Abstract
We analysed patterns in the incidence of cervical intraepithelial neoplasia grades 2 and 3 (CIN2, CIN3) and adenocarcinoma in situ (AIS) by age and histology in 1992–2016 in Norway and described changes in screening tests. Incident cases of CIN2, CIN3, AIS and cervical cancer were identified in the Cancer Registry of Norway, as were all women with at least one screening test. The annual percentage change statistic was used to assess point estimates and changes in age‐specific and age‐standardised incidence rates (IR). Women aged 25–29 years had the highest incidence of cervical precancerous lesions (CIN2: 192.9/10, CIN3: 737.2/10, AIS: 32.5/105 in 2016). The IR of CIN2 increased for all screening ages (25–69 years) from 3.6% to 6.7% per year. CIN3 incidence increased by 1.6% (95% confidence interval [CI] 0.6–2.6) annually. A steep increase in AIS incidence was observed in all age groups (7.1% per year, 95% CI 5.3–8.8). Changes in screening tests and the histological verification of cervical precancerous lesions alone cannot explain the steady increase in incidence we observed over the 25‐year study period, and increased exposure to human papillomavirus (HPV) likely plays a role. Age‐appropriate treatment of screening‐detected cervical precancerous lesions is needed for effective cervical cancer control while avoiding overtreatment and related health risks. In order to perform an appropriate harm‐benefit evaluation of cervical cancer control efforts, detailed information on screening technology and background risks, including HPV vaccination status, is needed to create optimal public health policy.
Keywords: cervical precancerous lesions, trend analysis, cervical cancer screening
Short abstract
What's new?
In Norway, cervical cancer screening coverage has held steady around 65–68%, while incidence of cervical precancerous lesions has increased. Here, the authors analysed changes in incidence of cervical intraepithelial neoplasias grades 2 and 3 (CIN2 and 3) and adenocarcinoma in situ (AIS). They observed period effects of the same magnitude for all three, indicating that changes in the screening test accounts for part of the increase. Another likely cause, they report, is increased exposure to HPV. Public health policy, they conclude, should consider detailed information on screening technology and background risks, including HPV vaccination status.
Abbreviations
- AC
adenocarcinoma
- AIS
adenocarcinoma in situ
- APC
annual percentage change
- CIN2
cervical intraepithelial neoplasia grade 2
- CIN3
cervical intraepithelial neoplasia grade 3
- HPV
human papillomavirus
- hrHPV
high‐risk human papillomavirus
- LBC
liquid‐based cytology
- NCCSP
Norwegian Cervical Cancer Screening Program
- SCC
squamous cell carcinoma
Introduction
Cervical cancer is the 4th most common cancer among women globally,1 and prevention through screening is a major public health commitment for many countries.2 Cervical cancer screening detects precancerous lesions, and with treatment, cancer can be avoided. When screening coverage is high in a population, decreasing cervical cancer incidence and mortality rates are generally observed.3, 4 In 2016, the age‐standardised incidence rate (IR, World standard) for cervical cancer in Norway was 10.3/105; it was the 11th most common cancer among women overall and the 3rd most common among women aged 25–49 years.5 In 1992–1994, the Norwegian Cervical Cancer Screening Program (NCCSP) was based on opportunistic screening. At that time, screening coverage was 65.2%. This rate increased by 8.4% in the first years after the organised NCCSP was implemented,6 but it has not changed substantially since then, and in 2006–2015, the 3.5‐year screening coverage in Norway was 67.7%.7, 8, 9
Cervical squamous cell tumours and glandular tumours progress through precancerous phases after persistent infection with high‐risk human papillomavirus (HPV) types.10 Cervical intraepithelial neoplasia grades 2 and 3 (CIN2, CIN3) precede squamous cell carcinoma, and adenocarcinoma in situ (AIS) precedes adenocarcinoma.10 Eighty‐six percent, 93%, and 89% of women with CIN2, CIN3 and cervical cancer, respectively, are HPV‐positive, and HPV16 and 18 are the most prevalent HPV types in cervical cancer.11 In some screening programmes, HPV testing is about to replace cytology, which has been used for decades with great success. Indeed, in the Nordic countries, cytology‐based screening programmes have prevented at least 50% of expected cervical cancers.12
However, in 2006 more than 3,000 women in Norway were treated for cervical precancerous lesions, with the highest burden observed among those aged 20–29 years – a decade before the observed peak of cancer incidence at age 30–39 years.13 Precancerous lesions are asymptomatic, and their diagnosis is dependent on screening intensity and quality; but changes in background risk, i.e. exposure to HPV infection, also impact this burden. The observed recent increase in cervical adenocarcinomas and other HPV‐related cancers in Norway is believed to be related to an increase in HPV infection.14 Knowledge of the epidemiology of cervical precancerous lesions over time in countries with relatively stable screening attendance can be extremely useful in evaluating cervical cancer prevention efforts. Furthermore, in the near future, we expect to see the impact of HPV vaccination on cervical precancerous lesions, with modelling studies suggesting that there will be a dramatic reduction in such lesions.15
During the last years, the NCCSP Annual Reports7, 8, 9 have noted an increase in the incidence of cervical precancerous lesions. It is important to quantify this increase and reveal possible underlying mechanisms. In this study, we analysed patterns in the incidence of CIN2, CIN3 and AIS by age and histology during the period 1992–2016 in Norway and describe changes in screening tests.
