Abstract
Background and purpose
The aim of this study was to retrospectively investigate clinical and neuroimaging characteristics in the largest sample size of patients with corpus callosum infarction to date and then to follow up these patients for 1 year to clarify the prognosis of this rare stroke entity.
Methods
A total of 127 patients with acute callosal infarction out of 5584 acute ischaemic stroke patients were included in this study. The recruited patients were divided into a pure callosal infarction group and a complex callosal infarction group (coupled with other infarct locations simultaneously), and clinical and neuroimaging features were analyzed. Some of the patients were followed up for 1 year to evaluate recurrence rate and mortality.
Results
The incidence of acute callosal infarction was 2.3%. Most patients presented with advanced neurological dysfunction with or without mild to moderate motor or sensory disorders on admission. The negative rate of computed tomography scan was still 76.4% even at >24 h after onset. Large‐artery atherosclerosis was the most common etiological type. Compared with complex callosal infarction, the pure callosal infarction group had more mental disorders (P = 0.030). Compared with common basal ganglia infarction, the pure callosal infarction group had better short‐term recovery (P = 0.016) but higher 1‐year mortality (P = 0.037). Age and mental disorders were independent risk factors for death in callosal infarction.
Conclusions
Callosal infarction is a white matter stroke that occurs with low incidence. Elderly patients with vascular risk factors showed sudden mental or cognitive disorders and callosal infarction could not be excluded. More attention should be paid to the early diagnosis and secondary prevention of callosal infarction because of its poor long‐term outcome.
Keywords: clinical manifestations, corpus callosum infarction, etiology, neuroimaging, prognosis, thrombolysis
Introduction
The corpus callosum (CC) is the largest association fiber of white matter, which works as a pathway (sensory, movement, vision, hearing, emotion and cognition) or only as a liaison between the left and right cerebral hemispheres 1. The incidence of CC infarction is low because of the rich blood supply and sufficient collateral circulations 2, 3. The clinical manifestations of callosal infarction are complex and there is a lack of specificity because of its own physiological structure and function, and adjacent locations are frequently involved following ischaemia. These characteristics may result in, at least to some degree, misdiagnosis and delayed treatment for callosal infarction. Patients are often ignored and there is a delay in hospital referral especially when the onset is sudden, and mental or cognitive disorders are the early symptoms. It is a pity that many patients miss the thrombolytic time window of acute ischaemic stroke and cannot obtain timely therapy, which sometimes leads to poor prognosis.
The predilection sites of callosal infarction are different in different reports. This discrepancy may be related to the diverse susceptibility of cerebral vascular lesions, genetic or research method differences and insufficient sample size 4, 5, 6. To our knowledge, the vast majority of reports on callosal infarction are case reports. In the present study, a large sample of patients with acute callosal infarction was reviewed to investigate clinical features of callosal ischaemia, the causes of misdiagnosis and the prognosis of pure callosal infarction in the Han Chinese population. Our research provides a comprehensive overview of callosal infarction, which is helpful for clinicians in making early diagnosis, providing timely treatment and significantly improving prognosis.
Methods
Written informed consent was obtained from every patient on the use of case information on admission. The Changhai Hospital Ethics Committee approved the study. Relevant data were obtained from the clinical database of Changhai Hospital.
A total of 5584 acute ischaemic stroke patients were retrospectively collected in an inpatient setting at our hospital between January 2012 and December 2016. These patients had hyperintensity of diffusion‐weighted imaging (DWI) in brain magnetic resonance imaging (MRI) (Magneton Impact, Siemens, Berlin, Germany) scanned during hospitalization, indicating acute cerebral ischaemia. Among them, 127 patients with acute callosal infarction were recruited into our study (Fig. S1). Patients with previous mental illness and cognitive impairment, lesions of CC and severe stroke sequelae (modified Rankin scale score ≥3) were excluded.
The demographic data of 127 patients were collected. The time from onset to hospital, past medical history, neurological symptoms and signs, blood tests (lipid and glucose), therapeutic conditions and neuroimaging data were all analyzed. The neuroimaging information included computed tomography (CT) (Brilliance ICT, Philips, Amsterdam, the Netherlands), MRI, magnetic resonance angiography (MAGNETOM Skyra 3.0T, Siemens) and CT angiography (Aquillion One, Toshiba, Tokyo, Japan). The stenosis of cerebral arteries was assessed by the methods reported by Samuels et al. 7.
