Abstract
One‐third of the world's humans has latent tuberculosis infection (LTBI), representing a large pool of potentially active TB. Recent LTBI carries a higher risk of disease progression than remote LTBI. Recent studies suggest important roles of antibodies in TB pathology, prompting us to investigate serum antibody profiles in a cohort with LTBI. In this single‐center prospective observational study, we analyzed IgG‐antibody concentrations against five major Mycobacterium tuberculosis (Mtb) antigens (including 6 kDa early secretory antigenic target (ESAT6), CFP10, and antigen 85A, which are expressed mainly in the growth phase; and mycobacterial DNA‐binding protein 1 (MDP1) and alpha‐crystallin like protein (Acr), which are expressed in the dormant phases) in individuals with recent (n=13) or remote (n=12) LTBI, no Mtb infection (n=19), or active TB (n=15). Antibody titers against ESAT6 and MDP1 were significantly higher in individuals with recent LTBI than in those with no Mtb infection or remote LTBI. All pairwise antibody titers against these five major antigens were significantly correlated throughout the stages of Mtb infection. Five individuals with recent LTBI had significantly higher antibody titers against ESAT6 (P = 0.03), Ag85A (P = 0.048), Acr (P = 0.057), and MDP1 (P = 0.0001) than in individuals with remote LTBI; they were also outside the normal range (+2 SDs). One of these individuals was diagnosed with active pulmonary TB at 18‐month follow‐up examination. These findings indicated that concentrations of antibodies against both multiplying and dormant Mtb are higher in recent LTBI and that individuals with markedly higher antibody titers may be appropriate candidates for prophylactic therapy.
Keywords: Biomarker, latent tuberculosis infection, mycobacterial DNA‐binding protein 1, serum antibody
Abbreviations
- Acr
alpha‐crystallin like protein
- CFP
culture filtrate protein
- Dos S
sensor histidine kinase of DosR
- EIA
enzyme‐linked immunosorbent assay
- ESAT
early secretory antigenic target
- IGRA
interferon gamma release assay
- LAM
lipoarabinomannan
- LTBI
latent tuberculosis infection
- MAC
Mycobacterium avium complex
- MDP1
mycobacterial DNA binding protein 1
- Mtb
Mycobacterium tuberculosis
- TB
tuberculosis
- TBGL
tuberculous glycolipid
1. INTRODUCTION
In 2016, 10.4 million people newly developed tuberculosis (TB), in the vast majority of cases arising from LTBI.1 It is estimated that one‐third of all humans have asymptomatic Mtb, and are therefore at risk of disease progression. Individuals with past or old TB who have abnormal chest radiographs are at higher risk of disease progression.2 Individuals with LTBI are also asymptomatic, but have no abnormalities on chest radiographs. However, LTBI also carries the risk of TB progression depending on the time interval since infection. The American Thoracic Society classifies Mtb‐infected individuals as symptomatic or asymptomatic.2 They divide the asymptomatic group into the following subgroups: (i) past TB (inactive TB; class 4); (ii) LTBI (class 1), which is further divided into recent LTBI (<2 years since infection) or remote LTBI (>2 years since infection); and (iii) preclinical TB (including asymptomatic individuals who subsequently develop active TB). Identification of people at risk of TB progression is important regarding administration of appropriate preventative medication. However, it is difficult to differentiate recent LTBI or preclinical TB from remote LTBI because of the wide spectrum of poorly characterized clinical features of Mtb infection.3 Recently validated diagnostic methods, such as IGRAs, do not permit assessments of the risk of TB progression.4 Thus, distinguishing between stages of Mtb infection remains an important diagnostic challenge.
Recent studies suggest that antibodies play important roles in TB pathology and diagnosis.5, 6 We have previously reported that antibody titers against the following six of the 23 types of Mtb antigens, antigen 85A (Ag85A), 6 kDa ESAT6, 10 kDa CFP10, MDP1, Acr, and sensor histidine kinase of DosR (Dos S), are high in individuals with past TB.7 Antigen 85A is expressed in the growth phase of Mtb, whereas ESAT6 and CFP10 are expressed in the growth to stationary phases8, 9 and MDP1, Acr, and Dos S are upregulated in the stationary to dormant phases.10 These data suggest the coexistence of both growing and dormant Mtb in individuals with past TB and the utility of antibody responses in determining TB infection status. However, it is, still necessary to evaluate biomarker‐targeted antibody responses in individuals with recent LTBI and preclinical TB, both of whom are at higher risk of TB progression than individuals with remote LTBI.
