Abstract
Soft tissue sarcomas (STS) are rare tumors accounting for less than 1% of human cancers. While the highest incidence of sarcomas is observed in elderly, this population is often excluded or poorly represented in clinical trials. The present study reports on clinicopathological presentation, and outcome of sarcoma patients over 90 recorded in the Netsarc.org French national database. NETSARC (netsarc.org) is a network of 26 reference sarcoma centers with specialized multidisciplinary tumor board (MDTB), funded by the French National Cancer Institute to improve the outcome of sarcoma patients. Since 2010, presentation to an MDTB, second pathological review, and collection of sarcoma patient characteristics and follow‐up are collected in a database Information of patients registered from January 1, 2010, to December 31, 2016, in NETSARC were collected, analyzed and compared to the younger population. Patients with sarcomas aged >90 have almost exclusively sarcomas with complex genomics (92.0% vs. 66.3%), are less frequently metastatic (5.3% vs. 14·7%) at diagnosis, have more often superficial tumors (39.8% vs. 14.7%), as well as limbs and head and neck sites (75.2% vs. 38.7%) (all p < 0.001). Optimal diagnostic procedures and surgery were less frequently performed in patients over 90 (p < 0.001). These patients were less frequently operated in NETSARC centers, as compared to those of younger age groups including aged 80–90. However, local relapse‐free survival, metastatic relapse‐free survival and relapse‐free survival were not significantly different from those of younger patients, in the whole cohort, as well as in the subgroup of operated patients. As expected overall survival was worse in patients over 90 (p < 0.001). Patients over 90 who were not operated had worse overall survival than younger patients (9.9 vs. 27.3 months, p < 0.001). Patients with STS diagnosed after 90 have distinct clinicopathological features, but comparable relapse‐free survival, unless clinical practice guidelines recommendations are not applied. Standard management should be proposed to these patients if oncogeriatric status allows.
Keywords: sarcomas, registry, survival, relapse, progression, oncogeriatry, elderly patients, NETSARC
Short abstract
What's new?
While the highest incidence of soft‐tissue sarcoma (STS) is observed in the elderly, this population is often excluded or poorly represented in clinical trials. Therefore, little is known about the characteristics, treatment, and outcomes of STS in these patients. In this study, the authors analyzed numerous clinical characteristics of patients with sarcoma diagnosed at age 91 or older. They conclude that standard STS management and clinical practice guidelines should be followed for these patients if possible.
Abbreviations
- LMS
leiomyosarcomas
- MDT
multidisciplinary team
- MFS
myxofibrosarcomas
- PFS
progression‐free survival
- STS
soft tissue sarcomas
- UPS
undifferentiated pleomorphic sarcomas
Introduction
Soft tissue sarcomas (STS) are rare tumors accounting for less than 1% of human cancers, with a yearly incidence close to 5.9/100,000.1, 2, 3, 4, 5 Death due to tumor is frequent in this group of diseases.5 While the highest incidence of sarcomas is observed in patients aged between 75 and 84 years with a rate of 16%,1, 5 elderly patients are often excluded or very poorly represented in clinical trials.6 Therefore, little is known about characteristics, treatment and outcomes concerning patients aged over 65, in particular for those over 90 years with STS.6, 7
Sarcomas represent a heterogeneous group with over 80 different pathological subtypes.8 Sarcoma with “simple genomics” include those with specific translocations, well‐differentiated liposarcomas with 12q amplicon and GIST with tyrosine kinase mutation accounting for 12–20% of cases each; those with “complex genomic” profiles (e.g., undifferentiated pleomorphic sarcomas [UPS], leiomyosarcomas [LMS] and myxofibrosarcomas [MFS]) account for 50% of all STS.9, 10, 11
The objective of this work was to analyze the presentation and outcome of sarcoma patients over 90 years, a group of patients unreported in the literature, with the aim of providing data which could guide the management of the general elderly patient population with sarcomas.
