Abstract
Purpose
We determined the latencies of orienting responses during a preferential looking task in children with normal vision and in children with visual impairments between 6 and 12 years old, and assessed the feasibility of scoring grating detection in these populations with video‐based eye tracking.
Methods
Children performed a computerized preferential looking test, while a remote eye tracker measured the children's eye movements. The stimuli consisted of a 2 × 2 grid, with three uniform grey fields and one target field consisting of a black‐and‐white square wave grating. The grating was presented randomly at one of the four locations. The spatial frequencies (1.05, 2.11 and 7.02 cyc/deg) were randomly interleaved, with 10 trials per spatial frequency. Three different methods were used to score the accuracy of the responses: (1) primary saccade ends on target, (1) gaze 50% of the presentation time on target, and (3) a combination of method 1 and 2 (i.e. primary saccade ends on target, and/or gaze 50% of the presentation time on target).
Results
The combined scoring method was most reliable to determine whether children could resolve the gratings. Children with visual impairments had significantly lower accuracies than children with normal vision with all three scoring methods. In addition, saccade latencies decreased with age and were significantly longer (62 ± 15 ms) in children with visual impairments.
Conclusion
The use of eye tracking to assess grating detection with a preferential looking task in clinical populations provides valuable additional information, including objective detection measures and developmental delays in saccade latencies.
Keywords: case–control study, child development, orienting response, reaction times, visual acuity
Introduction
The most commonly used clinical method to assess visual acuity in infants, toddlers and non‐verbal children is the preferential looking technique (Lambert & Lyons 2016). This method is based on the phenomenon that infants, when simultaneously presented with a patterned target and a blank target, have a greater tendency to look at the pattern (Fantz 1963). The grating with the finest stripes that yields a consistent orienting response provides an estimate of the child's visual acuity. The standard diagnostic tool, which uses the preferential looking technique to assess visual acuity, is the Teller acuity card test (TAC) (McDonald et al. 1985; Teller et al. 1986). The advantage of the TAC is that it provides a fast and fairly reliable estimate of grating visual acuity (Courage & Adams 1990). However, the outcome of the TAC relies on a subjective assessment of whether the patient can see the grating (Courage & Adams 1990). This assessment is not only based on visual judgement of the eye and head movements; other factors such as verbal responses, facial expressions or pointing also influence the examiner's judgement. Thus, the outcome of the TAC depends on the experience of the clinician and on the cooperation and attention of the child (Lambert & Lyons 2016). Teller suggested already in 1983 that the analysis of the infant's head and eye movements could provide valuable and more objective information (Teller 1983). Remote eye trackers now offer the possibility to measure gaze during preferential looking paradigms, thereby allowing for more objective scoring of the evoked orienting responses (see e.g. Pel et al. 2010; Richmond & Nelson 2009). Recently, several researchers have attempted to assess visual acuity by combining such video‐based gaze measurements with computerized preferential looking tasks. The resulting visual acuity scores were compared with the outcome of traditional preferential looking tests, such as the TAC and the Keeler infant acuity cards (Hathibelagal et al. 2015; Jones et al. 2014; Sturm et al. 2011). Different measures have been adopted to quantify the visual scanning behaviour during these tasks and to assess whether the subject resolves the grating. These measures are all based on the assumption that the grating is resolved if the target pattern is fixated for a prolonged period of time. Two studies used a relative fixation time criterion by measuring the percentage of the time that a participant fixates the patterned target field during a trial (Hathibelagal et al. 2015; Sturm et al. 2011). A third study used an absolute fixation time criterion (at least 167 ms within the target area) to assess whether the grating was resolved (Jones et al. 2014). The visual acuity estimates based on prolonged viewing of the target corresponded well with the outcome of the traditional preferential looking tests in adults (Hathibelagal et al. 2015; Sturm et al. 2011) and in infants (Hathibelagal et al. 2015; Jones et al. 2014). In conclusion, computerized tests which combine preferential looking paradigms with eye tracking provide a rapid, automated, and more objective measure of grating acuity. In addition, it has been shown that eye tracking can provide additional information about other visual functions, such as visual field size, contrast sensitivity and colour perception (Kooiker et al. 2016; Murray et al. 2009).
Previous studies that have used eye tracking to evaluate preferential looking behaviour in computerized TAC tests have only assessed its potential to estimate the visual acuity of the participants (Hathibelagal et al. 2015; Jones et al. 2014; Sturm et al. 2011). However, as Teller stated: ‘… the quality and intensity of the infant's staring behaviour on each trial contains more information than one gets out of the single left‐right judgement imposed by the forced choice method’ (Teller 1983). Video‐based eye tracking techniques now allow for quantitative assessment of this behaviour. One of the important variables that can be assessed is the saccade latency. Saccades are the fast eye movements that change the line of sight from one point of fixation to another. Saccade latency is the interval between stimulus presentation and the onset of a saccade and this latency reflects visual processing, target selection and motor programming (Leigh & Kennard 2004; Leigh & Zee 2015). Furthermore, saccade latencies are abnormal in a range of disorders in which cortical areas associated with vision and eye movements are affected (Leigh & Kennard 2004; Leigh & Zee 2015). Therefore, quantifying the latencies and the accuracy of saccades evoked during computerized visual acuity tests could provide valuable insight into the development and integrity of the oculomotor and the visual system.
Saccade latencies can be influenced by a wide range of factors, such as the contrast and luminance of the target, the amplitude and direction of the saccade and the nature of the task (for an extensive overview, see Leigh & Zee 2015). Furthermore, the spatial frequency of the target influences saccade latency as well (Ludwig et al. 2004). This is relevant when assessing the latencies of orienting responses evoked during preferential looking tests, as the spatial frequency of the gratings is systematically varied in these tests. In addition, several studies in participants with normal vision have demonstrated that saccade latencies are longer in children than in adults, and that the latencies decrease with age through childhood, until adult levels are reached at approximately 10 to 12 years of age (Fukushima et al. 2000; Munoz et al. 1998; Salman et al. 2006; Yang et al. 2002). Therefore, reference values for saccade latencies during preferential looking paradigms have to be age‐ and target‐specific.
Although preferential looking tests are often used in children with visual impairment to assess visual acuity, previous studies which combined computerized TAC tests with eye tracking only tested adults and infants with normal vision but have not addressed ophthalmological abnormalities (Hathibelagal et al. 2015; Jones et al. 2014; Sturm et al. 2011). Video‐based eye tracking in participants with ophthalmological problems can be challenging because certain eyes are difficult to track, for instance due to the presence of nystagmus, or abnormal anatomical properties of the eyes (Huurneman & Boonstra 2016). Nonetheless, saccade latencies in participants with visual impairments have been assessed in other tasks. Recently, it has been shown that children (Huurneman et al. 2016) and adults (Dunn et al. 2015) with infantile nystagmus have longer saccade latencies, and that children with cerebral visual impairment (CVI) have delayed orienting responses towards cartoons (Kooiker et al. 2014; Pel et al. 2011). However, in these studies only one spatial frequency or one stimulus size was presented. Assessment of saccade latencies and saccade metrics in preferential looking tests provides measures of both speed and accuracy of the visual system for different spatial frequencies. It has been argued that such measures are key to better quantify visual impairment (Farzin & Norcia 2011).
