Abstract
Objective
To compare long‐term work loss in methotrexate‐refractory early rheumatoid arthritis (RA) patients randomized to the addition of infliximab or conventional combination treatment.
Methods
This study was a multicenter, 2‐arm, parallel, randomized, active‐controlled, open‐label trial. RA patients with <1‐year symptom duration were recruited from 15 rheumatology clinics in Sweden between 2002–2005. Patients who did not achieve low disease activity after 3–4 months of methotrexate therapy were randomized to the addition of infliximab or conventional combination treatment with sulfasalazine plus hydroxychloroquine. Yearly sick leave and disability pension days >7 years after randomization were retrieved from nationwide registers kept by the Swedish Social Insurance Agency.
Results
Of 210 working‐age patients, 109 were randomized to infliximab (mean age 48.4 years, 73% women) and 101 to conventional treatment (mean age 48.7 years, 77% women). The year before randomization, the mean number of annual work days lost was 127 in the infliximab arm and 118 in the conventional treatment group (mean difference 9 [95% confidence interval (95% CI) −23, 39]). Compared to the year before randomization, the mean changes at 7 years were −25 days in the infliximab and −26 days in the conventional treatment group (adjusted mean difference 10 [95% CI −25, 46]). The cumulative mean for work‐loss days was 846 in the infliximab group and 701 in the conventional treatment group (adjusted mean difference 104 [95% CI −56, 284]).
Conclusion
Long‐term work loss improved significantly in early RA patients randomized to infliximab plus methotrexate or conventional combination therapy. No difference was detected between strategies, and the level of work‐loss days remained twice that observed in the general population.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic disease characterized by systemic inflammation and joint destruction. An estimated 0.6% and 41 per 100,000 Americans per year are affected by RA 1, 2. Over the last 2 decades, many new antirheumatic drugs have been introduced, and together with treat‐to‐target strategies, the attitude has changed from reactive to preventive therapies aiming for remission and improved work ability. However, with the destructive nature of the disease, work disability is still highly prevalent among RA patients, already in the first years after disease onset 3, 4, 5, 6. Work loss has been reported to be the largest driver of societal costs in RA 7, 8 and has been estimated to incur annual costs of $11 billion (56% of total RA costs) in the US 9.
Box 1. Significance & Innovations.
In methotrexate‐refractory early rheumatoid arthritis (RA), work loss improved significantly over 7 years in patients treated with a strategy starting with the addition of infliximab or conventional combination therapy to methotrexate.
No difference in work‐loss days over 7 years between patients randomly allocated to infliximab plus methotrexate or conventional combination therapy could be detected, and any long‐term persisting effect of the small but statistically significant radiologic difference at 2 years favoring the infliximab treatment strategy did not translate into better work‐loss outcomes.
Based on the long‐term work‐loss findings from the Swedish Pharmacotherapy (SWEFOT) trial, and when taking into account the substantially higher cost of biologic agents, an attempt using a strategy with a combination of conventional disease‐modifying antirheumatic drugs appears imperative before starting infliximab treatment in methotrexate‐refractory early RA.
Nonrandomized patients who had a favorable response to methotrexate monotherapy reduced their work‐loss days to the same level as the general population within a year after treatment start.
Biologic tumor necrosis factor (TNF) inhibitors have shown superior efficacy regarding disease activity suppression compared to nonbiologic single disease‐modifying antirheumatic drug (DMARD) alternatives 10, and some studies, when using a single DMARD comparator, suggest that the high costs of biologic agents will be offset by improvements in work loss, especially in patients with early RA 11, 12, 13, 14, 15. However, already at the beginning of the biologic agent era, the randomized Finnish Rheumatoid Arthritis Combination Therapy Trial also reported a strategy of initial combination of conventional DMARDs to result in superior disease activity and work‐loss outcomes as compared to a single DMARD regimen in early RA 16, 17. Furthermore, as summarized in a recent review 18, the available randomized trials of biologic agent versus combination DMARD strategies in early RA report no differences in clinical outcomes and no or small differences in radiologic outcomes 19, 20. Rather than using single DMARD comparators, it thus appears clinically more relevant to compare a biologic agent combination alternative to a combination of conventional DMARDs in studies of work loss.
The randomized Swedish Pharmacotherapy (SWEFOT) trial was an investigator‐initiated study aiming to compare the TNF inhibitor infliximab, in addition to methotrexate (MTX), to a combination of conventional DMARDs in early RA patients with insufficient response to MTX. From the 2‐year results of this trial, we previously reported a small but statistically significant difference in radiographic outcomes favoring the infliximab group, while disease activity, quality of life, and work loss improved similarly in both treatment arms 21, 22, 23, 24. To the best of our knowledge, no randomized controlled trial has so far evaluated the long‐term effect of a biologic drug on work loss compared to combination DMARDs. While work loss may be more inert to treatment than clinical and radiologic outcomes, long‐term data are important, although they are likely to include challenges with low drug adherence several years after treatment allocation.
In addition to the head‐to‐head comparison, work‐loss outcomes in the nonrandomized patients who had a favorable response to MTX in the SWEFOT trial may add important data to the ongoing discussion of any potential treatment window and whether to use an initial aggressive treatment strategy or a step‐up approach 25, 26. Excellent clinical outcomes have previously been reported in this patient group 27, 28, but no study has investigated work‐loss outcomes in initial MTX responders in early RA.
