Abstract
In many countries, the monitoring of child growth does not occur in a regular manner, and instead, we may have to rely on sporadic observations that are subject to substantial measurement error. In these countries, it can be difficult to identify patterns of poor growth, and faltering children may miss out on essential health interventions. The contribution of this paper is to provide a framework for pooling together multiple datasets, thus allowing us to overcome the issue of sparse data and provide improved estimates of growth. We use data from multiple longitudinal growth studies to construct a common correlation matrix that can be used in estimation and prediction of child growth. We propose a novel 2‐stage approach: In stage 1, we construct a raw matrix via a set of univariate meta‐analyses, and in stage 2, we smooth this raw matrix to obtain a more realistic correlation matrix. The methodology is illustrated using data from 16 child growth studies from the Bill and Melinda Gates Foundation's Healthy Birth Growth and Development knowledge integration project and identifies strong correlation for both height and weight between the ages of 4 and 12 years. We use a case study to provide an example of how this matrix can be used to help compute growth measures.
Keywords: child health, correlation, growth, SDS
1. BACKGROUND
The study of physical growth in children is a challenging and complex topic that must consider a variety of genetic, physiological, and socio‐economic factors. There is a vast literature on child growth, and a thorough overview of the topic is provided in two books by J.M. Tanner.1, 2 This paper will focus on the estimation and prediction of growth based on data from longitudinal growth studies. There is a great deal of interest in being able to understand the factors that drive growth faltering in young children, in order to develop better preventative measures.3, 4, 5
To adequately monitor the health of young children, it is imperative that we are able to accurately model their growth across their formative years. In many countries, children are measured regularly to track their progress, but there are many places where such monitoring does not occur in a consistent or regular manner.6 In these countries, a child's height and weight may only be measured sporadically, and these measurements may be subject to a great deal of measurement error. It can thus be very challenging to estimate the growth pattern of children in these areas and to identify children whose growth may be faltering and who may require health interventions. By pooling together multiple datasets and drawing strength across studies, it may be possible to overcome data sparsity issues and provide substantially better estimates than would be possible from a single study.
To estimate a child's growth where measurements are sparse, it is important to have some measurement of how much correlation exists between a child's growth measures across different ages.7 For example, we would be keen to know whether a child's height at age 100 days is likely to be a good predictor for that same child's height at 300 days. If we know the correlation between these time points in a child's life, then we are able to make inference about what height a child might be after 300 days given that we know their height at 100 days. Such predictions can also help to quickly identify whether a child's growth is faltering—if their actual observed height at 300 days is substantially less than what was predicted, then this could indicate poor growth over that period,2, 8 which may be a result of an underlying health issue.9, 10, 11 This approach is common in child health monitoring; the World Health Organisation (WHO) have developed a set of widely used growth reference charts12 that show a healthy range of heights and weights for children at each age point, thus allowing parents and doctors to quickly identify when a child is developing more slowly than would be expected.
The existing WHO growth curves were built using a mixture of longitudinal data measured from birth to 24 months and cross‐sectional data from children aged 18 to 71 months. Therefore, these curves may not reflect longitudinal trends for children aged 24 months and older as accurately as would be the case with longitudinally observed data. The approach outlined in this paper incorporates both subject‐specific and marginal correlation data to provide an estimate of a child's growth curve. By using longitudinal data from low‐ and middle‐income countries, we aim to produce a more realistic, empirical representation of growth trajectories for these children. A well‐developed correlation matrix using growth data from resource‐poor settings would be a useful resource to help medical experts provide accurate growth estimation for children in such countries. Such a correlation matrix also helps facilitate the computation of conditional standard deviation scores (cSDS), which measure the relative change in height from one time point to the next (eg, a change in height between 100 and 300 days in the example above). In this paper, we propose a novel 2‐stage approach, which uses data from multiple studies to construct a common correlation matrix that can be used in estimation and prediction of child growth. The first stage involves the construction of a raw and often incomplete matrix via a set of elementwise univariate meta‐analyses. In stage 2, we smooth this raw matrix in order to obtain a valid and complete correlation matrix.
2. DATA
As part of the Bill and Melinda Gates Foundation's Healthy Birth Growth and Development knowledge integration project, we have access to 16 studies, which include sufficient longitudinal child growth data. Data from the following studies were used in this paper: Evaluation and Control of Neglected Mucosal Enteric Infections in Childhood (cntt, European Commission13); lower respiratory tract infection (LRTI), respiratory syncytial virus (RSV), and Influenza Cohort Study (grip, Iqbal et al14); PROVIDE Study PR‐10060, funded by NIH grant R01 AI043596 (prvd, Naylor et al15); Infant Growth in Peru (phua, Lopez de Romaña et al16); Respiratory Pathogens Birth Cohort (rspk, Iqbal et al14); Peru Zn Fortification (pzn, Brown et al17); Longitudinal study of bovine serum concentrate (BSC) in Guatemala (gbsc, Begin et al18); Medical Research Council (MRC) Keneba (knba, Hennig et al19); Study of Biomarkers for Environmental Enteropathy (ee, Iqbal et al14); Deuterium dilution study in Mali (mmam, Ackatia‐Armah et al20); Child Malnutrition and Infection Network (cmin, MAL‐ED Investigators21); Zn Trial in Burkina Faso (bfzn, Hess et al22); CMC Vellore Birth Cohort 2002 (cmc, Rehman et al23); NIH Birth Cohort Study, funded by NIH grant R01 AI043596 (nbrt, Mondal et al24); Longitudinal Growth Study in Bangladesh (bngd, Brown et al25); NIH Preschool Cohort Study, funded by NIH grant R01 AI043596 (npre, Haque et al26).
Table 1 provides a summary of the data within these studies. Within each study, a group of children have their height and weight measured at a number of different ages. The measurement ages and the number of measurements are not consistent from child to child, even within a study. For example, child A may be measured at ages 100, 200, and 300 days, while child B may be measured at ages 150 and 250 days. Each study covers a slightly different age range of children; for example, bfzn contains children aged between 168 and 927 days, while grip contains children aged between 1 and 521 days. It is also likely that the amount of measurement error will vary from one study to the next, since the studies were conducted independently in a variety of locations, using different study methodologies and measurement techniques. Our model includes a parameter that accounts for this difference in data quality between studies.
Table 1.