In this study, we conducted a hospital‐based survey at Toneyama National Hospital, Osaka, Japan in which, since 2000, patients with active TB have been receiving chemotherapy for approximately 2 months in a specialized negative‐pressure ward. Although staff always wear N95 masks in this ward, several staff members were infected with Mtb and their disease progressed to active TB in 2000‐2010. In 2010, healthcare workers at Toneyama National Hospital underwent extensive contact screening regarding transmission of TB. Individuals considered to have recent or remote LTBI on the basis of their IGRAs in an annual medical examination were selected from all staff members who had been in contact with patients with active TB. Serum antibody profiles against major Mtb proteins, including the six above‐listed antigens, were analyzed in individuals with recent LTBI, remote LTBI, no Mtb infection, and active TB to identify biomarkers to determine of TB progression.
2. MATERIALS AND METHODS
2.1. Subjects
With the aim of preventing nosocomial transmission of TB, IGRAs have been included in periodic medical examinations of healthcare workers since 2010. All staff members with positive reactions to BCG vaccination and who had been in contact with patients with TB underwent annual medical examinations, including chest X‐ray and irregular TB patient contact examinations, from 2011 to 2015. LTBI was diagnosed on the basis of positive IGRAs, with no clinical, bacteriological, or radiographic evidence of active disease. Individuals with LTBI were further categorized as having recent or remote LTBI according to the results of annual and irregular medical examinations. IGRAs to identify recent LTBI have been assessed yearly in new staff members and staff members who were previously IGRA‐negative. The specimens for serodiagnosis were collected from the staff and inpatients with TB after obtaining written informed consent. This study was approved by the Research and Ethical Committees of the National Toneyama Hospital (2009‐0920) and Osaka City University Graduate School of Medicine (1458).
Participants were divided into the following groups:.
-
1)
Recent LTBI group with positive IGRA (<2 years after infection). This group consisted of 13 individuals (aged 43 ± 10 years, male/female ratio 3/10) with positive conversion of IGRA, including 10 identified as a result of annual medical examinations in 2011 and 2012 and three identified in irregular contact examinations of staff members who had previously been IGRA‐negative.
-
2)
Remote LTBI group (>2 years after infection). This group consisted of 12 individuals (aged 50 ± 6 years, male/female ratio 3/9) who had been IGRA‐positive from 2 years before 2012.
-
3)
No Mtb infection group. This group consisted of 19 individuals (aged 44 ± 10 years, male/female ratio 5/14) who were IGRA‐negative results and had negative results on testing with three commercially available serodiagnostic tests (Determiner TBGL kit [Kyowa Medex, Tokyo, Japan], MycoDot kit [Mossman Associates, Blackstone, MA], and MAC EIA kit [Tauns Laboratory, Shizuoka, Japan]).
-
4)
Positive serodiagnosis group. This group consisted of 15 individuals (aged 44 ± 8 years, male/female ratio 5/10) who were IGRA‐negative and positive according to Determiner TBGL and/or MycoDot tests. One individual with a positive MAC EIA test result was excluded from this study.
-
5)
Active TB group. This group included 15 inpatients (aged 47 ± 18 years, male/female ratio 8/7) whose were diagnosed as having active TB by microbiologic examination of sputum specimens yielding positive cultures and positive RNA amplification tests specific for Mtb (TRC Test, TRCRapid‐160; Tosoh; Tokyo, Japan) in 2011.