The aim of this study was to compare the long‐term and objectively assessed sick leave and disability pension in MTX‐refractory early RA patients randomized to infliximab plus MTX or conventional combination therapy, who after the 2‐year trial period were treated according to best practice. A secondary aim was to evaluate work loss in the nonrandomized MTX responders in comparison with the general population.
PATIENTS AND METHODS
The SWEFOT trial has been described previously in more detail 29. Briefly, adult patients (ages ≥18 years) diagnosed with early RA (<1‐year symptom duration) were recruited from 15 rheumatology units in Sweden between 2002 and 2005. Key inclusion criteria were RA according to the revised American College of Rheumatology criteria 30; no previous DMARD use; no oral, or stable, glucocorticoid therapy for at least 4 weeks; and a Disease Activity Score based on 28‐joint count (DAS28) of >3.2 31.
Procedures
Run‐in period and randomization
At inclusion, all patients were prescribed MTX monotherapy (2.5‐mg tablets), with an initial dose of 10 mg weekly, increased every 2 weeks by 5‐mg increments to 20 mg a week. DAS28 was assessed at a followup visit after the 3 to 4‐month run‐in period. If the score was ≤3.2 (low disease activity), patients continued treatment with MTX and did not participate further in the trial. Patients who did not achieve low disease activity during the run‐in phase were randomized to the addition of either infliximab (3 mg/kg body weight, rounded up to the nearest 100‐mg increment, given intravenously at weeks 0, 2, and 6, and every 8 weeks thereafter) or conventional combination therapy with sulfasalazine (1,000 mg twice daily, given orally) and hydroxychloroquine (400 mg daily, given orally).
The computer‐generated random list for treatment allocation was kept at the study center. The statistician who prepared the list had no further role in the study. When a patient at the 3‐month visit was judged to be eligible for randomization, the investigator contacted the central study coordinator by telephone and requested randomization. Stratification or blocking was not used in the randomization process, and both doctors and patients were aware of the treatment allocation (addition of 2 oral drugs versus 1 infusion).
Treatment adjustments
In the trial protocol, dose and frequency adjustments were permitted for sulfasalazine plus hydroxychloroquine, but only frequency changes for infliximab. Sulfasalazine plus hydroxychloroquine could be discontinued and replaced by cyclosporin A (2.5 mg/kg daily in divided doses; increase allowed to 5 mg/kg daily), and infliximab could be discontinued and replaced by etanercept (50 mg weekly).
Followup and study population
In the present study, patients were followed for 7 years. During the first 2‐year trial period, included patients were scheduled for a visit at the rheumatology clinic at 7 different time points. From years 3 through 7, data on treatment were collected from the Swedish Rheumatology Quality Register 32, for both the randomized and the nonrandomized patients. Randomized patients could discontinue the assigned treatment at any time for lack of effectiveness, side effects, or by own choice. Treatment was decided by the responsible rheumatologist in case of discontinuation and after the 2‐year trial period, as well as after the run‐in period in nonrandomized patients who had a favorable response to MTX. The current analysis of the SWEFOT trial population included only early RA patients of working age (<64 years) at randomization.
Study outcome
The primary outcome of the SWEFOT study was achievement of a good response according to the European League Against Rheumatism criteria and has been reported elsewhere 29. The current study analyzed work‐loss change, measured as accumulated days over 7 years of followup, yearly days, and days per quarter, with sick leave and disability pension compensation (maximum 360 days per year and maximum 90 days per quarter). During the study period, sick leave episodes >14 days were generally not included (compensated by the employer), and for longer periods of absence due to illness, disability pension was granted by the Social Insurance Agency for individuals who were considered to have a persistent reduction of work ability with at least 25% due to illness. Secondary analyses of health economic outcomes were prespecified in the trial protocol. We used time at randomization, i.e., the start of biologic agent or conventional combination treatment, as baseline. To simplify comparisons to the randomized patients, for the nonrandomized MTX responders we used the end date of the run‐in period as baseline. Complete outcome data on a daily basis were available for all participants and time points, retrieved from the Swedish Social Insurance Agency, until emigration, death, their 65th birthday, or the end of followup.
General population comparator cohort
General population comparators were identified from the Swedish Register of the Total Population by sampling 5 sex‐, age‐, education‐, and county‐matched comparators per RA patient in the nonrandomized MTX responders. Thus, the comparator cohort were Swedish residents without RA at the matching date, and each individual in this comparison cohort was assigned the same index date as the corresponding RA patient.
Statistical analysis
For different reasons, mainly due to slower recruitment than anticipated, the initial design of 600 patients with the possibility to detect a difference of 15% in treatment response, measured by DAS28, at a statistical power of 90% (α = 0.05), the SWEFOT trial closed after enrollment of 487 patients 29. To detect the same difference of 15%, the statistical power would be reduced to approximately 75% (α = 0.05).
All working‐age patients who had undergone random allocation were analyzed using an intention‐to‐treat approach. A few patients never received their allocated treatment, and in this study, patients with <1 year into their allocated treatment were removed in a modified intention‐to‐treat analysis (Figure 1). Finally, with very few patients staying on their allocated treatment for the complete 7 years of followup, we created a modified per‐protocol group. Patients allocated to infliximab plus MTX and who were treated with any biologic agents during the complete 7 years of followup, with ≤90 days between stop and start date of any next biologic drug (for those who switched drugs), were included. For the participants allocated to conventional treatment, we included all patients who were not treated with any biologic agents during the complete followup in the modified per‐protocol analysis. Time to biologic drug discontinuation in the infliximab group and time to biologic drug initiation in the conventional treatment group, as well as in the nonrandomized MTX responders, was described using Kaplan‐Meier curves (Figure 2).