Summary of relevant studies within the HBGDki project
| Dataset | # Children | # Obs | Obs Per Child | Child Age (in Days) | ||||
|---|---|---|---|---|---|---|---|---|
| Min | Median | Max | Min | Median | Max | |||
| cntt | 197 | 4405 | 10 | 21 | 41 | 1 | 116 | 702 |
| grip | 203 | 1427 | 1 | 7 | 17 | 1 | 136 | 521 |
| prvd | 700 | 9741 | 1 | 16 | 16 | 1 | 175 | 756 |
| phua | 153 | 1839 | 1 | 13 | 16 | 1 | 185 | 679 |
| rspk | 278 | 3177 | 1 | 13 | 33 | 1 | 211 | 525 |
| pzn | 302 | 1140 | 2 | 4 | 4 | 153 | 265 | 457 |
| gbsc | 315 | 2548 | 1 | 10 | 13 | 119 | 269 | 493 |
| knba | 2954 | 41587 | 1 | 13 | 69 | 1 | 309 | 900 |
| ee | 380 | 8436 | 2 | 23 | 26 | 1 | 343 | 1175 |
| mmam | 289 | 577 | 1 | 2 | 2 | 186 | 423 | 1090 |
| cmin | 3125 | 35506 | 1 | 9 | 37 | 1 | 446 | 1846 |
| bfzn | 7637 | 18983 | 1 | 2 | 4 | 168 | 541 | 927 |
| cmc | 373 | 12478 | 23 | 34 | 37 | 1 | 558 | 1111 |
| nbrt | 629 | 11828 | 1 | 21 | 43 | 1 | 644 | 2199 |
| bngd | 197 | 2352 | 1 | 14 | 15 | 95 | 804 | 1903 |
| npre | 529 | 8656 | 1 | 16 | 30 | 731 | 3257 | 6696 |
Abbreviation: HBGDki, Healthy Birth Growth and Development knowledge integration.
3. METHODS
The heights and weights in every study were standardised using the WHO's Z‐scores.12 A Z‐score reflects a child's development relative to the global “healthy” average. In this paper, we will work with both height‐for‐age Z‐scores (HAZ) and weight‐for‐age Z‐scores (WAZ). Some applications also use weight‐for‐height Z‐scores, but those are not analysed in this study. One issue with standardisation across multiple geographical regions is that there may be differing secular trends in growth around the world, which may have an impact on estimation and prediction.27 However, standardisation of the data facilitates direct comparison between children of different ages, heights, and weights, which is important when comparing multiple studies.
The rate of a child's growth, often referred to as growth velocity, is commonly assessed via centile‐crossing approaches.28 Such approaches characterise a child's growth velocity based on the change in their relative height or weight between 2 time points. Across the study, the expected mean change in Z‐score will be 0 as long as an appropriate growth reference is used. If a child has a Z‐score of 0.2 at age 100 days, but a Z‐score of 0.5 at age 200 days, then that implies that they have grown quickly relative to their peers over that period. However, it is well known that such measures can be affected by regression to the mean since smaller children are more likely to grow relatively quickly and larger children are more likely to grow relatively slowly.29 The cSDS accounts for this by adjusting for the correlation between the 2 time points of interest.
Let Z 1 and Z 2 be the Z‐scores measured at ages t 1 and t 2, respectively, and let r 12 be the correlation between these 2 time points. Then, the cSDS between these time points, denoted Z ⟨2|1⟩, is given by
| (1) |
Note that this velocity does not directly depend on the time points t 1 and t 2, but instead on the correlation between them, r 12. It is therefore crucial that we are able to accurately estimate this correlation term for all pairs of possible time points to facilitate the calculation of growth velocities. To compute such correlations, it is first necessary to discretise continuous time into a series of age groups. The nature of this discretisation will be dependent on both the context of the study and the computational time available. In practice, it should be sufficient to construct the matrix based on age groups within which the correlation is likely to be constant and stable, based on existing knowledge of growth patterns.
Developing an overall correlation matrix based on these studies is difficult. Ideally, one might wish to combine all of the data into 1 large dataset and then construct the underlying correlation structure in a single stage using some form of multilevel model. However, the sheer volume of data within these studies makes this approach computationally challenging; we have over 100 000 observations taken from over 15 000 children. Instead, we consider a 2‐stage approach where we fit a separate correlation matrix for each study and then combine these matrices into one larger matrix. One important advantage of this 2‐stage approach is its ability to easily handle new datasets. If we wish to integrate the results from a new study into our matrix, then we simply have to construct the correlation matrix for that study and then update our final matrix. Under a 1‐stage model, we would have to repeat the entire analysis each time a new study was added.
It is fairly straightforward to compute study‐specific correlation matrices in studies that have a regular and structured observation schedule.7 However, it is more challenging to do so in studies such as ours, which have a sparse and/or irregular data structure. One possibility is to assume that the correlation matrix takes a parametric form with a few unspecified parameters, eg, Argyle et al.30 However, model misspecification can be a potential issue. In recent years, functional data analysis has been widely used for analysing longitudinal data.31 The advantage of functional data analysis is that it only requires that the correlation matrix is smooth across age, which seems reasonable for child growth curves, and does not impose any parametric form on the correlation matrix. Under the functional data model, a smooth correlation matrix can be obtained by conducting a bivariate smoothing of empirical covariances; see Appendix A for details. To obtain smooth correlation matrices, we used the fast covariance estimation method proposed in Xiao et al,32 which was specifically designed for longitudinal data. The fast covariance estimation method was implemented in the face R package.33 This method allowed us to estimate smooth covariance matrices for both height and weight Z‐scores in each of the 16 studies.
We then have 16 HAZ correlation matrices and 16 WAZ correlation matrices that we would like to combine to give a single HAZ and a single WAZ matrix, which can describe all children across all 16 studies. However, each individual matrix only describes the correlations for children within the age range of that particular study. The differences between the 16 studies mean that each of these 16 matrices covers a different age range. Figure 1 displays a heat map of all the study‐specific correlation matrices across the 0‐ to 6570‐day age range (this corresponds to 0 to 18 years). The colour corresponds to the number of studies that cover that particular age range, with darker red corresponding to more studies being available and lighter yellow meaning that fewer studies are available. We can see that we have lots of data available for the younger age groups, but less data are available for the older ages. Only 1 study (npre) covers the area in the top right corner of the matrix (roughly 4000‐6570 days), while there is no information available for the areas in the top left and bottom right of the plot (this corresponds to the correlation between very young children and older teenagers).
