
Table 1.
Test battery and underlying mechanism for all sessions 0 (baseline), 1 and 2 covering patients’ complaints in body representation
| Testa | Mechanism/aim | Session | Impaired/not impaired |
|---|---|---|---|
| Draw‐a‐person task | Semantic knowledge of body | 1 | Not impaired |
| Subjective sense of ownership (visual analogue scale) | Subjective sense of ownership | 1 and 2 | Impaired |
| Tactile pointing task | Metric aspects of body | 1 | Not impaired |
| Body localization task | Structural body representation | 1 | Not impaired |
| Implicit relative position sense task | Spatial configuration of body | 1 | Not impaired |
| Rubber hand illusion | Body ownership | 1 and 2 | See results |
| Finger gnosis | Structural body representation | 0 | Not impairedb |
| Proprioception | Primary somatosensory function | 0 | Not impaired |
| Two‐point discrimination | Primary somatosensory function | 0 | Not impaired |
See Appendix S1 for more detailed information on stimuli, test and test procedures. At the time of resection, there were no neuropsychological (i.e., memory, executive functioning, visuoperception, language) and psychiatric deficits (formally tested).
See Appendix S1 for more detailed information on stimuli, test and test procedures.
There were subtle signs of finger agnosia shortly after the resection.
Finally, we analysed whether the difference between session 1 and 2 for all the conditions (i.e., synchronous, asynchronous, visual) in SA was significantly greater than the difference observed in controls. Here, we found that only for the proprioceptive drift in the visual‐only condition SA's difference was significantly greater than controls t = −4.131, p = .01, indicating a significant drop in the second session for the visual condition only for SA.
Discussion and conclusion
Our observations suggest that right temporoparietal lesions can lead to bilateral body ownership deficits. The problems in body ownership could not explained by impairments in the primary somatosensory or motor functioning, nor by a general spatial perceptual deficit. Although the patient SA did show navigation deficits, his performance on several other spatial tasks was unimpaired. Furthermore, there were no indications that the structural, semantic or spatial body representation was impaired. Analyses confirm that SA had a heightened susceptibility to gain ownership over a foreign hand. This is in line with previous studies that found a stronger illusion for the contralesional hand after acquired brain injury (Burin et al., 2015; Llorens et al., 2017; Van Stralen, van Zandvoort, Kappelle, & Dijkerman, 2013; White & Aimola Davies, 2017; Zeller et al., 2011). Previous studies have suggested that a stronger illusion in patients with body ownership impairments is a result of a problem in the integration of contralesional afferent and efferent motor signals, as patients with body ownership deficits usually suffer from sensorimotor impairments (Burin et al., 2015). However, patient SA did not suffer from sensorimotor deficits, suggesting that body ownership impairments are not a consequence of a disturbed processing of motor signals. Indeed, a previous study of our laboratory also found a stronger RHI in a patient with body ownership impairments, but without sensorimotor impairments (Van Stralen et al., 2013). Secondly, the finding that the RHI was most pronounced during visual exposure (as opposed to the synchronous and asynchronous stimulation), and that SA did not differentiate between synchronous and asynchronous stimulation suggests that SA did not benefit from multisensory information, but may rely on vision, that is, ‘what he sees’ instead when processing bodily information. Previous studies have also found that asynchronous stimulation, usually considered as a control condition, elicited the RHI to a similar extent as synchronous stimulation in stroke patients (Van Stralen et al., 2013; White & Aimola Davies, 2017). The current study, as well as a previous study on a patient with body ownership impairment (Van Stralen et al., 2013) shows that visual exposure seems to elicit a stronger illusion opposed to multimodal (synchronous and asynchronous) stimulation. It remains inconclusive where this suboptimal integration stems from. White et al. (2017) propose a plausible explanation in patients with hemiplegia and state that these patients might have a problem in detecting asynchrony, that is, somatosensory (as opposed to visual) signals are delayed and as a resultant more weight is given to visual information. Despite intact primary sensory signals, SA's results do follow a similar pattern where he relies more on what he sees rather than the combination of what he sees and feels, indicating suboptimal multisensory integration. Multisensory integration and body ownership have been associated with the posterior parietal cortex (see Stein & Stanford, 2008; Tsakiris, 2010, for a review), which is in accordance with site of meningioma and resection. Simple exercises involving visual input about the body from a third person perspective combined with tactile stimulation seem to improve body awareness. Furthermore, interoceptive signals, such as affective touch, are able to boost feelings of ownership (Van Stralen et al., 2014) and have been associated with the right insular cortex. In patient SA, affective touch seemed to additionally reinstate body ownership in SA and might facilitate limb ownership in general.
Supporting information
Appendix S1. Stimuli, tests and procedures.
Appendix S2. Cognitive profile.
Acknowledgements
This study was supported by a Vici grant (453‐10‐003) from NWO (Netherlands Organization for Scientific Research) to HCD.
Note
One control was excluded, because he did not fully understand the test instructions, the questionnaires and the subsequent exercises.