Figure 1.
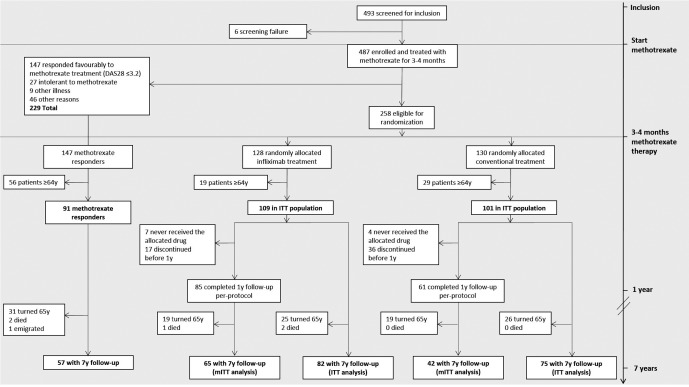
Flow chart of the Swedish Pharmacotherapy trial and number of included study subjects in the present study. DAS28 = Disease Activity Score based on 28‐joint count; ITT = intention‐to‐treat; y = year; mITT = modified intention‐to‐treat.
Figure 2.
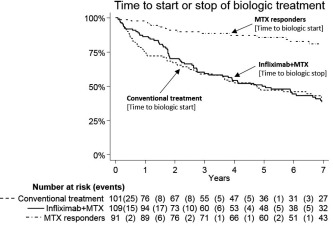
Time to discontinuation of biologic agent treatment in infliximab plus methotrexate (MTX) (switching from infliximab to another biologic agent within 90 days was not considered as a discontinuation), and time to biologic agent treatment start in conventional treatment and MTX responders. Conventional treatment: sulfasalazine and hydroxychloroquine plus MTX; MTX responder: nonrandomized patients who had a favorable Disease Activity Score based on 28‐joint count response to MTX after the run‐in period.
The year before randomization, the 95% confidence intervals (95% CIs) of the between‐group differences in the non‐normally distributed study outcome were estimated using bias‐corrected and accelerated nonparametric bootstrapping 33, 34, with 1,000 replicas the size of the original study. Differences between treatment arms after randomization were analyzed by analysis of covariance 35, with adjustment for days of work loss during the year before randomization. The same analysis was repeated for the modified intention‐to‐treat and the modified per‐protocol sample at 7 years after randomization. Data were analyzed using SAS software, version 9.4. Proportions of patients with sick leave and disability pension days were compared using the chi‐square test. Reported P values are 2‐sided, and P values less than 0.05 were considered to indicate statistical significance.
RESULTS
A total of 493 patients were recruited from October 2002 to December 2005, with 487 patients enrolled in the study 29. Of 258 patients undergoing random allocation, 210 were ages <64 years, of whom 109 were randomized to biologic agent treatment and 101 to conventional treatment (Figure 1). The baseline characteristics of randomized patients ages <64 years were similar between the treatment groups (Table 1).
Table 1.
Characteristics of study subjects and general population comparatorsa
| Variable | Infliximab treatment (n = 109) | Conventional treatment (n = 101) | MTX responders (n = 91) | General population (n = 455) |
|---|---|---|---|---|
| Women, no. (%) | 80 (73) | 78 (77) | 58 (64) | 290 (64) |
| Age (range 19–64), years | 48.4 ± 11.1 | 48.7 ± 11.6 | 49.8 ± 12.2 | 49.8 ± 12.1 |
| Rheumatoid factor positive, no. (%) | 78 (72) | 69 (68) | 71 (78) | – |
| Smoking, no. (%)b | 31 (28) | 24 (24) | 18 (20) | – |
| Symptom duration, months | ||||
| At run‐in | 7.0 ± 3.5 | 6.6 ± 3.1 | 6.4 ± 3.1 | – |
| At start of followupc | 10.4 ± 3.4 | 10.0 ± 3.2 | 9.6 ± 3.1 | – |
| DAS28 | ||||
| At run‐in | 5.8 ± 0.9 | 6.0 ± 1.0 | 5.2 ± 0.9 | – |
| At start of followupc | 4.9 ± 1.0 | 4.8 ± 1.0 | 2.4 ± 0.7 | – |
| HAQ | ||||
| At run‐in | 1.2 ± 0.6 | 1.3 ± 0.6 | 1.0 ± 0.5 | – |
| At start of followupc | 0.9 ± 0.5 | 1.0 ± 0.5 | 0.3 ± 0.4 | – |
| Education level, no. (%) | ||||
| ≤9 years | 14 (13) | 17 (17) | 19 (21) | 95 (21) |
| 10–12 years | 68 (62) | 56 (55) | 44 (48) | 220 (48) |
| >12 years | 27 (25) | 28 (28) | 28 (31) | 140 (31) |
| Work loss 1 year before start of followupc | ||||
| Total days | 126.8 ± 112.9 | 117.9 ± 112.0 | 73.8 ± 103.8 | 61.9 ± 121.7 |
| Sick leave days | 104.3 ± 98.4 | 82.5 ± 86.6 | 51.1 ± 75.8 | 17.1 ± 56.2 |
| Disability pension days | 22.4 ± 75.2 | 35.3 ± 98.3 | 22.7 ± 84.4 | 44.8 ± 111.0 |
| Unemployment and income, no. (%)d | ||||
| Any compensated days | 11 (10) | 10 (10) | 9 (10) | 39 (9) |
| Any income from paid work | 97 (89) | 83 (82) | 79 (87) | 357 (78) |
Values are mean ± SD unless indicated otherwise. Infliximab treatment: infliximab plus methotrexate (MTX). Conventional treatment: sulfasalazine and hydroxychloroquine plus MTX. DAS28 = 28‐joint count disease activity score; HAQ = Health Assessment Questionnaire.