Methods
We obtained information on cervical precancerous lesions and invasive cervical carcinomas diagnosed in 1992–2016 from four different registries or databases: the Norwegian Cytology Registry, Histology Registry, CIN Registry and the main incidence database of the Cancer Registry of Norway (CRN).16 Since 1953, the CRN has registered all new cases of cancer and high‐grade cervical precancerous lesions. Registration of CIN2 started in 1997 when the treatment database was established. The CRN receives information on cancer cases from hospitals, pathology laboratories, and clinicians; and it receives information on the population from the National Registry. Reporting is compulsory by law, which has ensured the high quality of CRN data.17 The registries/databases above were linked using the unique national identification number assigned to each resident in Norway at birth or immigration.
In 1995, the NCCSP became an organised program and began to send out reminders to attend screening to all women aged 25–69 years with no screening test within the recommended interval of 3 years.16 Initially, the NCCSP conducted screening based on conventional cytology, but since 2006, liquid‐based cytology (LBC) has been gradually replacing conventional cytology. The most common medium used for LBC is PreservCyt (Hologic, Inc, Marlborough, MA), while a few laboratories are equipped to process SurePath (Becton, Dickinson and Company, Franklin Lakes, NJ). Since 2005, HPV testing has also been used to follow up women with low‐grade cervical lesions, and it is mandatory to report all test results to the HPV register. From 2015, HPV testing started to gradually replace LBC as a primary screening test for women aged 34–69.16
Information on diagnosis code, region, and date of diagnosis was extracted from the CRN for all histologically confirmed cases of cervical precancerous lesions and cancer registered between 1 January 1992 and 31 December 2016 with topography code C53, according to the International Classification of Diseases for Oncology—3rd edition (ICD‐O‐3).18
According to the WHO Classification of Tumours of the Female Reproductive Organs19 and the ICD‐O‐3,18 cases of cervical precancerous lesions were classified into two mutually exclusive groups: i) CIN2/3 (8,077/2) and ii) AIS (8,140/2). The CIN2/3 group was further separated into CIN2 and CIN3 according to the Norwegian modification of the SNOMED 2002 classification.20 Different morphological subtypes of cervical cancer were combined into one diagnostic category.21
Statistical analysis
Incident cervical precancerous lesions were defined as a new diagnosis of CIN2, CIN3, or AIS in a woman with no history of histologically confirmed cervical abnormalities for the past 2 calendar years prior to the index diagnosis. If a woman had multiple new diagnoses within 2 years, she contributed only once with the most severe diagnosis,13 i.e. women with multiple new diagnosis are counted multiple times only when the time between lesions exceeded 2 years. Women with cervical cancer before 1992 were excluded. For the trend analysis, incident cases and female population numbers were categorised into nine age groups (0–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–59, 60–69, ≥70 years). Due to CIN2 registration start in 1997, we started to assess CIN2 incidence trends from 2002 onwards, assuming that 6 year is sufficient time to achieve registration completeness. To detect possible regional differences, all data extractions were repeated by region: Southeast, West, Middle and North Norway.
We used the Norwegian Cytology Registry and the National Registry to obtain the total population and the screened population during the study period. Women were included in the screened population if they had at least one cytology result recorded in the last 3.5 years. Number and type of cervical screening tests over the study period were extracted from the Norwegian Cytology Registry.
We calculated age‐specific IRs by diagnosis for the total and screened population. The age‐standardised IR for Norway as a whole and for each of the four regions was calculated using the World standard population.22
We assessed changes in IRs by a log‐linear model with period as a continuous variable. To distinguish between period and cohort effects, an age‐period‐cohort model was estimated.23 Age‐period‐cohort models estimate the effects of age, period and cohort by introducing some kind of constraint to the model, often related to the so‐called linear drift term. We used restricted cubic spline functions to model the three covariates. When making plots of incidence rate ratios as functions of time and birth cohorts, all of the linear drift was allocated to each of the effects, i.e. all the drift was assumed to be in the period effect when plotting incidence rate ratios for time, and vice‐versa for the cohort effects. This was achieved by using different parameterizations for the APC‐model, one where the effect of cohort was constrained to be zero on average on the log scale with zero slope and one where the effect of period was constrained to be zero on average on the log scale with zero slope. The APC‐model was compared to simpler age‐period and age‐cohort models by comparing AIC and BIC. For period effects, the reference diagnosis year was set to 2004, i.e., the middle point of our study period. For cohort effects, women born in 1970 were used as a reference cohort. There were several reasons why this birth cohort was chosen as a reference. Firstly, the sharpest decline in total fertility rates in Norway took place in the 1970s. Parity stabilised before the end of the 1970s and remained at a relatively low level for the next decade. In addition, time trends of smoking remained unchanged in Norway from 1970 to the end of the 1990s. Moreover, women born in 1970 reached screening target ages in 1995, when the programme officially set its screening start age at 25 years.
Results
We observed a total of 12,455 CIN2, 69,207 CIN3, 2,234 AIS and 7,859 cancer cases from 1992 to 2016. In 2016, we observed the highest CIN3/AIS incidence among women aged 26 years (IR 1140.4/105) (Fig. 1). CIN3/AIS incidence decreased with increasing age, but it was always higher than that of CIN2. CIN2 reached its peak in the same age group as CIN3/AIS, but with smaller magnitude (IR 258.7/105). The age‐specific IR of cervical cancer fluctuated from 2.8 to 43.3/10,5 and the peak occurred at 38 years of age. When we repeated the analysis in the screened population, the incidence pattern was similar for CIN3/AIS and cancer, while the IR of CIN2 was highest in women aged 22 years.