Of 127 patients, 21 with pure callosal infarction and 21 with callosal infarction coupled with other location infarcts simultaneously (complex callosal infarction) were selected (Fig. 1). During the same period, 21 patients with acute basal ganglia infarction were matched according to gender and age. Clinical data were analyzed among the three groups at the time of discharge (Fig. S2). A total of 21 of 127 patients with callosal infarction did not complete 1‐year follow‐up and were excluded. The remaining 106 patients and 21 patients with basal ganglia infarction were followed up to 1 year in the outpatient department to evaluate recurrence rate and mortality (Fig. S3).
Figure 1.
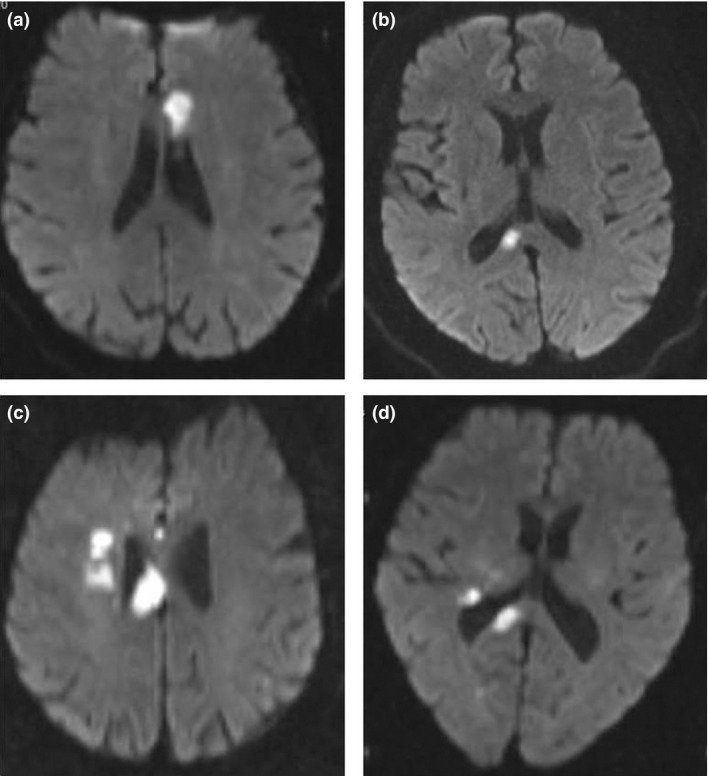
Representative images of pure and complex callosal infarction. (a) Pure genu infarction and (b) pure splenium infarction of the corpus callosum. (c,d) Complex callosal infarction. Part c shows infarction of body coupled with basal ganglia region. Part d shows infarction of splenium coupled with basal ganglia region.
For descriptive analyses, continuous data are expressed as median, upper (Q3) and lower (Q1) quartile or mean and SD; categorical data are expressed as n (%). For statistical test analysis, χ 2 test or Fisher exact test was used to compare categorical variables and one‐way anova or Kruskal–Wallis H‐test was used for continuous variables; Wilcoxon rank sum test was used to compare ordered variables wherever appropriate. Post hoc tests were used when P < 0.05. Partition of χ 2 test was used to compare categorical variables, Nemenyi test was used to compare categorical variables and Wilcoxon rank sum test was used to compare the ordered variables. To explore the risk factor of death, univariate analysis was used, including Student's t‐test, χ 2 test and Wilcoxon rank sum test. The univariate analysis P‐values of risk factors were included in a logistic regression model. Stepwise variable selection method was performed to select the possible risk factors. All tests were two‐sided. All analyses were performed using SAS (version 9.4; SAS Institute, Inc., Cary, NC, USA).
Results
Patient profile
The incidence of acute callosal infarction was 2.3% (127/5584). The age of the 127 patients ranged from 35 to 91 (mean 64.89 ± 12.61) years. A total of 79 patients were male. A significant difference was noted between men and women in the onset age (61.49 ± 12.00 vs. 70.48 ± 11.67 years, P = 0.000).
The top vascular risk factor for callosal infarction in the patients’ past medical history was hypertension (n = 94, 74.0%), followed by dyslipidaemia (high‐density lipoprotein <1.0 mmol/L or low‐density lipoprotein >3.6 mmol/L) (n = 54, 42.5%) and diabetes mellitus (n = 51, 40.2%). Other potential risk factors included smoking (n = 48, 37.8%), past history of cerebral infarction (n = 14, 11.0%), non‐neuroendocrine tumors (n = 13, 10.2%), atrial fibrillation (n = 5, 3.9%) and hyperuricemia (n = 2, 1.6%).