2.2. Commercially available IGRAs and serological tests
IGRAs was performed using QFT‐Gold according to the manufacturer's instructions (Cellestis, Carnegie, Australia) with CFP10 and ESAT6 of Mtb as antigens and a cutoff value for a positive IFN‐γ test of ≥0.35 IU/mL. Anti‐TBGL antibodies were measured by ELISA using a Determiner TBGL Kit with tuberculous glycolipids, including cord factor as an antigen. Anti‐LAM antibodies were detected using a MycoDot kit. Antibody detection was achieved using representative nitrocellulose discs for dot‐blot assays, the color intensities of the test reactions being compared with those of references. Kits for anti‐LAM and anti‐TBGL antibody testing were specific for IgG responses. The manufacturers' instructions for test performance were followed for all commercially available assays used in this study. Results of the TBGL test are expressed as U/mL and of the LAM test using a 5‐point scale. The cutoff points were 2 U/mL for anti‐TBGL antibodies and 1 + for anti‐LAM antibodies, in accordance with the standards set by the manufacturer of each test. GPL serum core IgA antibody titers were measured using a MAC EIA kit according to the manufacturer's instructions. All specimens were assayed without prior knowledge of the clinical status. Materials and reagents have been described in detail in a previous paper.7
2.3. Recombinant protein preparation
Recombinant Mtb antigens, including ESAT6 (Rv3875), CFP10 (Rv3874), MDP1 (Rv2986c), HBHA (Rv0475), Acr (Rv2031c), HrpA (Rv0251c), DosS (Rv3132), Ag85A (Rv3804c), and Ag85B (Rv1886c), were expressed in Escherichia coli BL21 (DE3) using a pET21b vector and purified by affinity chromatography, as described previously.7
2.4. ELISA
Titers of IgG antibodies against recombinant Mtb proteins were determined by ELISA as follows. Ninety‐six well microplates (Sumilon Type H; LMS, Tokyo, Japan) were coated with one of the recombinant antigen in bicarbonate buffer, pH 9.6 (0.5 μg/well) overnight at 4°C. The plates were then blocked with PBS containing 0.05% Tween 20 and 5% skim milk for 12 hours at 4°C and washed four times with PBS containing 0.05% Tween 20. Human serum samples diluted 1:100 in PBS containing 0.05% Tween 20 and 0.5% skim milk were incubated for 12 hours at 4°C. After washing, HRP‐conjugated anti‐human IgG antibodies were added at a 1:5000 dilution. After 1 hour of incubation at 37°C, the plates were washed four times, and 100 μL of SureBlue reserve‐TMB Microwell Peroxidase Substrate (KPL, Gaithersburg, MD) added to each well. The reactions were stopped after 3 minutes by the addition of 50 μL of 0.1 M HCl, after which the plates were read at 450 nm using a Multiskan (Thermo Fisher Scientific K.K., Yokohama, Japan). Samples were analyzed in triplicate. The normal range for each antibody titer was defined as less than two SDs above the mean titer of the group with no Mtb infection.
2.5. Statistical analyses
Statistical analyses were performed using JMP 9 (SAS Institute, Cary, NC). Antibody titers were obtained by delta OD and are reported as means ± SD. Logarithm‐converted antibody titers were used for statistical analyses and correlations between antibody titers were analyzed. The Kruskal–Wallis test was used to determine differences in antibody titers between groups. Differences between pairs of groups were analyzed using the Tukey–Kramer honest significant difference post hoc test. Relationships between antibody titers were assessed using Pearson correlation coefficients and linear regression analyses. Differences were considered significant when P < 0.05. The cut‐off values for positivity of each antibody were defined as two SDs above the mean titer of the group with no Mtb infection.
3. RESULTS
3.1. Enrollment of individuals with recent LTBI
Thirteen individuals with recent LTBI diagnosed on the basis of results of annual medical examinations and irregular screenings from 2011 to 2015 were enrolled (Table 1). No individuals with positive IGRA conversion were identified in 2013, 2014, or 2015. All staff, including those in the recent LTBI group, reported no respiratory symptoms and had no radiographic evidence of current disease. Serum specimens obtained in 2011 and 2012 were used to measure IgG antibody titers against Mtb antigens.
Table 1.
Enrollment of healthcare workers with recent LTBI
| IGRA results | Number of HCWs | Number of TB inpatients/year | ||||
|---|---|---|---|---|---|---|
| Positive | Positive conversion | Negative | Indeterminate | |||
| Annual examinations | ||||||
| 2010 | 15 (9.2%) | 131 (80.4%) | 17 (10.4%) | 163 | 489 | |
| 2011 | 9 (6.7%) | 5 | 108 (80%) | 18 (13.3%) | 135 | 335 |
| 2012 | 13 (8.7%) | 5 | 117 (78%) | 20 (13.3) | 150 | 257 |
| 2013 | 6 (2.9%) | 0 | 199 (94.7%) | 5 (2.4%) | 210 | 215 |
| 2014 | 2 (1.1%) | 0 | 178 (96.7%) | 4 (2.2%) | 184 | 208 |
| 2015 | 0 | 0 | 148 (99.3%) | 1 (0.7%) | 149 | 160 |
| Irregular screenings | ||||||
| 2011–2012 | 3 | 3 | 15 |
Abbreviations: HCWs: Health Care Workers.
3.2. Comparisons of antibody titers
Comparisons of IgG antibody titers against Mtb antigens in healthcare workers and inpatients with active TB are summarized in Table 2. There are no significant differences in age between the five groups. Antibody titers against the five selected antigens were significantly higher in patients with active TB than in the group of non‐Mtb‐infected persons (P < 0.0001). The rates of positivity for antibodies against ESAT6, CFP10, Ag85A, Acr, and MDP1 were 60%, 33%, 67%, 80%, and 80%, respectively. Two patients (13%) were negative results for all five tested antigens. The antibody titers against four antigens, the exception being CFP10, were also significantly higher than those in the recent and remote LTBI groups. However, there was no significant difference in antibody titers against ESAT6 between the active TB and recent LTBI groups.
