Summary
The extracytoplasmic function (ECF) σ factor, σE, is a key regulator of the cell envelope stress response in Streptomyces coelicolor. Although its role in maintaining cell wall integrity has been known for over a decade, a comprehensive analysis of the genes under its control has not been undertaken. Here, using a combination of chromatin immunoprecipitation‐sequencing (ChIP‐seq), microarray transcriptional profiling and bioinformatic analysis, we attempt to define the σE regulon. Approximately half of the genes identified encode proteins implicated in cell envelope function. Seventeen novel targets were validated by S1 nuclease mapping or in vitro transcription, establishing a σE‐binding consensus. Subsequently, we used bioinformatic analysis to look for conservation of the σE target promoters identified in S. coelicolor across 19 Streptomyces species. Key proteins under σE control across the genus include the actin homolog MreB, three penicillin‐binding proteins, two L,D‐transpeptidases, a LytR‐CpsA‐Psr‐family protein predicted to be involved in cell wall teichoic acid deposition and a predicted MprF protein, which adds lysyl groups to phosphatidylglycerol to neutralize membrane surface charge. Taken together, these analyses provide biological insight into the σE‐mediated cell envelope stress response in the genus Streptomyces.
Introduction
The bacterial cell envelope, made up of the cell wall and cell membranes, is critical in counteracting the high intracellular osmotic pressure to maintain cell shape (Silhavy et al., 2010). It also provides an essential defensive barrier against various environmental stress agents. The cell envelope facilitates the ability of the cell to monitor the external environment and modulate cell behaviour in response (Jordan et al., 2008). Numerous antibiotics target the bacterial cell envelope. For example, penicillin and other β‐lactams mimic the D‐alanyl‐D‐alanine (D‐ala‐D‐ala) terminus of the pentapeptide side chain of peptidoglycan and thus block the activity of penicillin‐binding proteins (PBPs) in the elongation and cross‐linking of peptidoglycan precursors. Furthermore, vancomycin and other glycopeptide antibiotics bind to the D‐ala‐D‐ala terminus and thereby inhibit peptidoglycan cross‐linking (Kahne et al., 2005).
Bacteria employ two major types of signalling system to sense and respond to environmental stresses: two‐component systems and extracytoplasmic function (ECF) σ factors (Raivio, 2005; Jordan et al., 2008; Mitrophanov and Groisman, 2008; Capra and Laub, 2012). These two systems are functionally analogous in that they generally consist of a membrane protein (a sensor kinase or an anti‐σ factor) that acts as a stress sensor and a transcription factor (a response regulator or a σ factor) that modulates gene expression in response. In the case of two‐component systems, the inducing signal leads to the autophosphorylation of a membrane‐bound sensor kinase. As a result, the kinase phosphorylates its cognate response regulator, which then activates transcription of the genes involved in the cellular response (Mitrophanov and Groisman, 2008; Capra and Laub, 2012). Similarly, ECF σ factors typically control the cellular stress response via an interaction with a cognate anti‐σ factor, which is usually a transmembrane protein (Mascher, 2013). In the absence of the signal, the anti‐σ factor sequesters its cognate ECF σ factor to the membrane, inhibiting its activity. The inducing signal inactivates the anti‐σ factor, either by causing a conformational change in the protein, or by proteolysis (Mascher, 2013). In either case, the result is the release of the ECF σ factor, which is then able to direct RNA polymerase (RNAP) to its target promoters and elicit a specific transcriptional response. Streptomyces coelicolor σE, the subject of this work, is unusual in that it is not regulated by an anti‐σ. Instead, S. coelicolor σE activity is controlled at the level of transcription of its structural gene (sigE) by a two‐component system, CseBC (see below) (Fig. 1).
Figure 1.
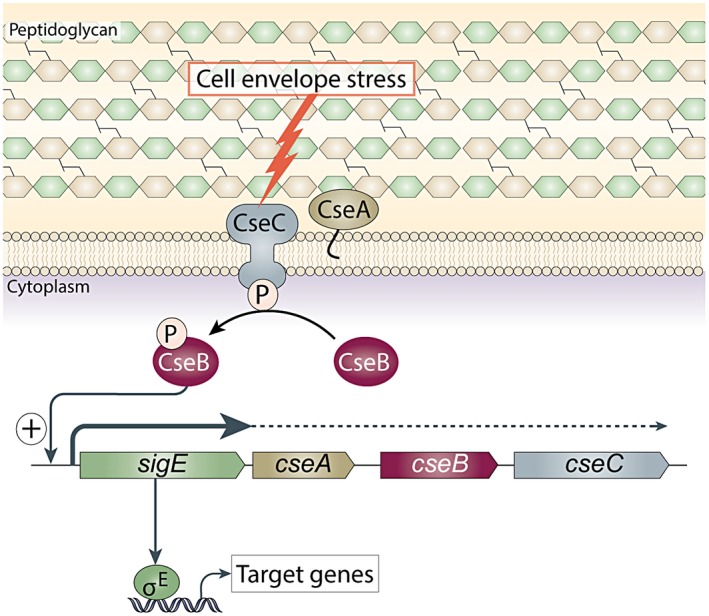
Model for the σE cell envelope stress response. Expression of the gene encoding σE (sigE) is regulated at the level of transcription by the CseB/CseC two‐component signal transduction system. In response to signals originating in the cell envelope when it is under stress, the sensor kinase, CseC, becomes autophosphorylated and transfers this phosphate to the response regulator, CseB. Phospho‐CseB activates the promoter of the sigE operon, and σE is recruited by core RNA polymerase to transcribe its regulon. Note that >90% transcription from the sigE promoter terminates just downstream of sigE and that the promoter of the sigE gene itself is not a σE target. CseA is a lipoprotein localised to the extracytoplasmic face of the cell membrane and loss of the CseA results in upregulation of the sigE promoter.
The roles of the two‐component system CpxAR and the ECF σ factor σE in the cell envelope stress response of Escherichia coli have been well established (Ruiz and Silhavy, 2005; Guest and Raivio, 2016). The CpxAR system is induced by a variety of cell envelope stresses including alkaline pH, increased osmolality, overexpression of the outer membrane protein NplE, altered membrane composition, and the accumulation of pilus subunits or misfolded MalE aggregates (Guest and Raivio, 2016). Activation of CpxAR results in the elevated expression of target genes that are involved in envelope protein folding and degradation, such as the periplasmic protease DegP, the periplasmic disulfide oxidoreductase DsbA and the foldase chaperone PpiA (Guest and Raivio, 2016). The E. coli ECF σ factor σE mainly responds to stresses that affect the folding of outer membrane proteins (OMPs) such as heat shock (Rouviere et al., 1995). In line with this, mutations in the OMP folding chaperone also induce the σE stress response (Missiakas et al., 1996). The σE regulon includes a variety of genes involved in OMP folding (Dartigalongue et al., 2001; Rhodius et al., 2005) and several small RNAs that downregulate OMP expression, thereby reducing the flow of OMPs to the cell envelope (Johansen et al., 2006; Thompson et al., 2007; Udekwu and Wagner, 2007).
In Bacillus subtilis, four two‐component systems (LiaRS, BceRS, YvcPQ, YxdJK) and at least four of its seven ECF σ factors, σM, σX, σV, σW, have roles in the response to cell envelope stress (Jordan et al., 2008; Hastie et al., 2014; 2016; Lewerke et al., 2018). For example, BceRS is strongly induced by bacitricin and is involved in bacitracin detoxification (Mascher et al., 2003). σM is activated by a wide variety of sources of envelope stress such as vancomycin, bacitracin, phosphomycin and cationic antimicrobial peptides (Mascher et al., 2003; Thackray and Moir, 2003; Kingston et al., 2013). Much effort has also been made to define the regulatory networks linked to these signalling systems. σM contributes to the transcription of genes whose functions are related to transcriptional control, cell wall biosynthesis, cell shape determination, cell division, DNA monitoring and repair, and detoxification (Eiamphungporn and Helmann, 2008). Approximately 57 genes (30 operons) are direct targets of σM under antibiotic stress conditions, including several targets that also belong to the σX and/or σW regulons (Eiamphungporn and Helmann, 2008).
Streptomyces coelicolor is a soil dwelling, saprophytic actinobacterium with a complex differentiating life cycle involving filamentous growth and sporulation (Flärdh and Buttner, 2009), and it is a well‐established model organism in which to study signal transduction in the Streptomyces genus (Hutchings et al., 2004). S. coelicolor encodes 67 paired two‐component systems (Hutchings et al., 2004), and 51 ECF σ factors (collected from Mist2 database, http://mistdb.com/) (Ulrich and Zhulin, 2010). Of these, only the two‐component systems VanRS and CseBC and the ECF σ factor σE have so far been shown to play a role in the cell envelope stress response. VanRS controls the expression of an inducible vancomycin resistance cluster of seven genes (vanSRJKHAX) (Hong et al., 2004; Hutchings et al., 2006a), and vancomycin activates the VanRS two‐component system directly by binding to the sensor kinase VanS (Koteva et al., 2010). Expression of the vanHAX genes reprograms cell wall biosynthesis such that the stem pentapeptide of peptidoglycan precursors terminate in D‐alanyl‐D‐lactate (D‐Ala‐D‐Lac), rather than in D‐Ala‐D‐Ala (Hong et al., 2005). The affinity of vancomycin for precursors terminating in D‐Ala‐D‐Lac is ~1000‐fold lower than for precursors terminating D‐Ala‐D‐Ala (Bugg et al., 1991), thus rendering S. coelicolor resistant. VanRS responds specifically to glycopeptide antibiotics like vancomycin, ristocetin, chloroeremomycin and A47934, but not to other cell envelope‐specific antibiotics with different modes of action like the phosphoglycolipid moenomycin A, the peptide bacitracin and the cyclic depsipeptide ramoplanin (Hong et al., 2004; Hutchings et al., 2006a). In contrast, the expression of S. coelicolor σE is induced by a diverse range of antibiotics that target the cell wall, including penicillins, cephalosporins, glycopeptides, moenomycin A, bacitracin and ramoplanin (Hong et al., 2002). A sigE mutant shows a 50‐fold increase in sensitivity to the cell wall hydrolytic enzyme lysozyme and a subtle alteration in its cell wall muropeptide profile (Paget et al., 1999a). In addition, sigE mutants require high levels of magnesium for normal growth and development and overproduce actinorhodin and form crenelated colonies in its absence (Paget et al., 1999a). It therefore seems likely that Mg2+ stabilizes the defect in the cell envelope of sigE mutants, thereby suppressing the phenotype. High levels of magnesium are known to suppress a wide range of cell envelope defects in bacteria (Formstone and Errington, 2005). Thus, while the VanRS system is dedicated to glycopeptide resistance, σE seems to play a much more general role in the response of S. coelicolor to cell envelope stress.
The initial characterization of the S. coelicolor sigE gene led directly to the discovery of the ECF subfamily of σ factors 25 years ago (Lonetto et al., 1994). The sigE gene is located in a four‐gene operon, sigE cseA cseB cseC, with cseA encoding a lipoprotein, cseB encoding a response regulator and cseC encoding a membrane‐anchored sensor kinase (Fig. 1). Approximately 90% of transcription terminates directly downstream of the sigE gene and transcription of sigE is completely dependent on the two‐component system, CseBC (Paget et al., 1999b; Hong et al., 2002). By analogy with other two‐component systems, it seems likely that in response to cell envelope stress, the sensor kinase CseC autophosphorylates before phosphorylating its cognate response regulator, CseB, that in turn directs the transcription of sigE (Paget et al., 1999b; Hong et al., 2002) (Fig. 1). The sigE promoter seems to be the sole target of CseB since the sigE mutant and cseB mutant show the same phenotype and constitutive expression of σE complements the lysozyme sensitivity of S. coelicolor lacking CseB (Paget et al., 1999b). The function of the lipoprotein CseA remains unknown, but deletion of cseA results in upregulation of the sigE promoter, raising the speculative possibility that CseA might modulate the activity of the signal transduction system by interacting with the extracytoplasmic domain of the CseC sensor kinase (Hutchings et al., 2006b). The absence of an anti‐σ and the requirement of a two‐component system for transcription of sigE sets this system apart from other well‐characterized ECF σ factor regulatory mechanisms. Thus, despite having the same name, S. coelicolor σE is distinct from both E. coli σE and Mycobacterium tuberculosis σE, which instead employ an anti‐σ factor to control ECF σ factor activity (Sineva et al., 2017).
Despite the critical role of σE in modulating the cell envelope stress response in S. coelicolor, only two in vivo targets have so far been described: the hrdD gene, encoding another σ factor (Paget et al., 1999a), the function of which is poorly understood (Buttner et al., 1990; Strakova et al., 2014), and the 12‐gene cwg operon, predicted to be involved in the biosynthesis of a cell wall glycan (Hong et al., 2002). To gain a broader picture of the physiological function of σE in the cell envelope stress response in Streptomyces, here we use a combination of ChIP‐seq, microarray transcriptional profiling and bioinformatic analysis to define the regulon of genes under σE control. Over 50 targets were found to be directly involved in cell envelope‐related functions and many other targets are implicated in signal transduction systems. Finally, we used bioinformatic analysis to identify S. coelicolor σE target promoters that are conserved across the Streptomyces genus. The σE‐directed cell envelope stress response characterized here is likely to be specific to the streptomycetes, because the sigE‐cseABC operon appears to be absent outside this genus (http://www.microbesonline.org/ [Dehal et al., 2010]).