References
- Baier, B. , & Karnath, H.‐O. (2008). Tight link between our sense of limb ownership and self‐awareness of actions. Stroke, 39, 486–488. 10.1161/STROKEAHA.107.495606 [DOI] [PubMed] [Google Scholar]
- Botvinick, M. , & Cohen, J. (1998). Rubber hands ‘feel’ touch that eyes see. Nature, 391, 756 10.1038/35784 [DOI] [PubMed] [Google Scholar]
- Burin, D. , Livelli, A. , Garbarini, F. , Fossataro, C. , Folegatti, A. , Gindri, P. , … Pia, L. (2015). Are movements necessary for the sense of body ownership? Evidence of the rubber hand illusion in pure hemiplegic patients. PLoS One, 16, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford, J. R. , & Garthwaite, P. H. (2007). Comparison of a single case to a control or normative sample in neuropsychology: Development of a Bayesian approach. Cognitive Neuropsychology, 24, 343–372. [DOI] [PubMed] [Google Scholar]
- Crawford, J. R. , & Howell, D. C. (1998). Comparing an individual's test score against norms derived from small samples. The Clinical Neuropsychologist, 12, 482–486. 10.1076/clin.12.4.482.7241 [DOI] [Google Scholar]
- Crucianelli, L. , Metcalf, N. K. , Fotopoulou, A. , & Jenkinson, P. M. (2013). Bodily pleasure matters: Velocity of touch modulates body ownership during the rubber hand illusion. Frontiers in Psychology, 4, 703 10.3389/fpsyg.2013.00703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkerman, H. C. , & de Haan, E. H. (2007). Somatosensory processes subserving perception and action. Behavioural and Brain Sciences, 30, 189–201. 10.1017/S0140525X07001392 [DOI] [PubMed] [Google Scholar]
- Ehrsson, H. H. , Spence, C. , & Passingham, R. E. (2004). That's my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science, 305, 875–877. 10.1126/science.1097011 [DOI] [PubMed] [Google Scholar]
- Ferri, F. , Chiarelli, A. M. , Merla, A. , Gallese, V. , & Constantini, M. (2013). The body beyond the body: Expectation of a sensory event is enough to induce ownership over a fake hand. Proceedings of the Royal Society of London B: Biological Sciences, 280, 20131140 10.1098/rspb.2013.1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotopoulou, A. , Jenkinson, P. M. , Tsakiris, M. , Haggard, P. , Rudd, A. , & Kopelman, M. (2011). Mirror‐view reverses somatoparaphrenia: Dissociation between first‐ and third‐person perspectives on body ownership. Neuropsychologia, 49, 3946–3955. 10.1016/j.neuropsychologia.2011.10.011 [DOI] [PubMed] [Google Scholar]
- Gandola, M. , Invernizzi, P. , Sedda, A. , Ferrè, E. , Sterzi, R. , Sberna, M. , … Bottini, G. (2012). An anatomical account of somatoparaphrenia et. al. Cortex, 48, 1165–1178. 10.1016/j.cortex.2011.06.012 [DOI] [PubMed] [Google Scholar]
- Kammers, M. P. M. , de Vignemont, F. , Verhagen, L. , & Dijkerman, H. C. (2009). The rubber hand illusion in action. Neuropsychologia, 47, 204–211. 10.1016/j.neuropsychologia.2008.07.028 [DOI] [PubMed] [Google Scholar]
- Llorens, R. , Borrego, A. , Palomo, P. , Cebolla, A. , Noé, E. I. , Badia, S. B. , & Baños, R. (2017). Body schema plasticity after stroke: Subjective and neurophysiological correlates of the rubber hand illusion. Neuropsychologia, 96, 61–69. 10.1016/j.neuropsychologia.2017.01.007 [DOI] [PubMed] [Google Scholar]
- Longo, M. R. , Cardozo, S. , & Haggard, P. (2008). Visual enhancement of touch and the bodily self. Consciousness and Cognition, 17, 1181–1191. 10.1016/j.concog.2008.01.001 [DOI] [PubMed] [Google Scholar]
- Nightingale, S. (1982). Somatoparaphrenia: A case report. Cortex, 18, 463–467. 10.1016/S0010-9452(82)80043-9 [DOI] [PubMed] [Google Scholar]
- Stein, B. E. , & Stanford, T. R. (2008). Multisensory integration: Current issues from the perspective of the single neuron. Nature reviews. Neuroscience, 9, 255 10.1038/nrn2331 [DOI] [PubMed] [Google Scholar]
- Tsakiris, M. (2010). My body in the brain: A neurocognitive model of body‐ownership. Neuropsychologia, 48, 703–712. 10.1016/j.neuropsychologia.2009.09.034 [DOI] [PubMed] [Google Scholar]
- Vallar, G. , & Ronchi, R. (2009). Somatoparaphrenia: A body delusion. Experimental Brain Research, 192, 533–551. 10.1007/s00221-008-1562-y [DOI] [PubMed] [Google Scholar]
- Van Stralen, H. E. , Van Zandvoort, M. J. E. , Hoppenbrouwers, S. S. , & Dijkerman, C. (2014). Affective touch modulates the rubber hand illusion. Cognition, 131(1), 147–158. 10.1016/j.cognition.2013.11.020 [DOI] [PubMed] [Google Scholar]
- Van Stralen, H. E. , van Zandvoort, M. J. E. , Kappelle, L. J. , & Dijkerman, H. C. (2013). The rubber hand illusion in a patient with hand disownership. Perception, 42, 991–993. 10.1068/p7583 [DOI] [PubMed] [Google Scholar]
- White, R. C. , & Aimola Davies, A. M. (2017). Asynchrony in the rubber hand paradigm: Unexpected illusions following stroke. Cortex, 93, 224–226. 10.1016/j.cortex.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Winward, C. E. , Halligan, P. W. , & Wade, D. T. (2002). The Rivermead Assessment of Somatosensory Performance (RASP): Standardization and reliability data. Clinical Rehabilitation, 16, 523–533. 10.1191/0269215502cr522oa [DOI] [PubMed] [Google Scholar]
- Zeller, D. , Gross, C. , Bartsch, A. , Johansen‐Berg, H. , & Classen, J. (2011). Ventral premotor cortex may be required for dynamic changes in the feeling of limb ownership: A lesion study. Journal of Neuroscience, 31, 4852–4857. 10.1523/JNEUROSCI.5154-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Stimuli, tests and procedures.
Appendix S2. Cognitive profile.