Figure 1.
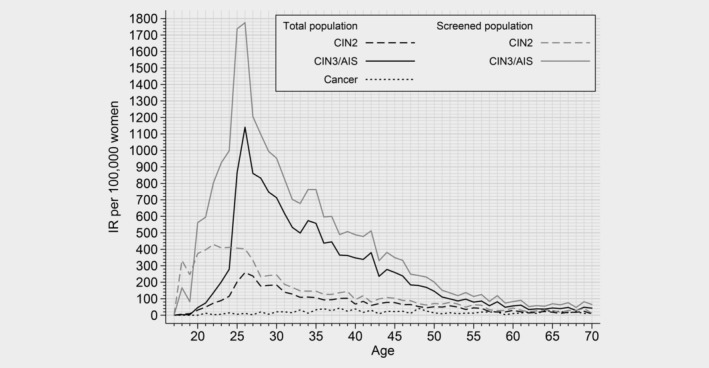
Cervical intraepithelial neoplasia grade 2 (CIN2), Cervical intraepithelial neoplasia grade 3/adenocarcinoma in situ (CIN3/AIS) and cervical cancer age‐specific incidence rate per 100,000 women and per 100,000 screened women by age in Norway in 2016.
Each year, the age‐standardised IR of CIN2 increased by 4.7% (95% CI 2.7; 6.8), that of CIN3 increased by 1.6% (95% CI 0.6; 2.6), and that of AIS increased by 7.1% (95% CI 5.3; 8.8) (Table 1). The corresponding age‐standardised incidence of CIN2 in 2002 to 2016 increased from 29.5 to 50.1/10.5 In 1992 to 2016, the age‐standardised IR of CIN3 increased from 61.2 to 171.0/10,5 and that of AIS increased from 0.9 to 8.7/105 (Supporting Information Fig. 1).
Table 1.
Annual percentage change (APC) from 1992 to 2016 with 95% confidence intervals (95% CI) and p‐values by age groups and regions in Norway
| CIN21 | CIN3 | AIS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2002–2016 | 1992–2016 | 1992–2016 | |||||||
| APC | 95% CI | p‐value | APC | 95% CI | p‐value | APC | 95% CI | p‐value | |
| Total Norway | 4.7 | 2.7; 6.8 | <0.00 | 1.6 | 0.6; 2.6 | <0.00 | 7.1 | 5.3; 8.8 | <0.00 |
| Age group | |||||||||
| −24 | 0.5 | −2.3; 3.2 | 0.5 | −1.1 | −2.4; 0.2 | 0.1 | 9.9 | 6.4;13.5 | <0.00 |
| 25–29 | 6.7 | 4.3; 9.1 | <0.00 | 3.3 | 2.0; 4.7 | <0.00 | 11.0 | 8.5; 13.5 | <0.00 |
| 30–34 | 5.0 | 2.2; 7.9 | <0.00 | 1.4 | 0.4; 2.3 | <0.00 | 5.0 | 2.3; 7.8 | <0.00 |
| 35–39 | 5.0 | 2.5; 7.5 | <0.00 | 1.2 | 0.4; 2.0 | <0.00 | 5.7 | 3.7; 7.7 | <0.00 |
| 40–44 | 5.3 | 3.9; 6.7 | <0.00 | 1.4 | 0.5; 2.3 | <0.00 | 8.0 | 6.1; 9.9 | <0.00 |
| 45–49 | 5.6 | 3.7; 7.6 | <0.00 | 1.3 | 0.5; 2.2 | <0.00 | 5.5 | 2.7; 8.4 | <0.00 |
| 50–59 | 5.3 | 3.5; 7.2 | <0.00 | −0.0 | −1.1; 1.1 | 1.00 | 1.1 | −1.2; 3.4 | 0.4 |
| 60–69 | 3.6 | 1.6; 5.7 | <0.00 | −0.6 | −1.8; 0.7 | 0.4 | 3.6 | 0.9; 6.3 | <0.00 |
| 70+ | −0.1 | −4.4; 4.3 | 0.9 | −1.4 | −2.5; −0.4 | <0.00 | 7.9 | 3.6; 12.2 | <0.00 |
| Region | |||||||||
| Soul h‐East | 3.2 | 1.4; 4.9 | <0.00 | 1.4 | 0.4; 2.5 | <0.00 | 6.8 | 4.7; 8.8 | <0.00 |
| West | 4.2 | 1.5; 6.9 | <0.00 | 2.4 | 1.4; 3.4 | <0.00 | 9.1 | 6.6; 11.5 | <0.00 |
| Middle | 2.7 | −0.2; 5.6 | 0.1 | 1.4 | 0.3; 2.6 | <0.00 | 3.7 | 1.4; 6.0 | <0.00 |
| North | 10.5 | 7.8; 13.2 | <0.00 | 0.7 | −0.5; 2.0 | 0.2 | 5.8 | 3.2; 8.6 | <0.00 |
CIN2 ‐ cervical intraepithelial neoplasia grade 2.
CIN3 ‐ cervical intraepithelial neoplasia grade 3.
AIS ‐ adenocarcinoma in situ.
Due to registration start in 1997, CIN2 incidence trend will be assesed from 2002 onwards.