Results and discussion
Identification of the σE regulon
To define the genes under direct control of σE, we used chromatin immunoprecipitation coupled with high‐throughput sequencing (ChIP‐seq). To do this, we first constructed a strain of S. coelicolor that lacked sigE at its native locus but expressed an N‐terminally triple‐FLAG‐tagged version of σE from the ΦBT1 integration site. As shown in Fig. S1, expression of 3 × FLAG‐σE in trans, under control of its native promoter, restores the resistance of S. coelicolor to lysozyme to wild‐type levels. Furthermore, Western blot analysis showed that vancomycin induced expression of 3 × FLAG‐σE (Fig. 2A) in the same way that it induces expression of native σE in wild‐type S. coelicolor (Hong et al., 2002).
Figure 2.
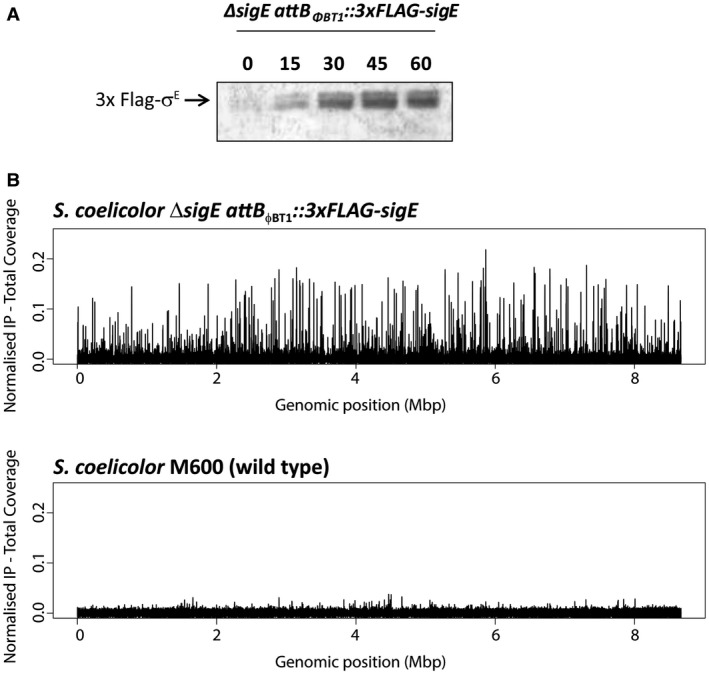
A. Western blot analysis of S. coelicolor ΔsigE attBΦBT1::3 × FLAG‐sigE grown in NMMP liquid cultures and sampled after 0, 15, 30, 45 and 60 minutes treatment with 10 µg/ml vancomycin. Total protein (10 µg) was loaded per lane and 3 × FLAG‐σE was detected using anti‐σE polyclonal antibody. B. Chromosome‐wide distribution of σE‐binding sites in S. coelicolor identified by ChIP‐seq analysis. ChIP‐seq was conducted using M2 anti‐FLAG antibody on the ΔsigE attBΦBT1::3 × FLAG‐sigE strain after 30 minutes treatment with 10 µg/ml vancomycin. The wild‐type strain (expressing non‐tagged σE from the native locus) analysed under the same conditions was used as a negative control.
ChIP‐seq was conducted using M2 anti‐FLAG antibody after 30 minutes of treatment with vancomycin to induce 3 × FLAG‐σE expression. The congenic wild‐type S. coelicolor strain M600 was used as a negative control to eliminate any false signals that might arise from cross‐reaction of the anti‐FLAG antibody with other DNA‐binding proteins. In addition, total (non‐immunoprecipitated) input DNA was also subjected to sequencing. This additional control enables non‐uniform shearing of the chromosome to be taken into account (Teytelman et al., 2009). Using P < 10−4 as the threshold for significance, a total of ~200 peaks were detected in the FLAG‐tagged SigE strain (Fig. 2B and Table S1). Notably, only a few small peaks were detected in the wild‐type M600 control strain expressing the non‐tagged version of σE (Fig. 2B). Next, we looked for candidate σE target promoter sequences for each ChIP‐seq target, based on the conservation of AAC and TC, respectively, in the −35 and −10 regions of the two previously characterised σE target promoters, hrdD and cwg (Paget et al., 1999a; Hong et al., 2002). Restricting our search to within 400 bp of the start codon of the downstream gene, we identified 91 putative σE target promoters through this route (Table 1).
Table 1.
The σE regulon in S. coelicolor
| σE target gene | Product | Predicted promoter sequence | Distance to start codon (bp) |
|---|---|---|---|
| sco0662‐0664 | Membrane transport protein; 2‐hydroxyacid dehydrogenase; Hypothetical protein | AGCAACCTCGGCTACAACATGGT‐GGTCTT | 360 |
| sco0736 | L,D‐transpeptidase | CGCAACCAAAGCCGCCGGACGGC‐GGTCTA | 70 |
| sco0849‐0848 | Membrane protein; Putative oxidoreductase | AGGAACGGATAGGTGTTCTTCGC‐CATCCC | 306 |
| sco0877‐0879 | LuxR‐type transcriptional regulator; Hydrolase; AAA domain protein | GCCAACGAGGGCCGGACGCCGGC‐CGTCCC | 18 |
| sco1023‐1024 | Membrane protein; Hypothetical protein | GGGAACCCCGTCCTGGGTCCGCG‐CGTTGG | 97 |
| sco1168 | Hypothetical protein | CTGAACCTCACGCGCGCGGAGCA‐CGTCGT | 249 |
| sco1647 | Hypothetical protein (Pfam: Pup_ligase) | ACCAACCCCGACGACTGGGCCCG‐CATCTC | 362 |
| sco1738 | Hypothetical protein | TCGCACGACGCCTGCGCGCACAC‐CGTCTT | 384 |
| sco1755 | Hypothetical protein | GACAACCAGAAGGCAGGGTTCCG‐GGTCTA | 38 |
| sco1875 | HMW PBP | GGGAACGACCGCCGCCCGCCCGTTCGTCCT | 18 |
| sco2055 | Membrane protein | CGGAACCAATTCTCTCAGACCCG‐CGTCCG | 171 |
| sco2168‐2167 (pspA) | Phage shock protein A homolog; Hypothetical protein | GGGAACGATCCGGCAACGCCGGT‐CGTCTG | 262 |
| sco2255 | Membrane protein | CGGAACTCCGCGGGACGGCCCGTACGTCCT | 105 |
| sco2294‐2293 | Putative AraC family transcription regulator; Hypothetical protein (Pfam: EamA) | GGACACCGCGGGATTCCTCTGAT‐CGTCTG | 23 |
| sco2334 | Membrane protein | GCCAACGTTTCCGTTCGAATTAT‐CGTCTT | 54 |
| sco2368 | Hypothetical protein | GGCAACGTCTCGCGCGCCTACGG‐CGTCTT | 317 |
| sco2419‐2410 | Operon of membrane proteins | TACGACCACTACTTCAACCTCTT‐CCTCTC | 224 |
| sco2611‐2609 (mreBCD) | Lateral cell wall biosynthesis | GGGAACGGATCCCACCGTTGGCC‐CGTCTC | 157 |
| sco2629 | Membrane protein | TTCAACTACAAGTTCCCCGACAC‐GGTCTT | 171 |
| sco2807 | Membrane protein | GGCAACCCGAGGGGCGATGCCCG‐CGTCTA | 122 |
| sco2892 | Membrane protein (Pfam: Lipase_GDSL_2) | CGGAACGGAACACAAGTTCCCGG‐CGTCTG | 113 |
| sco2897 | HMW PBP, cell wall biosynthesis | GGGAACGGAACCCGCGGTGCGAG‐AGTCTT | 260 |
| sco2939 | Hypothetical protein | GGCAACGAGTGCGTCCCCCCACG‐CGTCCT | 36 |
| sco2974 (pkaA) | Ser/Thr protein kinase | GGCAACCACGGGACCGGGTCGAG‐CGTCTT | 108 |
| sco2975 | Hypothetical protein | CGTGACCGATCTCAAGCGGACGG‐CATTCG | 221 |
| sco3034 (whiB) | Sporulation regulatory protein | CGGAACGGGATCGATCGCCGGGG‐CGTCCT | 238 |
| sco3044 | LytR‐CpsA‐Psr (LCP) family protein, wall teichoic acid deposition | AGTGACCTGAGGGGCCCCGCACG‐CGTCTG | 335 |
| sco3098 | Putative secreted protein (Pfam: Transglycosylase, LysM domain) | GTCAACCGCCGCGTGGTCCCCGT‐CGTCTT | 15 |
| sco3194 | L,D‐transpeptidase, lipoprotein | GGGAACCCCACGGGCCGCCGGGCACCTCTA | 46 |
| hrdD (sco3202) | RNA polymerase sigma factor | GGCAACCCTCAGGCGGTACGGGC‐CGTCTT | 375 |
| sco3342‐3341 | Glycine‐rich secreted protein; Hypothetical protein | GGGAACGGTGTGCCGGGCCGAGCGGCTCTT | 74 |
| sco3396 | Hypothetical protein (Pfam: Esterase) | CGGAACCTCGCCCGACATTTCCT‐CATCTG | 151 |
| sco3397 (mprF) | Putative MprF lysylphosphatidylglycerol synthase, membrane protein | GTGAACCTCTCCCTCCGAGACAC‐CGTCCT | 95 |
| sco3419 | Hypothetical protein | CTCAACGGCGACACCATGCTGGA‐CGCCTT | 137 |
| sco3424 | Putative regulator, similar to AbaA and BldB | GGGAACGACTTCTCGGGCCCCGG‐CGTCGT | 164 |
| sco3481 | Hypothetical protein | TGGAACGACTACCTGGTCGCCAC‐CGTCTT | 207 |
| sco3548 | Putative anti‐sigma factor | TGCAACCAGGAGCGCATTCTCAA‐GATCTT | 182 |
| sco3559 | Oxidoreductase | GGGCACGGCGCCGGGTTGCGTAG‐GGTCTT | 4 |
| sco3712 | Putative hydrolase, similar to polysaccharide deacetylase | GGGATCCCGCGGCGGGTTTCTCC‐CGTCCT | 5 |
| sco3728 | Membrane protein | GGGAACGGATCGGCGGCCGGCAG‐CGTCGT | 46 |
| sco3761 | Hypothetical protein | GGGAACCTCGGCATGACCGTGTT‐CGTCTC | 47 |
| sco3900‐3899 | Hypothetical protein (Pfam: PadR); Hypothetical protein | CAAAACCCCCGCGGCCCGAAGTT‐CACCTC | 142 |
| sco3972 | Hypothetical protein (Prim‐Pol domain) | TGGAACCCGGCGACGGACCCGGG‐CGTCCT | 317 |
| sco4042 | Membrane protein (Pfam: LytR_C) | TCGAACCTCGGAACGTCGACTGATCATCTA | 60 |
| sco4069 | Membrane protein | CCGAACCCGGCAGGCCCCGGCTC‐CGTCTC | 259 |
| sco4120 | Hypothetical protein | AGGAACTCCCCCGGCCACCGGGG‐CGTCTG | 145 |
| sco4133 | Membrane protein | TGGAACGTATCAACGGGGACCGTGCGTTCC | 84 |
| sco4134 | Putative lipoprotein | GGGAACCCGCGCCCCCACACCCC‐CGTCTC | 33 |