Missing data on smoking for 5 patients in the infliximab group, 1 patient in the conventional treatment group, and 18 (20%) among the MTX responders.
Day of randomization for randomized patients, end date of the run‐in period for MTX responders as well as for their matched general population comparators.
From paid work the calendar year before start of followup.
Sick leave and disability pension before randomization
The mean days on sick leave and disability pension in the intention‐to‐treat analysis the year before randomization was 127 (median 112) in the infliximab arm and 118 (median 105) in the conventional treatment group (mean difference 9 [95% CI −23, 39]). The corresponding mean among the patients who completed 7 years of followup in the intention‐to‐treat analysis was 131 (n = 82, median 110) in the infliximab group and 107 (n = 75, median 92) in the conventional treatment group (mean difference 24 [95% CI −11, 59]) (Table 2). The mean number of days peaked at the run‐in phase in both the future infliximab and conventional treatment groups, with 54 and 51 days per quarter, respectively (mean difference 3 [95% CI −7, 12]) (Figure 3), with almost all additional days due to sick leave in both treatment arms. The proportion of patients who had any sick leave or disability pension days the quarter before randomization was 77% (n = 84) in the infliximab and 74% (n = 75) in the conventional treatment group (P = 0.64), while patients who had full‐time disability pension (90 days of 90) were 3 (3%) and 7 (7%) in the infliximab and the conventional treatment groups, respectively (P = 0.16) (Supplementary Figures 1–4, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22899/abstract).
Table 2.
Change from baseline in annual days on sick leave and disability pension 7 years after randomization between infliximab and conventional treatmenta
| Method | No. infliximab/conventional | Baseline | 7 years | Change vs. baseline (SE) | Adjusted difference (95% CI)b | |||
|---|---|---|---|---|---|---|---|---|
| Infliximab | Conventional | Infliximab | Conventional | Infliximab | Conventional | |||
| Main analysis | ||||||||
| Intention‐to‐treat | 82/75 | 131 ± 114 | 107 ± 108 | 107 ± 132 | 81 ± 126 | −25 (13) | −26 (12) | 10 (−25, 46) |
| Alternative analyses | ||||||||
| Modified intention‐to treatc | 65/42 | 129 ± 115 | 103 ± 103 | 95 ± 122 | 90 ± 135 | −34 (16) | −14 (16) | −10 (−55, 38) |
| Modified per‐protocol | 32/27 | 125 ± 113 | 112 ± 105 | 108 ± 137 | 94 ± 152 | −18 (23) | −18 (26) | 5 (−58, 80) |
Values are mean ± SD unless indicated otherwise. Intention‐to‐treat analysis included all randomized patients of working age. Modified intention‐to‐treat analysis included all randomized patients of working age who completed 1 year according to protocol. Modified per‐protocol analysis included all randomized patients of working age who were treated with any biologic drug (for patients allocated to the infliximab group), and patients who did not receive any biologic drug (in the conventional treatment group) for the complete 7 years of followup period. Infliximab treatment: infliximab plus methotrexate. Conventional treatment: sulfasalazine and hydroxychloroquine plus methotrexate.
Adjusted for work‐loss days the year before randomization (WorkDaysLost7y = α + β1 × group + β2 × WorkDaysLostbaseline + ɛ). Confidence intervals were estimated using nonparametric bootstrapping.
≥1 year on allocated drug.
Figure 3.
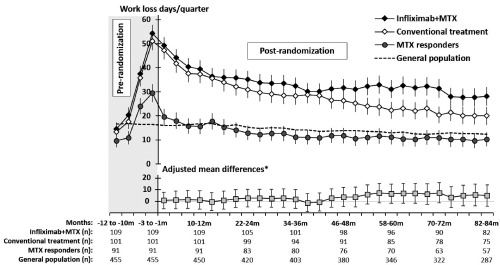
Mean days on sick leave and disability pension per 90‐day period in relation to day of randomization for randomized patients, end date of the run‐in period for nonrandomized methotrexate (MTX) responders as well as for their general population comparators (upper chart), and adjusted mean differences between infliximab plus MTX and conventional treatment (lower chart). Error bars indicate standard errors (upper chart) and 95% confidence intervals (lower chart). * = adjusted for work‐loss days the year before randomization. General population: comparators matched 5:1 by age, sex, education level, and place of residence; conventional treatment: sulfasalazine and hydroxychloroquine plus methotrexate; MTX responders: nonrandomized patients who had a favorable Disease Activity Score based on 28‐joint count response to MTX after the run‐in period.
Nonrandomized MTX responders versus matched general population
At 10–12 months before the end of run‐in, the overall mean days per quarter of sick leave and disability pension in the nonrandomized group started to increase from a statistically nonsignificant lower level as compared to the general population comparators (10 versus 17 days per quarter, respectively; mean difference −7 [95% CI −12, 0]), and increased to 29 days per quarter during the run‐in period (mean difference 13 [95% CI 6, 22]) (Figure 3 and Supplementary Figures 5 and 6, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22899/abstract).