Figure 1.
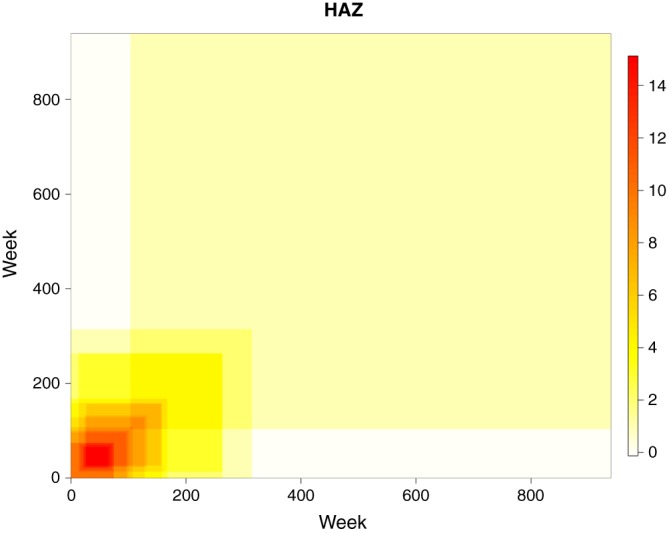
A heat map showing the extent of the correlation matrices for individual studies. The colour corresponds to the number of matrices that cover that particular age range. Darker red means more studies are available, while paler yellow means fewer studies are available. HAZ, height‐for‐age Z‐scores [Colour figure can be viewed at wileyonlinelibrary.com]
There are 2 basic approaches to meta‐analysing correlation matrices. The first is the univariate approach, where the meta‐analysis of the matrix is conducted on an elementwise basis; for example, a 10×10 matrix would require 100 separate meta‐analyses.34 The second is a multivariate approach, which attempts to combine the matrices as a whole.35 The multivariate approach has a number of advantages, most notably in terms of being able to maintain the smoothness of the correlation matrix,36 but the methodology has been developed to handle a set of homogeneous matrices, and it is not clear how to adapt this methodology to deal with our case where each matrix covers a slightly different range. With that in mind, we adopt a univariate approach for our meta‐analysis.
4. STATISTICAL METHODOLOGY
Let A=a 1,…,a r be the range of ages across which we wish to estimate correlations, with A typically defined by the age range of the children across all studies. We thus wish to estimate the combined correlation matrix H, where H is an r×r matrix with entry h ij corresponding to the correlation between children aged a i and a j.
Suppose that we have K studies, indexed by k=1,…,K. Let n k be the number of children who were observed in study k, and let be the range of ages across which we have estimated correlations for study k, where s k is the number of discrete timepoints within the scope of the study. Assume that we have the set of matrices C={C 1,…,C K}, where C k is the s k×s k correlation matrix from study k, with c ijk corresponding to the correlation between children aged b ki and b kj for study k. Since B k and s k may vary from study to study, the corresponding correlation matrices also differ in terms of size and scope, and it is thus important that we retain consistent indexing when estimating H. Thus, we construct a set of matrices , where each is an r×r matrix with entries defined as follows:
We now have a set of K consistently indexed study‐specific correlation matrices, and we wish to combine these into a single matrix H. This is achieved via a 2‐stage process: First, we conduct univariate meta‐analysis to construct a rough, incomplete correlation matrix, and then we apply a bivariate smoother to produce a more realistic estimate of H.
4.1. Univariate meta‐analysis
The univariate meta‐analysis approach allows us to use a set of elementwise calculations to construct a rough estimate for the combined correlation matrix, based on a set of elementwise calculations. Here, each element is computed separately based on the set of values . Our univariate meta‐analysis is based on the random effects approach outlined by Hedges and Olkin.34 The random effects approach has an underlying assumption that the set of observed correlation matrices form a sample from a larger population of correlation matrices, which is an entirely reasonable assumption in our context since we only observe a small sample of all the children in the world. This model makes an assumption that each of the correlations come from a normal distribution, and therefore, a Fisher transform is applied to each correlation to ensure normality.37 The set of Fisher‐transformed matrices is denoted by .
Since our study‐specific correlation matrices , and thus our Fisher‐transformed matrices were themselves estimated via a model, we assume that each observed value is an unbiased estimate of the true study‐specific correlation Θ ijk. We assume the model
where . By ensuring that each correlation observation has its own error term, we can account for the differences in data quality between studies. We further assume that each of these study‐specific correlations Θ ijk comes from a normal distribution with mean m ij and a variance τ 2. Here, τ 2 represents the level of heterogeneity between the studies. This leads to the random effects model
where φ ijk∼N(0,τ 2).
We obtain our estimate via a weighted average of the true study‐specific terms Θ ijk based on the random effects model. Here, we use inverse‐variance weights,34 given by
This weighting structure ensures that as the uncertainty associated with a correlation observation increases, the weight given to that observation decreases. Our weighted average takes the form
Applying this approach to each possible pair (i,j), we obtain a matrix, M. An inverse Fisher transformation is then applied to M to obtain the final rough correlation matrix H.
Note that in practice, we do not always have estimated correlations for all k. In the case where the correlation between ages i and j was not estimated in a specific study, this study is not included in the estimation of . In the case where the correlation between ages i and j was not estimated in any studies, we do not obtain an estimate for and must compute this using the smoothing technique outlined in the next section.
4.2. Smoothing
The matrix obtained in stage 1 provides a rough estimate of the correlation structure but has 2 major flaws. The matrix may be incomplete due to a lack of data covering a pair of ages (see Figure 1 for an illustration), which means it cannot be used as a correlation matrix. Additionally, the matrix is likely to be subject to large discontinuities in the correlation surface due to the univariate nature of the estimation procedure. These 2 issues can be addressed by smoothing the matrix to provide a more realistic correlation surface.
To obtain smooth estimates of correlation functions on the off‐diagonals and also to fill in unobserved correlations, we conduct a bivariate smoothing on the existing correlations. Let be the estimated correlations from stage 1, where is the estimated correlation at ages a i and a j and δ ij is 1 if exists and 0 otherwise.
We first conduct a Fisher transformation of these correlations, . Then, we conduct a bivariate smoothing of g ij under the working model g ij=g(a i,a j)+ε ij, where g(a i,a j) is a bivariate smooth function and ε ij is independent Gaussian random variables. We use the spline smoother outlined in Marx and Eilers,38 with a constraint added to ensure the symmetry of our eventual correlation function. We obtain an estimated function and apply the inverse Fisher transform to to obtain our correlation estimate . This process is described in more detail in Appendix B.