| sco4159‐4158 | GlnR transcriptional regulatory protein | GCGAACCGGGCACGACCACAAAC‐CGTCCC | 16 |
| sco4253 | Hypothetical protein | AGAAACGCCGGGCGTCCGCCAGG‐GGTCTT | 158 |
| sco4263 | Transcriptional regulator | CACCACCGTTCACCGCAGTCGTT‐CGTCTG | 38 |
| sco4289 | Secreted protein | GACAACGTCACGGACGGTTCCCC‐CGCCTG | 110 |
| sco4439 | LMW PBP; cell wall biosynthesis | TGGAACCAGTAGGTATGTCGTTCTCGTCTT | 222 |
| sco4468‐4467 | Hypothetical proteins | GACAACCGCCCCCAACGCCGTGC‐CGTCTG | 169 |
| sco4471 | Lipoprotein | CGGAACCCGCTCGTTCGTCGCGT‐CCTCTC | 38 |
| sco4494 | Hypothetical protein | AGTAACCGGGGCGTACCGTTGACCCGTCTG | 19 |
| sco4582 | Membrane protein | GGCAACCCGACCGGAACCTGTGC‐CCTCCC | 345 |
| sco4613 | Membrane protein | CGCAACCACCCGGCGCGGTCGGAACGTCCT | 88 |
| sco4651 | Putative lipoprotein | AGAAACCACAAGATCGTTCGAAC‐CGTTTC | 105 |
| sco4847 | LMW PBP; cell wall biosynthesis | CGCAACCCGATGACCCCGACGAC‐CGTCCC | 271 |
| sco4849 | Membrane protein | GAGGACGTCACGGACGCCCTGAG‐CGTCCC | 20 |
| sco4904 | Membrane protein (Pfam: VanZ) | CGGAACCGCACACGGCGGGGGGCGCGTCTA | 7 |
| sco4934 | L,D‐transpeptidase, lipoprotein | GGCAACCGCCGCCCGGGGTTTCGTCGTCTC | 172 |
| sco4968 | Membrane protein | CGGAACGGCGTACCAGCCGCTGAAGGTCTA | 347 |
| sco5030 | Membrane protein | CTCAACCTCGCGCAGCCCCTCAC‐CGTCTT | 94 |
| sco5039 | HMW PBP, cell wall biosynthesis | CACAACCTTGAACCCCGCTCGTA‐CGTCGG | 335 |
| sco5049 | Hypothetical protein | GCGAACTGTCCGACTTGAATTTCACCTTTC | 212 |
| sco5213 | Membrane protein | GCGAACCGGCTCCGGGTCCTCGA‐CGTCTT | 198 |
| sco5255 | Signal peptidase protein | GCAAACAGGCGGGAAAGCATGAAGCGTTCC | 132 |
| sco5310 | Hypothetical protein | GGGAACGGGCCGCCACGCGCGCA‐CGTTCT | 124 |
| sco5358 | LytR‐CpsA‐Psr (LCP) family protein, wall teichoic acid deposition | TGCAACCCTGTCCCGAGTTCCGC‐CGTCTG | 108 |
| sco5535‐5336 (accB‐accE) | Acetyl‐CoA carboxylase complex subunits | TGTGACCTCTACAAGCCAGAGGC‐CCTCTG | 117 |
| sco5705 | Hypothetical protein | GCGAACGCGCTCTCCCCGGCCCG‐CGTCTC | 304 |
| sco5742 | Membrane protein | GGGCACCTGAAGGGGCGTTCGTT‐CGTCTG | 49 |
| sco5856 | Membrane protein | CGGAACTAATGGTTTCGGCCGCA‐CGTCCC | 52 |
| sco5981 | Hypothetical protein | GCGAACCCTCAGCCTCCTCAGAC‐CCTCTT | 29 |
| sco6028 | Putative ribonuclease | CGGAACGTTCCGTCGGCGGGCTC‐CGTCGA | 122 |
| sco6130 | Hypothetical protein (HATPase domain) | CTCCACCCCCGTCCCCACGTGAG‐CGCCTC | 21 |
| sco6178‐6177 | Putative deacetylase; Hypothetical protein | ATGAACCGCGTATATACACGCAG‐CGTATA | 50 |
| sco6179‐6190 (cwg operon) | Cell wall glycan synthesis | CGCAACCTGGTCCCCGTTTTCGT‐CGTCTT | 147 |
| sco6262‐6263 | Putative helicase; Hypothetical protein | CGAGACCACCGGTGCCGGTCTCGACGTCTT | 389 |
| sco6357‐6353 | 3 membrane proteins; Response regulator; Sensor kinase | GGGAACGTTCCTCACTCCGCCAT‐CGTCTA | 88 |
| sco6379 | Membrane protein | TGGAACGGTCCTCACCCCGCTGC‐CGTCTA | 88 |
| sco6750 | Putative IPP isomerase | GCGGACGGCCCGGGGGCGCGCACGCACCGG | 234 |
| sco6773 | Putative peptidase (Lysin motif domain) | GGGAACCCTTCGCTTGTCCCTGTGCGTCTT | 224 |
| sco6832‐6833 | Methylmalonyl‐CoA mutase; isobutyryl‐CoA mutase | GGCGACCGTGCTGCGGAGCCCAA‐CATCTT | 242 |
| sco6979‐6982 | Solute‐binding lipoprotein; ABC transporter membrane protein; ABC transporter membrane ATP binding protein; Hypothetical protein | CTCAACCTCCGCCAGGGGTACGCCCGTCTG | 322 |
| sco7233 | Membrane protein | GGCAACCCGAAGGATCTCCATCC‐CCTCCT | 69 |
| sco7657‐7658 | Membrane protein; Hypothetical protein | GACAACCGGGCATCCGAGCGCTC‐CCTCTC | 75 |
| sco7730 | Hypothetical protein | CGAGACCGACGCCCCGGCGCGGACCATCCT | 245 |
| scot11 | tRNA‐Met | GGGAACCGCGCGGCACGCTGCGG‐AGTCCT | 107 |
To determine how σE influences the expression of its target genes, S. coelicolor M600 and the congenic sigE mutant were subjected in parallel to time‐resolved, genome‐wide transcriptional profiling following treatment with vancomycin. Note that the transcriptional profiling data for the wild type (but not for the sigE mutant) has been published previously (Hesketh et al., 2011). Some σE ChIP‐seq targets were vancomycin inducible in wild‐type S. coelicolor and were completely depended on sigE for expression (Figs 3 and S2). However, other σE ChIP‐seq targets were vancomycin inducible in the wild type and retained vancomycin inducibility to varying degrees in the sigE mutant (Figs 4, 5 and S2). This phenomenon was investigated further by analysing the transcription of a selection of genes using S1 nuclease protection assays, covering the full range of σE ChIP‐seq target genes all the way from those showing complete dependence on sigE, such as sco3396, mprF (sco3397), sco4263 and sco7657 (Fig. 3), to those showing little or none, such as sco3194 (Fig. 5).
Figure 3.
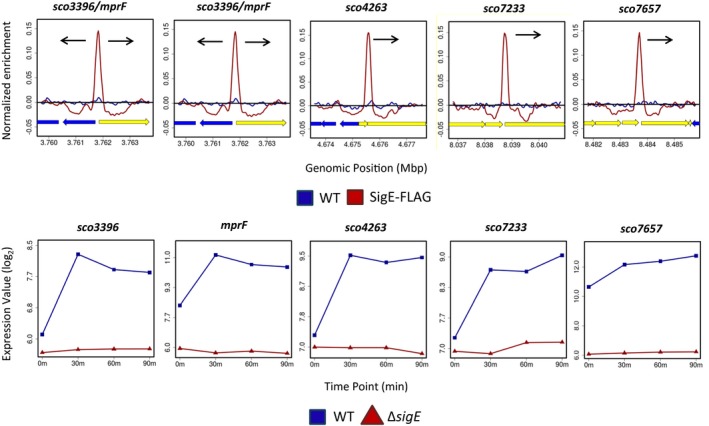
ChIP‐seq (above) and microarray transcriptional profiling data (below) for the Class I σE target genes sco3396, mprF (sco3397), sco4263, sco7233 and sco7657. Class I targets have a single promoter that is completely dependent on σE for its transcription (see, for example, Fig. 6A). Colour‐coding of the ChIP samples is as follows: S. coelicolor M600 (WT, blue), ΔsigE attBΦBT1::3 × FLAG‐sigE (SigE‐FLAG, red). Plots span approximately 3 kb of DNA sequence. Genes running left to right are shown in yellow, and genes running right to left are shown in blue. The black arrow indicates the gene subject to σE‐dependent transcription. Colour‐coding of the microarray data is as follows: S. coelicolor M600 (WT, blue squares), sigE null mutant J2130 (∆sigE, red triangles). In each panel, the x‐axis indicates the time in minutes (0, 30, 60 or 90) after the addition of 10 µg/ml vancomycin, and the y‐axis indicates the per gene normalized transcript abundance (log2).
Figure 4.
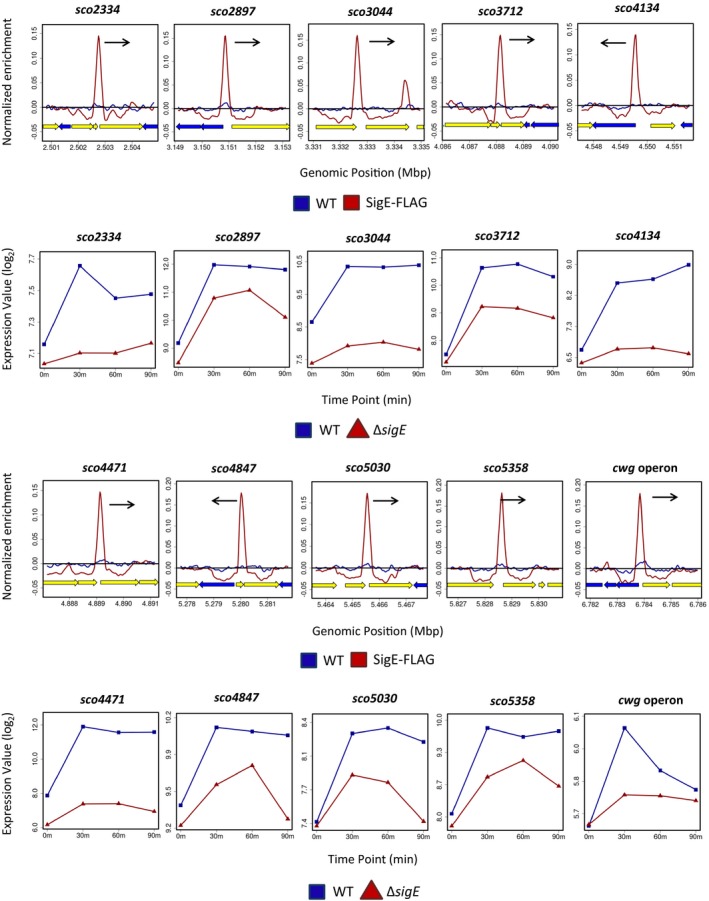
ChIP‐seq (above) and microarray transcriptional profiling data (below) for the Class II σE target genes sco2334, sco2897, sco3044, sco3712, sco4134, sco4471, sco4847, sco5030, sco5358 and the 12‐gene cwg operon (sco6179‐6190). Class II targets have a single promoter that is partially dependent on σE for its transcription (see, for example, Fig. 6B). In the ChIP‐seq panels, the black arrows indicate the genes subject to σE‐dependent transcription. In the microarray transcriptional profiling panels, the x‐axis indicates the time in minutes (0, 30, 60 or 90) after the addition of 10 µg/ml vancomycin, and the y‐axis indicates the per gene normalized transcript abundance (log2). See the legend to Fig. 3 for explanation of the colour‐coding.
Figure 5.
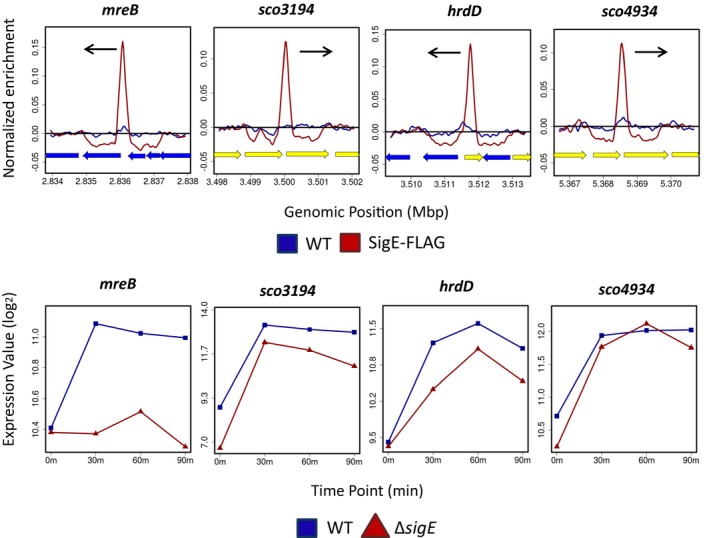
ChIP‐seq (above) and microarray transcriptional profiling data (below) for the Class III σE target genes mreB (sco2611), sco3194, hrdD (sco3202) and sco4934. Class III targets have multiple promoters, one of which is partially or wholly dependent on σE (see, for example, Fig. 6C). In the ChIP‐seq panels, the black arrows indicate the genes subject to σE‐dependent transcription. In the microarray transcriptional profiling panels, the x‐axis indicates the time in minutes (0, 30, 60 or 90) after the addition of 10 µg/ml vancomycin, and the y‐axis indicates the per gene normalized transcript abundance (log2). See the legend to Figure 3 for explanation of the colour‐coding.