The highest incidence of CIN2, CIN3 and AIS was observed in the age group 25–29 years (Fig. 2). During the study period, CIN2 incidence showed a continually significant, increasing trend in all screening age groups (25–69 years) while AIS incidence increased significantly up to 45–49 years and from 60 to 69 to 70+ years (Table 1). The incidence of CIN3 increased to a lower extent than that of AIS and CIN2, with the sharpest increase in the age group 25–29 years (APC: 3.3; 95% CI 2.0; 4.7). We observed a decrease in the incidence of cervical cancer in the three oldest age groups (≥50 years) (Fig. 2). Otherwise, cancer incidence remained stable across age groups over time. When we looked at regional incidence, the age‐standardised IR of AIS increased significantly in all Norwegian regions (Table 1, Supporting Information Fig. 1). In general, incidence patterns were rather similar throughout the four regions except for CIN2, which showed higher IRs in the North of Norway compared to the country as a whole (Supporting Information Fig. 1).
Figure 2.
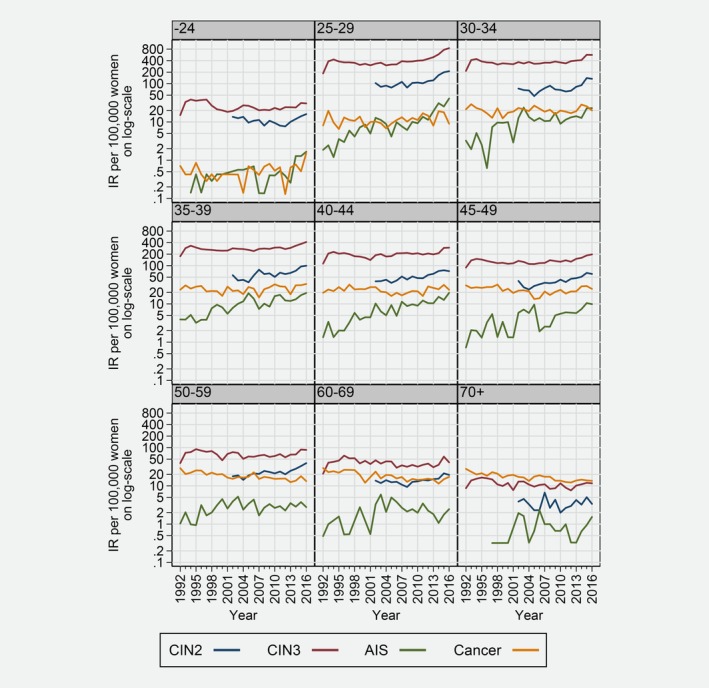
Cervical intraepithelial neoplasia grade 2 (CIN2), cervical intraepithelial neoplasia grade 3 (CIN3), and adenocarcinoma in situ (AIS) incidence rate per 100,000 on the log scale by nine age groups from 1992 to 2016.
Using AIC/BIC criteria to compare the age‐period‐cohort model with both an age‐period model, and an age‐cohort model, it is confirmed that the APC‐model is a better fit to the data. The age‐period‐cohort model detected both cohort and period effects for all cervical precancerous lesions (Fig. 3). Comparing women screened in 2016 had a twofold higher incidence rate ratio of receiving a diagnosis of cervical precancerous lesions than women screened in 2004. Women born in 1996 had a 15‐fold higher incidence rate ratio for AIS, a fivefold higher incidence rate ratio for CIN2, and twofold higher incidence rate ratio for CIN3 than women born in 1970.
Figure 3.
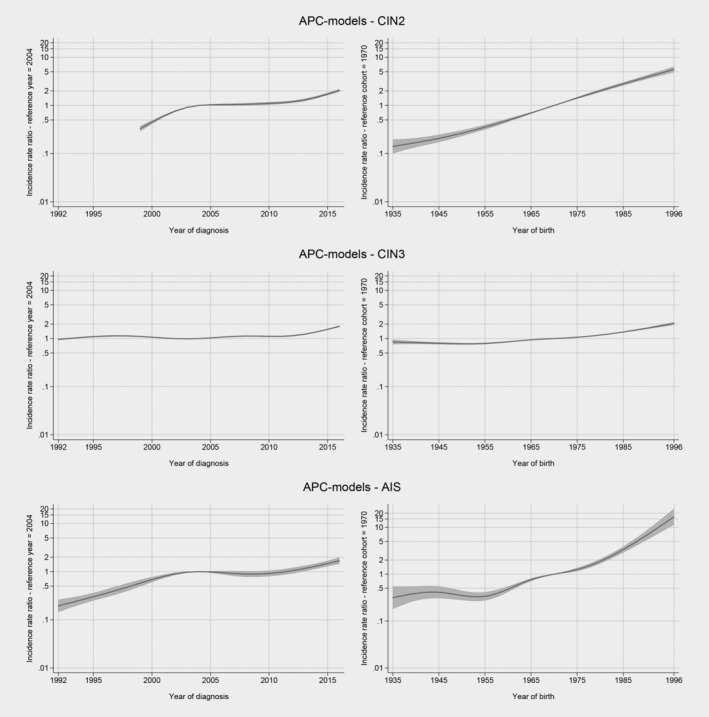
Period and cohort analysis for cervical intraepithelial neoplasia grade 2 (CIN2), cervical intraepithelial neoplasia grade 3 (CIN3), and adenocarcinoma in situ (AIS).