Sick leave and disability pension after randomization
The mean cumulative work‐loss days over 7 years in patients <64 years at randomization was 846 in the infliximab group (n = 109) and 701 in the conventional treatment group (n = 101, adjusted mean difference 104 [95% CI −56, 284]) (Figure 4). At 7 years after randomization, mean days per year had decreased to 107 (mean change −25 days) in the infliximab group and to 81 (mean change −26 days) in the conventional treatment group (adjusted mean difference 10, favoring conventional treatment [95% CI −25, 46]) (Table 2).
Figure 4.
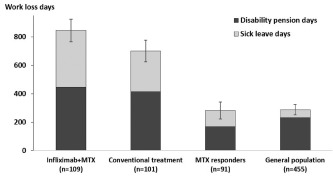
Mean accumulated sick leave and disability pension days over 7 years for randomized patients as well as for the nonrandomized methotrexate (MTX) responders and their general population comparators. Error bars indicate standard errors. General population: comparators matched 5:1 by age, sex, education level, and place of residence; conventional treatment: sulfasalazine and hydroxychloroquine plus methotrexate; MTX responders: nonrandomized patients who had a favorable Disease Activity Score based on 28‐joint count response to MTX after the run‐in period.
The corresponding mean days per quarter of sick leave and disability pension at 82–84 months after randomization, when compared to 1–3 months before randomization, was 28 (mean change −27 days) and 20 (mean change −28 days) in the infliximab and the conventional treatment group, respectively (adjusted mean difference 5, favoring conventional treatment [95% CI −5, 15]) (Figure 3). The proportion of patients who had any sick leave or disability pension days at 82–84 months after randomization was 46% (n = 38) in the infliximab arm and 35% (n = 26) in the conventional treatment arm (P = 0.14), while the proportion of patients on full‐time disability pension was 15% (n = 12) and 13% (n = 10) in the infliximab and the conventional treatment groups, respectively (P = 0.81) (Supplementary Figures 2 and 4, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22899/abstract). We also analyzed the adjusted mean differences between the infliximab and the conventional treatment groups in the other 27 quarters during followup and could not detect any statistically significant difference in any of the 3‐month periods (Figure 3).
Nonrandomized MTX responders versus matched general population within 1 year
The nonrandomized patients decreased their sick leave and disability pension days per quarter to the same level as the general population comparators within 1 year after inclusion in the trial (16 days in the nonrandomized group versus 16 days in the general population, mean difference −1 [95% CI −7, 7]) (Figure 3 and Supplementary Figures 5 and 6, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22899/abstract).
Alternative analyses
Modified intention‐to‐treat analysis
Of the 210 randomized patients, 65 (60%) who were randomized to infliximab and 42 (42%) to conventional treatment remained >1 year on the per‐protocol treatment to which they were initially allocated. While no difference could be detected between the treatment arms, a numerically greater decrease in sick leave and disability pension days per year was observed in the infliximab group (−34 in the infliximab versus −14 in the conventional group, adjusted mean difference −10 [95% CI −55, 38]) (Table 2).
Modified per‐protocol analysis
Of the 109 patients randomly allocated to infliximab, 32 (29%) were treated with any biologic drug during the complete 7 years of followup. Of the 101 patients randomized to conventional treatment, we identified 27 patients (27%) who were not treated with any biologic drug during the followup period (Figure 2). In this patient group, as compared to the main analysis findings, we found a similar point estimate of the adjusted mean difference between the treatment arms (Table 2).
DISCUSSION
In this study we investigated work loss by comparing 2 treatments strategies, rather than 2 specific drug regimens, over 7 years in real world clinical practice, and observed that work loss in MTX‐refractory early RA patients improved significantly, with the largest improvement during the first 3 years. However, no difference, per quarter or cumulatively, between patients randomly allocated to infliximab plus MTX or conventional combination therapy could be detected. Nonrandomized patients who responded sufficiently to the initial MTX therapy (Figure 1), had fewer work‐loss days already before the run‐in phase of the trial, as compared to the future randomized patients, and reduced their work‐loss days to the same level as the general population within 1 year after start of MTX treatment.
The results in this study are in line with the 2‐year findings from the SWEFOT trial, where a substantial and similar improvement was found in sick leave and disability pension days in both treatment arms at 21 months after randomization 21. Five other recent randomized, controlled trials have compared a biologic agent treatment strategy to a combination of nonbiologic DMARDs. The TEAR (US), BeSt (The Netherlands), and the NEO‐RACo trials (Finland) contrasted these treatment alternatives in early RA patients 19, 20, 36, and found no difference in disease activity, and no or a small difference in radiographic progression. In established RA, randomized trials from the US (RACAT) and the UK (TACIT) have shown that a combination of DMARDs was noninferior to a biologic agent strategy in combination with MTX regarding disease activity, radiographic progression, and health assessment questionnaire scores 37, 38.
These findings are consistent with the results from the SWEFOT study. However, none of these other trials have so far published data on work loss. In contrast, several studies have reported superior work‐loss improvements in early RA patients randomized to receive an initial biologic agent in combination with MTX, when compared to initial MTX monotherapy 11, 12, 13, 14, 15. Based on the short‐term and long‐term findings of work loss from the SWEFOT trial, and when taking into account the substantially higher cost of biologic agents, an attempt using a strategy with a combination of conventional DMARDs appears imperative before starting infliximab treatment in MTX‐refractory early RA.