Validation and classification of σE targets by S1 nuclease mapping
The promoters of 17 σE target genes [sco2334, mreB (sco2611), sco2897, sco3044, sco3194, sco3396, mprF (sco3397), sco3712, sco4134, sco4263, sco4471, sco4847, sco4934, sco5030, sco5358, sco7233 and sco7657] were characterised using S1 nuclease protection assays. The results confirmed that the genes identified by ChIP‐seq do indeed dependent upon σE for their expression (Fig. 6 and data not shown). This was further confirmed by in vitro transcription experiments using purified σE and the promoters of mreB, sco2334, sco3194, sco3396 and sco4471 (Fig. S3 and data not shown). Subsequently, we divided the 17 σE target genes into three classes, based on the number of promoters upstream of each gene and their dependence on σE, as determined by S1 nuclease protection assays (Fig. 6) and the time‐resolved, genome‐wide transcriptional profiling (Figs 3, 4, 5 and S2). Class I genes (sco3396, mprF, sco4263, sco7233, sco7657) represent targets that have a single promoter that is completely dependent on σE for its expression (Fig. 6A). In line with the results of the S1 nuclease protection assays, microarray transcriptional profiling showed that the transcription of Class I targets is induced in the presence of vancomycin in the wild type and is entirely dependent upon sigE (Fig. 3). Class II genes [sco2334, sco2897, sco3044, sco3712, sco4134, sco4471, sco4847, sco5030, sco5358 and the 12‐gene cwg operon previously characterised as a σE target (Hong et al., 2002)] represent targets that have a single promoter that is partially dependent on σE (Fig. 6B). Once again, in agreement with the S1 nuclease protection assays, microarray transcriptional profiling showed clear induction by vancomycin and partial dependence upon sigE (Fig. 4). Finally, Class III genes (mreB, sco3194, hrdD and sco4934) represent targets that have more than one promoter, one of which is partially or wholly dependent on σE for its expression (Fig. 6C and data not shown). The multiple promoters of S. coelicolor hrdD and mreB were characterised by S1 nuclease mapping previously (Buttner et al., 1990; Burger et al., 2000). The transcription of these four genes is increased on addition of vancomycin but the dependence on sigE is subtle (especially for sco3194 and sco4934). (Fig. 5). Looking across all three classes of σE target gene, the differences in the number of promoters and the extent of dependence of the σE target promoter on σE allows target genes to be expressed with a wide range of induction ratios.
Figure 6.
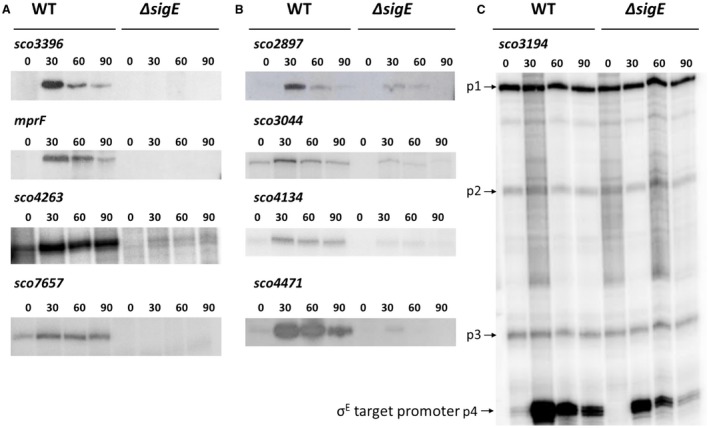
Examples of S1 nuclease protection assays of σE target genes, divided into three classes. A. Class I genes, having a single promoter that is completely dependent on σE. B. Class II genes, having a single promoter that is partially dependent on σE. (C) Class III genes, having multiple promoters, one of which is partially dependent on σE. RNA was prepared from S. coelicolor M600 (WT) and the sigE null mutant J2130 (ΔsigE) after 0, 30, 60 and 90 minutes treatment with 10 µg/ml vancomycin. In (C), p4 is the σE target promoter of the sco3194 gene.
The S1 mapping data were then used to identify the −10 and −35 recognition sequences for the 17 novel targets tested, additionally including the previously characterised hrdD and cwg promoters (Buttner et al., 1990; Hong et al., 2002) (Fig. 7). Based on these validated promoter sequences, a σE consensus was generated (Fig. 7) using WebLogo (Crooks et al., 2004). It is noteworthy that no unambiguous distinction exists between the predicted −35 and −10 binding motifs of those promoters that are completely dependent on σE and those that exhibit partial dependence, although it seems that the latter class are significantly enriched for a G at position 2 of the −10.
Figure 7.
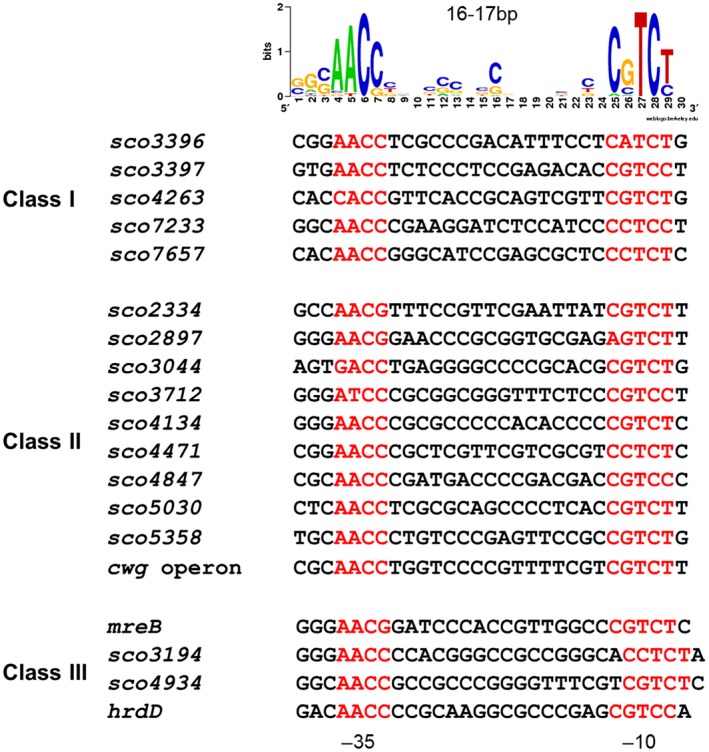
Alignment of the −10 and −35 recognition sequences of the 17 σE target promoters characterised by S1 mapping, additionally including the previously characterised hrdD and cwg promoters (Buttner et al., 1990; Hong et al., 2002). The target genes are divided into Class I (one promoter, completely dependent upon σE), Class II (one promoter, partially dependent upon σE) and Class III targets (multiple promoters, one at least partially dependent upon σE). The corresponding σE consensus sequence, generated using WebLogo (Crooks et al., 2004), is shown above the alignment.
The majority of the σE target promoters tested by S1 mapping are only partially dependent on sigE, suggesting that there are additional ECF σ factors that also recognize these promoters. Further, most of these promoters remain vancomycin‐inducible in the sigE mutant, implying the additional ECF σ factors involved also respond to cell envelope stress. Overall, these results suggest there is a network of two or more ECF σ factors that cooperate with σE to maintain cell envelope integrity in S. coelicolor, which is perhaps unsurprising given there are 51 ECF σ factors in this species. Overlapping promoter specificity between different ECF σ factors has been described in several bacterial genera. For example, multiple ECF σ factors are involved in the cell envelope stress response in B. subtilis, and three of them, σM, σW and σX, can all contribute to the transcription of a common promoter from the same start site (Mascher et al., 2007; Kingston et al., 2013). The predicted consensus binding motifs for these ECF σ factors are highly similar, with some target promoters belonging to one regulon or the other and other target promoters belonging to more than one regulon (Mascher et al., 2007). It has been shown that single nucleotide changes in the −10 motif can determine whether a given promoter is recognised by σX, σW, or both (Qiu and Helmann, 2001). In addition, it has also been shown that the presence or absence of a homopolymeric T‐tract between the −35 and −10 elements contributes to promoter selectivity between σM, σW, σX and σV in Bacillus (Gaballa et al., 2018). Finally, there is also a clear analogy with the oxidative stress response in Streptomyces. Many promoters in S. coelicolor that are recognised by the oxidative stress response σ factor, σR, retain some activity in a sigR null mutant and these promoters are frequently still induced by oxidative stress in that background, implying that there is also a network of related ECF σ factors that coordinate the response to oxidative damage in Streptomyces (Paget et al., 2001; Kim et al., 2012).
Genes of the σE regulon
Over half of the genes under control of σE encode proteins relating to the cell envelope (Table 1). These proteins include those involved in cell wall peptidoglycan assembly, cell wall teichoic acid deposition, lateral cell wall synthesis and sporulation, as well as membrane modification and maintenance of integrity (Table 1). A further 15 σE target genes encode proteins involved in signal transduction and gene regulation (including the σ factor, HrdD) emphasizing the pleiotropic role of σE. Indeed, HrdD itself is predicted to regulate the expression of over 80 genes, including a further 31 genes that themselves encode regulatory proteins (Strakova et al., 2014). Hesketh et al. (2015) used mass spectrometry to analyse changes in the S. coelicolor proteome upon vancomycin‐induced stress. In line with the work presented here, they identified several proteins encoded by σE target genes that increased in abundance in response to vancomycin treatment, including σHrdD and the products of sco1647, sco2368 and sco4494.
Cell wall peptidoglycan elongation and assembly
Six σE target genes encode pencillin‐binding proteins (PBPs). PBPs are involved in the final stage of peptidoglycan synthesis, catalysing its polymerization and cross‐linking outside the membrane (Macheboeuf et al., 2006; Sauvage et al., 2008). PBPs are broadly divided into two classes: the high molecular weight (HMW) PBPs and the low molecular weight (LMW) PBPs. Based on their structure and specific catalytic activity, the HMM PBPs are further subdivided into two classes: A and B (Macheboeuf et al., 2006). Class A enzymes have an N‐terminal glycosyltransferase domain involved in glycan chain elongation and a C‐terminal transpeptidase domain involved in cross‐linking the pentapeptide stems of the glycan units (Macheboeuf et al., 2006; Sauvage et al., 2008). This class of PBP is critical for cell growth in some bacteria such as in E. coli, where deletion of the two class A PBPs (PBP1a and PBP1b) is lethal (Denome et al., 1999). Similarly, in Streptococcus pneumoniae, deletion of the class A PBPs, PBP1a and PBP2a, appears to be lethal (Hoskins et al., 1999). The σE target genes sco2897, sco3901 and sco5039 (sco3901 is a σE target in ChIP‐seq but the bioinformatically predicted σE‐binding site is >400 bp upstream, Table S1) encode proteins belonging to this subclass and they are the only three class A HMW PBPs among more than 20 PBPs in S. coelicolor. It has been shown that deletion of any of these three PBPs results in decreased vancomycin resistance (Hesketh et al., 2011).
The σE target sco1875 encodes a class B HMW PBP and a sco1875 mutant exhibits increased sensitivity towards both vancomycin and bacitracin (Hesketh et al., 2011). In contrast to class A HMW PBPs, class B HMW PBPs do not include an N‐terminal glycosyltransferase domain, but rather an N‐terminal domain thought to be involved in cell morphogenesis via interaction with partner proteins (Macheboeuf et al., 2006; Sauvage et al., 2008). For example, in E. coli, the class B PBP FtsI is recruited by the cell division protein FtsW to the site of cell division (Mercer and Weiss, 2002). In M. tuberculosis, a class B HMW PBP (PBPA) is required for cell division and maintenance of cell shape, and phosphorylation of PBPA by the Ser/Thr kinase PknB is suggested to regulate the positioning of PBPA at the cell septum, thereby modulating peptidoglycan synthesis (Dasgupta et al., 2006). Some class B HMW PBPs such as PBP2a from methicillin‐resistant S. aureus (MRSA) (Chambers, 1997; Lim and Strynadka, 2002; Katayama et al., 2004) and PBP5fm from Enterococcus faecium (Fontana et al., 1994; Sauvage et al., 2002) have a low affinity for penicillin and thus give rise to β‐lactam resistance.
The σE targets sco4439 and sco4847 encode putative D‐ala‐D‐ala carboxypeptidases. These are LMW PBPs involved in the cleavage of the terminal alanine of the pentapeptide stems of the glycan chain and thus modulate peptidoglycan maturation or recycling (Macheboeuf et al., 2006; Sauvage et al., 2008).
Among the six σE target genes that encode PBPs, sco2897 and sco4847 are induced by vancomycin (Fig. 4) and have been confirmed by S1 nuclease protection assays to be transcribed from a single promoter that is partially dependent on σE (Fig. 6B and data not shown). Microarray transcriptional profiling also shows that sco1875, sco4439 and sco5039 are induced by vancomycin and that transcription is partially dependent on σE (Fig. S2). These findings suggest that σE‐directed PBP expression is likely to be an important component of the response to cell envelope damage in Streptomyces.
An alternative pathway to peptidoglycan cross‐linking
The target of β‐lactam antibiotics is the D,D‐transpeptidase activity of HMW PBPs, responsible for the synthesis of 4 → 3 cross‐links between peptide side chains in the peptidoglycan of bacterial cell walls. The σE targets sco3194, sco4934 (both encoding lipoproteins, the latter secreted through the Tat pathway; Thompson et al., 2010) and sco0736 encode proteins that contain a L,D‐transpeptidase catalytic domain (Pfam: YkuD). Such proteins cross‐link peptidoglycan by forming 3 → 3 cross‐links between peptide side chains (Hugonnet et al., 2014). This bypasses the typical 4 → 3 transpeptidase activity of PBPs, thus promoting resistance to β‐lactams (Biarrotte‐Sorin et al., 2006). The peptidoglycan of M. tuberculosis is rich in 3 → 3 cross links, which are suggested to play a role in the adaptive response of the bacteria during stationary phase (Lavollay et al., 2008). L,D‐transpeptidase activity is also employed by E. coli in the attachment of Braun’s lipoprotein (BLP) to the peptidoglycan (Magnet et al., 2007). BLP is involved in cell envelope integrity through the connection of the outer membrane to the peptidoglycan layer (Yem and Wu, 1978; Hayashi and Wu, 1990). Transcription of sco0736, sco3194 and sco4934 is highly induced by vancomycin and partially dependent on σE (Figs 5, 6C, S2, and Table S1).