Patients and Methods
NETSARC network
NETSARC is the French reference network for the management of soft tissue and visceral sarcomas, collecting clinical, centrally reviewed pathology, therapeutics and outcome of all sarcoma patients in 26 centers. The registry was approved in October 2009 by the INCa (Institut National du Cancer) and the competent authorities (CNIL) in 2010.5 These databases have been approved by the French Ethics Committee and Agency in charge of noninterventional trials: Comité consultatif sur le traitement de l'information en matière de recherche dans le domaine de la Santé (CCTIRS: number of approval 09.594) and Commission Nationale Informatique et Liberté (CNIL: number of approval 909,510). In the present work, we used information of patients registered from January 1, 2010, to December 2016. Mean follow‐up of this series is 17 months. Patient's data (gender, histology, grade, depth, size, localization, treatment, relapse and survival) were collected from the NETSARC database (https://netsarc.org).
Statistical analysis
Collected data were analyzed using IBM SPSS Statistics version 20 (IBM, Paris, France). Chi‐square and Fisher exact tests were performed to analyze the data sets of the different age groups. Survival was plotted according to the Kaplan–Meier method, compared to the log‐rank test. Cox model was used for multivariate analysis of prognostic factors.
Results
Population
A total of 12,835 incident patients with sarcomas, with 113 (0.9%) patients aged >90 were registered in the national NETSARC sarcoma database between January 1, 2010, and December 31, 2016. Patients’ characteristics are described in Table 1. Sarcomas with complex genomics were overrepresented in patients over 90 when compared to patients under 90 (92.0% vs. 66.3%, p < 0.001). Undifferentiated pleomorphic sarcoma (UPS), LMS and MFS were the most represented histological subtypes in patients over 90 (Table 1). Limb and head and neck sites, and superficial locations were overrepresented in patients over 90 (p ≤ 0.001). Male patients were overrepresented in patients aged >90 while representing close to 25% of French citizen aged >90.
Table 1.
Patient characteristics
| Age at diagnosis | p | ||
|---|---|---|---|
| Diagnosis at ≤90 n (%) n = 12,722 (99.4%) | Diagnosis at >90 n (%) n = 113 (0.6%) | ||
| Age at diagnosis (years) | |||
| Median | 62 | 92 | |
| Range | 0–90 | 91‐101 | |
| Sex | NS1 | ||
| Female | 6,283 (49·4%) | 62 (54·9%) | |
| Male | 6,439 (50·6%) | 51 (45·1%) | |
| Localization at diagnosis | |||
| Trunk | 6,706 (52·7%) | 25 (22·1%) | ≤0.0011 |
| Limb | 4,218 (33·2%) | 67 (59·3%) | |
| Superior limb | 1,092 (8·6%) | 22 (19·5%) | |
| Inferior limb | 3,126 (2·6%) | 45 (3·8%) | |
| Head & neck | 710 (5·5%) | 18 (15·9%) | |
| Unknown | 1,088 (8·6%) | 3 (2·7%) | |
| Histology | |||
| Complex genomics | 8,431 (66·3%) | 104 (92·0%) | ≤0.0011 |