With respect to the nonrandomized patients who responded favorably to MTX, their work‐loss days were at a similar level as the general population comparators within a year after MTX initiation. While immediate initiation of aggressive therapy may be warranted in some patients, a strategy starting with MTX results in approximately one‐third of patients achieving low disease activity or remission within a year 27, 29, 36. Patients who had an insufficient response to MTX and were randomized to a more aggressive treatment alternative increased their work‐loss days from the same level as the general population 1 year before randomization, and remained at around twice as high as the general population 7 years after randomization. This persisting gap highlights, also when using long‐term work loss as an outcome, the need for earlier diagnosis and a method to discriminate between patients who will have a favorable or insufficient MTX response.
In addition, adjustments in the working environment may also be considered to halt the reduction of work ability. Although work place adjustments are likely to imply an additional cost, as long as unmet needs of earlier diagnosis and prediction of response to MTX exist, adjustments for individuals’ needs may be an important intervention for the benefit of both personal finances and self‐esteem, as well as to reduce the gap of work‐loss days to the general population.
Via linkage to nationwide registers of work‐loss compensation at the Social Insurance Agency, we had access to objectively assessed data on sick leave and disability pension on a daily basis, both before the trial and to more than 7 years after the trial began, for all patients who were initially enrolled in the SWEFOT trial, as well as for general population comparators. While work loss may be more inert to treatment than clinical outcomes, the access to long‐term data is important.
The main limitation of the SWEFOT trial was that both patients and physicians were aware of the treatment allocation. Blinding assessment of disease activity was considered in the trial design, but was deemed unfeasible due to limited personnel resources at smaller participating units. Although work loss was objectively assessed using registers, the allocated treatment may have influenced the expectations of work ability from both patients and their rheumatologists.
A limitation for the analysis of quarterly work loss at 7 years was the small sample of randomized working‐age patients at 7 years of followup (turning 65 years was by far the most common reason why patients were removed from the analysis). A small sample, in addition to the power calculation in the trial design of detecting between‐group difference in disease activity and not work loss, increases the risk of type II errors. However, in the analysis of accumulated work‐loss days, all randomized patients ages <64 years were included.
Work loss over 7 years improved significantly in MTX‐refractory early RA patients randomly allocated to either of 2 treatment strategies, starting with infliximab plus MTX or conventional combination therapy. However, no difference between the 2 strategies could be detected. Patients who responded favorably to MTX monotherapy reduced their work‐loss days to the same level as the general population within a year after MTX initiation, while randomized patients remained at a level twice that in the general population, indicating the need for more effective treatments and more attention to improve work participation in RA.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Eriksson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Petersson, Ernestam, van Vollenhoven, Neovius.
Acquisition of data
Eriksson, Petersson, Ernestam, van Vollenhoven, Neovius.
Analysis and interpretation of data
Eriksson, Wallman, Miller, Petersson, Ernestam, Vivar, van Vollenhoven, Neovius.
ROLE OF THE STUDY SPONSOR
Schering‐Plough/Merck Sharp and Dohme had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Publication of this article was not contingent upon approval by Schering‐Plough/Merck Sharp and Dohme.
Supporting information
Supplementary Figure 1 Mean days of sick leave and disability pension per 90‐day‐period in relation to randomization in patients allocated to infliximab+methotrexate (MTX)
Supplementary Figure 2 Number of patients with no (0 days), part time (1‐89 days), and full time (90 days) sick leave or disability pension compensation per 90‐day‐period in patients allocated to infliximab+methotrexate
Supplementary Figure 3 Mean days of sick leave and disability pension per 90‐day‐period in relation to randomization in patients allocated to conventional combination therapy
Supplementary Figure 4 Number of patients with no (0 days), part time (1‐89 days), and full time (90 days) sick leave or disability pension compensation per 90‐day‐period in patients allocated to conventional combination therapy
Supplementary Figure 5 Mean days of sick leave and disability pension per 90 days period in relation to end date of the run‐in period in methotrexate (MTX) responders
Supplementary Figure 6 Number of patients with no (0 days), part time (1‐89 days), and full time (90 days) sick leave or disability pension compensation per 90 days period in methotrexate (MTX) responders
ACKNOWLEDGMENTS
The authors thank all patients, colleagues, and staff who made the SWEFOT trial possible.
ClinicalTrials.gov identifier: NCT00764725, and WHO database: CT20080004.
Sponsored by the Swedish Rheumatism Association, Stockholm County, and Schering‐Plough/Merck Sharp and Dohme.
Dr. Eriksson's work was supported by Novo Nordisk and Combine Sweden (less than $10,000 each), and he has served as a consultant to AbbVie (less than $10,000).
Dr. Wallman has served on an advisory board for Novartis.
Dr. Van Vollenhoven has received research support and honoraria from Abbott, GSK/HGS, MSD, Pfizer, Roche, and UCB Pharma (less than $10,000 each).
Dr. Neovius has served on advisory boards for Pfizer and Abbott, he has participated in research projects fully or partly funded by Schering‐Plough, AstraZeneca, Novo Nordisk, Pfizer, and Roche (less than $10,000 each), and he has served as a consultant to Pfizer, Sanofi‐Aventis, and Abbott (less than $10,000 each).