Cell wall teichoic acid deposition
The σE targets sco3044 and sco5358 encode proteins in the LytR‐CpsA‐Psr (LCP) family and expression of sco3044 in particular depends heavily on σE (Figs 4 and 6B). LCP proteins are involved in the attachment of wall teichoic acid (WTA) and capsular polysaccharides to the peptidoglycan of the bacterial cell wall (Kawai et al., 2011). WTA can constitute up to 60% of the Gram‐positive cell wall and has roles in the regulation of cell division, cell shape determination, antibiotic resistance and pathogenesis (Brown et al., 2013). In B. subtilis, there are three LCP homologs and deletion of all three genes results in a failure to deposit WTA at the cell envelope (Kawai et al., 2011). Similarly, deletion of all three LCP genes in Staphylococcus aureus leads to release of WTA into the extracellular medium (Chan et al., 2013) and abnormalities in septum placement and cell separation (Over et al., 2011). Transcription of the LCP gene msrR in S. aureus is induced by cell wall disrupting agents such as β‐lactams, glycopeptides and lysostaphin, and deletion of msrR results in increased sensitivity to methicillin and teicoplanin (Rossi et al., 2003). These observations implicate the σE response in the maintenance of cell wall components other than peptidoglycan in Steptomyces.
The cytoskeleton, cell wall synthesis and sporulation
Unexpectedly, mreB was found to be a σE target (Fig. 5). MreB is an actin homolog that acts in rod‐shaped bacteria like E. coli, B. subtilis and Caulobacter crescentus as a cytoskeletal element to direct peptidoglycan biosynthesis in the lateral wall (Errington, 2015). However, in contrast to rod‐shaped bacteria, Streptomyces hyphae do not grow by inserting new cell wall material in the lateral wall, but rather by tip extension and by initiating new branches de novo. This polar mode of growth does not require MreB and is instead directed by a polarisome complex involving DivIVA, Scy and FilP (Bush et al., 2015). Rather, MreB appears to direct spore wall thickening, localizing under the membrane at spore septa at cell division before spreading around the immature spore (Mazza et al., 2006; Kleinschnitz et al., 2011). However, mreB is abundantly transcribed during vegetative growth (Fig. 5) (Burger et al., 2000), suggesting that MreB might have an additional role unconnected to sporulation. S. coelicolor mreB mutants sporulate poorly and overproduce actinorhodin (Mazza et al., 2006), and the sigE null mutant exhibits similar characteristics (Paget et al., 1999a).
This study also identified the whiB gene, encoding the key developmental transcription factor WhiB, as a σE target. WhiB is essential for the initiation of sporulation septation in Streptomyces (Bush et al., 2015; 2016; Bush, 2018), and in M. tuberculosis the expression of the WhiB ortholog WhiB2 is induced by cell wall‐inhibiting agents (isoniazid, ethambutol and cycloserine) (Geiman et al., 2006). S. coelicolor whiB has two promoters, and the upstream promoter was previously shown to be recognised by σE in a run‐off assay (Soliveri et al., 1992; Kang et al., 1997). The transcription of whiB is highly induced by vancomycin in a σE‐dependent manner (Table 1). Unexpectedly, these observations suggest that whiB might play a significant role in the σE‐mediated cell envelope stress response.
Membrane modification
The σE target sco3397 encodes a homolog of B. subtilis MprF (multiple peptide resistance factor) (24% identity, 50% similarity) (Table 1). Transcription of sco3397 is highly induced by vancomycin and is completely dependent on σE (Figs 3 and 6A). MprF proteins are lysylphosphatidylglycerol synthases that catalyse the transfer of L‐lysine from lysyl‐tRNA to the negatively charged lipid phosphatidylglycerol, thus neutralizing the membrane surface charge. This enhances resistance to cationic antimicrobial peptides (CAMPs) and antibiotics through repulsion (Ernst et al., 2009). MprF has also been shown to affect resistance to vancomycin and daptomycin in S. aureus (Ruzin et al., 2003; Nishi et al., 2004; Friedman et al., 2006). In S. coelicolor, a sco3397 mutant shows markedly increased sensitivity towards both vancomycin and bacitracin (Hesketh et al., 2011), in line with a role in the cell envelope stress response. In addition to Sco3397, there is a second homolog of B. subtilis MprF in S. coelicolor (Sco6384), but the sco6384 gene is not a σE target.
Maintenance of membrane integrity
The σE target sco2168 encodes a PspA (phage shock protein A) homolog (Vrancken et al., 2008). PspA is the major effector of the phage shock protein (Psp) system present in many bacteria. The Psp system plays a role in the adaptive response to multiple extracytoplasmic stresses, blocking stress‐induced membrane damage and the resulting dissipation of the proton‐motive force (Joly et al., 2010). In Streptomyces lividans, the pspA gene is strongly induced under stress conditions that attack membrane activity and is essential for growth and survival under most of these conditions (Vrancken et al., 2008). Both PspA and its paralog LiaH are induced as part of the cell envelope stress response in B. subtilis (Jordan et al., 2008). While PspA is under control of σW, LiaH is the primary target of the LiaRS two‐component system and is strongly induced by antibiotics targeting the membrane‐anchored steps of cell wall biosynthesis (Wiegert et al., 2001; Wolf et al., 2010).
The σE target sco4471 encodes a novel lipoprotein that contributes to lysozyme resistance
The σE target gene sco4471 encodes a lipoprotein (Thompson et al., 2010) and is heavily but not completely dependent on sigE for its transcription (Figs 4 and 6B). Furthermore, sco4471 expression increases dramatically in response to induction, being more than 20‐fold higher in wild‐type S. coelicolor than the sigE mutant after treatment with vancomycin (Fig. 4 and Table S1). Deletion of sco4471 resulted in a fourfold increase in sensitivity to lysozyme compared to wild type (Fig. S4), suggesting that loss of sco4471 expression contributes to the ~50‐fold increase in lysozyme sensitivity seen in the sigE mutant relative to the wild type (Paget et al., 1999a). As shown in Fig. S4, the sco4471 mutant also displays minor abnormalities in spore size and shape.
Conservation of σE target promoters across the Streptomyces genus
Following our identification of the genes under σE control in S. coelicolor, we searched bioinformatically to determine if the promoters of these σE target genes were conserved across the panel of 19 Streptomyces species listed in Table S2. Given the high conservation of the σ2 and σ4 domains (that bind the −10 and −35 promoter elements respectively) in the σE orthologs across the 19 species (Fig. S5), we anticipated that these 19 σE orthologs would recognize highly similar or identical promoter motifs to S. coelicolor σE. Accordingly, two promoter position weight matrices (PWMs) with a 16 bp or 17 bp spacer between the −35 region and −10 region were generated from 19 validated σE S. coelicolor target promoter sequences. These two PWMs were then used to predict all possible σE‐binding sites that lie within 10–200 bp of the start codon of the downstream gene across the 19 genomes. In S. coelicolor, this prediction detected each of the 19 in vitro validated σE targets and over 70% of the targets identified in our ChIP‐seq experiments (Table 1), suggesting suitable parameters for accurate prediction.
This analysis predicts that 21 of the 91 σE target promoters identified in S. coelicolor are conserved across at least 9 of the 19 Streptomyces genomes (Fig. 8). These 21 genes (equivalent to sco0736, sco1875, sco2255, sco2419, mreB, sco2807, sco2892, pkaA, sco3396, mprF, sco4120, sco4134, sco4439, sco4471, sco4613, sco4934, sco5030, sco5039, sco5358, sco5742, sco7657) include 9 targets (mreB, sco3396, mprF, sco4134, sco4471, sco4934, sco5030, sco5358 and sco7657) validated in our S1 mapping and in vitro transcription experiments. mreB is present in all 19 predicted σE regulons, and the gene encoding the lipoprotein that contributes to lysozyme resistance, sco4471, is present in 18/19 predicted σE regulons. Also among the products of these 21 genes are the PBPs Sco1875, Sco4439 and Sco5039, the L,D‐transpeptidases Sco0736 and Sco4934, the putative MprF protein Sco3397, and the LytR‐CpsA‐Psr family protein Sco5358. sco2255, sco2892, sco3396, sco4134 and sco7657 encode cell envelope‐associated enzymes, whereas sco2419, sco2807, sco4613, sco5030 and sco5742 encode cell envelope proteins of completely undefined function (Table 1). Finally, PkaA (Sco2974) is a Ser/Thr protein kinase and Sco4120 is predicted to be a regulatory protein (Table 1).
Figure 8.
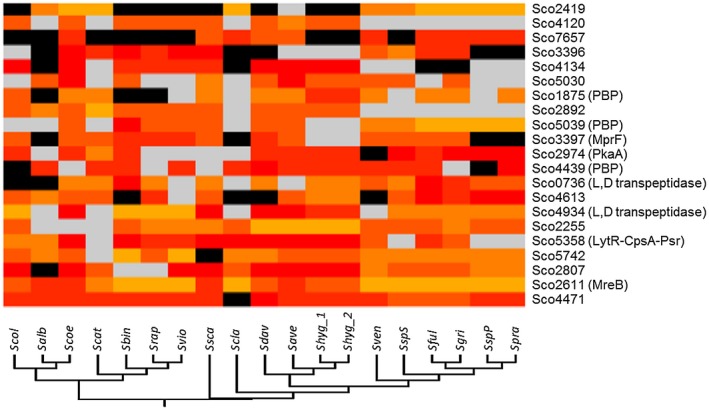
Bioinformatic analysis of the conservation of S. coelicolor σE target promoters across 19 Streptomyces genomes, showing the 21 S. coelicolor σE target promoters that are predicted to be conserved in at least 9 Streptomyces genomes. Black indicates no ortholog of the target gene is found in the designated genome. Grey indicates the ortholog of the target is found, but the σE‐binding consensus is not present between within 200 bp upstream of the open reading frame. Yellow, orange and red indicate that an ortholog of the target is found and that there is a σE‐binding consensus within 200 bp upstream of the open reading frame. The σE‐binding consensus of each target was predicted by the Virtual Footprint version 3.0 tool incorporated into the PRODORIC server (http://www.prodoric.de/vfp/vfp_regulon.php) (Münch et al., 2005; Grote et al., 2009) and a PRODORIC score was given to reflect the quality of the prediction. The yellow to red linear gradient indicates the Prodoric score of the σE‐binding site from the minimum value to the maximum value. The abbreviations used for each species are the same as those listed in Table S2. The phylogenetic relationship between these Streptomyces strains is shown by the phylogenetic tree of their 16s rDNA at the bottom.
Conclusions
This study reveals the complex regulatory network activated by σE in response to cell envelope‐induced stress (Fig. 9). In particular, it shows that key proteins under σE control include the actin homolog MreB, multiple PBP and L,D‐transpeptidases, a LytR‐CpsA‐Psr‐family protein involved in cell wall teichoic acid deposition, PspA, involved in the maintenance of membrane integrity, and a putative MprF protein, predicted to add lysyl groups to phosphatidylglycerol to neutralize membrane surface charge, potentially contributing to resistance to cationic antimicrobial peptides and antibiotics (Fig. 9).
Figure 9.
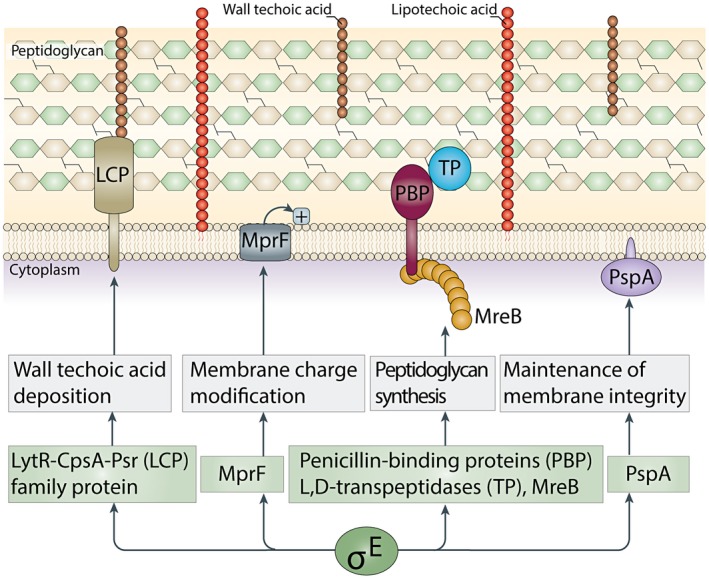
Mechanisms underlying the σE‐dependent cell envelope stress response. Key proteins under σE control include the actin homolog MreB, multiple PBPs and L,D‐transpeptidases, a LytR‐CpsA‐Psr family protein predicted to be involved in cell wall teichoic acid deposition, PspA, involved in the maintenance of membrane integrity, and a predicted MprF protein that adds lysyl groups to phosphatidylglycerol to neutralize membrane surface charge, potentially contributing to resistance to cationic antimicrobial peptides and antibiotics.
Experimental proceedures
Bacterial strains, plasmids and oligonucleotides
Bacterial strains, plasmids and primers in this study are listed in Table S3.