To ensure the integrity of the correlation matrix, we remove the diagonal elements (which are 1s) prior to the smoothing and then normalise the smoothed correlations to ensure that the final matrix has 1s on the diagonal. Specifically, let be the estimated correlation matrix after smoothing; then, the final matrix is , where .
5. APPLICATION
We applied the methodology outlined in Section 4 to the set of 16 studies outlined in Section 3. The aim was to construct a large combined correlation matrix that covers the entire range of the set of studies (0 to 6570 days). Doing this on a day‐by‐day basis would have required a 6571×6571 matrix, which is extremely large and would require a great deal of computational time. We thus simplified matters by computing the correlations on a week‐by‐week basis, which corresponds to a more manageable 940×940 matrix. By doing so, we are making an assumption that correlation is stable and constant within a week, which seems reasonable. The choice of weekly age groups was made to ensure a sufficient level of detail for very young children, where rapid growth changes can occur. For older children, weekly age groupings are less necessary, but we have retained them to ensure a consistent structure.
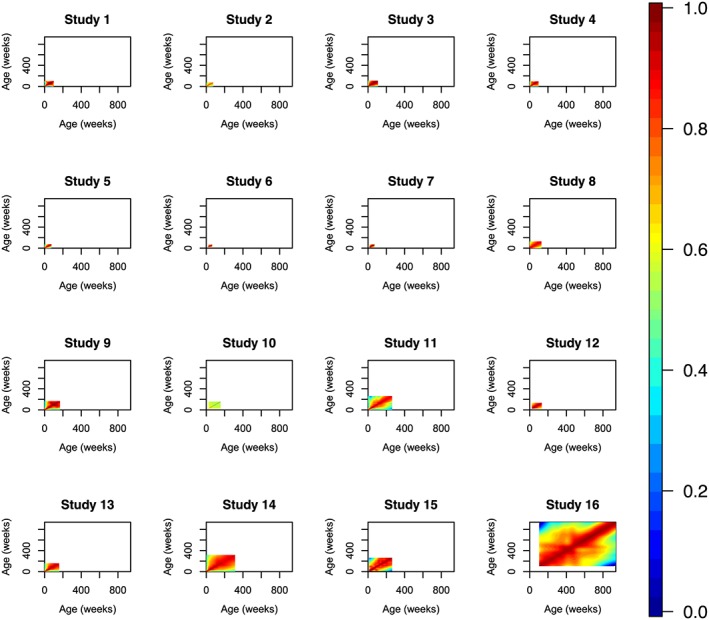
The univariate meta‐analysis step outlined in section 4.1 was conducted for HAZ and WAZ in turn. The first step of this process was to construct separate HAZ and WAZ correlation matrices for each of our 16 studies, and these are illustrated in Figures 2 and 3. These matrices were then combined using the using the metacor function, which is part of the meta R package.39 We computed each entry of our 940×940 weekly correlation matrix in turn. In keeping with the methodology outlined in section 4.1, each entry of only took into account the studies that provided a correlation estimate for that age range. That means that some of the correlation parameters are estimated based on as many as 15 datasets, while others are estimated based on just 1 dataset, as illustrated in Figure 1. This also means that a small number of entries could not be estimated at this stage due to a lack of data in the initial studies.
Figure 2.

Week‐by‐week height‐for‐age Z‐scores correlation matrices for each of the 16 studies [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 3.
Week‐by‐week weight‐for‐age Z‐scores correlation matrices for each of the 16 studies [Colour figure can be viewed at wileyonlinelibrary.com]
The raw HAZ and WAZ correlation estimates obtained via this simple meta‐analysis are displayed in the left panels of Figures 4 and 5, respectively. Dark red corresponds to high correlation, while blue corresponds to lower correlation. Unsurprisingly, it appears that the correlations are higher for low age differences and get lower as the difference between the time points increases. We can also note that the matrices produced are far from smooth and thus may not provide an entirely realistic representation of the true correlation structure. The white regions correspond to the ages between which we were unable to compute correlations.
Figure 4.
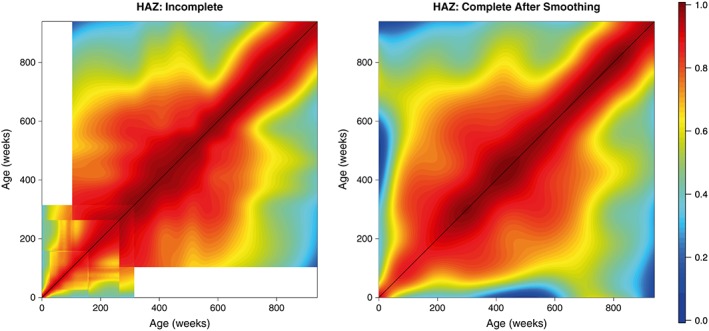
Week‐by‐week height‐for‐age Z‐scores (HAZ) correlation matrix obtained via meta‐analysis of 16 studies. The left panel displays the unsmoothed estimates obtained from the univariate meta‐analysis, while the right panel displays the final smoothed matrix. Dark red corresponds to high correlation, while blue corresponds to lower correlation [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 5.

Week‐by‐week weight‐for‐age Z‐scores (WAZ) correlation matrix obtained via meta‐analysis of 16 Gates studies. The left panel displays the unsmoothed estimates obtained from the univariate meta‐analysis, while the right panel displays the final smoothed matrix. Dark red corresponds to high correlation, while blue corresponds to lower correlation [Colour figure can be viewed at wileyonlinelibrary.com]
These matrices were then smoothed as outlined in section 4.2 to provide more realistic estimates of the correlation surface. These smoothed matrices are displayed in the right panels of Figures 4 and 5. In both cases, we obtain matrices that possess the necessary characteristics of a correlation matrix—they are complete, smooth, and symmetric; all values lie within the range 0 to 1; and all diagonal entires are equal to 1. We also obtain lower and upper confidence surfaces for both HAZ and WAZ by smoothing the incomplete lower and upper confidence surfaces obtained from our univariate meta‐analyses; these are displayed in Figures 6 and 7. Additionally, Figure 8 displays the uncertainty surface for each of our estimated correlation matrices, obtained by subtracting the lower bounds from the upper bounds.
Figure 6.