The aim of the present study is to determine the latencies of orienting responses during a preferential looking task in children with normal vision and in children with visual impairments between 6 and 12 years old, and to assess the feasibility of scoring grating detection in these populations with video‐based eye tracking. Towards that end, we compared different detection scoring methods and identified factors which could reduce the chance of successful eye tracking. We also compared the latencies of stimulus‐evoked primary saccades for the two populations to determine whether the onset of the orienting responses was delayed in the children with visual impairments.
Materials and Methods
Participants
The current study was part of a larger project in which we assessed visual processing speed in children with normal vision (Barsingerhorn et al. 2018a) and in children with visual impairments (Barsingerhorn et al. 2018b). The children who participated in the present study also participated in those previous studies. Eighty‐eight children (9.6 ± 1.8 years) with normal vision (NV), 15 children (9.0 ± 1.6 years) with cerebral visual impairment (CVI) and 19 children (9.0 ± 2.4 years) with visual impairment due to congenital or acquired disorders of the eye without additional impairments (mental or neurological) (VIo) participated. The children with VIo and CVI were recruited through Bartiméus, a specialised Dutch institute for visually impaired people. The children with NV were recruited from primary schools in the surrounding region. For the children with NV and VIo, the following inclusion criteria were applied: age 6 to 12 years old, birth at term, normal birth weight, no perinatal complications and normal development. In addition, children with NV had to have a crowded visual acuity of 0.1 logMAR or better. Additional criteria for children with VIo were as follows: a crowded distant visual acuity (DVA) between 0.2 and 1.3 logMAR and a congenital or acquired ocular abnormality without mental or neurological impairment. For the children with CVI, inclusion criteria were as follows: being diagnosed with CVI by experienced paediatric ophthalmologists at the institute for the visually impaired based on a thorough ophthalmological examination and detailed patient history, age 6 to 12 years, and having a crowded distance visual acuity of 1.3 LogMAR or better. All children performed the Freiburg visual acuity test (FrACT, Bach 1996) to verify whether they met the acuity inclusion criterion. Children who did not have the mental and motor skills to understand and execute the tasks were excluded. Clinical characteristics and visual acuities of the children with VIo and CVI are presented in Table S1.
The study was conducted according to the principles of the Declaration of Helsinki and was approved by the local ethics committee (CMO Arnhem‐Nijmegen, The Netherlands). All parents or legal guardians provided informed consent in writing before the start of the study.
Procedure
The children were seated unrestrained at approximately 65 cm from a 23‐inch LCD screen (Dell U2412M, 1920 × 1200 pixels, pixel pitch 0.27 mm) and were instructed to keep their back against the back of the chair to keep the distance to the screen constant during the experiment. This was checked during the experiment and the instruction was repeated if children leaned towards the screen. Similar to Sturm et al. (2011), each stimulus consisted of four fields on a black background, arranged in a 2 × 2 grid (Fig. 1). One field contained a black‐and‐white square wave grating while the other three were uniform grey fields. The grating was presented randomly at one of the four locations. A four alternative choice design was used to decrease the possibility of false positives, that is, that the children did not actually perceive the location of the target, but looked at the target location by chance (see e.g. Sturm et al. 2011; Teller 1979 for similar approaches). Each field subtended 9.6 × 9.6 deg and the centre of each field was positioned 10.2 deg from the centre of the screen. Three different spatial frequencies were used: 1.05, 2.11 and 7.02 cyc/deg. This corresponds to 1.45, 1.15 and 0.63 LogMAR. The luminance of the background and the black stripes was 2 cd/m2 and the luminance of the white stripes was 236 cd/m2, as measured with a luminance metre (Minolta LS‐100; Minolta Co. Ltd., Osaka, Japan). The mean luminance of the grey fields (~118 cd/m2) was matched to the space‐average luminance of the grating to prevent that the participants could detect the position of the grating based on differences in luminance. In contrast to the previous studies that have used eye tracking during a preferential looking task (Hathibelagal et al. 2015; Jones et al. 2014; Sturm et al. 2011), we added a high‐contrast fixation dot (98.2% Michelson) at the centre of the screen before each trial. The fixation dot was presented for a random duration of 320–640 ms, after which the fixation dot disappeared and the stimulus was presented for 3 s (Fig. 1). The three spatial frequencies were presented in pseudo‐random order with 10 trials per frequency, resulting in a total of 30 trials. The children were instructed to fixate the fixation dot at the start of each trial and to look at the target as soon as the fixation dot disappeared.
Figure 1.
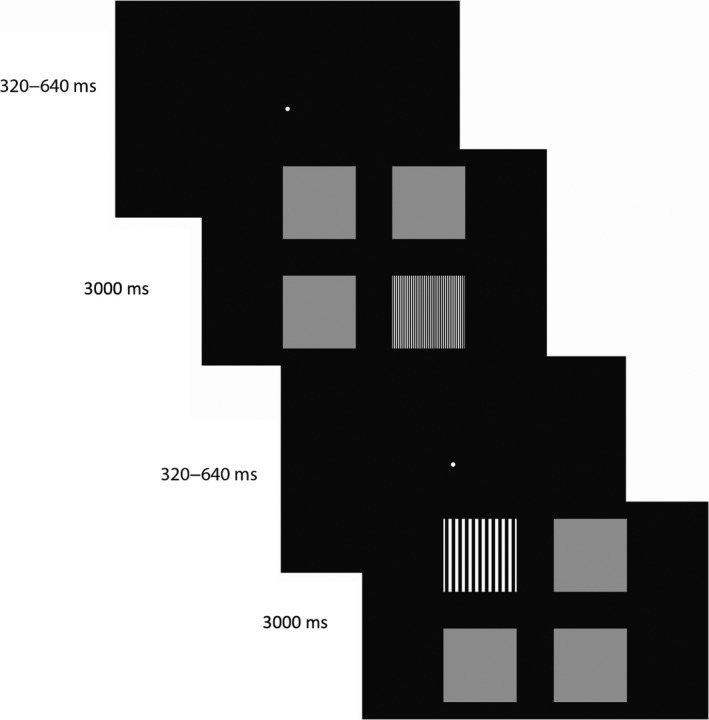
Stimuli of the preferential looking task. Each trial started with a central fixation dot which was presented for a random duration between 320 and 640 ms. As soon as the fixation spot disappeared, the stimulus array appeared for a duration of 3000 ms. The children were instructed to fixate the fixation dot at the start of each trial and to look at the square wave grating target as soon as the fixation dot disappeared. The target (three different spatial frequencies) appeared randomly at one of the four positions, the other fields were grey.