Table 2.
Comparison of IgG antibody titers against M. tuberculosis (Mtb) antigens between healthcare workers and patients with active TB
| Healthcare workers | |||||
|---|---|---|---|---|---|
| Active TB | No Mtb infection | Recent LTBI | Remote LTBI | Serodiagnosis (+) | |
| Number (M:F) | 15 (8:7) | 19 (5:14) | 13 (3:10) | 12 (3:9) | 15 (5:10) |
| Age, y | 47 ± 18.1 (23–78) | 44 ± 9.8 (27–58) | 43 ± 10.3 (24–58) | 50 ± 6.4 (38–58) | 44 ± 7.5 (28–57) |
| Antigens | Antibody titers (ΔOD) | ||||
| ESAT6 | 0.49 ± 0.56 | 0.04 ± 0.02# | 0.31 ± 0.41 | 0.07 ± 0.07* | 0.04 ± 0.02$ |
| CFP10 | 0.48 ± 0.49 | 0.12 ± 0.1# | 0.24 ± 0.24 | 0.24 ± 0.24 | 0.21 ± 0.16 |
| Acr | 0.84 ± 0.51 | 0.15 ± 0.07# | 0.38 ± 0.28* | 0.26 ± 0.15# | 0.25 ± 0.18$ |
| MDP1 | 1.52 ± 0.86 | 0.27 ± 0.12# | 0.86 ± 0.43* | 0.33 ± 0.15# | 0.38 ± 0.13# |
| Ag85A | 1.93 ± 0.87 | 0.76 ± 0.29# | 1.01 ± 0.87* | 1.02 ± 0.44$ | 0.91 ± 0.47& |
| QFT‐Gold | (‐):15, ( ± ):4, ( + ):0 | (‐):0, ( ± ):0, ( + ):13 | (‐):0, ( ± ):0, ( + ):12 | (‐):13, ( ± ):2, ( + ):0 | |
| TBGL (unit/mL) | 0.20 ± 0.34 | 1.62 ± 2.06 | 1.88 ± 2.59 | 5.08 ± 6.44 | |
| LAM | (‐):19, ( + ):0 | (‐):9, ( + ):4 | (‐):9, ( + ):3 | (‐):5, ( + ):10 | |
| GPL‐core (unit/mL) | 0.01 ± 0.02 | 0.05 ± 0.05 | 0.01 ± 0.01 | 0.03 ± 0.03 | |
Abbreviations: ΔOD, optical density (average ± SD); M:F, male:female.
P‐values between active TB and other groups:
ESAT6 (#: P = 0.0006, *: P = 0.0071, $: P = 0.0014)
CFT10 (#: P = 0.0033).
Acr (#: P < 0.0001, *: P = 0.0005)
MDP1 (#: P < 0.0001, *: P = 0.0015)
Ag85A (#: P < 0.0001, *: P = 0.0017, $: P = 0.0027, &: P < 0.0001)
Individuals with recent LTBI had significantly higher logarithm‐converted antibody titers against ESAT6 than those in the groups with no Mtb infection or remote LTBI (P < 0.0001; P = 0.0004, respectively); the same was true for MDP1 (P < 0.0001; P = 0.0003, respectively) (Figure 1). In the recent LTBI group, the proportions with antibodies against ESAT6, CFP10, Ag85A, Acr, and MDP1 were 85%, 8%, 38%, 31%, and 77%, respectively. Two patients(15%) had no absorbance for antibodies against any of the five antigens. No increases in antibody titers against other antigens (DosS, HBHA, HrpA, and Ag85B) were detected in the recent LTBI group (data not shown).
Figure 1.
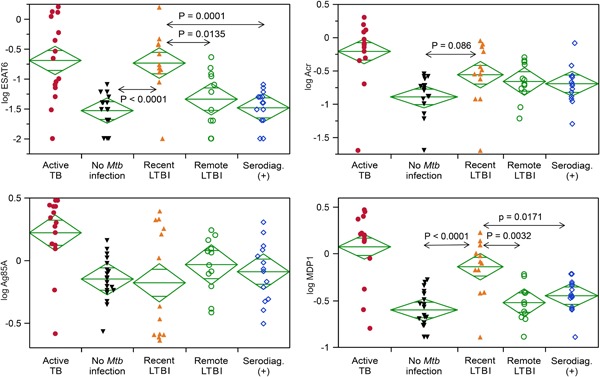
Comparative analysis of IgG antibody titers against four Mtb antigens among the five study groups. Individuals with recent LTBI had significantly higher logarithm‐converted antibody titers against ESAT6 (P < 0.0001; P = 0.0004) and MDP1 (P < 0.0001; P = 0.0003) than those in the groups with no Mtb infection or remote LTBI. ESAT, early secretory antigenic target; LTBI, latent tuberculosis infection; MDP1, mycobacterial DNA‐binding protein 1; Mtb, Mycobacterium tuberculosis [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Relationships between logarithm‐converted antibody titers against the five selected antigens
There were significant correlations between all pairs of antibody titers against ESAT6, CFP10, Ag85A, Acr, and MDP1 (all P < 0.0001) (Figure 2). Although some antibodies were not detected in some patients, the antibody titers in patients with active TB were the highest (right‐upper area in Figure 2), followed by the antibody titers in individuals with recent LTBI. The antibody titers of individuals in the group with no Mtb infection were generally low.