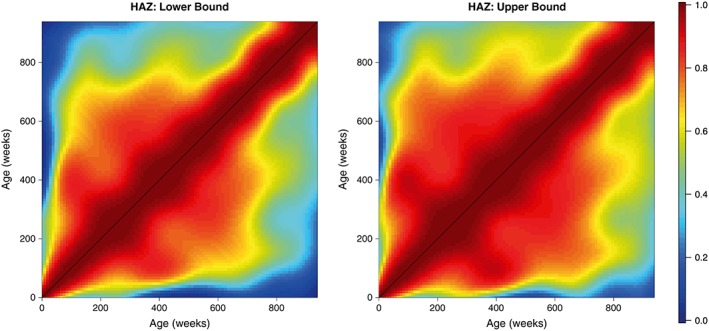
The lower and upper bounds of the height‐for‐age Z‐scores (HAZ) correlation matrix, calculated by smoothing the incomplete lower and upper confidence surfaces [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 7.
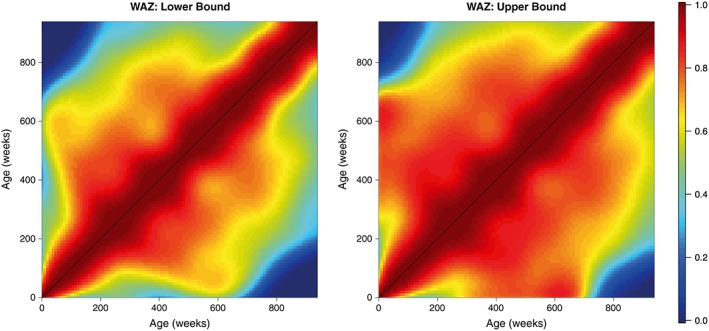
The lower and upper bounds of the weight‐for‐age Z‐scores (WAZ) correlation matrix, calculated by smoothing the incomplete lower and upper confidence surfaces [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 8.
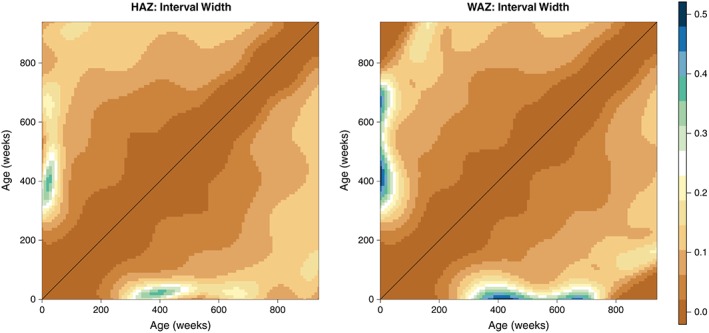
The uncertainty surface for our estimated height‐for‐age Z‐scores (HAZ) and weight‐for‐age Z‐scores (WAZ) matrices, displayed as the difference between lower bounds and upper bounds [Colour figure can be viewed at wileyonlinelibrary.com]
We identify strong correlations over small age gaps, but these correlations reduce for larger age gaps. There appears to be very little correlation between one's height (or weight) as a baby and one's height (or weight) as an adult, which is not hugely surprising, but is still an interesting result. Reasonably strong correlations exist between the ages of 200 and 600 weeks (roughly 4 and 12 years), which suggests that children remain fairly stable in terms of height and weight prior to puberty. This is in line with existing results that note that after a child's initial early development, their growth remains roughly constant until puberty.40 These results suggest that growth in the early part of a child's life (up to 4 years) is crucial in a child's future development, since height and weight at age 4 appear to be excellent predictors for height and weight at age 12.
6. CASE STUDY—COMPUTING GROWTH VELOCITIES
In this section, we provide a case study that shows how our correlation matrix can be used to compute growth velocities. Recall from Equation 1 that the computation of the cSDS, Z ⟨2|1⟩, is dependent on r 12, a measure of the correlation between time points t 1 and t 2. Using the combined correlation matrix computed in Section 5, we can extract the estimated correlation r 12 for any pair of time points t 1, t 2 in the range 0 to 6570 days. This will be illustrated using the data from cntt.
The study outlined in cntt was conducted in 2 peri‐urban shanty towns with high population density, just outside Lima, Peru. These peri‐urban communities are composed of 50 000 residents, the majority of whom are immigrants from rural areas. In the last 2 decades, this area has undergone many economic and social developments. The study contains 197 children with anthropometric measurements taken from birth. The median number of observations per child was 23, with a total of 4405 data points obtained.
We will focus on 1 randomly selected child from this study and will show how the correlation matrix can be used to compute growth velocities. The selected child was observed 25 times during the study, and their Z‐scores are plotted as blue dots in Figure 9. Let Z 1,…Z 25 be the set of HAZ scores obtained for this child at timepoints t 1,…t 25. Suppose we wish to measure the growth velocity for this child between their first and last measurements. Here, Z 1=−0.58, where t 1=62 days, and Z 25=0.43, with t 25=365 days. To compute the cSDS, Z ⟨25|1⟩, we also need r 1,25, which is the correlation between 62 and 365 days. This correlation can be obtained from our combined correlation matrix as 0.66. Using these values, we can compute the cSDS as follows:
Figure 9.
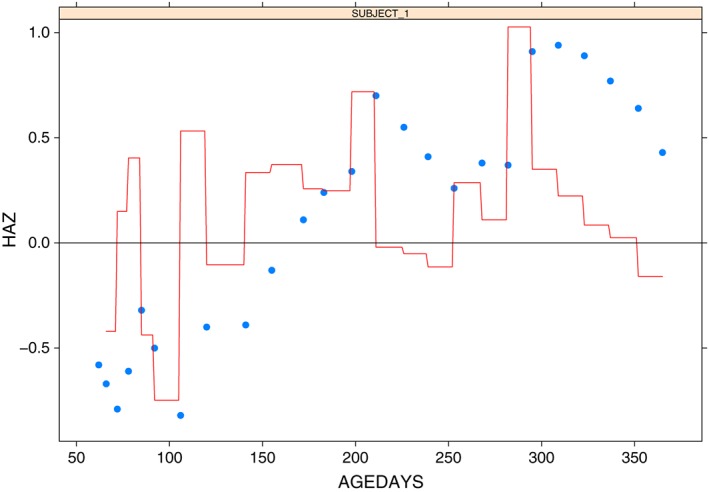
Visualisation of Z‐scores and conditional standard deviation score (cSDS) for a single child from cntt. The blue points represent observed Z‐scores, and the red line displays the cSDS computed between each pair of consecutive points. HAZ, height‐for‐age Z‐scores [Colour figure can be viewed at wileyonlinelibrary.com]
This implies an overall improvement in the velocity score of around 1 standard deviation over the period of observation. Recall that the cSDS uses the correlation to account for regression to the mean, and therefore, this computed score should be independent of the child's initial height. It may also be of interest to calculate what we refer to as dynamic cSDS by computing the cSDS between each pair of consecutive observations, thus identifying changes in the child's rate of growth over the observation period. Figure 9 provides a display of this dynamic cSDS; the blue points represent the observed Z‐scores, and the red line corresponds to the dynamic cSDS. We can see that there is a general trend of growth over time, but we also note that there are some periods where the child's Z‐score decreases. By developing the growth matrix in Section 5, we have allowed users the flexibility to measure growth in both the short and long terms, thus allowing them to look at overall trends and also identify short periods of poor growth, which may merit further investigation.