Custom matlab software (version 2013b; MathWorks, Inc., Natick, MA, USA) with the Psychophysics Toolbox (version 3.0.12) was used to generate the stimuli. The software was executed on a laptop (Dell M3800; Dell Inc., Round Rock, TX, USA) equipped with an OpenGL graphics card (Nvidia Quadro K1100M; Santa Clara, CA, USA).
Eye tracker
We used a stereoscopic eye tracking system with two USB 3.0 cameras and two infrared light sources in a fully calibrated configuration with respect to the stimulus screen (Barsingerhorn et al. 2018c). In this way, we could obtain calibrated measures of the two‐dimensional (2D) orientation the optical axes of the two eyes and their three‐dimensional (3D) location in space. During data collection, each camera tracked both eyes with an average frame rate of ~300 Hz. The image coordinate of the pupils and the image coordinates of the reflections of the infrared light sources were extracted online and stored on disk for offline reconstruction of gaze (Barsingerhorn et al. 2018c; Chen et al. 2008; Guestrin & Eizenman 2006; Zhu & Ji 2005). After combining the asynchronously sampled data from the two cameras, the final gaze position signals had an average refresh rate of ~500 Hz. The spatial accuracy of these signals was ~0.7 degrees in both directions (Barsingerhorn et al. 2018c). The advantage of this stereoscopic system is that a one‐point calibration procedure is enough to obtain calibrated measures of gaze under head‐free conditions. Only the subject‐specific angles between the optical and visual axes must be determined for a given participant. This one‐point calibration can even be performed under head‐free conditions, because the orientation of the visual axes can be computed from the known 3D location of the calibration target being fixated and the measured 3D position of the eyes. In the current study, we used the gaze position data when participants where fixating the central fixation dot during the preferential looking task as the one‐point calibration. Fixation periods were identified by the experimenter by using a mouse tool which marked the beginning and end of stable fixation of the fixation dot. Therefore, there was no separate calibration necessary before the start of the test.
Data processing
The data were analysed in Matlab. The sampling rate of the gaze position signals was variable because the two cameras of the stereo eye tracker ran asynchronously (Barsingerhorn et al. 2018c). Therefore, the data of the stereo eye tracker were resampled to a fixed sampling rate of 500 Hz using linear interpolation to facilitate the saccade detection based on velocity and acceleration thresholds. Saccades were detected with a velocity threshold criterion of 25°/s and an acceleration threshold criterion of 3000°/s2 for saccade onsets and offsets. All saccade markings were visually checked and corrected if necessary. Subsequently, saccade latency was determined as the difference between stimulus onset and the onset of the saccade. Primary saccades had to start within 80 to 900 ms after stimulus onset. Participants were excluded from the analysis if no saccades were found in more than one third of the trials.
The median latency of the primary saccades was determined for each participant and for each spatial frequency. Subsequently, primary saccades were categorized into correct, goal‐directed saccades and incorrect saccades. Only trials in which the starting position of the primary saccade fell within a square window of 2.75 × 2.75 deg centred at the fixation dot were included in this analysis. This excluded trials in which children already fixated the location of the target pattern by chance before the stimulus onset. Correct saccades were those primary saccades that had an amplitude of >1.5 deg and landed on the target, or within 1.4 deg from the target boundary. Incorrect saccades were saccades of >1.5 deg which landed outside this target window. We determined the median saccade latencies for the correct and the incorrect saccades separately.
The accuracy of the responses was assessed with different scoring methods to account for the variance in response patterns observed during the experiment. Figure 2 illustrates the variability in response patterns by showing four trials of a child with normal vision (Fig. 2A) and four trials of a child with visual impairment due to albinism and with a nystagmus (Fig. 2B). We compared three methods to establish which method is most reliable in discriminating whether the children could resolve the gratings. In the first method, we scored the accuracy based on the end‐point of the primary saccades. For each participant and for each spatial frequency, we calculated the percentage of correct primary saccades, that is, trials in which the primary saccade landed on target or within 1.4 deg from the target boundary (Figs 2A1 & 2B1). This analysis provides insight in the accuracy of the first orienting response. However, if the primary saccade was in the wrong direction, this did not necessarily mean that the participant could not resolve the grating. In a large number of these trials, the participants seemed to have guessed the potential location of the grating but corrected their initial error by making a second, goal‐directed saccade to fixate the grating (Fig. 2A2). In addition, the children did not always fixate the fixation dot at the time the stimulus appeared, but their first saccade was directed towards the grating (Figs 2A4 & 2B2–B4). It also happed that the participant's gaze was already at the location of the grating at the time the stimulus appeared, after which the subject continued to fixate this target. To account for these behaviours, we used a second method to assess whether participants successfully located the target position. In this second method, accuracy scores are based on prolonged viewing of the target (Hathibelagal et al. 2015; Sturm et al. 2011). Only trials in which the gaze position was available for at least 2 out of 3 seconds were included in the analysis. The gaze position was considered to be on target if it fell within the target area (see Fig. 2), that is, on target or within 1.4 degrees from the target boundary. We calculated the percentage of presentation time in which the gaze position fell within the target area. To limit the possibility of false positives, we considered that the participant could resolve the grating if the relative fixation time (RTF) exceeded 50%, that is, if the gaze of the participant was on target during 50% of its presentation time. Subsequently, for each participant and each spatial frequency, we calculated the proportion of trials in which the participant resolved the grating according to this RTF criterion. The examples presented in Fig. 2A are all responses in which the child correctly located the grating. However, the trials in Fig 2A2 and Fig. 2A4 would be considered inaccurate with the first method and accurate with the second method, while the trial in Fig. 2A3 (goal‐directed saccade after which the gaze returned to the centre of the screen) would be considered inaccurate with the second method and accurate with the first method. Therefore, we combined the two previous methods for the third method. In this case, a response was considered correct if (i) the primary saccade was goal‐directed, or if (ii) the RTF exceeded 50%. As a result, all examples in Figure 2A would be correctly scored as accurate responses with the third method.
Figure 2.