Figure 2.
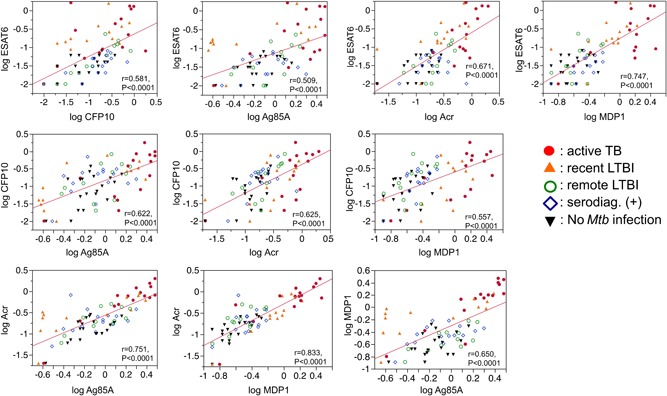
Relationships between logarithm‐converted antibody titers against the five selected antigens in all participants. There were significant correlations between all pairs of antibody titers against ESAT6, CFP10, Ag85A, Acr, and MDP1 (all P < 0.0001). Each symbol indicates an individual from one of the five groups [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Detection of preclinical TB
In five individuals with recent LTBI (including the one indicated by an arrow and case numbers 1, 2, 3, and 4 in Figure 3), antibody titers against ESAT6 (P = 0.03), Ag85A (P = 0.048), Acr (P = 0.057), and MDP1 (P = 0.0001) were significantly higher than those in the remote LTBI group. These antibody titers were also above their normal ranges. The individual preclinical TB indicated with an arrow in Figure 3 was diagnosed as having active pulmonary TB with pleurisy by chest X‐ray examination 19 months after measurement of serum antibody concentrations (Figure 4).
Figure 3.
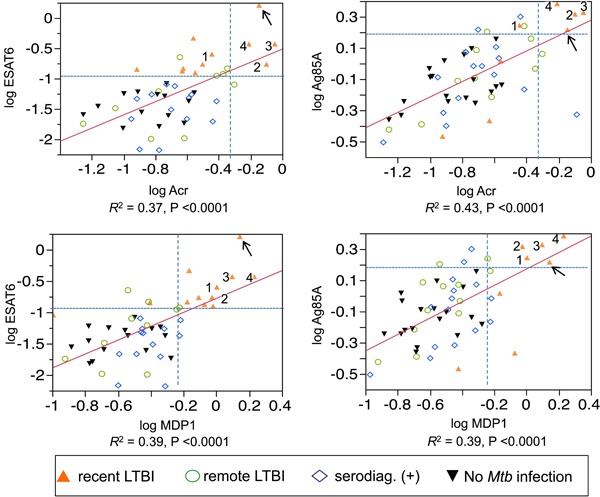
Antibody titers against four antigens are shown for all healthcare workers, except for those whose antibody titers were below the detection level. The cutoff values (dotted lines) for positivity were defined as two SDs above the mean titer in the group without Mtb infection. Arrow indicates an individual with preclinical TB. Numbers 1, 2, 3, and 4 indicate cases 1, 2, 3, and 4 in the recent LTBI group, respectively. In these five individuals, the antibody titers against ESAT6 (P = 0.03), Ag85A (P = 0.048), Acr (P = 0.057), and MDP1 (P = 0.0001) were significantly higher than those in the remote LTBI group [Color figure can be viewed at wileyonlinelibrary.com]
Figure 4.