To further illustrate the importance of our matrix in computing these scores, we randomly selected another 10 children from the study and computed the cSDS between their first and last observations. These results are displayed in Table 2. We note here that the inclusion of the correlation term to account for regression to the mean has a big impact on the scores. For example, child A has a very slight increase in Z‐score between ages 1 and 2 and yet ends up with a cSDS of −0.60. This is because one would expect a much more substantial improvement in child A as a result of regression to the mean, and a failure to improve may be an indication of ongoing poor growth. Compare this to child B, who has a very similar cSDS value despite a Z‐score decrease of 0.99. Child B's height is well above average to begin with, and therefore, a relative decrease is of much less concern than it would be for a child who had started off low and dropped further.
Table 2.
Table of cSDS values for 10 randomly selected children
| Child | Age 1, t 1 | Age 2, t 2 | Z 1 | Z 2 | Correlation, r 12 | cSDS, Z ⟨2|1⟩ |
|---|---|---|---|---|---|---|
| A | 41 | 428 | −1.23 | −1.21 | 0.59 | −0.60 |
| B | 15 | 365 | 1.18 | 0.19 | 0.60 | −0.66 |
| C | 35 | 308 | −2.37 | −0.45 | 0.67 | 1.56 |
| D | 36 | 397 | −3.56 | −1.18 | 0.60 | 1.21 |
| E | 86 | 702 | −2.58 | −0.03 | 0.52 | 1.54 |
| F | 44 | 265 | −0.75 | −0.53 | 0.72 | 0.01 |
| G | 57 | 183 | −40.45 | −1.14 | 0.80 | −1.30 |
| H | 22 | 182 | −1.16 | −0.65 | 0.78 | 0.41 |
| J | 29 | 182 | −2.11 | −0.79 | 0.79 | 1.41 |
| K | 29 | 308 | −0.58 | −0.71 | 0.67 | −0.43 |
Abbreviation: cSDS, conditional standard deviation score. The bold text was used to identify the cSDS column as being the most important.
7. DISCUSSION
In this paper, we have outlined a method for obtaining a single correlation matrix by combining a set of matrices from different studies. The innovation of this approach lies in its ability to combine a set of matrices that are heterogeneous in terms of the age range over which they are measured, while still maintaining the symmetry and completeness of the final correlation matrix. We achieve this via a 2‐stage process—first, we construct a raw, incomplete matrix by conducting a set of entrywise univariate meta‐analyses of the correlation matrices, and then we smooth the resulting matrix in order to produce a complete and valid correlation matrix. This represents a quick and relatively simple solution to a challenging problem. We appreciate that a multivariate solution may offer a number of advantages over this 2‐stage approach, but we also believe that adopting such an approach would come at the cost of sacrificing the simplicity and speed of our approach.
The motivation for this methodology was to provide a correlation matrix that explained the growth pattern of young children in resource‐poor settings. These studies are typically from countries with a low Human Development Index, and in most cases, the populations of these studies exhibit growth that is substantially lower than the global average. Existing charts and models for childhood growth have been designed to compare children with the global average, and these may not be appropriate for the children in our studies. We have thus produced a pair of correlation matrices for HAZ and WAZ within our study populations. This facilitates the calculation of centile‐crossing velocity scores such as the cSDS. These matrices also provide a very useful tool for health professionals who wish to monitor the growth and development of children in these countries. This work considers height and weight separately and constructs separate height × age and weight × age correlation matrices. However, it may be of interest in future to combine these into a single height × weight × age matrix, which could describe all possible growth relationships.
Supporting information
Supporting info item
Supporting info item
Supporting info item
Supporting info item
ACKNOWLEDGEMENTS
The HBGDki initiative was supported by the Bill and Melinda Gates Foundation. The article contents are the sole responsibility of the authors and may not necessarily represent the official views of the Bill & Melinda Gates Foundation or other agencies that may have supported the primary data studies included in the HBGDki knowledge base. The authors thank all study subjects, data contributors, and primary funding agencies for all data used in this study.
We would especially like to thank Christine M. McDonald, Children's Hospital Oakland Research Institute, Oakland, California, United States of America; Andrew M Prentice, PhD, MRC Keneba, MRC Unit The Gambia, Banjul, Gambia; Bakary Sonko, MRC Keneba, MRC Unit The Gambia, Banjul, Gambia; William A. Petri, Jr, MD, PhD, Chief, Infectious Diseases & International Health, University of Virginia, United States of America; Uma Nayak, PhD, Public Health Sciences, University of Virginia School of Medicine, United States of America; Gagandeep Kang, Christian Medical College, Vellore, India; Prasanna S. Premkumar, Christian Medical College, Vellore, India; Kenneth H Brown, Program in International and Community Nutrition and Department of Nutrition, University of California, Davis, United States of America; Janet M Peerson, Program in International and Community Nutrition and Department of Nutrition, University of California, Davis, United States of America; Asad Ali, Associate Professor, Department of Pediatrics and Child Health, Aga Khan University, Karachi, Pakistan; Imran Ahmed, Department of Pathology and Laboratory Medicine, Aga Khan University, Karachi, Pakistan; Stacey L. Knobler, Fogarty International Center, National Institutes of Health, Bethesda, Maryland, United States of America for their contributions to the data collection, management, and contribution. In addition, we would like to thank Diego Bassani, Jonathan French, Ted Grasela, Ryan Hafen, Jonas Haggstrom, Tom Peppard, Amy Racine, Daniel Roth, Louise Ryan, Elly Trepman, and Stef van Buuren for their help in pushing this work forward at various stages of its development.