Clinical manifestations
The shortest period from the onset of symptoms to the hospital was 0.3 h and the longest was 480 h with a median time of 24 h. On admission, 43.3% (55/127) of patients had cortical and psychiatric dysfunction, including cognitive decline (33/127, 26.0%), mental disorders (27/127, 21.3%) and sleep disturbance (13/127, 10.2%). A total of 73.2% (93/127) of patients had limb paralysis and 38.6% (49/127) had central palsy of the face. A total of 93 patients with limb paralysis were evaluated by the Lovett muscle rating scale and 91 (97.8%) of them had muscle strength greater than or equal to the fourth level. A total of 20.5% (26/127) of patients had sensory disorders, 9.4% (12/127) had hemianopia and 7.9% (10/127) had ataxia (Table 1)
Table 1.
Clinical manifestations of the patients with corpus callosal infarction
| Clinical manifestations | |
|---|---|
| Cognitive impairment | 33 (26.0) |
| Mental disorder | 27 (21.3) |
| Sleep disorder | 13 (10.2) |
| Aphasia | 22 (17.3) |
| Sensory abnormality | 26 (20.5) |
| Central facial paralysis | 49 (38.6) |
| Limbs paralysis | 93 (73.2) |
| Hemianopia | 12 (9.4) |
| Ataxia | 10 (7.9) |
| Alien hand syndrome | 1 (0.8) |
Data are given as n (%).
Imaging features
Infarction sites were assessed by DWI by MRI. Of 127 patients, 52 (40.9%) had callosal splenium infarction, followed by genu (27/127, 21.3%) and body infarction (8/127, 6.3%). A total of 92 were scanned by brain magnetic resonance angiography or CT angiography, including the anterior cerebral artery, middle cerebral artery, posterior cerebral artery (PCA), internal carotid artery, vertebral artery and basilar artery. For large cerebral vessels, there were 28 cases (30.4%) with anterior cerebral artery stenosis, 41 (44.6%) with middle cerebral artery stenosis, 44 (47.8%) with PCA stenosis, 48 (52.2%) with internal carotid artery stenosis, 32 (34.8%) with vertebral artery stenosis and 21 (22.8%) with basilar artery stenosis. For large cerebral vessels, only 8 of 92 (8.7%) patients had no vascular stenosis. The number of vascular stenoses was ≥2 in 63 (68.5%) patients. One patient had stenosis in the aforementioned six vessels.
On admission, all patients were scanned immediately by brain CT. The results of CT were compared with DWI in the same patient and callosal infarction was not detected in 105 patients, i.e. the false‐negative rate of CT scan for callosal infarction was 82.7% (105/127) in our study. Even at >24 h after onset, the CT false‐negative rate was still 76.4% (97/127).
Etiological type
Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification was used to assess the etiology of 127 patients with callosal infarction. A total of 97 (76.4%) had large‐artery atherosclerosis, 18 (14.2%) had small‐artery occlusion, 7 (5.5%) had stroke of other undetermined etiology and 4 (3.1%) had acute stroke of other etiology. Only one patient (0.8%) had cardiac embolism.
Clinical profile of pure and complex callosal infarctions
There was a significant difference in incidence between the pure and complex callosal infarction groups. No difference was found in admission time, vascular risk factors, sites and number of cerebral vascular stenoses, and neurological function recovery at the time of discharge between the two groups, whereas more patients in the pure callosal infarction group had mental disorders (Table 2).
Table 2.