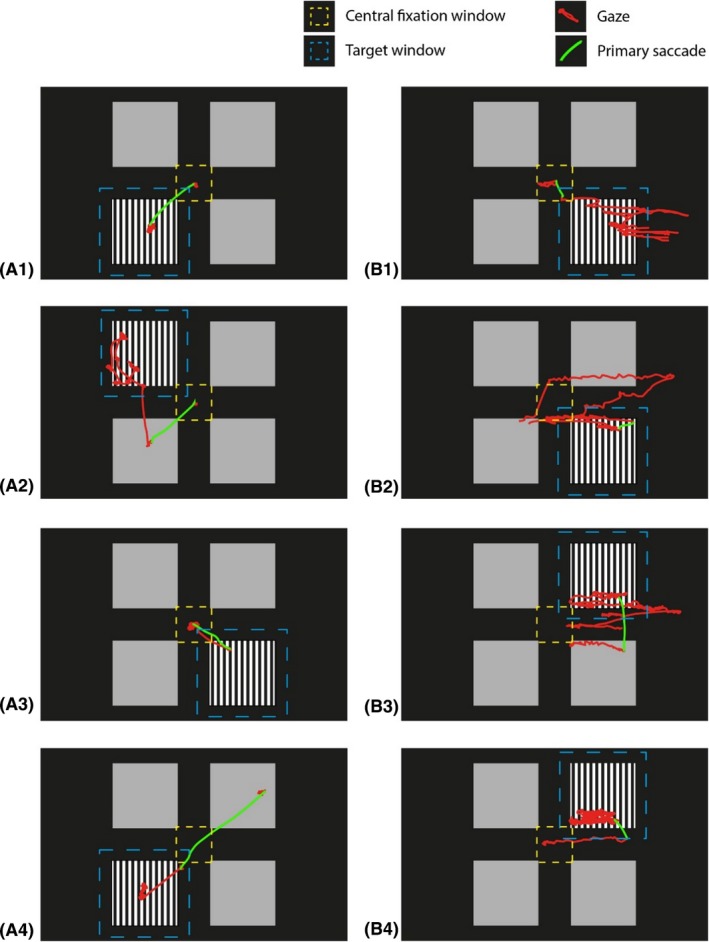
Illustration of observed response patterns and scoring criteria. (A) Four trials of a child with normal vision (B) four trials of a child with visual impairment due to albinism and with a nystagmus. The target window is indicated with the blue dashed lines, the central fixation window is indicated with the yellow dashed lines, the gaze coordinates are plotted as red lines and the primary saccade is plotted in green.
Since the gratings were randomly presented at four different positions on the screen, we considered that the participant could resolve the grating if he or she correctly looked at the grating in more than 62.5% of the trials (i.e. halfway between 25% chance level performance and 100% correct performance).
Statistical analysis
We assessed whether the latencies of the primary saccades change with age, whether the latencies depend on the spatial frequency of the grating, and whether the developmental effects are equal for the three spatial frequencies for children with normal vision. A repeated measures anova with age and spatial frequency as independent variables and the saccade latency of the primary saccade as dependent variable was performed. We also applied the repeated measures anova separately for the saccade latencies of the correct and incorrect primary saccades. For all repeated measures anovas age was centred on the age of 9, the middle of the inclusion range. Children were only included in the repeated measures anovas if latencies were available for all three grating frequencies. Subsequently, we used the multiple linear regression models from the repeated measures anovas to determine the upper 95th percentile in the data from the children with normal vision. The onset of the saccades of an individual child with visual impairments was considered to be delayed if the median latency exceeded the upper 95th percentile of the normative data. Alpha (type 1 error) was set on 0.05 for all statistical group comparisons.
Results
The flow chart in Fig. 3 shows the number of children who participated, the number of children from whom we could collect eye tracking data, and the number of children in whom the quality of this data was deemed sufficient for analysis. For participants in whom eye tracking was not possible, we reported the main reason why the eye tracking failed. For 71/88 children with normal vision and 15/34 children with visual impairments (nine children with VI and six children with CVI), we collected eye tracking recordings. For 56/88 children with normal vision and 13/34 of the children with visual impairments (seven children with VI and six children with CVI), the quality of the eye movement data was sufficient for the analyses (Fig. 3). In supplemental Table S1, we indicated for all children with visual impairments whether we were able to record their eye movements. The main reason why eye tracking failed was the presence of eyeglasses. This was particularly problematic in the children with visual impairments, as most of these children wore prescription glasses. However, the presence of eyeglasses did not necessarily mean that eye tracking was impossible. Eight of the thirteen children with visual impairments in whom we were able to collect valid eye tracking data wore glasses. In addition, in five children with normal vision and prescription glasses we could collect eye tracking data as well. Factors that influenced the success of eye tracking in children with eyeglasses were related to the size and colour of the frame, and thickness of the glasses. Smaller frames, black frames and thick glasses made detection of the eyes and/or extraction of the relevant image features difficult.
Figure 3.
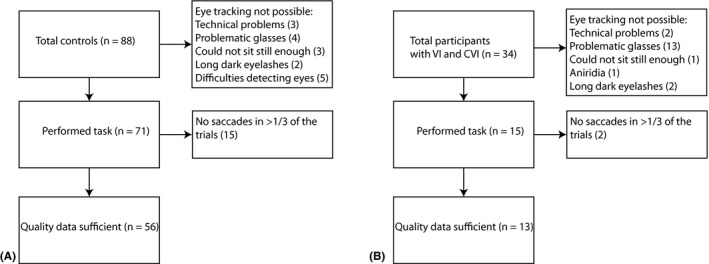
Flow chart showing the total number participants, the number of children in which we could collect eye tracking data, and the number of children in which the quality of the eye tracking data was deemed sufficient for analysis for (A) children with normal vision, and (B) children with visual impairments. In case eye tracking was not possible, the main reason why the eye tracking failed is listed.
Accuracy
The grating‐detection accuracy of the children with normal vision is presented in Fig. 4A for each of the three different scoring methods. Because the spatial frequencies of the gratings were at least 0.6 LogMAR above their visual acuity (as measured with the Freiburg acuity test), the children with normal vision should have been able to resolve gratings of all three spatial frequencies. Therefore, children with normal vision were expected to look correctly at the grating in more than 62.5% of the trials. The accuracy of the responses was determined with three different methods, to establish which method is most reliable in discriminating whether the children could resolve the gratings. To that end, we determined the number of children who did not reach the 62.5% threshold.
Figure 4.