Until 2004, only a few labs used LBC, and conventional Pap smear was the prevailing screening test in Norway. Since 2006, the proportion of screening performed with LBC has increased remarkably, escalating from 2010, (Fig. 4). In 2016, 86.5% of all the screening tests were LBC. The absolute number of screening tests taken every year has been gradually decreasing while the population size has been stable.
Figure 4.
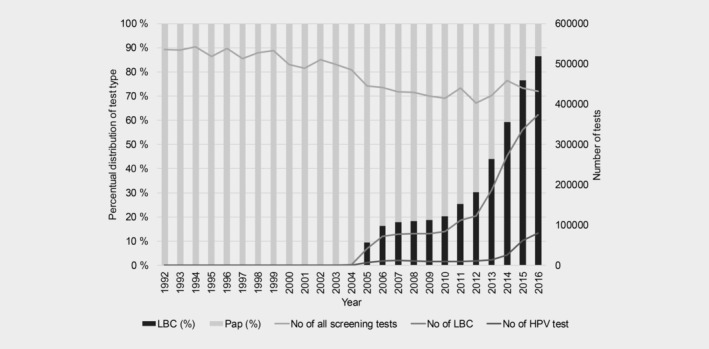
Numbers and proportions of different screening test used from 1992 to 2016.
Discussion
This study is the first to report changes in the incidence of cervical precancerous lesions since the start of the NCCSP in 1992. We observed an increasing trend in the incidence of all precancerous lesions in most age groups. The increase in CIN2 and AIS was particularly profound in women aged 50 years or younger. Cancer incidence was stable, except in the three oldest age groups, in which incidence decreased. It is noteworthy that the after some increase in early years,6 overall screening coverage has remained stable, while the number of screening tests administered every year has been decreasing, highlighting the effectiveness of the NCCSP.9
The incidence of precancerous lesions increased especially in women aged <50 years. With the year 2004 as a reference, period effects of equivalent magnitude for CIN2, CIN3 and AIS were observed, indicating that recent changes in the NCCSP might partially explain this increase (Fig. 4). Indeed, LBC has gradually replaced conventional cytology as a primary screening test, and in 2016 close to 90% of screening tests were LBC (Fig. 4). LBC has the potential to be more sensitive than conventional cytology, although a few studies have claimed only minor differences in sensitivity between these tests.24, 25 In addition, the HPV test, which was introduced in 2005 for triage of women with low‐grade cytological abnormalities (atypical cells of undetermined significance or low‐grade squamous intraepithelial lesions), has improved the sensitivity of the NCCSP and detection of precancerous lesions.26 Increasing patterns of cervical precancerous lesions, similar to those we observed in Norway, have also been described in nationwide studies from Denmark27 and the Netherlands.28 The observed changes were attributed mostly to the increased use of LBC. However, these countries also started to use automation‐assisted LBC reading, while in Norway all cytology tests are manually read and interpreted. In addition, direct comparisons are difficult due to differences in national clinical management algorithms, LBC platforms and HPV tests used. Our study lacks information on changes in test collection methods and laboratory routines, which may also contribute to the changes in incidence. Still, if there have been changes in test collection or laboratory routines, they were not limited to a specific region of Norway (Supporting Information Fig. 1). In conclusion, described changes in technologies used in the NCCSP clearly affect its overall performance and partly explain the observed increase in the incidence of precancerous cervical lesions, but there may also be other causes that influence this incidence.
The age‐period‐cohort model has the advantage of separating the nonlinear period and cohort effects. The period effect showed a continuous increase in the incidence of AIS and CIN2 among women born after 1970. Women born in 1996 had a 15‐fold higher incidence rate ratio for AIS and fivefold higher incidence rate ratio of CIN2 as compared to those born in 1970. CIN3, the most common precancerous lesion, was twice as common in the 1996 cohort. Of note, the vast majority of these women have not been vaccinated against HPV, as the catch‐up HPV vaccination programme started in Norway in late 2016. The observed increase was prominent in younger age groups and may be attributed to the increased background risks for HPV infection. Age at first sexual intercourse has decreased, while number of lifetime sexual partners has increased, leading to higher exposure to sexually transmitted infections, including HPV.29, 30, 31 The incidence of chlamydia, the second most common sexually transmitted disease in Norway, has also been increasing, particularly among women and men aged younger than 24 years.32 In older age groups, the incidence of chlamydia has been stable, corresponding well to our finding of an age‐specific increase in the incidence of cervical precancerous lesions during the same time period.32
The relatively high proportion of cervical precancerous lesions can be attributed to infection with oncogenic HPV types.11 HPV is the most common sexually transmitted virus in the world, and most sexually active people become infected during their lifetime. Worldwide, IRs of HPV‐related cancers (except squamous cell cervical cancer, which is preventable by screening) are increasing,33 which allows us to attribute some of the observed increasing trends of cervical precancerous lesions to HPV infection. Taking into account the timing of changes in the NCCSP and changes in sexual behaviour, we suggest that the steady increase in the incidence of cervical precancerous lesions over the study period is likely explained by the cohort effect, while the period effect mainly contributes to the observed increase during the latest study period.
The age‐specific IR of cervical precancerous lesions for the total and screened populations revealed that CIN2 was most common among women who were below 25 years of age, i.e., women who were not invited to attend screening by the NCCSP, but attended screening opportunistically. Close to 100,000 women under 25 years of age attended screening in 2014–2016 (data not shown), suggesting that screening in these ages was not restricted to high‐risk groups.