Construction of a 3 × FLAG‐σE‐complemented S. coelicolor strain
In order to engineer an S. coelicolor strain expressing a form of σE with an N‐terminal, triple‐FLAG tag (DYKDHDGDYKDHDIDYKDDDDK), a pMS82‐derived construct was created via a two‐step fusion PCR approach. In the first step, the cosmid STE94 was used as a template for two separate PCR reactions. The first reaction amplified the promoter region of the sigE gene using the primer pair P13NFLAGSigE and P23NFLAGSigE. The second reaction amplified the coding region of the sigE gene using the primer pair P33NFLAGSigE and P43NFLAGSigE. Together the P23NFLAGSigE andP33NFLAGSigE primers contain the sequence encoding the triple‐FLAG tag via a 24bp overlapping section. In the second step, the primers P13NFLAGSigE and P43NFLAGSigE were used to amplify the entire sigE gene and its promoter, fusing the two products from step 1 together and incorporating the 3 × FLAG tag sequence between them. The P13NFLAGSigE and P43NFLAGSigE primers additionally contain the HindIII and KpnI sites, respectively, to enable cloning into HindIII, KpnI‐cut pMS82. The resulting vector was then introduced into the ΔsigE mutant J2130 (Paget et al., 1999a) by conjugation using the dam dcm hsdS E. coli strain ET12567 containing pUZ8002.
Lysozyme sensitivity tests
Lysozyme sensitivity tests for the wild‐type strain M600, the sigE mutant J2130 and the 3 × FLAG‐σE‐complemented sigE mutant strain were performed as described previously (Paget et al., 1999a). Briefly, 2×106 spores of S. coelicolor were spread onto a Difco Nutient Agar (DNA) plate to make a confluent lawn. 5 µl of 1 mg/ml lysozyme was then diluted in a twofold series and spotted onto the freshly spread spore lawns before incubation at 30°C for 2 days. Lysozyme sensitivity tests were carried out on the wild‐type strain M600 and the ∆sco4471 mutant in the same way but using lysozyme concentrations ranging from 3.75 to 0.0075 mg/ml, generated as a twofold dilution series.
Western blot analysis
The 3 × FLAG‐sigE complemented sigE mutant was incubated in 5 ml TES buffer (250 mM N‐[tris(hydroxymethyl)methyl]‐2‐aminoethanesulfonic acid, pH7.2) for 10 minutes at 50°C and germination carried out in 5 ml 2 × PG (0.5 ml of 10% yeast extract, 0.25 ml of 20% casamino acids, 0.05 ml of 1M CaCl2 and 4.2 ml of H2O) medium for 2–3 hours. Following this, germinated spores were span down at 4500 × g for 10 minutes and inoculated into 50 ml NMMP medium in 250 ml canonical flasks with springs to achieve a final OD450 of 0.010, then grown at 30°C, shaking at 250 rpm. At OD450 ~ 0.6, vancomycin was added to a final concentration of 10 µg/ml and samples were collected at 15 minutes intervals for 1 hour.
For Western blotting, for each time point, 5 ml culture was taken and spun down at 3000 rpm for 1 minutes. Cells were washed in 5 ml ice‐cold sonication buffer [20 mM Tris pH 8.0, 5 mM EDTA, 1 × EDTA‐free protease inhibitors (Roche)] and finally resuspended in 1 ml before sonication (5 × 5 seconds on, 15 seconds off) at 4.5 micron amplitude. Lysates were then centrifuged at 16,000 × g for 15 minutes at 4°C to remove cell debris. Total protein concentration was determined using the Bradford assay (Biorad). Equal amounts of total protein from each sample were loaded on a 12.5% polyacrylamide SDS‐PAGE gel. After electrophoresis, transfer was carried out to a Hybond‐C Extra nylon membrane (Amersham Pharmacia Biotech) using the Invitrogen XCell II Blot system. For detection of 3 × FLAG‐σE, anti‐σE polyclonal antibody raised in rabbit was diluted in a ratio of 1:300. 3 × FLAG‐σE was visualised via an anti‐rabbit IgG alkaline phosphatase secondary antibody (sigma A8025), diluted 3:5000 and detected directly on the membrane using the SigmaFast system (Sigma) that uses BCIP/NBT (5‐Bromo‐4‐chloro‐3‐indolyl phosphate/Nitro blue tetrazolium) as a substrate.
RNA isolation and DNA microarray analysis
RNA isolation from S. coelicolor was performed as described previously (Hong et al., 2002). Total RNA was isolated from mycelium harvested from 5 ml liquid cultures using an RNeasy Midi Kit (Qiagen) according to the manufacturer’s instruction with some modifications. The cell pellet was resuspended in TE buffer containing lysozyme (10 mM Tris, pH 8, 1mM EDTA, 15 mg/ml lysozyme) and incubated at room temperature for 60 minutes. RLT buffer (Qiagen) was added (4 ml) and samples were sonicated 3 cycles ON‐OFF on ice at 18 micron amplitude and for 20 seconds. Samples were then extracted twice with Phenol:Chloroform:Isoamyl Alcohol 25:24:1 saturated with 10 mM Tris, pH 8.0, 1 mM EDTA (2 ml) and once with chloroform (4 ml). Extracts were mixed with 100% ethanol and applied to RNeasy Midi columns. Purified RNA was eluted with 300 µl RNase‐free water. Affymetrix Gene Chip hybridization and data collection were essentially as described before (Hesketh et al., 2009; Bibb et al., 2012). The CEL files received from the scanner were read into the R package for statistical computing (R Core Team, 2012) using the ReadAffy function of the affy package (Gautier et al., 2004). The rma function of the affy package was used to compute an ExpressionSet object from the CEL files. This ExpressionSet object contains the expression values (log2) for each gene in each CEL file. The function lmFit of the limma package (Smyth, 2005) along with a suitable design matrix, was used to combine replicate arrays into single coefficients of expressions for each gene at each time point or strain into an MArrayLM object. Expression values were retrieved from the MArrayLM object and subjected to a per gene normalization to the median before being used to generate the graphs shown in this paper.
Chromatin immunoprecipitation sequencing
Spores of the S. coelicolor wild‐type strain M600 and the congenic 3 × FLAG‐σE‐complemented sigE mutant spores were germinated and grown as described for the Western blot analysis. For the Chromatin immunoprecipitation (ChIP), the cell envelope stress response was induced by treatment with vancomycin to a final concentration of 10 µg/ml and for 30 minutes. Following this, formaldehyde was added to cultures at a final concentration of 1% (v/v) and incubation was continued at 30°C with shaking for a further 30 minutes. Glycine was then added to a final concentration of 125 mM to stop the cross‐linking. Cells were then harvested, lysed, sonicated and the immunoprecipitation conducted via M2 (Sigma Aldrich A2220) gel suspension. Subsequent steps were conducted as described by Bush et al. (2013). Notably, for each tested strain, while immunoprecipitated DNA was used as a ChIP (input) sample, the non‐immunoprecipitated total DNA was used as a reference sample. Sequence analysis was conducted as described by Bush et al. (2013).
Data availablilty
The anti‐FLAG‐σE ChIP‐seq data and microarray transcriptional profiling data have been deposited at the MIAME‐compliant ArrayExpress database (https://www.ebi.ac.uk/arrayexpress/) under accession numbers E‐MTAB‐7827 (ChIP‐seq data) and E‐MTAB‐7814 (microarray transcriptional profiling data).
S1 nuclease mapping
To generate the probes, a reverse primer within 80 bp downstream of the startcodon of each gene was first labelled with [ɣ‐32P] ATP. Amplification was then conducted from a template using the labelled reverse primer and a forward primer 400 bp upstream of the start codon. For all assays, 30 μg of RNA and 25 pmol of labelled probe were dissolved in 20 μl of sodium TCA buffer and hybridized at 45°C overnight after denaturation at 65°C for 15 minutes. Primer sequences used in S1 nuclease mapping are listed in Table S3. Sequencing ladders were generated using Sequenase™ Version 2.0 DNA Sequencing Kit (USB Europe GMBH).
Purification of σE and in vitro transcription assays
σE was overexpressed and purified to homogeneity as described previously (Paget et al., 1999a). Run‐off transcription assays were performed using [α‐32P]‐CTP (Perkin Elmer) at 3000 Ci mmol−1 as described previously (Buttner et al., 1987). Reaction mixtures contained 1.25 pmol of E. coli core RNA polymerase (Epicentre Technologies) and 6 pmol of σE. Transcripts were analysed on a 6% (w/v) polyacrylamide‐7 M urea gel using a heat‐denatured, 32P‐labelled HpaII digest of pBR322 as size standards. Cold RNase‐free PCR probes generated as for the S1 mapping experiments were used as templates.
Construction of a sco4471 mutant
A sco4471 mutant in which the coding region was replaced with an apramycin resistance (apr) cassette was generated using ‘Redirect’ PCR targeting (Gust et al., 2003). Cosmid D65 was introduced into E. coli BW25113 containing pIJ790 and the relevant gene was replaced with the apr‐oriT cassette amplified from pIJ773 using the primer pair SCO4471KOFW and SCO4471KORV (Table S3). The resulting disrupted cosmid was confirmed by restriction digestion and by PCR analysis using appropriate flanking primers (Table S3) and introduced into S. coelicolor by conjugation via the methylation‐deficient E. coli strain ET12567 (dam dcm hsdS) carrying the driver plasmid pUZ8002. Null mutant derivatives, generated by double crossing over, were identified by their apramycin‐resistant, kanamycin‐sensitive phenotypes, and their chromosomal structures were confirmed by PCR analysis using appropriate flanking primers (Table S3) and by Southern hybridization.
Scanning electron microscopy of the S. coelicolor sco4471 mutant
For scanning electron microscopy, 5‐day‐old colonies were mounted on the surface of an aluminium stub with optimal cutting temperature compound (BDH Laboratory Supplies, Poole, England). The stub was then immediately plunged into liquid nitrogen slush at approximately −210°C to cryopreserve the material and transferred to the cryostage of an ALTO 2500 cryotransfer system (Gatan, Oxford, England) attached to a Zeiss Supra 55 VP field emission gun scanning electron microscope (Zeiss SMT, Germany). The surface frost was sublimated at −95°C for 3 minutes before the sample was sputter‐coated with platinum for 2 minutes at 10 mA at below −110°C. After sputter‐coating, the sample was moved onto the cryo stage in the main chamber of the microscope, held at approximately −130°C. The sample was imaged at 3kV and digital TIFF files were stored.
Prediction of the promoter motif associated with each ChIP‐seq target
Initially, at least 200 bp sequences surrounding the highest enriched ‘25 bp’ genomic region of all the ChIP‐seq targets were extracted. Then, over‐represented 2‐block motifs mimicking a typical promoter with conserved ‘‐35’ and ‘‐10’ regions were identified in the forward strand of these sequences by the BioProspector program using the parameters: ‘W = 4’, ‘w = 5’, ‘G = 17’, ‘g = 16’ and ‘G‐g = 1 bp’ (‘W’ and ‘w’ stand for the length of the upstream and downstream motifs respectively; ‘G’ and ‘g’ stand for the maximum and minimum distances between the two blocks respectively) (Liu et al., 2001). The 2‐block motifs were obtained from iterative searches using all combinations of these parameters. After 40 reinitializations, the highest scoring motifs were then selected to represent the σE‐binding sites since they highly resemble the previous reported σE promoter motif.
Bioinformatic analysis of the conservation of S. coelicolor σE target promoters across 19 Streptomyces genomes
Two promoter PWMs, PWM_19_16 and PWM_19_17, were built from the 19 validated S. coelicolor σE promoter sequences shown in Figure 7 by restricting the spacer between the −35 region and −10 region to be 16 bp and 17 bp respectively. In the case of PWM_19_16, one base was removed from the non‐conserved region of the sco3194 and sco4934 promoters respectively, whereas, in the case of PWM_19_17, one base was added into the non‐conserved region of each promoter with 16 bp between the −35 region and −10 region. Then, these promoter PWMs were used to search for putative σE‐binding sites from 19 Streptomyces spp. chromosome sequences using the Virtual Footprint version 3.0 tool incorporated into the PRODORIC server (http://www.prodoric.de/vfp/vfp_regulon.php) (Münch et al., 2005; Grote et al., 2009) with the parameters: ‘Non‐Occurrence Penalty = None’, ‘Sensitivity = 1’, ‘Core Sensitivity/Size = 1/6’. Searches were restricted to sequences between 10 and 200 bp upstream from the start codon of the closest predicted coding sequence. Orthologues of these targets were searched for in each Streptomyces genome using BlastP. S. coelicolor σE target promoters predicted to be conserved in at least 9 of the 19 genomes analysed are shown in Figure 8.
Supporting information
Acknowledgements
We thank Kim Findlay for scanning electron microscopy and Susan Schlimpert for help in preparing the figures. This work was supported by a Dorothy Hodgkin Postgraduate Award to Ngat Tran from the BBSRC, by a postgraduate stipend to Xiaoluo Huang from China Scholarship Council, by BBSRC grant BB/N006852/1 to Mark Buttner in the framework of the ERASynBio initiative, by Deutsche Forschungsgemeinschaft (DFG) grant MA2837/2‐2 and German Federal Ministry of Education and Research (BMBF) grant 031L0010A (ERASynBio initiative framework) to Thorsten Mascher, and by BBSRC Institute Strategic Programme Grant BB/J004561/1 to the John Innes Centre. Daniela Pinto was supported by the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme (FP72007‐2013) under REA grant agreement no. 628509.