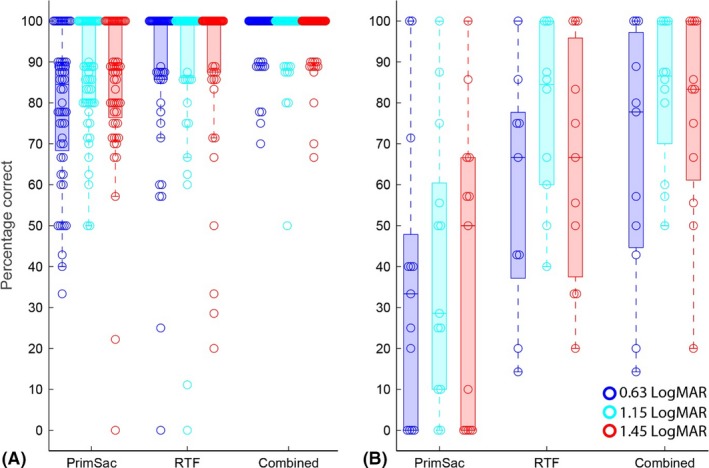
Boxplots of the accuracy of the responses determined with three different scoring methods: 1. Primary saccade ends on the target area (PrimSac), 2. Relative fixation time (RTF) >50%, that is, if the gaze of the participant was on target during 50% of its presentation time, and 3. Primary saccade ends on the grating) or RTF exceeds 50% (Combined). (A) grating‐detection accuracy of the children with normal vision, and (B) grating‐detection accuracy of the children with visual impairments. The colours indicate the spatial frequency of the grating.
First, we scored the grating‐detection accuracy as the percentage of correct primary saccades, that is, trials in which the primary saccade landed on target or within 1.4 deg from the target boundary. With this method, 10/56 participants with normal vision did not reach 62.5% correct for the finest grating, 3/56 did not reach 62.5% correct for the middle grating, and 1/56 did not reach 62.5% correct on the coarsest grating. The median per cent correct was 85%, 89% and 89%, respectively. With the second scoring method, we considered that the participant could resolve the grating if the relative fixation time (RTF) exceeded 50%, that is, if the gaze of the participant was on target during 50% of its presentation time. This method resulted in a total of 6/56 children on the finest grating, 3/56 children on the middle grating and 4/56 children on the coarsest grating who did not reach beyond 62.5% correct. The median accuracy with this method was 100% for all three spatial frequencies. The third scoring method combines the two other methods, that is, a response was considered correct if the primary saccade was goal‐directed, or if the RTF exceeded 50%. This third method was most reliable to determine whether children with normal vision could resolve the gratings; only one child did not reach beyond the 62.5% threshold on the middle grating. Furthermore, the median accuracy was 100% for all spatial frequencies as well.
The accuracy of the responses of the children with visual impairments is presented in Fig. 4B. For only one of these children, the finest grating and the middle grating fell below its visual acuity (see Table S1). The coarsest grating was above visual acuity for all children, and therefore, they should have been able to resolve these gratings. However, for the finest and middle grating, 10/13 children with visual impairments did not reach the 62.5% correct threshold if the response accuracy was determined from the end‐points of the primary saccades alone. For the coarsest grating, the accuracy of the primary saccade fell below 62.5% correct in 9/13 children. The median accuracy of the first orienting response was only 33%, 28% and 50%, respectively. Mann–Whitney U‐tests revealed that the accuracy of the children with visual impairments was significantly lower than the accuracy of the children with normal vision for all three grating frequencies as scored by the end‐point of the primary saccades (z = 3.75, p < 0.001, z = 4.40, p < 0.001, and z = 4.27, p < 0.001, respectively). Similar to the results in the children with normal vision, the accuracy scores were higher if the scoring was based on sustained viewing of the target pattern (RTF). In this case, the number of children who did not score above the 62.5% correct threshold was 4/13 for the finest grating, 3/13 for the middle grating and 5/13 for the coarsest grating. The median accuracy based on prolonged viewing of the target pattern was 67%, 85% and 67%, respectively. Even though the accuracies of the children with visual impairments were higher with this method compared to the first method, the accuracies as scored with the second method were significantly lower than the accuracies of the children with normal vision (z = 3.44, p < 0.001, z = 2.62, p = 0.008, and z = 3.10, p = 0.002, respectively). As for the children with normal vision, the third scoring method yielded the best accuracies, with median scores of 78% for the finest, 87% for the middle grating and 83% for the coarsest grating. However, still 5/13 children with visual impairment did not reach the 62.5% correct threshold for the finest grating, and this number was 3/13 on the middle and coarsest grating. Furthermore, even with this method, the accuracies of the children with visual impairments were significantly lower than the accuracies of the children with normal vision (z = 4.02, p < 0.001, z = 3.82, p < 0.001, and z = 3.66, p < 0.001, respectively).
Saccade latencies
The saccade latencies of children with normal vision are presented in Fig. 5. The data are stratified by spatial frequency (colours) and plotted as a function of age. First, we analysed the latencies of all primary saccades, independent of whether they were directed towards the grating or not (Fig. 5A). A repeated measures anova (n = 56) revealed that the saccade latencies significantly decreased with age (main effect: F(1, 54) = 9.59, p = 0.003) and that this developmental effect did not differ significantly between the three spatial frequencies (interaction spatial frequency × age: F(2, 108) = 0.81, p = 0.45). However, a significant difference between the three spatial frequencies was found (main effect: F(2, 108) = 16.85, p < 0.001). Post hoc t‐tests showed that the saccade latencies were on average longer for the finest grating compared to the middle (difference 30 ± 6 ms, p < 0.001) and the coarsest grating (difference 31 ± 7 ms, p < 0.001). The average difference between the latencies for the middle and coarsest grating was not statistically significant (difference: 0.4 ± 5 ms, p = 0.99).
Figure 5.
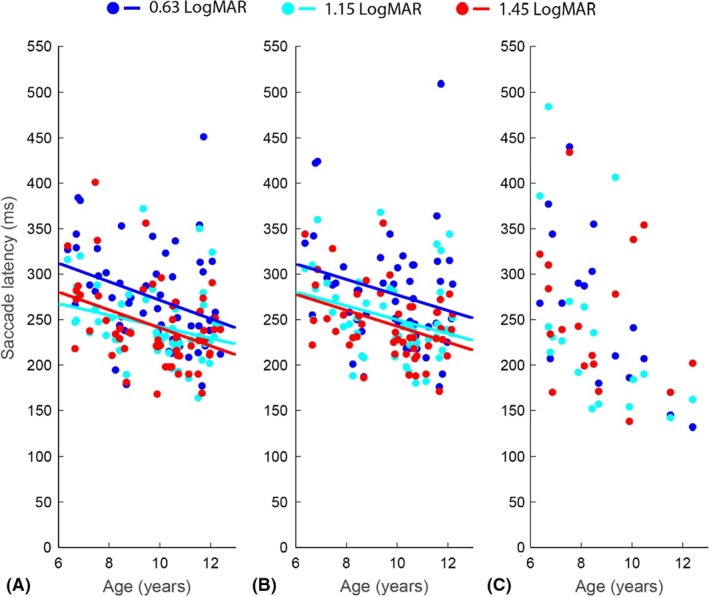
Median saccade latencies for the children with normal vision as a function of age and spatial frequency for: (A) all primary saccades. (B) the goal‐directed primary saccades, and (C) the incorrect primary saccades. The lines are the results of the repeated measures anova for the three different gratings.