The incidence of CIN3/AIS peaked at the age of 25–26 years (Fig. 1), reflecting the impact of the first screening round, which accounts for both incident and prevalent cases in the population. Obviously, the epidemiology of precancerous lesion is dependent on screening activity in a given population. Interestingly, we observed that CIN2 was more prevalent in younger ages compared to CIN3, in screened population. This observation is in line with natural history studies, which indicated that CIN2 is more likely to regress and is, therefore, more common at younger ages.34 While persistent HPV infection initiates the natural history of cervical cancer, and CIN3 is considered a real precancerous lesion, diagnosis of CIN2 is poorly reproducible. Furthermore, CIN2 is considered a biologically heterogeneous, borderline lesion between acute HPV infection and CIN3.35 Hence, our results support the consensus that regular screening at younger ages can be less effective and should be avoided,36 as it has been shown to lack additional benefit in preventing cervical cancer.37, 38 Moreover, resembling the age‐specific pattern of HPV infection observed worldwide,39 IRs of all precancerous lesions dropped remarkably after the age of 26 years and remained relatively stable after the age of 55 years (Fig. 2). Because exposure to new HPV infections is low at this age, our data suggest that changing to HPV‐based screening might improve detection of women with higher risk for precancerous lesions and consequently justify the extension of the screening interval without impacting the overall effectiveness of the NCCSP.40
In Norway, as in many countries, clinical guidelines support the treatment of CIN2 or worse.16 Management does not differentiate between CIN2 and CIN3, although the probability for regression, especially among young women, is remarkable for CIN2.41 Ideally, the treatment of young women should be balanced against the possible risks, such as preterm delivery.42 Based on Norwegian data, Bjørge et al. found that the risk of preterm birth was 1.8 times higher in women who gave birth after cervical conisation compared to women without the treatment.43 A study done in Denmark found that women who underwent conisation had more contact with their general practitioner and hospitals after cervical conisation than did women without the treatment.44 Although an assessment of the treatment of cervical precancerous lesions is beyond the scope of this study, we determined that estimated number of 3,000 treatments per year13 needs to be studied and, if necessary, re‐evaluated. Considering that in 2016, 6,242 women received a diagnosis of CIN2 or CIN3/AIS, this previously suggested number of treatments is a strong underestimation.
In the light of the continuing increase in the incidence of cervical precancerous lesions and the need for treatment, the question of availability of physicians and treatment facilities arises. In combination with an escalating background risk, the epidemic of cervical precancerous lesions in Norway is viable. Countries like Australia45 and the US46 are already benefiting from mass HPV vaccination, which they started a decade ago. In Norway, HPV vaccination started in 2009, and until 2016, only one birth cohort of girls per year was immunised. In 2016, at least one dose of HPV vaccine was administered in the school‐based programme (girls born in 2004) and catch‐up programme, with coverages of 89%47 and 38%,48 respectively. The first school‐based HPV vaccinated cohort will be of screening age in 2022. The optimal screening routine for HPV‐vaccinated women has yet to be decided. However, the impact of the HPV vaccine on asymptomatic precancerous lesions cannot be measured if the age of screening is postponed, as has been suggested by recent cost‐effectiveness modelling studies.15
Our results are to some extent in line with those of other European studies reporting trends in cervical precancerous lesions. In Denmark from 1997 to 2004, the incidence of CIN3 and AIS increased significantly (1.4% and 6.1%, respectively), followed by stable incidence in 2009–2012.27 In Ireland, incidence of CIN3 increased by 3.8% (95% CI: 2.9–4.7) annually and cancer incidence increased by 1.3% (95% CI: −0.1‐2.7) from 1994 to 2008.49 Contrary to Danish results, the Irish study described a pronounced increase in the incidence of CIN3 among women younger than 35 years.49 Nevertheless, the magnitude of reported APCs in other European studies was remarkably lower than that we observed in Norway. Still, the direct comparison of trends between studies is limited due to differences in study periods, data quality, analytical approaches and screening practices. For instance, whether simultaneous cervical AIS and endometrial cancer constitute one or two incident cases may have consequences when evaluating incidence patterns across countries.50 There is also a small number of studies from the US that investigated trends of precancerous cervical lesions. However, these studies showed a substantial decrease in incidence,46, 51, 52 which was explained by younger screening age and higher HPV vaccination coverage, and they are therefore incomparable with our results.
One of the main strengths of this study is the use of high‐quality data from the national, population‐based CRN. The CRN registers all cancer and precancerous cases in Norway with essential information, including morphology and histology codes. This allowed us to group precancerous lesions according to approved, recognised guidelines. Usage of the national identification number ensured that the number of incident cases concurred with the approved case definition. However, some limitations need to be considered. First, we lack information on changes in laboratory and diagnostic methods over time. Although the procedures are stated in the NCCSP quality manual, we have information that they have changed and/or may vary by laboratory. Second, cervical precancerous lesions are strongly related to HPV infection; however, we do not know the HPV status of women with cervical lesions. Comparison of HPV prevalence over the periods would have given us stronger evidence to support our hypothesis about the increase of background risk in the population. Information about changes in sexual behaviour in these birth cohorts would also have been very valuable. In the future, information on HPV vaccination status will be crucial for developing the best screening strategy. Finally, we did not have information on CIN2 incidence before 1997.