| UPS | 1,569 (12·3%) | 40 (35·4%) | |
| LMS | 2,762 (21·7%) | 16 (14·2%) | |
| Myxofibrosarcoma | 804 (6·3%) | 14 (12·4%) | |
| Angiosarcoma | 666 (5·2%) | 12 (10·6%) | |
| DDLPS | 1,506 (11·8%) | 11 (9·7%) | |
| Undifferentiated sarcoma | 768 (6·0%) | 10 (8·8%) | |
| Rhabdomyosarcoma | 98 (0·7%) | 1 (0·9%) | |
| Fibrosarcoma | 44 (0·3%) | 0 (0%) | |
| Liposarcoma pleomorphic | 174 (1·4%) | 0 (0%) | |
| Osteosarcoma | 40 (0%) | 0 (0%) | |
| GIST | 1,490 (11·7%) | 6 (5·3%) | |
| Translocation sarcoma | 2,801 (22·0%) | 3 (2·7%) | |
| Ewing | 769 (6·0%) | 1 (0·9%) | |
| Myxoid LPS | 484 (3·8%) | 1 (0·9%) | |
| Synovial sarcoma | 635 (5·0%) | 1 (0·9%) | |
| ESS | 273 (2·1%) | 0 (0%) | |
| Epithelioid hemangioendothelioma | 100 (0·9%) | 0 (0%) | |
| EMC | 89 (0·7%) | 0 (0%) | |
| SFT | 451 (3·5%) | 0 (0%) | |
| Grade | |||
| 1 | 1,086 (8·5%) | 6 (5·3%) | NS1 |
| 2 | 3,133 (24·6%) | 24 (2·2%) | |
| 3 | 3,800 (29·9%) | 39 (34·5%) | |
| Unknown | 4,703 (36·9%) | 44 (38·9%) | |
| Depth | <0.0011 | ||
| Superficial | 1873 (14·7%) | 45 (39·8%) | |
| Deep | 8,636 (67·9%) | 50 (44·2%) | |
| Superficial + deep | 806 (6·3%) | 13 (11·5%) | |
| Unknown | 1,407 (11·1%) | 5 (4·4%) | |
| Size | NS1 | ||
| <50 mm | 1,620 (12·7%) | 18 (15·9%) | |
| ≥50 mm | 3,924 (30·8%) | 29 (25·7%) | |
| Unknown | 7,178 (56·4%) | 66 (58·4%) | |
| Previous history of radiotherapy | NS1 | ||
| No | 12,388 (97·7%) | 110 (97·3%) | |
| Yes | 294 (2·3%) | 3 (2·7%) | |
| Previous history of cancer | NS1 | ||
| No | 8,563 (67·3%) | 68 (60·2%) | |
| Yes | 1,741 (13·7%) | 20 (17·7%) | |
| Unknown | 2,418 (19·0%) | 25 (22·1%) | |
| Known genetic predisposition | NS1 | ||
| No | 10,181 (80·0%) | 88 (77·9%) | |
| Yes | 123 (1·0%) | 0 (0%) | |
| Unknown | 2,418 (19·0%) | 25 (2·1%) | |
| Metastatic at diagnosis | 0.0091 | ||
| Yes | 1865 (14·7%) | 6 (5·3%) | |
| No | 9,664 (76·0%) | 89 (78·8%) | |
| Unknown | 1,193 (9·4%) | 18 (15·9%) | |
Italics refer to molecular subtypes of sarcomas.
Chi2 test.
(https://www.insee.fr/fr/statistiques/1892086?sommaire=1912926), male patients represented 45% of the patients in this age group. Patients >90 were also less frequently metastatic at initial diagnosis (p = 0·009), even when considering only patients who had a CT scan (data not shown). Prior history of cancers and radiotherapy were no more frequent in patients aged over 90 vs. younger patients (Table 1). Genetic predisposition was observed in none of the 113 patients over 90 vs. 123 reported in the remaining population.
Patient management
The adherence to ESMO clinical practice guidelines (2,3) was then analyzed (Table 2). While biopsy rate was similar, patients aged over 90 had less frequently appropriate pretreatment imaging than patients under 90 (56.6% vs. 75.1%, p ≤ 0.001). This was true also when comparing patients aged >90 to the group of 60–80 or 80–90 (Table 3).
Table 2.