The results of the anova on the reaction times of only the goal‐directed primary saccades (Fig. 5B) were very similar. Seven children had to be excluded from this anova because they did not make correct saccades towards all spatial frequencies. As a result, 49 out 56 children with normal vision were included in this analysis. The latencies of the correct saccades decreased significantly with age (main effect: F(1, 47) = 5.06, p = 0.03) and this developmental effect did not differ significantly between the gratings (interaction: F(2, 94) = 0.17, p = 0.85). Furthermore, a significant main effect of spatial frequencies was found (F(2, 94) = 13.56, p < 0.001): saccadic latencies were on average longer for the finest grating compared to the middle (difference 28 ± 6 ms, p < 0.001) and the coarsest grating (difference 35 ± 8 ms, p < 0.001). The difference between the middle and coarsest grating was not statistically significant (difference: 7 ± 5 ms, p = 0.39).
The saccade latencies of the incorrect primary saccades are presented in Fig. 5C. Only 18 children made saccades in the incorrect direction for all the spatial frequencies. Due to this low number of children, especially the low number of older children (two 10‐year‐olds, one 11‐year‐old and one 12‐year‐old), interpretation of age effects and effects of grating acuity could be spurious. Therefore, we did not perform a repeated measures anova on this data. Instead, we only compared the latencies of correct saccades to the latencies of all primary saccades. This repeated measures anova with the type of latency measure as an additional within‐subject factor showed that the latencies of correct saccades were on average 4 ± 2 ms faster than the latencies of all primary saccades (F(1, 47) = 5.7, p = 0.02), while the effects of age and spatial frequency were not significantly different between these two measures (p‐values >0.2).
The children with visual impairments had on average more trials in which they did not correctly fixate the fixation dot. Therefore, we only analysed the latencies of all primary saccades in the children with visual impairments. The saccade latencies of the children with visual impairments are presented in Fig. 6. In total, 8 out of 13 children with visual impairments scored ≥95th percentile of the saccade latencies of the children with normal vision for at least one of the spatial frequencies. Three children had longer latencies (≥95th percentile) on all spatial frequencies, one child on the finest and the middle grating, one child only on the finest grating, and three children only on the coarsest grating. To test whether on average the children with visual impairments had longer saccade latencies, we performed a repeated measures anova with age, spatial frequency and group (normal vision versus visual impairment) as the independent variables and the latency of the primary saccade as the dependent variable. The results revealed a significant effect of group (F(1, 65) = 16.26, p < 0.001): the saccade latencies of the children with visual impairment were on average 62 ± 15 ms longer.
Figure 6.
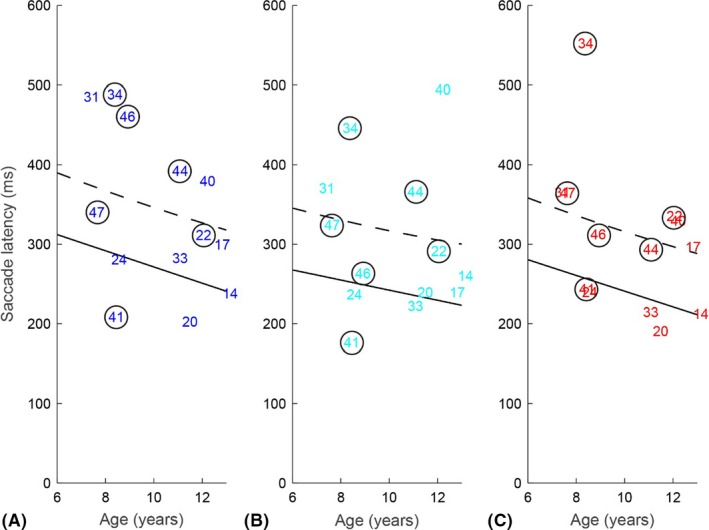
Median saccade latencies for the children with visual impairments (numbers correspond with individual participants, a circle indicates a child with CVI, Table S1) for (A) gratings of 0.63 LogMAR, (B) gratings of 1.15 LogMAR and (C) 1.45 LogMAR. The solid lines are the result of the repeated measures anova for the children with normal vision, the dashed black lines indicate the 95th percentile in children with normal vision. Latency data were pooled across correct and incorrect trials.
Discussion
The aim of the present study was to determine the latencies of saccadic eye movements evoked during a preferential looking task in children with normal vision and in children with visual impairments between 6 and 12 years, and to assess the feasibility of scoring grating acuity in these populations with video‐based eye tracking. In line with previous research (Fukushima et al. 2000; Munoz et al. 1998; Salman et al. 2006; Yang et al. 2002), the saccade latencies of the children with normal vision decreased with age. This developmental effect was similar for all spatial frequencies. Furthermore, both the latencies of all primary saccades and the latencies of the correct saccades were longer for the finest grating of 7 cyc/deg, even though this grating was still well above the children's visual acuity (at least 0.6 LogMAR). A similar effect of spatial frequency on saccade latencies has been found in adults; in adults latencies increased for higher spatial frequencies (Ludwig et al. 2004).
To determine whether the onset of the saccades of children with visual impairments was delayed, we compared their saccade latencies with the data from controls. In 8/13 children with visual impairments, the saccadic latencies were abnormally long (> 95th percentile of children with normal vision) for at least one of the spatial frequencies and on average the children with visual impairments were 62 ± 15 ms slower than children with normal vision. This corresponds well to previous studies, which revealed longer saccade latencies in children and adults with infantile nystagmus (Dunn et al. 2015; Huurneman et al. 2016), and in children with cerebral visual impairment (Pel et al. 2011). Apart from longer saccade latencies, children with visual impairments also have longer search times (Geldof et al. 2015; Huurneman et al. 2014; Tadin et al. 2012), lower reading speeds (Douglas et al. 2002; Gompel et al. 2004), and they need more time for the discrimination of optotypes (Barsingerhorn et al. 2018b) compared to children with normal vision.