The copyright line for this article was changed on 24 July 2019 after original online publication.
REFERENCES
- 1. Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part I. Arthritis Rheum 2008;58:15–25. [DOI] [PubMed] [Google Scholar]
- 2. Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum 2010;62:1576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allaire S, Wolfe F, Niu J, Lavalley MP. Contemporary prevalence and incidence of work disability associated with rheumatoid arthritis in the US. Arthritis Rheum 2008;59:474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neovius M, Simard JF, Askling J. How large are the productivity losses in contemporary patients with RA, and how soon in relation to diagnosis do they develop? Ann Rheum Dis 2011;70:1010–5. [DOI] [PubMed] [Google Scholar]
- 5. Neovius M, Simard JF, Klareskog L, Askling J, Group AS. Sick leave and disability pension before and after initiation of antirheumatic therapies in clinical practice. Ann Rheum Dis 2011;70:1407–14. [DOI] [PubMed] [Google Scholar]
- 6. Puolakka K, Kautiainen H, Pekurinen M, Mottonen T, Hannonen P, Korpela M, et al. Monetary value of lost productivity over a five year follow up in early rheumatoid arthritis estimated on the basis of official register data on patients' sickness absence and gross income: experience from the FIN‐RACo trial. Ann Rheum Dis 2006;65:899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boonen A, Severens JL. The burden of illness of rheumatoid arthritis. Clin Rheumatol 2011;30 Suppl 1:S3–8. [DOI] [PubMed] [Google Scholar]
- 8. Eriksson JK, Johansson K, Askling J, Neovius M. Costs for hospital care, drugs and lost work days in incident and prevalent rheumatoid arthritis: how large, and how are they distributed? Ann Rheum Dis 2015;74:648–54. [DOI] [PubMed] [Google Scholar]
- 9. Birnbaum H, Pike C, Kaufman R, Marynchenko M, Kidolezi Y, Cifaldi M. Societal cost of rheumatoid arthritis patients in the US. Curr Med Res Opin 2010;26:77–90. [DOI] [PubMed] [Google Scholar]
- 10. Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost‐effectiveness. Health Technol Assess 2006;10:iii‐iv, xi‐xiii, 1–229. [DOI] [PubMed] [Google Scholar]
- 11. Anis A, Zhang W, Emery P, Sun H, Singh A, Freundlich B, et al. The effect of etanercept on work productivity in patients with early active rheumatoid arthritis: results from the COMET study. Rheumatology (Oxford) 2009;48:1283–9. [DOI] [PubMed] [Google Scholar]
- 12. Bejarano V, Quinn M, Conaghan PG, Reece R, Keenan AM, Walker D, et al. Effect of the early use of the anti–tumor necrosis factor adalimumab on the prevention of job loss in patients with early rheumatoid arthritis. Arthritis Rheum 2008;59:1467–74. [DOI] [PubMed] [Google Scholar]
- 13. Kimel M, Cifaldi M, Chen N, Revicki D. Adalimumab plus methotrexate improved SF‐36 scores and reduced the effect of rheumatoid arthritis (RA) on work activity for patients with early RA. J Rheumatol 2008;35:206–15. [PubMed] [Google Scholar]
- 14. Smolen JS, Han C, van der Heijde D, Emery P, Bathon JM, Keystone E, et al. Infliximab treatment maintains employability in patients with early rheumatoid arthritis. Arthritis Rheum 2006;54:716–22. [DOI] [PubMed] [Google Scholar]
- 15. Van Vollenhoven RF, Cifaldi MA, Ray S, Chen N, Weisman MH. Improvement in work place and household productivity for patients with early rheumatoid arthritis treated with adalimumab plus methotrexate: work outcomes and their correlations with clinical and radiographic measures from a randomized controlled trial companion study. Arthritis Care Res (Hoboken) 2010;62:226–34. [DOI] [PubMed] [Google Scholar]
- 16. Mottonen T, Hannonen P, Leirisalo‐Repo M, Nissila M, Kautiainen H, Korpela M, et al, for the FIN‐RACo trial group. Comparison of combination therapy with single‐drug therapy in early rheumatoid arthritis: a randomised trial. Lancet 1999;353:1568–73. [DOI] [PubMed] [Google Scholar]
- 17. Puolakka K, Kautiainen H, Mottonen T, Hannonen P, Korpela M, Julkunen H, et al. Impact of initial aggressive drug treatment with a combination of disease‐modifying antirheumatic drugs on the development of work disability in early rheumatoid arthritis: a five‐year randomized followup trial. Arthritis Rheum 2004;50:55–62. [DOI] [PubMed] [Google Scholar]
- 18. Sethi MK, O'Dell JR. Combination conventional DMARDs compared to biologicals: what is the evidence? Cur Opin Rheumatol 2015;27:183–8. [DOI] [PubMed] [Google Scholar]
- 19. Moreland LW, O'Dell JR, Paulus HE, Curtis JR, Bathon JM, StClair EW, et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheum 2012;64:2824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rantalaiho V, Kautiainen H, Korpela M, Hannonen P, Kaipiainen‐Seppanen O, Mottonen T, et al. Targeted treatment with a combination of traditional DMARDs produces excellent clinical and radiographic long‐term outcomes in early rheumatoid arthritis regardless of initial infliximab: the 5‐year followup results of a randomised clinical trial, the NEO‐RACo trial. Ann Rheum Dis 2014;73:1954–61. [DOI] [PubMed] [Google Scholar]
- 21. Eriksson JK, Neovius M, Bratt J, Petersson IF, van Vollenhoven RF, Geborek P, et al. Biological vs. conventional combination treatment and work loss in early rheumatoid arthritis: a randomized trial. JAMA Intern Med 2013;173:1407–14. [DOI] [PubMed] [Google Scholar]