Disease management in patients aged above and under 90
| Age at diagnosis | p | ||
|---|---|---|---|
| Diagnosis at ≤90 n (%) n = 12,712 | Diagnosis at >90 n (%) n = 113 | ||
| Disease management | |||
| Biopsy performed before surgery | NS1 | ||
| Yes | 8,213 (64·6%) | 72 (63·7%) | |
| No | 3,552 (27·9%) | 32 (28·3%) | |
| Unknown | 957 (7·5%) | 9 (8·0%) | |
| Imaging performed before surgery | ≤0.0011 | ||
| Yes | 9,552 (75·1%) | 64 (56·6%) | |
| No | 700 (5·5%) | 14 (12·4%) | |
| Unknown | 2,470 (19·4%) | 35 (31·0%) | |
| Neoadjuvant treatment before surgery | 0.0451 | ||
| Yes | 835/3565 (23·4%) | 3/34 (8·8%) | |
| No | 2730/3565 (76·6%) | 31/34 (91·2%) | |
| Total | 3,565 | 34 | |
| Surgery performed | ≤0.0011 | ||
| Yes | 9,988 (78·5%) | 68 (60·2%) | |
| No | 1,014 (8·0%) | 23 (20·4%) | |
| Unknown | 1,720 (13·5%) | 22 (19·5%) | |
| Excision margins of first surgery | 0.091 | ||
| R0 | 3,910 (30·7%) | 26 (23%) | |
| R1 | 2,608 (20·5%) | 20 (17·7%) | |
| R2 | 990 (7·8%) | 9 (8%) | |
| Unknown | 5,214 (41·0%) | 58 (51·3%) | |
| Reexcision after first surgery | NS1 | ||
| No | 11,380 (89·5%) | 105 (92·9%) | |
| Yes | 1,342 (10·5%) | 8 (7·1%) | |
| Excision margins at second surgery | NS1 | ||
| R0 | 993 (7·8%) | 5 (4·4%) | |
| R1 | 213 (1·7%) | 2 (1·8%) | |
| R2 | 39 (0·3%) | 0 | |
| Unknown | 97/1342 | 1/8 | |
Chi‐square or Fisher's exact test.
Table 3.
Sarcoma patient characteristics and management across age groups
| Age | Age at diagnosis (% of the age groups within the series) | ||||||
|---|---|---|---|---|---|---|---|
| 0–17 n = 367 (2.9%) | 18–30 n = 772 (6.0%) | 31–60 n = 4,451 (34.7%) | 61–79 n = 5,789 (45.1%) | 80–90 n = 1,343 (10.5%) | >90 n = 113 (0.6%) | p value1 | |
| Histology | |||||||
| Complex genomics | |||||||
| UPS | 2 (0.5%) | 23 (2.9%) | 427 (9.6%) | 774 (13.4%) | 273 (20.3%) | 40 (35·4%) | <0.001 |
| LMS | 7 (1.9%) | 44 (5.7%) | 1,038 (23.3%) | 1,359 (23.5%) | 314 (23.4%) | 16 (14·2%) | <0.001 |
| Myxofibrosarcoma | 2 (0.5%) | 8 (1.0%) | 214 (4.8%) | 445 (7.7%) | 135 (10.1%) | 14 (12·4%) | <0.001 |
| Angiosarcoma | 8 (2.2%) | 35 (4.5%) | 194 (4.4%) | 324 (5.6%) | 105 (7.8%) | 12 (10·6%) | <0.001 |
| DDLPS | 0 (0%) | 10 (1.3%) | 412 (9.3%) | 892 (15.4%) | 192 (14.3%) | 11 (9·7%) | <0.001 |
| Undifferentiated sarcoma | 11 (2.9%) | 40 (5.2%) | 245 (13.2%) | 369 (6.4%) | 103 (7.7%) | 10 (8·8%) | 0.002 |
| Pleomorphic RMS | 0 (0.0%) | 6 (0.8%) | 23 (0.7%) | 32 (0.5%) | 6 (0.4%) | 1 (0.9%) | 0.64 |
| GIST | 2 (0.5%) | 20 (2.6%) | 496 (11.1%) | 859 (14.8%) | 113 (8.4%) | 6 (5.3%) | <0.001 |
| Translocation sarcoma | |||||||
| Ewing | 85 (23.2%) | 94 (12.2%) | 93 (2.1%) | 34 (0.5%) | 5 (0.4%) | 1 (0·9%) | <0.001 |
| Myxoid LPS | 7 (1.9%) | 61 (7.9%) | 307 (6.8%) | 99 (1.7%) | 10 (0.7%) | 1 (0·9%) | <0.001 |
| Synovial sarcoma | 39 (10.6%) | 144 (18.6%) | 322 (7.2%) | 114 (1.9%) | 16 (1.2%) | 1 (0·9%) | <0.001 |