Previously, it has been argued that eye tracking in participants with ophthalmological problems can be challenging, because their eyes can be more difficult to track (Huurneman & Boonstra 2016). This is in line with our experience. For 19/34 of children with visual impairments, we could not obtain reliable eye tracking data. The limited number of children with a certain diagnosis makes it difficult to draw conclusions on whether certain diagnoses or clinical characteristics (e.g. nystagmus, astigmatism and high hyperopia) result in difficulties with eye tracking. The problems with eye tracking in clinical populations could largely differ between different eye trackers. Commercially available eye trackers might use different algorithms to detect the eyes, which might be more robust against anatomical abnormalities and/or eyeglasses. However, in a recent study on oculomotor behaviour of children with infantile nystagmus, eye tracking with an Eyelink 1000 plus was only successful in 47–72% of the participants (Huurneman et al. 2016). Thus, also commercial eye trackers appear to have problems with tracking the eyes of clinical populations.
Because our goal was to assess the feasibility of including saccade latencies as an outcome measure of a preferential looking task in children with visual impairments, we only used three spatial frequencies. As a result, we could not assess grating acuity of the children. Based on previous research, it is expected that children will exhibit more search behaviour if the gratings approach the discrimination threshold (Sturm et al. 2011). Furthermore, only high‐contrast gratings were used. The advantage of computerized preferential looking tasks is that it is possible to adjust the contrast of the gratings. It is likely that decreased contrast levels will result in longer saccade latencies (Ludwig et al. 2004). We could not assess whether the saccade latencies would increase for gratings with lower contrast levels, or for finer gratings towards the discrimination threshold. Recent studies on the time children need to discriminate optotypes revealed that the button‐press reaction times increased as the optotypes approached the discrimination threshold in children with normal vision and in children with visual impairments (Barsingerhorn et al. 2018a,b). In addition, because in these studies the optotypes ranged from below visual acuity to well above threshold, the reaction times could be corrected for reduced visual acuity. The results revealed that 40% of the children with visual impairments needed more time to discern optotypes (Barsingerhorn et al. 2018b) than one might expect from their reduced visual acuity alone.
The accuracy of the grating‐discrimination responses depended on the method used to assess whether the children could resolve the grating. Because the gratings were all well above threshold in the children with normal vision, we expected that these children could easily resolve the grating. However, it turned out that the primary saccade was not always in the correct direction. For about 18% of the children with normal vision, their visual acuity would have been profoundly underestimated if this criterion would have been used to determine the visual acuity. The use of a 2 × 2 grid with three grey fields and one target field instead of a uniform grey background with one grating as used in the original Teller Acuity Cards could have attributed to the relatively high number of primary saccades in the wrong direction. However, similar results have been found in traditional preferential looking tests. If only the direction of infants’ first fixation was used to determine response accuracy, their visual acuity appeared consistently lower compared to scores based on prolonged target fixation (Atkinson et al. 1977). In the current study, the accuracy scores based on prolonged target fixation also appeared to be more reliable to detect whether a child did successfully locate the target grating. However, some children appeared to make goal‐directed saccades, after which they redirected their gaze towards the centre of the screen to prepare for the next trial, fixating only briefly on the grating. In those cases, the relative fixation time on target does not adequately score the subject's visual acuity. The most representative accuracy scores were obtained with a novel scoring method, which combines the two previous scoring criteria. For the children with visual impairments, the highest accuracies were also obtained with this combined method. However, their accuracies were significantly lower than the accuracies of the children with normal vision, even though the gratings were in general above visual acuity for both groups. This could indicate that in children with visual impairments other criteria are needed to reliably estimate their visual acuity based on eye tracking data in computerized preferential looking tasks. An alternative could be to use a less strict criterion for detection than 62.5% correct, or to add an expert human observer for these children. Similar recommendations have been made to assess grating detection in infants. Given their low guess rates and high lapse rates the ideal threshold is often <50% correct (Jones et al. 2015). Studies with more children with visual impairments and a wider range of grating frequencies are necessary to estimate the best criteria for this population.
The low success rates of eye tracking in children with visual impairments and the low accuracies of their orienting responses are limiting factors for the use of eye tracking during preferential looking tasks in clinical settings. However, although it might not be possible to solely rely on eye tracking to assess visual acuity with a preferential looking task for all participants, if eye tracking is possible, it does provide valuable additional information, such as saccade latencies. The present study revealed that most children with visual impairments had longer saccade latencies than children with normal vision.
Supporting information
Table S1. Clinical characteristics of the children with their participant number
Acknowledgments
The authors are grateful to the parents and children for their participation and to Bartiméus Sonneheerdt for the use of the examination room.
This research was supported by the RadboudUMC (ADB, JG), Stichting Bartiméus (FNB), the European Union Program FP7‐PEOPLE‐2013‐ITN ‘HealthPAC’, grant 604063 ‐ IDP (JG), and the ODAS foundation (ADB, grant 2012‐35 awarded to FNB and JG).
References
- Atkinson J, Braddick O & Moar K (1977): Development of contrast sensitivity over the first 3 months of life in the human infant. Vision Res 17: 1037–1044. [DOI] [PubMed] [Google Scholar]
- Bach M (1996): The Freiburg Visual Acuity Test ‐ Automatic measurement of visual acuity. Optom Vis Sci 73: 49–53. [DOI] [PubMed] [Google Scholar]
- Barsingerhorn AD, Boonstra FN & Goossens J (2018a): Development of symbol discrimination speed in children with normal vision. Investig Ophthalmol Vis Sci 59: 3973–3983. [DOI] [PubMed] [Google Scholar]
- Barsingerhorn AD, Boonstra FN & Goossens J (2018b): Symbol discrimination speed in children with visual impairments. Investig Ophthalmol Vis Sci 59: 3963–3972. [DOI] [PubMed] [Google Scholar]