In conclusion, we observed a strong, increasing trend in the incidence of cervical precancerous lesions from 1992 to 2016 in Norway. We consider that the combination of changes in screening technologies, particularly the widespread use of LBC since 2010, and the gradually increasing background risk have caused the current disease burden. It is highly likely that without screening, the cancer rates would have been much higher, and we expect to observe a further decline in cervical cancer incidence within the next 10 years.
Supporting information
Supplementary Figure S1
Conflict of interest: The authors declare they have no competing interests.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 2. Basu P, Ponti A, Anttila A, et al. Status of implementation and organization of cancer screening in the European Union member states‐summary results from the second European screening report. Int J Cancer 2018;142:44–56. [DOI] [PubMed] [Google Scholar]
- 3. IARC . European guidelines for quality assurance in cervical cancer screening, Second edn., Luxembourg: Office for Official Publications of the European Communities, 2008. [Google Scholar]
- 4. Landy R, Pesola F, Castanon A, et al. Impact of cervical screening on cervical cancer mortality: estimation using stage‐specific results from a nested case‐control study. Br J Cancer 2016;115:1140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer in Norway 2016 ‐ Cancer incidence, mortality, survival and prevalence in Norway. The Cancer Registry of Norway, 2017.
- 6. Nygard JF, Skare GB, Thoresen SO. The cervical cancer screening programme in Norway, 1992‐2000: changes in pap smear coverage and incidence of cervical cancer. J Med Screen 2002;9:86–91. [DOI] [PubMed] [Google Scholar]
- 7.Annual report 2013–2014. Cervical Cancer screening [In Norwegian]. The Cancer Registry of Norway, 2015.
- 8.Annual report 2015. Cervical Cancer screening [In Norwegian]. The Cancer Registry of Norway, 2016.
- 9.Annual report 2016. Cervical Cancer screening [In Norwegian]. The Cancer Registry of Norway, 2018.
- 10. Schiffman M, Wentzensen N. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol Biomarkers Prev 2013;22:553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guan P, Howell‐Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV‐positive women: a meta‐analysis from cervical infection to cancer. Int J Cancer 2012;131:2349–59. [DOI] [PubMed] [Google Scholar]
- 12. Vaccarella S, Franceschi S, Engholm G, et al. 50 years of screening in the Nordic countries: quantifying the effects on cervical cancer incidence. Br J Cancer 2014;111:965–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nygard M, Hansen BT, Dillner J, et al. Targeting human papillomavirus to reduce the burden of cervical, vulvar and vaginal cancer and pre‐invasive neoplasia: establishing the baseline for surveillance. PLoS One 2014;9:e88323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hansen BT, Campbell S, Nygard M. Long‐term incidence trends of HPV‐related cancers, and cases preventable by HPV vaccination: a registry‐based study in Norway. BMJ Open 2018;8:e019005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pedersen K, Burger EA, Nygard M, et al. Adapting cervical cancer screening for women vaccinated against human papillomavirus infections: the value of stratifying guidelines. Eur J Cancer 2018;91:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quality assurance manual in Cervical Cancer Screening Programme [In Norwegian]. The Cancer Registry of Norway, 2012.
- 17. Larsen IK, Smastuen M, Johannesen TB, et al. Data quality at the cancer registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer 2009;45:1218–31. [DOI] [PubMed] [Google Scholar]
- 18. WHO . International classification of diseases for oncology (ICD‐O), 3rd edn. edition, 1st revision, Malta: World Health Organization, 2013. [Google Scholar]
- 19. IARC . WHO classification of Tumours of the female reproductive organs, Fourth edn. Geneva: WHO, 2014. [Google Scholar]
- 20. Norwegian SNOMED Coding System, available at: https://kreftregisteret.no/Registrene/Innrapportering/Rapportering‐av‐patologiinformasjon/. Retrieved June 6, 2018.