| Other/not specified | 203 (55.3%) | 287 (37.2%) | 1,080 (24.2%) | 488 (8.4%) | 71 (5.3%) | 0 (0%) | <0.001 |
| Gender | |||||||
| Female | 153 (41.7%) | 362 (46.9%) | 2,355 (52.9%) | 2,759 (47.7%) | 654 (48.7%) | 62 (54.9%) | <0.001 |
| Male | 214 (58.3%) | 410 (51.3%) | 2096 (47.1%) | 3,030 (52.3%) | 689 (51.3%) | 51 (45.1%) | |
| Grade | |||||||
| 1 | 9 (2.5%) | 68 (8.8%) | 513 (11.5%) | 418 (7.2%) | 78 (5.8%) | 6 (5.3%) | <0.001 |
| 2 | 28 (7.6%) | 130 (16.8%) | 1,081 (24.3%) | 1,541 (26.6%) | 353 (26.3%) | 24 (21.2%) | |
| 3 | 136 (37.1%) | 195 (25.3%) | 1,219 (27.4%) | 1,735 (30.0%) | 515 (38.3%) | 39 (34.5%) | |
| UNK/NA | 194 (52.8%) | 379 (49.1%) | 1,638 (36.8%) | 2095 (36.2%) | 397 (29.6) | 44 (38.9%) | |
| Depth | |||||||
| Deep | 176 (47.9%) | 488 (63.2%) | 3,523 (79.2%) | 4,361 (75.3%) | 894 (66.5%) | 63 (55.7%) | <0.001 |
| Superficial | 15 (4.1%) | 70 (9.1%) | 543 (12.2%) | 896 (15.5%) | 349 (26.0%) | 45 (39.8%) | |
| Unknown | 176 (48.0%) | 214 (27.7%) | 385 (8.6%) | 532 (9.2%) | 240 (17.8%) | 5 (4.4%) | |
| Size in mm (median) | 69.1 | 72.7 | 70.2 | 75.5 | 69.7 | 8.0 | 0.002 |
| Metastatic at diagnosis | |||||||
| Yes | 96 (26.1%) | 155 (20.1%) | 658 (14.7%) | 831 (14.3%) | 146 (10.8%) | 6 (5.3%) | <0.001 |
| No | 233 (63.5%) | 557 (72.2%) | 3,406 (76.5%) | 4,430 (76.5%) | 1,038 (77.3%) | 89 (78.8%) | |
| Unknown | 38 (10.4%) | 60 (7.8%) | 387 (8.7%) | 528 (9.1%) | 159 (11.8%) | 18 (15.9%) | |
| Biopsy before | |||||||
| Yes | 299 (81.5%) | 555 (71.9%) | 763 (62.1%) | 3,690 (63.7%) | 906 (87.5%) | 72 (63.7%) | <0.001 |
| No | 35 (9.5%) | 162 (21.0%) | 1,365 (30.7%) | 1,644 (28.4%) | 346 (25.8%) | 32 (28.3%) | |
| Unknown | 33(9.0%) | 55 (7.1%) | 323 (7.3%) | 455 (7.9%) | 91 (6.8%) | 9 (8.0%) | |
| Imaging < surgery | |||||||
| Yes | 311 (84.7%) | 639 (82.8%) | 3,406 (76.5%) | 4,281 (74.0%) | 915 (68.1%) | 64 (66.6%) | <0.001 |
| No | 8 (2.2%) | 29 (3.8%) | 199 (4.5%) | 326 (5.8%) | 128 (9.5%) | 14 (12.4%) | |
| Unknown | 48 (13.1%) | 104 (13.5%) | 846 (19.0%) | 1,172 (20.2%) | 300 (22.3%) | 35 (31.0%) | |
| Neoadjuvant treat | |||||||
| Yes | 82 (22.3%) | 82 (10.6%) | 310 (7.0%) | 325 (5.6%) | 36 (2.7%) | 3 (2.7%) | <0.001 |
| No | 25 (6.8%) | 139 (18.0%) | 981 (22.0%) | 1,308 (22.6%) | 277 (20.6%) | 31 (27.4%) | |
| Total | 260 (70.8%) | 551 (71.4%) | 3,160 (71.0%) | 4,156 (71.8%) | 1,030 (76.7%) | 79 (69.9%) | |
| Surgery in NETSARC | |||||||
| Yes | 146 (39.8%) | 291 (37.7%) | 1,529 (34.4%) | 1849 (31.9%) | 351 (26.1%) | 16 (14.2%) | <0.001 |
| No/No surgery/UNK | 221 (60.2%) | 481 (62.3%) | 2,922 (65.6%) | 3,940 (68.1%) | 992 (73.9%) | 97 (85.8%) | |
| Excision margins of last surgery | |||||||
| R0 | 122 (33.2%) | 307 (39.8%) | 1,799 (40.4%) | 2,229 (38.5%) | 392 (29.2%) | 29 (25.7%) | <0.001 |
| R1 | 47 (12.5%) | 105 (13.6%) | 721 (16.2%) | 997 (17.2%) | 254 (18.9%) | 18 (15.9%) | |