References
- Biarrotte‐Sorin, S. , Hugonnet, J.‐E. , Delfosse, V. , Mainardi, J.‐L. , Gutmann, L. , Arthur, M. et al. (2006) Crystal structure of a novel β‐lactam‐insensitive peptidoglycan transpeptidase. Journal of Molecular Biology, 359, 533–538. [DOI] [PubMed] [Google Scholar]
- Bibb, M.J. , Domonkos, Á. , Chandra, G. and Buttner, M.J. (2012) Expression of the chaplin and rodlin hydrophobic sheath proteins in Streptomyces venezuelae is controlled by σBldN and a cognate anti‐sigma factor, RsbN. Molecular Microbiology, 84, 1033–1049. [DOI] [PubMed] [Google Scholar]
- Brown, S. , Santa Maria, J.P. and Walker, S. (2013) Wall teichoic acids of gram‐positive bacteria. Annual Review of Microbiology, 67, 313–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg, T.D.H. , Wright, G.D. , Dutka‐Malen, S. , Arthur, M. , Courvalin, P. and Walsh, C.T. (1991) Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry, 30, 10408–10415. [DOI] [PubMed] [Google Scholar]
- Burger, A. , Sichler, K. , Kelemen, G. , Buttner, M.J. and Wohlleben, W. (2000) Identification and characterization of the mre gene region of S. coelicolor A3(2). Molecular and General Genetics, 263, 1053–1060. [DOI] [PubMed] [Google Scholar]
- Bush, M.J. (2018) The actinobacterial WhiB‐like (Wbl) family of transcription factors. Molecular Microbiology, 110, 663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush, M.J. , Bibb, M.J. , Chandra, G. , Findlay, K.C. and Buttner, M.J. (2013) Genes required for aerial growth, cell division, and chromosome segregation are targets of WhiA before sporulation in Streptomyces venezuelae . mBio, 4, e00684‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush, M.J. , Chandra, G. , Bibb, M.J. , Findlay, K.C. and Buttner, M.J. (2016) Genome‐wide chromatin immunoprecipitation sequencing analysis shows that WhiB is a transcription factor that co‐controls its regulon with WhiA to initiate developmental cell division in Streptomyces . mBio, 7, e00523‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush, M.J. , Tschowri, N. , Schlimpert, S. , Flärdh, K. and Buttner, M.J. (2015) c‐di‐GMP signalling and the regulation of developmental transitions in streptomycetes. Nature Reviews Microbiology, 13, 749–760. [DOI] [PubMed] [Google Scholar]
- Buttner, M.J. , Chater, K.F. and Bibb, M.J. (1990) Cloning, disruption, and transcriptional analysis of three RNA polymerase sigma factor genes of Streptomyces coelicolor A3(2). Journal of Bacteriology, 172, 3367–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner, M.J. , Fearnley, I.M. and Bibb, M.J. (1987) The agarase gene (dagA) of Streptomyces coelicolor A3(2): nucleotide sequence and transcriptional analysis. Molecular and General Genetics, 209, 101–109. [DOI] [PubMed] [Google Scholar]
- Capra, E.J. and Laub, M.T. (2012) Evolution of two‐component signal transduction systems. Annual Review of Microbiology, 66, 325–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers, H.F. (1997) Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clinical Microbiology Reviews, 10, 781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, Y.G. , Frankel, M.B. , Dengler, V. , Schneewind, O. and Missiakas, D. (2013) Staphylococcus aureus mutants lacking the LytR‐CpsA‐Psr family of enzymes release cell wall teichoic acids into the extracellular medium. Journal of Bacteriology, 195, 4650–4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks, G.E. , Hon, G. , Chandonia, J.‐M. and Brenner, S.E. (2004) WebLogo: a sequence logo generator. Genome Research, 14, 1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartigalongue, C. , Missiakas, D. and Raina, S. (2001) Characterization of the Escherichia coli σE Regulon. Journal of Biological Chemistry, 276, 20866–20875. [DOI] [PubMed] [Google Scholar]
- Dasgupta, A. , Datta, P. , Kundu, M. and Basu, J. (2006) The serine/threonine kinase PknB of Mycobacterium tuberculosis phosphorylates PBPA, a penicillin‐binding protein required for cell division. Microbiology, 152, 493–504. [DOI] [PubMed] [Google Scholar]
- Dehal, P.S. , Joachimiak, M.P. , Price, M.N. , Bates, J.T. , Baumohl, J.K. , Chivian, D. et al. (2010) MicrobesOnline: an integrated portal for comparative and functional genomics. Nucleic Acids Research, 38, D396–D400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denome, S.A. , Elf, P.K. , Henderson, T.A. , Nelson, D.E. and Young, K.D. (1999) Escherichia coli mutants lacking all possible combinations of eight penicillin binding proteins: viability, characteristics, and implications for peptidoglycan synthesis. Journal of Bacteriology, 181, 3981–3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiamphungporn, W. and Helmann, J.D. (2008) The Bacillus subtilis σM regulon and its contribution to cell envelope stress responses. Molecular Microbiology, 67, 830–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst, C.M. , Staubitz, P. , Mishra, N.N. , Yang, S.‐J. , Hornig, G. , Kalbacher, H. et al. (2009) The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion. PLoS Path, 5, e1000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington, J. (2015) Bacterial morphogenesis and the enigmatic MreB helix. Nature Reviews Microbiology, 13, 241–248. [DOI] [PubMed] [Google Scholar]
- Flärdh, K. and Buttner, M.J. (2009) Streptomyces morphogenetics: dissecting differentiation in a filamentous bacterium. Nature Reviews Microbiology, 7, 36–49. [DOI] [PubMed] [Google Scholar]
- Fontana, R. , Aldegheri, M. , Ligozzi, M. , Lopez, H. , Sucari, A. and Satta, G. (1994) Overproduction of a low‐affinity penicillin‐binding protein and high‐level ampicillin resistance in Enterococcus faecium . Antimicrobial Agents and Chemotherapy, 38, 1980–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formstone, A. and Errington, J. (2005) A magnesium‐dependent mreB null mutant: implications for the role of mreB in Bacillus subtilis . Molecular Microbiology, 55, 1646–1657. [DOI] [PubMed] [Google Scholar]
- Friedman, L. , Alder, J.D. and Silverman, J.A. (2006) Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus . Antimicrobial Agents and Chemotherapy, 50, 2137–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaballa, A. , Guariglia‐Oropeza, V. , Dürr, F. , Butcher, B.G. , Chen, A.Y. , Chandrangsu, P. et al. (2018) Modulation of extracytoplasmic function (ECF) sigma factor promoter selectivity by spacer region sequence. Nucleic Acids Research, 46, 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier, L. , Cope, L. , Bolstad, B.M. and Irizarry, R.A. (2004) affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics, 20, 307–315. [DOI] [PubMed] [Google Scholar]
- Geiman, D.E. , Raghunand, T.R. , Agarwal, N. and Bishai, W.R. (2006) Differential gene expression in response to exposure to antimycobacterial agents and other stress conditions among seven Mycobacterium tuberculosis whiB‐like genes. Antimicrobial Agents and Chemotherapy, 50, 2836–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote, A. , Klein, J. , Retter, I. , Haddad, I. , Behling, S. , Bunk, B. et al. (2009) PRODORIC (release 2009): a database and tool platform for the analysis of gene regulation in prokaryotes. Nucleic Acids Research, 37, D61–D65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest, R.L. and Raivio, T.L. (2016) Role of the Gram‐negative envelope stress response in the presence of antimicrobial agents. Trends in Microbiology, 24, 377–390. [DOI] [PubMed] [Google Scholar]
- Gust, B. , Challis, G.L. , Fowler, K. , Kieser, T. and Chater, K.F. (2003) PCR‐targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proceedings of the National Academy of Sciences of the United States of America, 100, 1541–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie, J.L. , Williams, K.B. , Bohr, L.L. , Houtman, J.C. , Gakhar, L. and Ellermeier, C.D. (2016) The anti‐sigma factor RsiV is a bacterial receptor for lysozyme: co‐crystal structure determination and demonstration that binding of lysozyme to RsiV Is required for σV activation. PLoS Genetics, 12, e1006287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie, J.L. , Williams, K.B. , Sepúlveda, C. , Houtman, J.C. , Forest, K.T. and Ellermeier, C.D. (2014) Evidence of a bacterial receptor for lysozyme: binding of lysozyme to the anti‐σ factor RsiV controls activation of the ECF σ factor σV . PLoS Genetics, 10, e1004643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, S. and Wu, H.C. (1990) Lipoproteins in bacteria. Journal of Bioenergetics and Biomembranes, 22, 451–471. [DOI] [PubMed] [Google Scholar]
- Hesketh, A. , Deery, M. and Hong, H.‐J. (2015) High‐resolution mass spectrometry based proteomic analysis of the response to vancomycin‐induced cell wall stress in Streptomyces coelicolor A3(2). Journal of Proteome Research, 14, 2915–2928. [DOI] [PubMed] [Google Scholar]
- Hesketh, A. , Hill, C. , Mokhtar, J. , Novotna, G. , Tran, N. , Bibb, M. et al. (2011) Genome‐wide dynamics of a bacterial response to antibiotics that target the cell envelope. BMC Genomics, 12, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh, A. , Kock, H. , Mootien, S. and Bibb, M. (2009) The role of absC, a novel regulatory gene for secondary metabolism, in zinc‐dependent antibiotic production in Streptomyces coelicolor A3(2). Molecular Microbiology, 74, 1427–1444. [DOI] [PubMed] [Google Scholar]
- Hong, H.‐J. , Hutchings, M.I. , Hill, L.M. and Buttner, M.J. (2005) The role of the novel Fem protein VanK in vancomycin resistance in Streptomyces coelicolor . Journal of Biological Chemistry, 280, 13055–13061. [DOI] [PubMed] [Google Scholar]
- Hong, H.J. , Hutchings, M.I. , Neu, J.M. , Wright, G.D. , Paget, M.S.P. and Buttner, M.J. (2004) Characterization of an inducible vancomycin resistance system in Streptomyces coelicolor reveals a novel gene (vanK) required for drug resistance. Molecular Microbiology, 52, 1107–1121. [DOI] [PubMed] [Google Scholar]
- Hong, H.J. , Paget, M.S.P. and Buttner, M.J. (2002) A signal transduction system in Streptomyces coelicolor that activates the expression of a putative cell wall glycan operon in response to vancomycin and other cell wall‐specific antibiotics. Molecular Microbiology, 44, 1199–1211. [DOI] [PubMed] [Google Scholar]
- Hoskins, J. , Matsushima, P. , Mullen, D.L. , Tang, J. , Zhao, G. , Meier, T.I. et al. (1999) Gene disruption studies of penicillin‐binding proteins 1a, 1b, and 2a in Streptococcus pneumoniae . Journal of Bacteriology, 181, 6552–6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugonnet, J.E. , Haddache, N. , Veckerlé, C. , Dubost, L. , Marie, A. , Shikura, N. et al. (2014) Peptidoglycan cross‐linking in glycopeptide‐resistant Actinomycetales . Antimicrobial Agents and Chemotherapy, 58, 1749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings, M.I. , Hong, H.J. and Buttner, M.J. (2006a) The vancomycin resistance VanRS two‐component signal transduction system of Streptomyces coelicolor . Molecular Microbiology, 59, 923–935. [DOI] [PubMed] [Google Scholar]
- Hutchings, M.I. , Hong, H.‐J. , Leibovitz, E. , Sutcliffe, I.C. and Buttner, M.J. (2006b) The σE cell envelope stress response of Streptomyces coelicolor is influenced by a novel lipoprotein, CseA. Journal of Bacteriology, 188, 7222–7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings, M.I. , Hoskisson, P.A. , Chandra, G. and Buttner, M.J. (2004) Sensing and responding to diverse extracellular signals? Analysis of the sensor kinases and response regulators of Streptomyces coelicolor A3 (2). Microbiology, 150, 2795–2806. [DOI] [PubMed] [Google Scholar]
- Johansen, J. , Rasmussen, A.A. , Overgaard, M. and Valentin‐Hansen, P. (2006) Conserved small non‐coding RNAs that belong to the σE regulon: role in down‐regulation of outer membrane proteins. Journal of Molecular Biology, 364, 1–8. [DOI] [PubMed] [Google Scholar]
- Joly, N. , Engl, C. , Jovanovic, G. , Huvet, M. , Toni, T. , Sheng, X. et al. (2010) Managing membrane stress: the phage shock protein (Psp) response, from molecular mechanisms to physiology. FEMS Microbiology Reviews, 34, 797–827. [DOI] [PubMed] [Google Scholar]
- Jordan, S. , Hutchings, M.I. and Mascher, T. (2008) Cell envelope stress response in Gram‐positive bacteria. FEMS Microbiology Reviews, 32, 107–146. [DOI] [PubMed] [Google Scholar]
- Kahne, D. , Leimkuhler, C. , Lu, W. and Walsh, C. (2005) Glycopeptide and lipoglycopeptide antibiotics. Chemical Reviews, 105, 425–448. [DOI] [PubMed] [Google Scholar]