Comparison of clinical features in the three groups
| Pure callosal infarction (n = 21) | Complex callosal infarction (n = 21) | Basal ganglia infarction (n = 21) | P‐value | P ▵value | P*value | |
|---|---|---|---|---|---|---|
| Time from onset to hospital (h) | 144 (1.75, 270) | 24 (8.5, 42) | 7 (4.25, 12) | 0.024 | 0.705 | 0.030 |
| Thrombolytic therapy | 2 (9.5%) | 2 (9.5%) | 7 (33.3%) | 0.087 | 1.000 | 0.133 |
| Hypertension | 15 (71.4%) | 18 (85.7%) | 16 (76.2%) | 0.645 | 0.452 | 0.726 |
| Diabetes mellitus | 12 (57.1%) | 10 (47.6%) | 2 (9.5%) | 0.004 | 0.537 | 0.001 |
| Hyperlipemia | 7 (36.8%) | 11 (52.4%) | 6 (28.6%) | 0.249 | 0.212 | 0.739 |
| Prior stroke | 6 (28.6%) | 2 (9.5%) | 0 (0) | 0.022 | 0.239 | 0.028 |
| Smoking | 8 (38.1%) | 9 (42.9%) | 9 (42.9%) | 0.938 | 0.753 | 0.753 |
| Cancer | 3 (14.3%) | 0 (0) | 1 (4.8%) | 0.312 | 0.231 | 0.599 |
| Number of risk factors | 3 (2, 4) | 3 (1, 4) | 2 (1, 2) | 0.048 | 0.129 | 0.083 |
| Clinical manifestations | ||||||
| Cognitive impairment | 6 (28.6%) | 5 (23.8%) | 0 (0) | 0.030 | 0.726 | 0.028 |
| Mental disorder | 8 (38.1%) | 2 (9.5%) | 0 (0) | 0.002 | 0.030 | 0.006 |
| Sleep disorder | 3 (14.3%) | 0 (0) | 0 (0) | 0.101 | 0.231 | 0.231 |
| Aphasia | 1 (4.8%) | 2 (9.5%) | 3 (14.3%) | 0.864 | 1.000 | 0.599 |
| Sensory abnormality | 3 (14.3%) | 5 (23.8%) | 11 (52.4%) | 0.021 | 0.694 | 0.009 |
| Central facial paralysis | 7 (33.3%) | 10 (47.6%) | 16 (76.2%) | 0.019 | 0.346 | 0.005 |
| Limb paralysis | 12 (57.1%) | 13 (61.9%) | 21 (100.0%) | 0.003 | 0.753 | 0.003 |
| Discharge conditions | ||||||
| Number of paralytic limbs | 1 (0, 2) | 1 (0, 2) | 2 (2, 2) | 0.000 | 0.664 | 0.001 |
| Muscle strength grade | 5 (4, 5) | 4 (4, 5) | 4 (3, 4) | 0.000 | 0.085 | 0.000 |
| NIHSS score | 1 (1, 2) | 1 (1, 2) | 4 (3, 5) | 0.006 | 0.030 | 0.016 |
P ▵value, pure callosal infarction compared with complex callosal infarction; P*value, pure callosal infarction compared with basal ganglia infarction. NIHSS, National Institutes of Health Stroke Scale. Data are given as n (%), median, upper (Q3) and lower (Q1) quartile or mean and SD.
Compared with basal ganglia infarction, the patients with pure callosal infarction had longer admission time, more vascular risk factors and there were more patients with diabetes mellitus or past cerebral infarction. For clinical symptoms and signs, there was more cognitive impairment or mental disorders in the pure callosal infarction group than in the basal ganglia infarction group, whereas there was less motor and sensory impairment. For assessment of large cerebral vessels, the number of vascular stenoses was apparently more in pure callosal infarction with a statistical difference for PCA and basilar artery. When discharged, the patients with pure callosal infarction had fewer paralytic limbs, higher grade of muscle strength and lower National Institutes of Health Stroke Scale score (Table 2).
Prognosis and analyses of death risk factors in patients with callosal infarction
Three patients with pure callosal infarction and 18 with complex callosal infarction were excluded because of the lack of 1‐year follow‐up. Eight patients with complex callosal infarction were lost during follow‐up. A total of 18 patients with pure callosal infarction, 80 with complex callosal infarction and 21 with basal ganglia infarction were followed up for 1 year. No difference was found in the 1‐year recurrence rate of ischaemic stroke among the three groups (22.2%, 13.8% and 9.5% for pure callosal infarction, complex callosal infarction and basal ganglia infarction, respectively; P = 0.513). Compared with the basal ganglia infarction group, mortality was higher in the pure (22.2% vs.0%, P = 0.037) and complex (15% vs.0%, P = 0.067) callosal infarction groups. Compared with the complex callosal infarction group, the patients with pure callosal infarction had higher 1‐year recurrence rate (P = 0.467) and mortality (P = 0.485) with no statistical significance.