| R2 | 9 (2.5%) | 42 (5.4%) | 197 (4.4%) | 235 (4.1%) | 58 (4.3%) | 7 (6.2%) | |
| Other/Unknown | 189 (51.5%) | 318 (41.2%) | 1,734 (38.9%) | 2,328 (40.2%) | 639 (47.6%) | 59 (52.2%) | |
Bold indicates significant p value (p < 0.05).
Chi2 test.
Surgery was less frequently performed in patients over 90 years (60.2% vs. 78.3%, p ≤ 0.001); they also had less frequently neoadjuvant treatment. This did not result in significant differences in terms of resection margins at first or at second surgery (Table 2). Resection rates (Table 2) and final result of the surgical removal (Table 3) were also not significantly different when comparing only patients for whom the R criterion was documented.
Patients over 90 as compared to other age groups
Table 3 presents an analytic description of histologies, clinical presentations and management within different age subgroups. As expected, histological subtypes were extremely different across age groups, in particular across the extremes. It is interesting to note that differences were also observed in the three elder groups, on histologies, depth, grade and metastasis. As compared to the 80–90 years group, histotypes, depth, metastasis at diagnosis and gender of patient with sarcoma aged >90 were different (Table 3). Regarding patient management, compliance to CPGs was the lowest in patients aged >90, together with the final quality of surgery (lowest R0 rate, highest R2 rate), with fewer patients operated in reference centers. Overall a significant trend of decrease of compliance to guidelines, management in reference centers and final quality of surgery over age groups was observed, from the children and adolescent/young adults to the older age group (Table 3).
Relapse and survival
Local progression‐free survival (PFS), metastatic PFS and PFS were not significantly different in the two populations (Figs. 1 a–1 c). Relapse‐free survival of patients aged over 90 vs. those aged 90 or under was also similar when the analysis was conducted only with operated patients (Fig. 1 d). No difference was observed either for local relapse‐free survival not for metastatic relapse‐free survival for operated patients (data not shown). As expected, overall survival was worse in patients >90 (Fig. 1 e). Importantly, the overall survival of patients who were not operated was significantly worse in patients aged over 90 vs. younger patients (9.9 vs. 27.1 months, p < 0.001) with no patient alive at 3 years in the older group vs. 40% in the younger one (Fig. 1 f). Using Cox model, with grade, size, depth, site, gender, presentation to a multidisciplinary team (MDT) prior to treatment5 and surgery in expert center12 and age >90 as tested variables, age >90 was not identified as an independent prognostic factor for either relapse‐free survival or PFS (data not shown).
Figure 1.