- 22. Karlsson JA, Neovius M, Nilsson JA, Petersson IF, Bratt J, van Vollenhoven RF, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in early rheumatoid arthritis: 2‐year quality‐of‐life results of the randomised, controlled, SWEFOT trial. Ann Rheum Dis 2013;72:1927–33. [DOI] [PubMed] [Google Scholar]
- 23. Van Vollenhoven RF, Geborek P, Forslind K, Albertsson K, Ernestam S, Petersson IF, et al. Conventional combination treatment versus biological treatment in methotrexate‐refractory early rheumatoid arthritis: 2 year followup of the randomised, non‐blinded, parallel‐group Swefot trial. Lancet 2012;379:1712–20. [DOI] [PubMed] [Google Scholar]
- 24. Eriksson JK, Karlsson JA, Bratt J, Petersson IF, van Vollenhoven RF, Ernestam S, et al. Cost‐effectiveness of infliximab versus conventional combination treatment in methotrexate‐refractory early rheumatoid arthritis: 2‐year results of the register‐enriched randomised controlled SWEFOT trial. Ann Rheum Dis 2015;74:1094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Nies JA, Krabben A, Schoones JW, Huizinga TW, Kloppenburg M, van der Helm‐van Mil AH. What is the evidence for the presence of a therapeutic window of opportunity in rheumatoid arthritis? A systematic literature review. Ann Rheum Dis 2014;73:861–70. [DOI] [PubMed] [Google Scholar]
- 26. Raza K, Filer A. The therapeutic window of opportunity in rheumatoid arthritis: does it ever close? Ann Rheum Dis 2015;74:793–4. [DOI] [PubMed] [Google Scholar]
- 27. O'Dell JR, Curtis JR, Mikuls TR, Cofield SS, Bridges SL Jr, Ranganath VK, et al. Validation of the methotrexate‐first strategy in patients with early, poor‐prognosis rheumatoid arthritis: results from a two‐year randomized, double‐blind trial. Arthritis Rheum 2013;65:1985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rezaei H, Saevarsdottir S, Forslind K, Albertsson K, Wallin H, Bratt J, et al. In early rheumatoid arthritis, patients with a good initial response to methotrexate have excellent 2‐year clinical outcomes, but radiological progression is not fully prevented: data from the methotrexate responders population in the SWEFOT trial. Ann Rheum Dis 2012;71:186–91. [DOI] [PubMed] [Google Scholar]
- 29. Van Vollenhoven RF, Ernestam S, Geborek P, Petersson IF, Coster L, Waltbrand E, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1‐year results of a randomised trial. Lancet 2009;374:459–66. [DOI] [PubMed] [Google Scholar]
- 30. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 31. Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty‐eight‐joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 32. Eriksson JK, Askling J, Arkema EV. The Swedish Rheumatology Quality Register: optimisation of rheumatic disease assessments using register‐enriched data. Clin Exp Rheumatol 2014;32 Suppl 85:S147–9. [PubMed] [Google Scholar]
- 33. Efron B. Better bootstrap confidence intervals. J Am Stat Assoc 1987;82:171–85. [Google Scholar]
- 34. Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? BMJ 2000;320:1197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vickers AJ, Altman DG. Statistics notes: Analysing controlled trials with baseline and follow up measurements. BMJ 2001;323:1123–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goekoop‐Ruiterman YP, de Vries‐Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJ, Hazes JM, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2008;58 Suppl:S126–35. [DOI] [PubMed] [Google Scholar]
- 37. O'Dell JR, Mikuls TR, Taylor TH, Ahluwalia V, Brophy M, Warren SR, et al. Therapies for active rheumatoid arthritis after methotrexate failure. N Engl J Med 2013;369:307–18. [DOI] [PubMed] [Google Scholar]
- 38. Scott DL, Ibrahim F, Farewell V, O'Keeffe AG, Walker D, Kelly C, et al. Tumour necrosis factor inhibitors versus combination intensive therapy with conventional disease modifying anti‐rheumatic drugs in established rheumatoid arthritis: TACIT non‐inferiority randomised controlled trial. BMJ 2015;350:h1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Mean days of sick leave and disability pension per 90‐day‐period in relation to randomization in patients allocated to infliximab+methotrexate (MTX)
Supplementary Figure 2 Number of patients with no (0 days), part time (1‐89 days), and full time (90 days) sick leave or disability pension compensation per 90‐day‐period in patients allocated to infliximab+methotrexate
Supplementary Figure 3 Mean days of sick leave and disability pension per 90‐day‐period in relation to randomization in patients allocated to conventional combination therapy
Supplementary Figure 4 Number of patients with no (0 days), part time (1‐89 days), and full time (90 days) sick leave or disability pension compensation per 90‐day‐period in patients allocated to conventional combination therapy
Supplementary Figure 5 Mean days of sick leave and disability pension per 90 days period in relation to end date of the run‐in period in methotrexate (MTX) responders
Supplementary Figure 6 Number of patients with no (0 days), part time (1‐89 days), and full time (90 days) sick leave or disability pension compensation per 90 days period in methotrexate (MTX) responders