APPENDIX A. FUNCTIONAL DATA ANALYSIS FOR LONGITUDINAL DATA
Consider the longitudinal data of the form {(t ij,Z ij),1≤j≤m i,1≤i≤n}, where Z ij (can be either HAZ or WAZ) is measured at time t ij, m i is the number of measurements for subject i, and n is the number of subjects. Then, the functional data model31, 41 for Z ij is
| (A1) |
Here, μ(t) is a fixed smooth mean function of t; b i(t) is a random function modelled by a zero‐mean Gaussian process with covariance operator ; and ε ij is white noise with variance . It is assumed that b i(t) and ε ij are independent within and across subjects.
Under model (A1), for a new subject with observations Z(t), the correlation between Z(t 1) and Z(t 2) at 2 time points t 1 and t 2 is
| (A2) |
where 1{·} is an indicator function that is equal to 1 if the statement inside the bracket is true and 0 otherwise. Thus, to estimate r(s,t), we just plug in estimates of and into (A2).
An estimate of μ(t) can be obtained by univariate smoothing of Z ij, ignoring within‐subject correlations; denote the estimate by . Then, empirical estimates of the covariance function can be constructed by . A bivariate smoothing of provides a smooth estimate of the covariance function and also an estimate of .
APPENDIX B. BIVARIATE P‐SPLINE SMOOTHER
To obtain smooth estimates of correlation functions on the off‐diagonals and also to fill in unobserved correlations, we conduct a bivariate smoothing on the existing correlations. Let be the estimated correlations from stage 1, where is the estimated correlation at ages a i and a j and δ ij is 1 if exists and 0 otherwise.
We first conduct a Fisher transformation of these correlations, . Then, we conduct a bivariate smoothing of g ij under the working model g ij=g(a i,a j)+ε ij, where g(a i,a j) is a bivariate smooth function and ε ij is independent Gaussian random variables. We use the spline smoother outlined in Marx and Eilers,38 with a constraint added to ensure the symmetry of our eventual correlation function. We obtain an estimated function and apply the inverse Fisher transform to to obtain our correlation estimate .
The bivariate P‐spline smoother approximates g(a i,a j) by a set of tensor‐product splines G(a i,a j)= ∑1≤κ≤c,1≤ℓ≤c θ κℓ B κ(a i)B ℓ(a j), where Θ=(θ κℓ)1≤κ≤c,1≤ℓ≤c is a coefficient matrix; {B 1(·),…,B c(·)} is the collection of univariate B‐spline basis functions; and c is the number of basis functions. Twenty cubic B‐splines is used on each dimension so that the potentially complex nature of correlations can be captured by a large number of basis functions (400 in total). The following constraint on Θ is also enforced:
With this constraint, G(a i,a j) is always symmetric, a desired property for estimates of correlation functions.
With a large number of basis functions, estimating Θ by least squares tends to overfit. Thus, following Marx and Eilers,38 we estimate Θ by minimising the penalised least squares:
where P(Θ) is the penalty used in Marx and Eilers38 and is essentially equivalent to the penalty and λ is a tuning parameter that balances model fit and smoothness of estimate and can be selected by cross‐validation methods. Then, we estimate g(a i,a j) by and apply the inverse Fisher transform to to obtain .
We note that this penalised least squares formula incorporates a constant variance term τ 2, thus implying that the variance across the studies is independent of time. The model could be extended to incorporate a term , which allows for different variances across different combinations of time points i and j. This approach has not been considered here but could merit further exploration in the future.
To ensure the integrity of the correlation matrix, we remove the diagonal elements (which are 1s) prior to the smoothing and then normalise the smoothed correlations to ensure that the final matrix has 1s on the diagonal. Specifically, let be the estimated correlation matrix after smoothing; then, the final matrix is , where .
Anderson C, Xiao L, Checkley W. Using data from multiple studies to develop a child growth correlation matrix. Statistics in Medicine. 2019;38:3540–3554. 10.1002/sim.7696
REFERENCES
- 1. Tanner JM. Fetus Into Growth: Physical Growth from Conception to Maturity. Cambridge, Mass.: Harvard University Press; 1979. [Google Scholar]
- 2. Tanner JM. A History of the Study of Human Growth. Cambridge: Cambridge University Press; 1981. [Google Scholar]
- 3. Allen LH. Nutritional influences on linear growth: a general review. Eur J Clin Nutr. 1994;48:S75‐89. [PubMed] [Google Scholar]
- 4. Victora CG, de Onis M, Hallal PC, Blössner M, Shrimpton R. Worldwide timing of growth faltering: Revisiting implications for interventions. Pediatrics. 2010;125(3):e473‐80. [DOI] [PubMed] [Google Scholar]
- 5. Prentice AM, Moore SE, Fulford AJ. Growth faltering in low‐income countries. Nutrition and Growth. 2013;106:90‐9. [DOI] [PubMed] [Google Scholar]
- 6. de Onis M, Wijnhoven TMA, Onyango AW. Worldwide practices in child growth monitoring. J Pediatr. 2004;144:461‐465. [DOI] [PubMed] [Google Scholar]
- 7. Cole TJ. Conditional reference charts to assess weight gain in British infants. Arch Dis Child. 1995;73:8‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shields B, Wacogne I, Wright CM. Weight faltering and failure to thrive in infancy and early childhood. BMJ. 2012;345:e5931. [DOI] [PubMed] [Google Scholar]
- 9. Checkley W, Epstein LD, Gilman RH, Black RE, Cabrera L, Sterling CR. Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch‐up growth. Am J Epidemiol. 1998;148:497‐506. [DOI] [PubMed] [Google Scholar]
- 10. Checkley W, Epstein LD, Gilman RH, Cabrera L, Black RE. Effects of acute diarrhea on linear growth in Peruvian children. Am J Epidemiol. 2003;157:166‐175. [DOI] [PubMed] [Google Scholar]
- 11. Lundeen EA, Behrman JR, Crookston BT, et al. Growth faltering and recovery in children aged 1‐8 years in four low‐ and middle‐income countries: Young Lives. Public Health Nutr. 2014;17:2131‐2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. WHO Multicentre Growth Reference Study Group . Length/height‐for‐age, weight‐for‐age, weight‐for‐length, weight‐for‐height and body mass index‐for‐age: Methods and development. Technical Report, World Health Organisation; 2006.