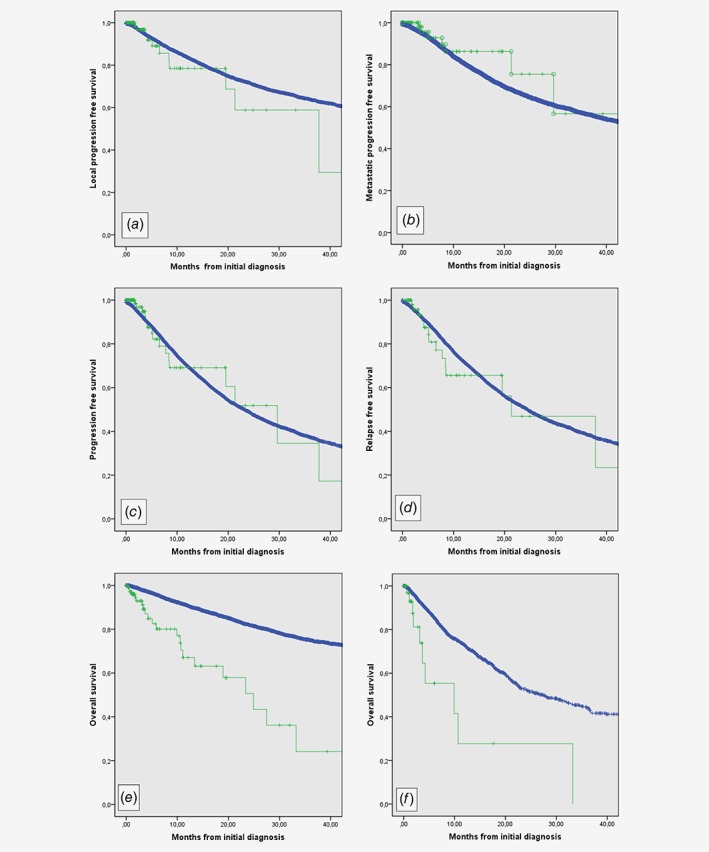
Progression, relapse and survival in sarcoma patients aged over or under 90. (a) Local progression‐free survival of patients aged over 90 (green) and younger (blue). (b) Metastatic progression‐free survival of patients aged over 90 (green) and younger (blue). (c) Progression‐free survival of patients aged over 90 (green) and younger (blue). (d) Relapse‐free survival of operated patients aged over 90 (green) and younger (blue). (e) Overall survival of patients aged over 90 (green) and younger (blue). (f) Overall survival of nonoperated patients aged over 90 (green) and younger (blue). [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
This analysis of a 6‐year nationwide registry of incident sarcoma patients includes over 12,000 patients with 113 (0.9%) patients aged >90. It identifies for the first time‐specific pathological and clinical characteristics of sarcomas diagnosed in patients over 90: these are almost exclusively sarcomas with complex genomics, more frequently superficial tumors, from limb sites or head and neck sites. Despite an obvious difference in life duration, sarcoma patients aged over 90 do not have a higher frequency of previous radiotherapy, previous cancers, and none genetic predisposing condition. Patients aged over 90 are also less frequently metastatic at initial presentation than the younger sarcoma patient population and relative to the sex ratio at this age in the French population, more frequently males. These clinicopathological and clinical specificities of sarcomas occurring at an older age are unexpected and had not been previously recognized to our knowledge. It is interesting to observe that these characteristics are not equally shared by the groups aged 60–80 or 80–90, but quite characteristic of this age group. Sarcomas occurring in higher age may result in more from accumulation of external oncogenic events over the lifetime; limb and head and neck sites may suggest exposure to the sun as risk factors to be tested. It is particularly notable that less than 3% of sarcoma in this population was translocation‐related sarcomas.