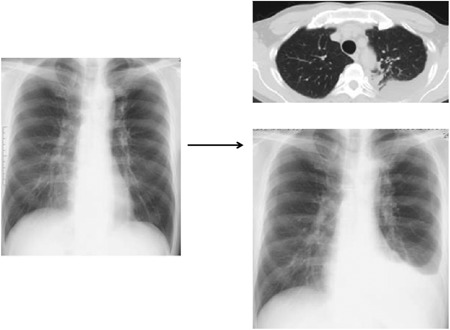
Detection of preclinical TB. An individual with recent LTBI was found to have progressed to having active pulmonary TB with pleurisy 19 months after measurement of serum antibody titers. Log‐corrected antibody titers for ESAT‐6, CFP10, MDP1, Acr, and Ag85A were 0.2, −0.57, 0.14, −0.14, and 0.22, respectively
4. DISCUSSION
To identify biomarkers of TB progression in asymptomatic individuals, we conducted a hospital‐based survey and compared biomarker‐targeted antibody responses between individuals with recent LTBI and remote LTBI, the former being at higher risk of TB progression than the latter.2 For controls, we also assessed sera of TB patients and staff members with no Mtb infection. Negative IGRA results alone are not sufficient to conclude there is no Mtb infection, as indicated by some patients with active TB reportedly being negative for IGRA.11 Therefore, to avoid false negatives in the present study, the group with no Mtb infection was required to be negative according to three commercially available serodiagnostic tests in addition to being negative for IGRA. However, we did not determine the sensitivity and specificity for individual antibodies because there were too few individuals with no Mtb infection (negative controls) and hospital staff are always at risk of infection with acid‐fast bacteria. Although TB‐specific antigens expressed in the growth or dormant phases are required for an accurate diagnosis of recent LTBI, ESAT6 and CFT10 are present in Mycobacterium kansasii and MDP1 is present in M. avium and other mycobacteria. The sensitivity of each anti‐TB antibody is not high enough to make a definite diagnosis of recent LTBI. Therefore, a diagnostic antibody panel using a combination of multiple antigens (TB‐specific antigens, nontuberculous mycobacteria [NTM]‐specific antigens, latent infection‐related antigens, and common antigens) is necessary for the identification of appropriate candidates for prophylactic therapy.11
In our study, antibody titers against ESAT6 and MDP1 were significantly higher in recent LTBI than in remote LTBI. Several individuals in the recent LTBI group had high antibody titers against Ag85A or Acr. The antibody titers to DosS, HBHA, HrpA, and Ag85B were under the detection limits in most participants in this study; however, other useful biomarkers for detecting LTBI, such as Mtb proteins Rv0222 and Rv3403c, Mtb thymidylate kinase antigen, and LAM, have been reported.12, 13, 14, 15 ESTA6 and Ag85A are expressed in growing bacilli, and Acr and MDP1 are upregulated in the stationary to dormant phases. The antibody responses against these antigens, which are associated with different growth states and correlated with bacterial burden,16, 17, 18 were strongest in patients with active TB, followed by those with recent LTBI (Figure 2). These results suggest that growing and dormant bacilli coexist in individuals with Mtb infection and that some, but not all, major antigens are useful antibody targets for determining the risk of TB progression. While TB granulomas differ in cellular composition and structure between patients with LTBI and those with active TB,19 different types of granulomatous lesions may be found in the same individual, either with active TB20 or with LTBI.21 In our previous study, immunohistochemistry revealed the colocalization of Ag85A, an antigen expressed by growing Mtb, and MDP1, an antigen expressed by stationary to dormant Mtb, inside tuberculous granuloma lesions in an asymptomatic individual with past TB, showing that Mtb in lesions can express both Ag85A and MDP1.7 It is conceivable that there are heterogeneous populations of dormant and multiplying tubercle bacilli within granulomatous lesions throughout the stages of TB infection, as observed in the analysis of past TB.
Identifying and treating (eg, isoniazid preventive therapy against growing bacilli, chemical prophylaxis) Mtb‐infected individuals with a risk of progression to active disease is crucial in achieving TB control.22, 23, 24 The five asymptomatic individuals with recent LTBI shown in Figure 3 had much higher antibody titers against all four antigens than those in the groups with remote LTBI and no Mtb infection. One individual among the five cases was later found to have developed active disease and was classified as preclinical TB. However, the present findings need validation in prospective, multicenter trials to determine whether such individuals are candidates for prophylactic therapy.
MDP1 has pleiotropic functions and is an essential protein for Mtb. It controls gene expression and suppresses mycobacterial multiplication under the hypoxic conditions of macrophages,25, 26 and also has ferritin‐like activity for controlling iron homeostasis.27 MDP1 stores iron inside bacteria and prevents the iron‐dependent generation of oxygen radicals.27 Iron is also essential for the survival and multiplication of Mtb inside macrophages. Our results and those of previous studies indicate that this protein is expressed more strongly in the latent stage than in the active disease state. This protein is likely involved in the decreased growth rate and long‐term persistence of Mtb in the latent state, thus accounting for IgG production in individuals with recent LTBI.