- 13. European commission: Community research and development information service. content project. https://cordis.europa.eu/project/rcn/81320_en.html.
- 14. Iqbal NT, Sadiq K, Syed S, et al. Promising biomarkers of environmental enteric dysfunction: a prospective cohort study in Pakistani children. Sci Rep. 2018;8(1):2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naylor C, Lu M, Haque R, et al. Environmental enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine. 2015;2(11):1759‐1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lopez de Romaña G, Brown KH, Black RE, Kanashiro HC. Longitudinal studies of infectious diseases and physical growth of infants in Huascar, an underprivileged peri‐urban community in Lima, Peru. Am J Epidemiol. 1989;129(4):769‐784. [DOI] [PubMed] [Google Scholar]
- 17. Brown KH, López de Romaña D, Arsenault JE, Peerson JM, Penny ME. Comparison of the effects of zinc delivered in a fortified food or a liquid supplement on the growth, morbidity, and plasma zinc concentrations of young Peruvian children. Am J Clin Nutr. 2007;85(2):538‐547. [DOI] [PubMed] [Google Scholar]
- 18. Begin F, Santizo MC, Peerson JM, Torun B, Brown KH. Effects of bovine serum concentrate, with or without supplemental micronutrients, on the growth, morbidity, and micronutrient status of young children in a low‐income, peri‐urban Guatemalan community. Eur J Clin Nutr. 2008;62(1):39‐50. [DOI] [PubMed] [Google Scholar]
- 19. Hennig BJ, Unger SA, Dondeh BL, et al. Cohort profile: the Kiang West Longitudinal Population Study (KWLPS)—a platform for integrated research and health care provision in rural gambia. Int J Epidemiol. 2015;46(2):e13‐e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ackatia‐Armah RS, McDonald CM, Doumbia S, Erhardt JG, Hamer DH, Brown KH. Malian children with moderate acute malnutrition who are treated with lipid‐based dietary supplements have greater weight gains and recovery rates than those treated with locally produced cereal‐legume products: a community‐based, cluster‐randomized trial. Am J Clin Nutr. 2015;101(3):632‐645. [DOI] [PubMed] [Google Scholar]
- 21. MAL‐ED Investigators . Childhood stunting in relation to the pre‐and postnatal environment during the first 2 years of life: the MAL‐ED longitudinal birth cohort study. PLoS Med. 2017;14(10):e1002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hess S, Abbeddou S, Yakes E, et al. Small‐quantity lipid‐based nutrient supplements together with malaria and diarrhea treatment improve growth and neurobehavioral development in young Burkinabe children. PLoS ONE. 2015;10:e0122242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rehman AM, Gladstone BP, Verghese VP, Muliyil J, Jaffar S, Kang G. Chronic growth faltering amongst a birth cohort of Indian children begins prior to weaning and is highly prevalent at three years of age. Nutr J. 2009;8(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mondal D, Minak J, Alam M, et al.. Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis. 2011;54(2):185‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown KH, Black RE, Becker S, Hoque A. Patterns of physical growth in a longitudinal study of young children in rural Bangladesh. Am J Clin Nutr. 1982;36(2):294‐302. [DOI] [PubMed] [Google Scholar]
- 26. Haque R, Ali IM, Sack RB, Farr BM, Ramakrishnan G, Petri Jr WA. Amebiasis and mucosal igA antibody against the Entamoeba histolytica adherence lectin in Bangladeshi children. J Infect Dis. 2001;183(12):1787‐1793. [DOI] [PubMed] [Google Scholar]
- 27. Cole TJ. Secular trends in growth. Proc Nutr Soc. 2000;59:317‐324. [DOI] [PubMed] [Google Scholar]
- 28. Cole TJ. Presenting information on growth distance and conditional velocity in one chart: practical issues of chart design. Stat Med. 1998;17:2697‐2707. [DOI] [PubMed] [Google Scholar]
- 29. Cole TJ. The development of growth references and growth charts. Ann Hum Biol. 2012;39:382‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Argyle J, Seheult AH, Wooff DA. Correlation models for monitoring child growth. Stat Med. 2008;27:888‐904. [DOI] [PubMed] [Google Scholar]
- 31. Yao F, Müller HG, Wang JL. Functional data analysis for sparse longitudinal data. J Amer Statist Assoc. 2005;100:577‐590. [Google Scholar]
- 32. Xiao L, Li C, Checkley W, Crainiceanu C. Fast covariance estimation for sparse functional data. Stat Comput. 2018:28:511‐522. https://link.springer.com/article/10.1007/s11222-017-9744-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiao L, Li C, Checkley W, Crainiceanu C. R package face: fast covariance estimation for sparse functional data. https://cran.r-project.org/web/packages/face/index.html; 2016. [DOI] [PMC free article] [PubMed]
- 34. Hedges LV, Olkin I. Statistical Methods for Meta‐Analysis. New York: Academic Press; 1985. [Google Scholar]
- 35. Cheung MWL, Chan W. Meta‐analytic structural equation modeling: a two‐stage approach. Psychol Methods. 2005;10:40‐64. [DOI] [PubMed] [Google Scholar]
- 36. Becker BJ. Model‐based meta‐analysis In: Cooper H, Hedges LV, Valentine JC, eds. The Handbook of Research Synthesis and Meta‐Analysis. New York, NY, US: Russell Sage Foundation; 2009:377‐395. [Google Scholar]
- 37. Cooper H, Hedges LV, Valentine JC. The Handbook of Research Synthesis and Meta‐Analysis. New York, NY, US: Russell Sage Foundation; 2009. [Google Scholar]
- 38. Marx BD, Eilers PHC. Multidimensional penalized signal regression. Technometrics. 2005;47:13‐22. [Google Scholar]
- 39. Schwarzer G. meta: General package for meta‐analysis. http://CRAN.R-project.org/package=meta, R package version 4.3‐2; 2015.
- 40. Rogol A, Clark PA, Roemmich JN. Growth and pubertal development in children and adolescents: effects of diet and physical activity. Am J Clin Nutr. 2000;72(2 Suppl):521S‐8S. [DOI] [PubMed] [Google Scholar]
- 41. Ramsay JO, Silverman BW. Functional Data Analysis. New York: Springer; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item
Supporting info item
Supporting info item
Supporting info item