Among 16 patients with callosal infarction who had died, the death of 11 was due to ischaemic stroke and 5 had undetermined etiology.
Multivariable analysis including baseline characteristics of 98 patients with callosal infarction was performed on the outcome of 1‐year mortality. Age and mental disorders were found to be independent death risk factors. Mortality in patients with mental disorders was 7.4 times that of those without mental disorders for callosal infarction (Table 3, Table S1).
Table 3.
Results of logistic regression for the risk factors of 1‐year mortality in patients with corpus callosum infarction
| Estimation | SE | OR | 95% CI | P‐value | |
|---|---|---|---|---|---|
| Age | 0.0691 | 0.0284 | 1.072 | [1.014–1.133] | 0.015 |
| Mental disorders | 2.0015 | 0.6501 | 7.400 | [2.070–26.462] | 0.002 |
CI, confidence interval; OR, odds ratio; SE, standard error.
Discussion
The blood supply of CC comes from both the anterior and posterior circulation and has developed collateral circulation, so the incidence of CC infarction is low. Most previous research is case reports 6, 8, 9 and the largest previous sample size was 59 patients 4. To the best of our knowledge, our study total of 127 patients with callosal infarction is the largest sample size so far. The etiology was mainly large‐artery atherosclerosis together with stenosis or occlusion of multiple large cerebral vessels. In the present study, the clinical manifestations of callosal infarction most often presented with mental or cognitive disorders with mild motor or sensory impairment. Negative brain CT scan even >24 h after onset did not exclude callosal infarction. We were surprised to find, by 1‐year follow‐up for those patients with callosal infarction and common basal ganglia infarction, that callosal infarction had a higher 1‐year recurrence rate of ischaemic stroke and higher mortality rate.
In our single‐center retrospective study, the incidence of callosal infarction was 2.3%, which is in the range reported in previous literature from China and elsewhere 4, 6, 10. Males had higher incidence and younger onset age than females, which may be related to larger overall CC size and hence requirement for larger blood supply in males 11. Hypertension, dyslipidemia and diabetes mellitus are the most common etiology of infarction. All of these demonstrated the similarity between callosal infarction and other ischaemic stroke 12, 13. However, callosal infarction had its own distinct characteristics.
The clinical manifestations of callosal infarction were variable. In addition to common motor and sensory deficits caused by stroke, higher neurological disorders often appeared as early symptoms. In our study, 43.3% of patients were presented with cognitive decline, mental disorders and sleep disturbance, which resulted in difficulty in early diagnosis and delayed hospital referral. We found that the average referral time was 24 h, which missed the thrombolytic time window. It is unfortunate that most patients did not receive timely and effective therapy because of under‐recognition. Especially for pure callosal infarction, our study showed that mental or cognitive disorders may be the only symptoms with no other positive neurological signs, which has seldom been reported before 6, 10, 14, 15. This may be a cause of misdiagnosis and delayed treatment.
In the present study, no statistical difference for thrombolytic therapy rate was found among the pure, complex and basal ganglia infarction groups, but a trend for higher thrombolytic rate in the basal ganglia infarction group was found. Hence, there is a clue to the National Institutes of Health Stroke Scale score, which is used extensively for thrombolytic assessment of ischaemic stroke. Psychiatric items are absent from the National Institutes of Health Stroke Scale, which leads to thrombolytic disqualification for some patients with callosal infarction because of low scores even when admitted within the time window. Our study showed that 1‐year mortality in patients with mental disorders was 7.4 times that of those without mental disorders for callosal infarction. This results in poor prognosis.
It is well known that CT is not sensitive to early ischaemic infarction within 24 h. We compared the results of CT and DWI by MRI on callosal infarction >24 h after onset and found that the CT false‐negative rate was still as high as 76.4%. This is partially responsible for the misdiagnosis in some primary healthcare institutions without MRI. Therefore, a negative result of CT scan at any point cannot eliminate callosal infarction and DWI in MRI is the current preferred diagnostic method.
Large‐artery atherosclerosis was found to be the main etiology of callosal infarction, whereas cardiac embolism was uncommon in our study. Furthermore, >90% of patients had stenosis or occlusion of large cerebral arteries and most of them had lesions in more than two vessels. This demonstrated that atherosclerosis was the main vascular risk factor for callosal infarction, in accordance with the conclusions of much previous literature 6, 16. However, some reports considered that embolism was the main cause 4, 17. Unfortunately, most of these studies did not perform detailed and visualized cerebral vascular evaluation. For example, Li and colleagues 4 considered that embolism was the most likely reason for callosal infarction because of the lesions affecting both hemispheres. Thus, comprehensive vascular assessment is necessary before determining the etiology of infarctions.