The Ag85 complex has mycolyl transferase enzymatic activity and mediates dynamic remodeling of mycolic acid‐containing glycolipids in the cell wall via its broad substrate specificity.28, 29, 30 These immune responses to glycerol monomycolate occur specifically in people with LTBI, but not in patients with active TB.31 Thus, Ag85‐dependent exchange of trehalose 6‐monomycolate to glycerol monomycolate is likely to occur in LTBI. It is not surprising that Ag85 may be useful for detecting asymptomatic Mtb infection with a risk of TB progression. It may be possible to identify individuals at high‐risk of preclinical or active TB by assessing IgA and/or IgG responses to the other Mtb protein antigens.32
Although ESAT6, CFP10, and Acr are Mtb‐specific antigens, antibodies against Ag85A and MDP1 are produced in the sera of patients with pulmonary MAC disease; these antigens exist in other acid‐fast bacilli (data not shown). A patient who was excluded on the basis of a MAC test result in this study was later diagnosed with MAC disease. To accurately detect recent LTBI, it is necessary to exclude NTM infection using biomarkers with antigenicity that is specific to other acid‐fast bacteria, such as MAC‐specific glycopeptide lipid.33
Accurate detection of recent LTBI/preclinical TB status and progression from LTBI to active TB disease can be achieved by assessing both humoral and cellular immune responses with combinations of characteristic antigens, including growth‐related ESAT6, CFP10, Ag85A, dormancy‐related MDP1, and Acr. Accurate detection will facilitate more effective preventive therapy and reduction in disease incidence, paving the way for eradication of TB. This study was performed at a single facility. Although cases with recent LTBI were accurately identified and followed regularly for 6 months, the sample size was small. Further larger multicenter trials are needed.
CONFLICTS OF INTERESTS
The authors declare that they have no conflict of interests.
ACKNOWLEDGMENTS
This study was supported by the Ministry of Health, Labor, and Welfare (Research on Emerging and Re‐emerging Infectious Diseases) and the Research Program on Emerging and Re‐emerging Infection from the Japan Agency for Medical Research and Development, AMED (2012‐Emerging and Re‐emerging Infection‐008 and JP18fk0108005). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Maekura R, Kitada S, Osada‐Oka M, et al. Serum antibody profiles in individuals with latent Mycobacterium tuberculosis infection. Microbiology and Immunology. 2019;63:130‐138. 10.1111/1348-0421.12674
The copyright line for this article was changed on 7 August 2019 after original online publication.
References
REFERENCES
- 1. World Health Organization. Global Tuberculosis Report 2016. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 2. Diagnostic standards and classification of tuberculosis in adults and children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;61:1376‐1395. [DOI] [PubMed] [Google Scholar]
- 3. Kunnath‐Velayudhan S, Gennaro ML. Immunodiagnosis of tuberculosis: a dynamic view of biomarker discovery. Clin Microbiol Rev. 2011;24:792‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Horsburgh CR, Jr. , Rubin EJ. Clinical practice. Latent tuberculosis infection in the United States. N Engl J Med. 2011;364:1441‐1448. [DOI] [PubMed] [Google Scholar]
- 5. Lu LL, Chung AW, Rosebrock TR, et al. A functional role for antibodies in tuberculosis. Cell. 2016;167:433‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zimmermann N, Thormann V, Hu B, et al. Human isotype‐dependent inhibitory antibody responses against Mycobacterium tuberculosis . EMBO Mol Med. 2016;8:1325‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Osada‐Oka M, Tateishi Y, Hirayama Y, et al. Antigen 85A and mycobacterial DNA‐binding protein 1 are targets of immunoglobulin G in individuals with past tuberculosis. Microbiol Immunol. 2013;57:30‐33. [DOI] [PubMed] [Google Scholar]
- 8. Elamin AA, Stehr M, Spallek R, Rohde M, Singh M. The Mycobacterium tuberculosis Ag85A is a novel diacylglycerol acyltransferase involved in lipid body formation. Mol Microbiol. 2011;81:1577‐1592. [DOI] [PubMed] [Google Scholar]
- 9. Rizvi N, Singh A, Yadav M, et al. Role of alpha‐crystallin, early‐secreted antigenic target 6‐kDa protein and culture filtrate protein 10 as novel diagnostic markers in osteoarticular tuberculosis. J Orthop Transl. 2016;6:18‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Belay M, Legesse M, Mihret A, et al. Pro‐ and anti‐inflammatory cytokines against Rv2031 are elevated during latent tuberculosis: a study in cohorts of tuberculosis patients, Household Contacts and Community Controls in an Endemic Setting. PLoS One. 2015;10:e0124134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maekura R, Kitada S, Tateishi Y, et al. Consideration of improvement measures from limitations of immunological tests—including interferon‐γ release and antibody‐based detection assays—for Mycobacterium tuberculosis infection. Kekkaku. 2017;92:551‐558. [Google Scholar]