- Barsingerhorn AD, Boonstra FN & Goossens J (2018c): The development and validation of a high‐speed stereoscopic eye tracker. Behav Res Methods 50: 2480–2497 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Tong Y, Gray W & Ji Q (2008): A robust 3D eye gaze tracking system using noise reduction. In: Proceedings of the Eye‐tracking Research Applications Conference (ETRA), Savannah, Georgia, 26–28 March 2008. [Google Scholar]
- Courage ML & Adams R (1990): Visual acuity assessment from birth to three years using the acuity card procedure: cross‐sectional and longitudinal samples. Optom Vis Sci 67: 713–718. [DOI] [PubMed] [Google Scholar]
- Douglas G, Grimley M, Hill E, Long R & Tobin M (2002): The use of the NARA for assessing the of children with low vision reading ability. Br J Vis Impair 20: 68–75. [Google Scholar]
- Dunn MJ, Margrain TH, Woodhouse JM & Erichsen JT (2015): Visual processing in infantile nystagmus is not slow. Investig Ophthalmol Vis Sci 56: 5094–5101. [DOI] [PubMed] [Google Scholar]
- Fantz RL (1963): Pattern vision in newborn infants. Science 140: 296–297. [DOI] [PubMed] [Google Scholar]
- Farzin F & Norcia A (2011): Impaired visual decision‐making in individuals with amblyopia. J Vis 11: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima J, Hatta T & Fukushima K (2000): Development of voluntary control of saccadic eye movements. I. Age‐related changes in normal children. Brain Dev 22: 173–180. [DOI] [PubMed] [Google Scholar]
- Geldof CJA, van Wassenaer‐Leemhuis AG, Dik M, Kok JH & Oosterlaan J (2015): A functional approach to cerebral visual impairments in very preterm/very‐low‐birth‐weight children. Pediatr Res 78: 190–197. [DOI] [PubMed] [Google Scholar]
- Gompel M, Van Bon WH & Schreuder R (2004): Reading by children with low vision. J Vis Impair Blind 2: 77–89. [Google Scholar]
- Guestrin ED & Eizenman M (2006): General theory of remote gaze estimation using the pupil center and corneal reflections. IEEE Trans Biomed Eng 53: 1124–1133. [DOI] [PubMed] [Google Scholar]
- Hathibelagal AR, Leat SJ, Irving EL, Nandakumar K & Eizenman M (2015): Measuring infant visual acuity with gaze tracker. Optom Vis Sci 92: 823–833. [DOI] [PubMed] [Google Scholar]
- Huurneman B & Boonstra FN (2016): Assessment of near visual acuity in 0–13 year olds with normal and low vision: a systematic review. BMC Ophthalmol 16: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huurneman B, Cox RFA, Vlaskamp BNS & Boonstra FN (2014): Crowded visual search in children with normal vision and children with visual impairment. Vision Res 96: 65–74. [DOI] [PubMed] [Google Scholar]
- Huurneman B, Boonstra FN & Goossens J (2016): Perceptual learning in children with infantile Nystagmus: effects on 2D Oculomotor behavior. Investig Ophthalmol Vis Sci 57: 4229–4238. [DOI] [PubMed] [Google Scholar]
- Jones PR, Kalwarowsky S, Wattam‐Bell J & Nardini M (2014): An automated test of infant visual acuity using remote eye‐tracking. Investig Ophthalmol Vis Sci 55: 2737. [DOI] [PubMed] [Google Scholar]
- Jones PR, Kalwarowsky S, Braddick OJ, Atkinson J & Nardini M (2015): Optimizing the rapid measurement of detection thresholds in infants. J Vis 15: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooiker MJG, Pel JJM & van der Steen J (2014): Viewing behavior and related clinical characteristics in a population of children with visual impairments in the Netherlands. Res Dev Disabil 35: 1393–1401. [DOI] [PubMed] [Google Scholar]
- Kooiker MJG, Pel JJM, Verbunt HJM, de Wit GC, van Genderen MM & van der Steen J (2016): Quantification of visual function assessment using remote eye tracking in children: validity and applicability. Acta Ophthalmol 94: 599–608. [DOI] [PubMed] [Google Scholar]
- Lambert SR & Lyons CJ (2016): Taylor and Hoyt's pediatric ophthalmology and strabismus. Edinburgh: Elsevier Health Sciences. [Google Scholar]
- Leigh RJ & Kennard C (2004): Using saccades as a research tool in the clinical neurosciences. Brain 127: 460–477. [DOI] [PubMed] [Google Scholar]
- Leigh RJ & Zee DS (2015): The neurology of eye movements. USA: Oxford University Press. [Google Scholar]
- Ludwig CJH, Gilchrist ID & McSorley E (2004): The influence of spatial frequency and contrast on saccade latencies. Vision Res 44: 2597–2604. [DOI] [PubMed] [Google Scholar]
- McDonald MA, Dobson V & Sebris SL (1985): The acuity card procedure: a rapid test of infant acuity. Investig Ophthalmol Vis Sci 26: 1158–1162. [PubMed] [Google Scholar]
- Munoz DP, Broughton JR, Goldring JE & Armstrong IT (1998): Age‐related performance of human subjects on saccadic eye movement tasks. Exp Brain Res 121: 391–400. [DOI] [PubMed] [Google Scholar]
- Murray IC, Fleck BW, Brash HM, MacRae ME, Tan LL & Minns RA (2009): Feasibility of saccadic vector optokinetic perimetry. a method of automated static perimetry for children using eye tracking. Ophthalmology 116: 2017–2026. [DOI] [PubMed] [Google Scholar]
- Pel JJM, Manders JCW & van der Steen J (2010): Assessment of visual orienting behaviour in young children using remote eye tracking: methodology and reliability. J Neurosci Methods 189: 252–256. [DOI] [PubMed] [Google Scholar]
- Pel JJM, Van Der Does L, Boot FH, De Faber JT, Van Der Steen‐Kant SP, Willemsen SP & van der Steen J (2011): Effects of visual processing and congenital nystagmus on visually guided ocular motor behaviour. Dev Med Child Neurol 53: 344–349. [DOI] [PubMed] [Google Scholar]
- Richmond J & Nelson CA (2009): Relational memory during infancy: evidence from eye tracking. Dev Sci 12: 549–556. [DOI] [PubMed] [Google Scholar]
- Salman MS, Sharpe JA, Eizenman M, Lillakas L, Westall C, To T, Dennis M & Steinbach MJ (2006): Saccades in children. Vision Res 46: 1432–1439. [DOI] [PubMed] [Google Scholar]
- Sturm V, Cassel D & Eizenman M (2011): Objective estimation of visual acuity with preferential looking. Investig Opthalmology Vis Sci 52: 708–713. [DOI] [PubMed] [Google Scholar]
- Tadin D, Nyquist JB, Lusk KE, Corn AL & Lappin JS (2012): Peripheral vision of youths with low vision: motion perception, crowding, and visual search. Investig Ophthalmol Vis Sci 53: 5860–5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teller DY (1979): The forced‐choice preferential looking procedure: a psychophysical technique for use with human infants. Infant Behav Dev 2: 135–153. [Google Scholar]
- Teller DY (1983): Measurement of visual acuity in human and monkey infants: the interface between laboratory and clinic. Behav Brain Res 10: 15–23. [DOI] [PubMed] [Google Scholar]
- Teller DY, McDonald MA, Preston K, Sebris SL & Dobson V (1986): Assessment of visual acuity in infants and children: the acuity card procedure. Dev Med Child Neurol 28: 779–789. [DOI] [PubMed] [Google Scholar]
- Yang Q, Bucci MP & Kapuola S (2002): The latency of saccades, vergence, and combined eye movements in children and in adults. Investig Ophthalmol Vis Sci 43: 2939–2949. [PubMed] [Google Scholar]
- Zhu Z & Ji Q (2005): Eye gaze tracking under natural head movements. IEEE Comput Soc Conf Comput Vis Pattern Recognit 91: 8–923. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical characteristics of the children with their participant number