The splenium was the location most susceptible to ischaemia in our population, which is in agreement with some literature from northeast China 4 and Greece 6. It was speculated that the supply area of PCA had less collateral circulation and higher ischaemic incidence. In contrast, body and genu have shown a higher risk of ischaemia in central China 18, America 5 and France 10. The diversity in the same race in different countries and in different regions of the same country may be due to sample size, ischaemic susceptibility of cerebral arteries and different in statistical methods. It is notable that our study has the largest sample size focusing on callosal infarction to date in an Asian population. Therefore, at least to some extent, our result is representative.
The patients with pure callosal infarction had lower incidence than those with complex callosal infarction. This is connected with anatomical structures of the callosal blood supply. The internal carotid system and vertebral basilar artery system are both involved in callosal blood circulation. Arteries of posterior circulation are smaller and mainly from vertical branches that obtain blood supply from bilateral‐vertebral arteries and constitute the pericallosal artery plexus 6, 19. Hence, the appearance of callosal infarction indicates extensive lesions of cerebral vessels frequently combined with infarction of other cerebral areas, which has been proved in the present study.
The patients with pure callosal infarction had better recovery and lower National Institutes of Health Stroke Scale scores than those with basal ganglia infarction at the time of discharge. This was partially explained by mild limb paralysis at admission and the rich collateral circulation and white matter structure of CC, which was less vulnerable to ischaemia than gray matter. However, the long‐term outcome was not positive. We have surprisingly found whether patients with pure or complex callosal infarction had higher 1‐year mortality than those with basal ganglia infarction, and mental disorder was an independent death risk factor for callosal infarction. The patients with callosal infarction had longer hospital admission, more vascular risk factors and lesions of large cerebral vessels. All of these were important causes of brain ischaemic recurrence, severe multiple locations and large cerebral infarctions. Unfortunately, some patients with callosal infarction pay little attention to the secondary prevention of stroke because of good recovery of neurological function at discharge. This indifference was, to some extent, responsible for the higher mortality of patients with callosal infarction. Therefore, it is necessary to pay great attention to vascular risk factor screening and secondary prevention of callosal infarction.
Limitations
Our study has some limitations. First, although the present study had the largest sample size by far, it was retrospective, mainly including patients from northeast China, which restricts the general application of some of our conclusions. Secondly, during the 1‐year follow‐up, the number of pure callosal infarctions was small and many factors contributing to recurrence of stroke and death had not been analyzed. Most of these are due to the low incidence of this rare stroke entity and difficulty of face‐to‐face data collection because of the walk‐in follow‐up in China. Therefore, prospective, large‐scale and multi‐center research is needed for further investigations.
Conclusions
In summary, our study has the largest sample size of callosal infarction so far. It is a rare stroke entity mainly presenting with higher neurological function disorders and, once damaged, often with multiple cerebral lesions. At the early‐onset stage, the false‐negative rate of CT scan is relatively high. In addition, its short‐term prognosis is good but long‐term outcome is poor. Therefore, more attention should be paid to the early diagnosis and secondary prevention of callosal infarction, which will improve the prognosis.
Disclosure of conflicts of interest
The authors declare no financial or other conflicts of interest.
Supporting information
Figure S1. Standard stroke investigation work‐up in our institution. CT, computed tomography; CTA, computed tomography angiography; DWI, diffusion‐weighted imaging; MRA, magnetic resonance angiography.
Figure S2. Flow chart of study design
Figure S3. Flow chart of follow‐up of the three groups
Table S1. Univariate analysis for risk factors of death in patients with callosal infarction
Acknowledgements
This study was supported by the National Nature Science Foundation of China (nos. 30900474 and 81571299), Talent Development Fund of Shanghai (no. 2017038) and Western Medicine Guidance Project from the Science and Technology Commission of Shanghai Municipality (no. 16411969900).