Less adequate management is offered to patients with STS over 90, in particular regarding pretreatment imaging and surgery. Of note, oncogeriatric assessment13 is not part of the NETSARC data set, and it is likely that coexisting conditions have largely contributed to these differences with younger patients. However, when patients over 90 are treated with surgery according to clinical practice guidelines (within NETSARC, the ESMO guidelines are used as reference),2, 3 relapse‐free survival and PFS remain similar to that of the younger population in univariate and also multivariate analysis where classical prognostic factors are introduced.2, 3, 5 This points to the need not to undertreat patients, including those aged over 90, and to adopt the classical CPGs for sarcoma management in patients at all age when oncogeriatric status allows. As expected, the overall survival of sarcoma patients aged over 90 is shorter than that of younger patients. Median overall survival is 25 months in this population of patients over 90 (whose median age is 92), an outcome which must be compared to the 50 months life expectancy for the general French population at the age of 90.14
To the best of our knowledge, this is the first series reporting on the different biology and natural history of patients with sarcomas occurring at a very high age. Sarcoma occurring after 90 has a specific biology and natural history, but not intrinsically a worse cancer‐specific prognosis. If fit according to geriatric assessment, this patient population, should be treated according to the general CPGs for sarcomas. It is reasonable to infer a similar statement for younger geriatric sarcoma patients.
Acknowledgements
The authors would like to thank the teams and leaders of the French National Cancer Institute (INCa) for the continuous support to the project.
Conflict of interests: Dr JEK reports travel support and honoraria from Pharmamar, travel support and advisory board for Astra‐Zeneca, travel support and advisory board for Tesaro, and advisory board for Clovis; Dr OM has acted as consultant for Amgen, Astra‐Zeneca, Bayer, Bristol‐Myers Squibb, Blueprint medicines, Eli‐Lilly, Merck, Novartis, Pfizer, Roche, Janssen, Servier, and Vifor Pharma. All other co‐authors report no competing interests or conflict of interests.
References
- 1. Ducimetiere F, Lurkin A, Ranchere‐Vincre D, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One 2011;6:e20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. ESMO/European Sarcoma Network Working Group . Soft tissue and visceral sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2014;25(Suppl 3):iii102–12. [DOI] [PubMed] [Google Scholar]
- 3. ESMO/European Sarcoma Network Working Group . Gastrointestinal stromal tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2014;25(Suppl 3):iii21–6. [DOI] [PubMed] [Google Scholar]
- 4. von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016;14:758–86. [DOI] [PubMed] [Google Scholar]
- 5. Blay JY, Soibinet P, Penel N, et al. Improved survival using specialized multidisciplinary board in sarcoma patients. Ann Oncol 2017;28:2852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer‐treatment trials. N Engl J Med 1999;341:2061–7. [DOI] [PubMed] [Google Scholar]
- 7. Garbay D, Maki RG, Blay JY, et al. Advanced soft‐tissue sarcoma in elderly patients: patterns of care and survival. Ann Oncol 2013;24:1924–30. [DOI] [PubMed] [Google Scholar]
- 8. Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. Pathology and genetics of tumours of soft tissue and bone. Lyon: World Health Organization, IARC Press, 2013. [Google Scholar]
- 9. Chibon F, Lagarde P, Salas S, et al. Validated prediction of clinical outcome in sarcomas and multiple types of cancer on the basis of a gene expression signature related to genome complexity. Nat Med 2010;16:781–7. [DOI] [PubMed] [Google Scholar]
- 10. Gibault L, Perot G, Chibon F, et al. New insights in sarcoma oncogenesis: a comprehensive analysis of a large series of 160 soft tissue sarcomas with complex genomics. J Pathol 2011;223:64–71. [DOI] [PubMed] [Google Scholar]
- 11. Taylor BS, Barretina J, Maki RG, et al. Advances in sarcoma genomics and new therapeutic targets. Nat Rev Cancer 2011;11:541–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blay J, Stoeckle E, Italiano A, et al. Improved overall and progression free survival after surgery in expert sites for sarcoma patients: a nationwide study of FSG‐GETO/NETSARC. Ann Oncol 2017;28:v521–38. [Google Scholar]
- 13. Owusu C, Berger NA. Comprehensive geriatric assessment in the older cancer patient: coming of age in clinical cancer care. Clin Pract (London, England) 2014;11:749–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mesle F. Esperance de vie et mortalité aux âges élevés, vol. 2 Paris: La Documentation Française: Retraite et Société, 2005. 89–113. [Google Scholar]