- Kang, J.‐G. , Hahn, M.‐Y. , Ishihama, A. and Roe, J.‐H. (1997) Identification of sigma factors for growth phase‐related promoter selectivity of RNA polymerases from Streptomyces coelicolor A3(2). Nucleic Acids Research, 25, 2566–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama, Y. , Zhang, H.‐Z. and Chambers, H.F. (2004) PBP 2a mutations producing very‐high‐level resistance to beta‐lactams. Antimicrobial Agents and Chemotherapy, 48, 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai, Y. , Marles‐Wright, J. , Cleverley, R.M. , Emmins, R. , Ishikawa, S. , Kuwano, M. et al. (2011) A widespread family of bacterial cell wall assembly proteins. EMBO Journal, 30, 4931–4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M.‐S. , Dufour, Y.S. , Yoo, J.S. , Cho, Y.‐B. , Park, J.‐H. , Nam, G.‐B. et al. (2012) Conservation of thiol‐oxidative stress responses regulated by SigR orthologues in actinomycetes. Molecular Microbiology, 85, 326–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston, A.W. , Liao, X. and Helmann, J.D. (2013) Contributions of the σW, σM and σX regulons to the lantibiotic resistome of Bacillus subtilis . Molecular Microbiology, 90, 502–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschnitz, E.M. , Heichlinger, A. , Schirner, K. , Winkler, J. , Latus, A. , Maldener, I. et al. (2011) Proteins encoded by the mre gene cluster in Streptomyces coelicolor A3(2) cooperate in spore wall synthesis. Molecular Microbiology, 79, 1367–1379. [DOI] [PubMed] [Google Scholar]
- Koteva, K. , Hong, H.‐J. , Wang, X.D. , Nazi, I. , Hughes, D. , Naldrett, M.J. et al. (2010) A vancomycin photoprobe identifies the His kinase VanSsc as a vancomycin receptor. Nature Chemical Biology, 6, 327–329. [DOI] [PubMed] [Google Scholar]
- Lavollay, M. , Arthur, M. , Fourgeaud, M. , Dubost, L. , Marie, A. , Veziris, N. et al. (2008) The peptidoglycan of stationary‐phase Mycobacterium tuberculosis predominantly contains cross‐links generated by L, D‐transpeptidation. Journal of Bacteriology, 190, 4360–4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerke, L.T. , Kies, P.J. , Müh, U. and Ellermeier, C.D. (2018) Bacterial sensing: a putative amphipathic helix in RsiV is the switch for activating σV in response to lysozyme. PLoS Genetics, 14, e1007527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, D. and Strynadka, N.C. (2002) Structural basis for the β lactam resistance of PBP2a from methicillin‐resistant Staphylococcus aureus . Nature Structural & Molecular Biology, 9, 870–876. [DOI] [PubMed] [Google Scholar]
- Liu, X. , Brutlag, D.L. and Liu, J.S. (2001) BioProspector: discovering conserved DNA motifs in upstream regulatory regions of co‐expressed genes. Pacific Symposium on Biocomputing, 2001, 127–138. [PubMed] [Google Scholar]
- Lonetto, M.A. , Brown, K.L. , Rudd, K.E. and Buttner, M.J. (1994) Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial RNA polymerase sigma factors involved in the regulation of extracytoplasmic functions. Proceedings of the National Academy of Sciences of the United States of America, 91, 7573–7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macheboeuf, P. , Contreras‐Martel, C. , Job, V. , Dideberg, O. and Dessen, A. (2006) Penicillin binding proteins: key players in bacterial cell cycle and drug resistance processes. FEMS Microbiology Reviews, 30, 673–691. [DOI] [PubMed] [Google Scholar]
- Magnet, S. , Bellais, S. , Dubost, L. , Fourgeaud, M. , Mainardi, J.‐L. , Petit‐Frère, S. et al. (2007) Identification of the L, D‐transpeptidases responsible for attachment of the Braun lipoprotein to Escherichia coli peptidoglycan. Journal of Bacteriology, 189, 3927–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher, T. (2013) Signaling diversity and evolution of extracytoplasmic function (ECF) σ factors. Current Opinion in Microbiology, 16, 148–155. [DOI] [PubMed] [Google Scholar]
- Mascher, T. , Hachmann, A.‐B. and Helmann, J.D. (2007) Regulatory overlap and functional redundancy among Bacillus subtilis extracytoplasmic function σ factors. Journal of Bacteriology, 189, 6919–6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher, T. , Margulis, N.G. , Wang, T. , Ye, R.W. and Helmann, J.D. (2003) Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon. Molecular Microbiology, 50, 1591–1604. [DOI] [PubMed] [Google Scholar]
- Mazza, P. , Noens, E.E. , Schirner, K. , Grantcharova, N. , Mommaas, A.M. , Koerten, H.K. et al. (2006) MreB of Streptomyces coelicolor is not essential for vegetative growth but is required for the integrity of aerial hyphae and spores. Molecular Microbiology, 60, 838–852. [DOI] [PubMed] [Google Scholar]
- Mercer, K.L. and Weiss, D.S. (2002) The Escherichia coli cell division protein FtsW is required to recruit its cognate transpeptidase, FtsI (PBP3), to the division site. Journal of Bacteriology, 184, 904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiakas, D. , Betton, J.M. and Raina, S. (1996) New components of protein folding in extracytoplasmic compartments of Escherichia coli SurA, FkpA and Skp/OmpH. Molecular Microbiology, 21, 871–884. [DOI] [PubMed] [Google Scholar]
- Mitrophanov, A.Y. and Groisman, E.A. (2008) Signal integration in bacterial two‐component regulatory systems. Genes & Development, 22, 2601–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch, R. , Hiller, K. , Grote, A. , Scheer, M. , Klein, J. , Schobert, M. et al. (2005) Virtual Footprint and PRODORIC: an integrative framework for regulon prediction in prokaryotes. Bioinformatics, 21, 4187–4189. [DOI] [PubMed] [Google Scholar]
- Nishi, H. , Komatsuzawa, H. , Fujiwara, T. , McCallum, N. and Sugai, M. (2004) Reduced content of lysyl‐phosphatidylglycerol in the cytoplasmic membrane affects susceptibility to moenomycin, as well as vancomycin, gentamicin, and antimicrobial peptides, in Staphylococcus aureus . Antimicrobial Agents and Chemotherapy, 48, 4800–4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Over, B. , Heusser, R. , McCallum, N. , Schulthess, B. , Kupferschmied, P. , Gaiani, J.M. et al. (2011) LytR‐CpsA‐Psr proteins in Staphylococcus aureus display partial functional redundancy and the deletion of all three severely impairs septum placement and cell separation. FEMS Microbiol Letts, 320, 142–151. [DOI] [PubMed] [Google Scholar]
- Paget, M.S. , Chamberlin, L. , Atrih, A. , Foster, S.J. and Buttner, M.J. (1999a) Evidence that the extracytoplasmic function sigma factor σE is required for normal cell wall structure in Streptomyces coelicolor A3(2). Journal of Bacteriology, 181, 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget, M.S. , Leibovitz, E. and Buttner, M.J. (1999b) A putative two‐component signal transduction system regulates σE, a sigma factor required for normal cell wall integrity in Streptomyces coelicolor A3(2). Molecular Microbiology, 33, 97–107. [DOI] [PubMed] [Google Scholar]
- Paget, M.S.B. , Molle, V. , Cohen, G. , Aharonowitz, Y. and Buttner, M.J. (2001) Defining the disulphide stress response in Streptomyces coelicolor A3(2): identification of the σR regulon. Molecular Microbiology, 42, 1007–1020. [DOI] [PubMed] [Google Scholar]
- Qiu, J. and Helmann, J.D. (2001) The ‐10 region is a key promoter specificity determinant for the Bacillus subtilis extracytoplasmic‐function σ factors σX and σW . Journal of Bacteriology, 183, 1921–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2012). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. ISBN 3‐900051‐07‐0. [Google Scholar]
- Raivio, T.L. (2005) MicroReview: envelope stress responses and Gram‐negative bacterial pathogenesis. Molecular Microbiology, 56, 1119–1128. [DOI] [PubMed] [Google Scholar]
- Rhodius, V.A. , Suh, W.C. , Nonaka, G. , West, J. and Gross, C.A. (2005) Conserved and variable functions of the σE stress response in related genomes. PLoS Biology, 4, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, J. , Bischoff, M. , Wada, A. and Berger‐Bächi, B. (2003) MsrR, a putative cell envelope‐associated element involved in Staphylococcus aureus sarA attenuation. Antimicrobial Agents and Chemotherapy, 47, 2558–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouviere, P. , De Las Penas, A. , Mecsas, J. , Lu, C.Z. , Rudd, K. and Gross, C. (1995) rpoE, the gene encoding the second heat‐shock sigma factor, σE, in Escherichia coli . EMBO Journal, 14, 1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz, N. and Silhavy, T.J. (2005) Sensing external stress: watchdogs of the Escherichia coli cell envelope. Current Opinion in Microbiology, 8, 122–126. [DOI] [PubMed] [Google Scholar]
- Ruzin, A. , Severin, A. , Moghazeh, S.L. , Etienne, J. , Bradford, P.A. , Projan, S.J. et al. (2003) Inactivation of mprF affects vancomycin susceptibility in Staphylococcus aureus . Biochimica et Biophysica Acta, 1621, 117–121. [DOI] [PubMed] [Google Scholar]
- Sauvage, E. , Kerff, F. , Fonze, E. , Herman, R. , Schoot, B. , Marquette, J.‐P. et al. (2002) The 2.4‐Å crystal structure of the penicillin‐resistant penicillin‐binding protein PBP5fm from Enterococcus faecium in complex with benzylpenicillin. Cellular and Molecular Life Sciences, 59, 1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage, E. , Kerff, F. , Terrak, M. , Ayala, J.A. and Charlier, P. (2008) The penicillin‐binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiology Reviews, 32, 234–258. [DOI] [PubMed] [Google Scholar]
- Silhavy, T.J. , Kahne, D. and Walker, S. (2010) The bacterial cell envelope. Cold Spring Harbor Perspectives in Biology, 2, a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sineva, E. , Savkina, M. and Ades, S.E. (2017) Themes and variations in gene regulation by extracytoplasmic function (ECF) sigma factors. Current Opinion in Microbiology, 36, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth, G.K. (2005) Limma: linear models for microarray data In: Gentleman R., Carey V.J., Huber W., Irizarry R.A., Dudoit S. (Eds.) Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Springer, New York: pp. 397–420. [Google Scholar]
- Soliveri, J. , Brown, K. , Buttner, M.J. and Chater, K.F. (1992) Two promoters for the whiB sporulation gene of Streptomyces coelicolor A3(2) and their activities in relation to development. Journal of Bacteriology, 174, 6215–6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakova, E. , Zikova, A. and Vohradsky, J. (2014) Inference of sigma factor controlled networks by using numerical modeling applied to microarray time series data of the germinating prokaryote. Nucleic Acids Research, 42, 748–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teytelman, L. , Özaydın, B. , Zill, O. , Lefrançois, P. , Snyder, M. , Rine, J. et al. (2009) Impact of chromatin structures on DNA processing for genomic analyses. PLoS ONE, 4, e6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray, P.D. and Moir, A. (2003) SigM, an extracytoplasmic function sigma factor of Bacillus subtilis, is activated in response to cell wall antibiotics, ethanol, heat, acid, and superoxide stress. Journal of Bacteriology, 185, 3491–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, K.M. , Rhodius, V.A. and Gottesman, S. (2007) σE regulates and is regulated by a small RNA in Escherichia coli . Journal of Bacteriology, 189, 4243–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, B.J. , Widdick, D.A. , Hicks, M.G. , Chandra, G. , Sutcliffe, I.C. , Palmer, T. et al. (2010) Investigating lipoprotein biogenesis and function in the model Gram‐positive bacterium Streptomyces coelicolor . Molecular Microbiology, 77, 943–956. [DOI] [PubMed] [Google Scholar]
- Udekwu, K.I. and Wagner, E.G.H. (2007) σE controls biogenesis of the antisense RNA MicA. Nucleic Acids Research, 35, 1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich, L.E. and Zhulin, I.B. (2010) The MiST2 database: a comprehensive genomics resource on microbial signal transduction. Nucleic Acids Research, 38, D401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrancken, K. , Van Mellaert, L. and Anné, J. (2008) Characterization of the Streptomyces lividans PspA response. Journal of Bacteriology, 190, 3475–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegert, T. , Homuth, G. , Versteeg, S. and Schumann, W. (2001) Alkaline shock induces the Bacillus subtilis σW regulon. Molecular Microbiology, 41, 59–71. [DOI] [PubMed] [Google Scholar]
- Wolf, D. , Kalamorz, F. , Wecke, T. , Juszczak, A. , Mäder, U. , Homuth, G. et al. (2010) In‐depth profiling of the LiaR response of Bacillus subtilis . Journal of Bacteriology, 192, 4680–4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yem, D.W. and Wu, H.C. (1978) Physiological characterization of an Escherichia coli mutant altered in the structure of murein lipoprotein. Journal of Bacteriology, 133, 1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