Contributor Information
S. Ding, Email: dingsuju@hotmail.com.
X. Bi, Email: bixiaoying2013@163.com.
References
- 1. Roy E, Hague C, Forster B, Colistro R, Andrews G. The corpus callosum: imaging the middle of the road. Can Assoc Radiol J 2014; 65: 141–147. [DOI] [PubMed] [Google Scholar]
- 2. Türe U, Yaşargil MG, Krisht AF. The arteries of the corpus callosum: a microsurgical anatomic study. Neurosurgery 1996; 39: 1075–1084. [DOI] [PubMed] [Google Scholar]
- 3. Wycoco V, Shroff M, Sudhakar S, Lee W. White matter anatomy: what the radiologist needs to know. Neuroimaging Clin N Am 2013; 23: 197–216. [DOI] [PubMed] [Google Scholar]
- 4. Li S, Sun X, Bai YM, et al Infarction of the corpus callosum: a retrospective clinical investigation. PLoS ONE 2015; 10: e0120409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kasow DL, Destian S, Braun C, Quintas JC, Kagetsu NJ, Johnson CE. Corpus callosum infarcts with atypical clinical and radiologic presentations. Am J Neuroradiol 2000; 21: 1876–1880. [PMC free article] [PubMed] [Google Scholar]
- 6. Chrysikopoulos H, Andreou J, Roussakis A, Pappas J. Infarction of the corpus callosum: computed tomography and magnetic resonance imaging. Eur J Radiol 1997; 25: 2–8. [DOI] [PubMed] [Google Scholar]
- 7. Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. Am J Neuroradiol 2000; 21: 643–646. [PMC free article] [PubMed] [Google Scholar]
- 8. Yang LL, Huang YN, Cui ZT. Clinical features of acute corpus callosum infarction patients. J Clin Neurol 2014; 7: 5160–5164. [PMC free article] [PubMed] [Google Scholar]
- 9. Gao X, Li B, Chu W, Sun X, Sun C. Alien hand syndrome following corpus callosum infarction: a case report and review of the literature. Exp Ther Med 2016; 12: 2129–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giroud M, Dumas R. Clinical and topographical range of callosal infarction: a clinical and radiological correlation study. J Neurol Neurosurg Psychiatry 1995; 59: 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bishop KM, Wahlsten D. Sex differences in the human CC: myth or reality? Neurosci Biobehav Rev 1997; 21: 581–601. [DOI] [PubMed] [Google Scholar]
- 12. Yao XY, Lin Y, Geng JL, et al Age‐ and gender‐specific prevalence of risk factors in patients with first‐ever ischemic stroke in China. Stroke Res Treat 2012; 2012: 136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldstein LB, Bushnell CD, Adams RJ, et al Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42: 517–584. [DOI] [PubMed] [Google Scholar]
- 14. Kaymakamzade B, Eker A. Acute infarction of corpus callosum due to transient obstructive hydrocephalus. Neurol Neurochir Pol 2016; 50: 280–283. [DOI] [PubMed] [Google Scholar]
- 15. Muangpaisan W, Srisajjakul S, Chiewvit P. The alien hand syndrome: report of a case and review of the literature. J Med Assoc Thai 2005; 88: 1447–1452. [PubMed] [Google Scholar]
- 16. Kazui S, Sawada T, Naritomi H, Kuriyama Y, Yamaguchi T. Angiographic evaluation of brain infarction limited to the anterior cerebral artery territory. Stroke 1993; 24: 549–553. [DOI] [PubMed] [Google Scholar]
- 17. Brandt T, Steinke W, Thie A, Pessin MS, Caplan LR. Posterior cerebral artery territory infarcts: clinical features, infarct topography, causes and outcome. Multicenter results and a review of the literature. Cerebrovasc Dis 2000; 10: 170–182. [DOI] [PubMed] [Google Scholar]
- 18. Wang LP, Wang WY, Li L, Zhang TY, Hu Y, Fang L. Clinical and radiological features of corpus callosum infarction. Pract J Card Cerebral Pneumal Vasc Dis 2013; 21: 57–58. [Google Scholar]
- 19. Kahilogullari G, Comert A, Arslan M, et al Callosal branches of the anterior cerebral artery: an anatomical report. Clin Anat 2008; 21: 383–388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Standard stroke investigation work‐up in our institution. CT, computed tomography; CTA, computed tomography angiography; DWI, diffusion‐weighted imaging; MRA, magnetic resonance angiography.
Figure S2. Flow chart of study design
Figure S3. Flow chart of follow‐up of the three groups
Table S1. Univariate analysis for risk factors of death in patients with callosal infarction