- 21. Lönnberg S, Hansen BT, Haldorsen T, et al. Cervical cancer prevented by screening: long‐term incidence trends by morphology in Norway. Int J Cancer 2015;137:1758–64. [DOI] [PubMed] [Google Scholar]
- 22. Doll R, Payne P, Waterhouse J. Cancer incidence in five continents: a technical report, Genève: International Union against Cancer, 1966. [Google Scholar]
- 23. Rutherford MJ, Lambert PC, Thompson JR. Age‐period‐cohort modeling. Stata J 2010;10:606–27. [Google Scholar]
- 24. Arbyn M, Bergeron C, Klinkhamer P, et al. Liquid compared with conventional cervical cytology: a systematic review and meta‐analysis. Obstet Gynecol 2008;111:167–77. [DOI] [PubMed] [Google Scholar]
- 25. Whitlock EP, Vesco KK, Eder M, et al. Liquid‐based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. preventive services task force. Ann Intern Med 2011;155:687–97. w214–5. [DOI] [PubMed] [Google Scholar]
- 26. Haldorsen T, Skare GB, Ursin G, et al. Results of delayed triage by HPV testing and cytology in the Norwegian cervical cancer screening Programme. Acta Oncol 2015;54:200–9. [DOI] [PubMed] [Google Scholar]
- 27. Baldur‐Felskov B, Munk C, Nielsen TS, et al. Trends in the incidence of cervical cancer and severe precancerous lesions in Denmark, 1997‐2012. Cancer Causes Control 2015;26:1105–16. [DOI] [PubMed] [Google Scholar]
- 28. Rozemeijer K, van Kemenade FJ, Penning C, et al. Exploring the trend of increased cervical intraepithelial neoplasia detection rates in The Netherlands. J Med Screen 2015;22:144–50. [DOI] [PubMed] [Google Scholar]
- 29. Stigum H, Samuelsen SO, Traeen B. Analysis of first coitus. Arch Sex Behav 2010;39:907–14. [DOI] [PubMed] [Google Scholar]
- 30. Liu G, Hariri S, Bradley H, et al. Trends and patterns of sexual behaviors among adolescents and adults aged 14 to 59 years, United States. Sex Transm Dis 2015;42:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guleria S, Faber MT, Hansen BT, et al. Self‐perceived risk of STIs in a population‐based study of Scandinavian women. Sex Transm Infect 2018;94:522–7. [DOI] [PubMed] [Google Scholar]
- 32. Norwegian Institute of Public Health . Annual report. Blood brone and sexually transmitted diseases in Norway 2016 [In Norwegian]. Oslo, Norway: Norwegian Institute of Public Health, 2017. [Google Scholar]
- 33. Serrano B, Brotons M, Bosch FX, et al. Epidemiology and burden of HPV‐related disease. Best Pract Res Clin Obstet Gynaecol 2018;47:14–26. [DOI] [PubMed] [Google Scholar]
- 34. Tainio K, Athanasiou A, Tikkinen KAO, et al. Clinical course of untreated cervical intraepithelial neoplasia grade 2 under active surveillance: systematic review and meta‐analysis. BMJ 2018;360:k499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schiffman M, Wentzensen N. From human papillomavirus to cervical cancer. Obstet Gynecol 2010;116:177–85. [DOI] [PubMed] [Google Scholar]
- 36. IARC, IARC Handbooks of Cancer Prevention . Cervix cancer screening, vol. 10, Lyon: IARCPress, 2005. [Google Scholar]
- 37. Makkonen P, Heinavaara S, Sarkeala T, et al. Impact of organized and opportunistic pap testing on the risk of cervical cancer in young women ‐ a case‐control study from Finland. Gynecol Oncol 2017;147:601–6. [DOI] [PubMed] [Google Scholar]
- 38. Lonnberg S, Anttila A, Luostarinen T, et al. Age‐specific effectiveness of the Finnish cervical cancer screening programme. Cancer Epidemiol Biomarkers Prev 2012;21:1354–61. [DOI] [PubMed] [Google Scholar]
- 39. Bruni L, Diaz M, Castellsague X, et al. Cervical human papillomavirus prevalence in 5 continents: meta‐analysis of 1 million women with normal cytological findings. J Infect Dis 2010;202:1789–99. [DOI] [PubMed] [Google Scholar]
- 40. von Karsa L, Arbyn M, De Vuyst H, et al. European guidelines for quality assurance in cervical cancer screening. Summary of the supplements on HPV screening and vaccination. Papillomavirus Res 2015;1:22–31. [Google Scholar]
- 41. McCredie MRE, Sharples KJ, Paul C, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol 2008;9:425–34. [DOI] [PubMed] [Google Scholar]
- 42. Kyrgiou M, Athanasiou A, Kalliala IEJ, et al. Obstetric outcomes after conservative treatment for cervical intraepithelial lesions and early invasive disease. Cochrane Database Syst Rev 2017;11:Cd012847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bjørge T, Skare GB, Bjørge L, et al. Adverse pregnancy outcomes after treatment for cervical intraepithelial neoplasia. Obst Gynec 2016;128:1265–73. [DOI] [PubMed] [Google Scholar]
- 44. Frederiksen ME, Vazquez‐Prada Baillet M, Jensen PT, et al. Conization and healthcare use: a population‐based register study. Eur J Cancer Prev 2019;28:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brotherton JM, Fridman M, May CL, et al. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet 2011;377:2085–92. [DOI] [PubMed] [Google Scholar]
- 46. Benard VB, Castle PE, Jenison SA, et al. Population‐based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol 2017;3:833–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Norwegian Institute of Public Health HPV vaccination coverage among girls born in 2004, available at: https://fhi.no/hn/helseregistre-og-registre/sysvak/dekkningsstatistikk/. Retrieved July 10, 2018.
- 48.Norwegian Institute of Public Health HPV vaccination coverage among women born 1991–1996, available at: http://norgeshelsa.no/norgeshelsa/. Retrieved July 10, 2018.
- 49. O'Brien KM, Sharp L. Trends in incidence of, and mortality from, cervical lesions in Ireland: baseline data for future evaluation of the national cervical screening programme. Cancer Epidemiol 2013;37:830–5. [DOI] [PubMed] [Google Scholar]
- 50. Leinonen MK, Hansen SA, Skare GB, et al. Low proportion of unreported cervical treatments in the cancer registry of Norway between 1998 and 2013. Acta Oncol 2018;57:1663–1670. [DOI] [PubMed] [Google Scholar]
- 51. Hariri S, Johnson ML, Bennett NM, et al. Population‐based trends in high‐grade cervical lesions in the early human papillomavirus vaccine era in the United States. Cancer 2015;121:2775–81. [DOI] [PubMed] [Google Scholar]
- 52. Watson M, Soman A, Flagg EW, et al. Surveillance of high‐grade cervical cancer precursors (CIN III/AIS) in four population‐based cancer registries, United States, 2009‐2012. Prev Med 2017;103:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1