- 12. López‐Ramos JE, Macías‐Segura N, Cuevas‐Cordoba B, et al. Improvement in the diagnosis of tuberculosis combining Mycobacterium tuberculosis immunodominant peptides and serum host biomarkers. Arch Med Res. 2018;49:147‐153. [DOI] [PubMed] [Google Scholar]
- 13. Ren N, JinLi J, Chen Y, et al. Identification of new diagnostic biomarkers for Mycobacterium tuberculosis and the potential application in the serodiagnosis of human tuberculosis. Microb Biotechnol. 2018;11:893‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wayengera M, Kateete DP, Asiimwe B, Joloba ML. Mycobacterium tuberculosis thymidylate kinase antigen assays for designating incipient, high‐risk latent M. tb infection. BMC Infect Dis. 2018;18:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hur YG, Kim A, Kang YA, et al. Evaluation of antigen‐specific immunoglobulin g responses in pulmonary tuberculosis patients and contacts. J Clin Microbiol. 2015;53:904‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silva VM, Kanaujia G, Gennaro ML, Menzies D. Factors associated with humoral response to ESAT‐6, 38 kDa and 14 kDa in patients with a spectrum of tuberculosis. Int J Tuberc Lung Dis. 2003;7:478‐484. [PubMed] [Google Scholar]
- 17. Steingart KR, Dendukuri N, Henry M, et al. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta‐analysis. Clin Vaccine Immunol. 2009;16:260‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wiker HG, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis . Microbiol Rev. 1992;56:648‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin PL, Rodgers M, Smith L, et al. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun. 2009;77:4631‐4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fenhalls G, Stevens L, Moses L, et al. In situ detection of Mycobacterium tuberculosis transcripts in human lung granulomas reveals differential gene expression in necrotic lesions. Infect Immun. 2002;70:6330‐6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barry CE, III , Boshoff HI, Dartois V, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7:845‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR. 2009;49:1‐54. [Google Scholar]
- 23. Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis: a general review. Adv Tuberc Res. 1970;26:28‐106. [PubMed] [Google Scholar]
- 24. Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis. 1999;3:847‐850. [PubMed] [Google Scholar]
- 25. Matsumoto S, Furugen M, Yukitake H, Yamada T. The gene encoding mycobacterial DNA‐binding protein I (MDPI) transformed rapidly growing bacteria to slowly growing bacteria. FEMS Microbiol Lett. 2000;182:297‐301. [DOI] [PubMed] [Google Scholar]
- 26. Niki M, Niki M, Tateishi Y, et al. A novel mechanism of growth phase‐dependent tolerance to isoniazid in mycobacteria. J Biol Chem. 2012;287:27743‐27752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takatsuka M, Osada‐Oka M, Satoh EF, et al. A histone‐like protein of Mycobacteria possesses ferritin superfamily protein‐like activity and protects against DNA damage by Fenton reaction. PLoS One. 2011;6:e20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Belisle JT, Vissa VD, Sievert T, Takayama K, Brennan PJ, Besra GS. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science. 1997;276:1420‐1422. [DOI] [PubMed] [Google Scholar]
- 29. Backus KM, Boshoff HI, Barry CS, et al. Uptake of unnatural trehalose analogs as a reporter for Mycobacterium tuberculosis . Nat Chem Biol. 2011;7:228‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matsunaga I, Naka T, Talekar RS, et al. Mycolyltransferase‐mediated glycolipid exchange in Mycobacteria. J Biol Chem. 2008;283:28835‐28841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Layre E, Collmann A, Bastian M, et al. Mycolic acids constitute a scaffold for mycobacterial lipid antigens stimulating CD1‐restricted T cells. Chem Biol. 2009;16:82‐92. [DOI] [PubMed] [Google Scholar]
- 32. Baumann R, Kaempfer S, Chegou NN, et al. A subgroup of latently Mycobacterium tuberculosis‐infected individuals is characterized by consistently elevated IgA responses to several mycobacterial antigens. Mediators Inflamm. 2015;2015. 364758‐10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kitada S, Kobayashi K, Ichiyama S, et al. Serodiagnosis of Mycobacterium avium‐complex pulmonary disease using an enzyme immunoassay kit. Am J Respir Crit Care Med. 2008;177:793‐797. [DOI] [PubMed] [Google Scholar]


