Abstract
Introduction
Severe burn injuries are known to initiate a profound systemic inflammatory response (SIRS) that may lead to burn shock and other SIRS related complications. Damage associated molecular patterns (DAMPs) are important early signaling molecules that initiate SIRS after burn injury. Mitochondrial DAMPs (mtDAMPs) – such as mitochondrial DNA (mtDNA) - are thought to be the most critical of these early signaling molecules. Previous work in a rodent model has shown that application of a topical immune modulator (p38 MAPK inhibitor) applied directly to the burn wound decreases cytokine expression, reduces pulmonary inflammation and edema and improves survival. Our group has demonstrated that tranexamic acid (TXA) – in addition to its use as an anti-fibrinolytic – has cell protective in vitro effects. We hypothesized that administration of TXA after burn injury would attenuate mtDAMP release and reduce lung inflammation.
Methods
C57/BL6 male mice were subjected to a 40% TBSA scald burn by immersion in an 80°C water bath. Sham animals underwent the same procedure, but were immersed in room temperature water. All animals were resuscitated according to the Parkland formula (3cc × %TBSA × Weight [Kg]) by intraperitoneal injection (IP). One treatment group received the topical application of 1mM solution of p38 MAPK inhibitor after burn injury. The other treatment group received an IP administration of TXA (100 mg TXA per 1 kg weight) after burn injury. Animals were sacrificed at 5 hours after injury or sham treatment. Plasma was collected by cardiac puncture. MtDNA levels in plasma were determined by qPCR. Syndecan-1 levels in plasma were determined by ELISA. Lungs were harvested, formalin fixed and paraffin embedded. Sections of lungs were deparaffinized and stained for Mac1 antigen to detect macrophages. Numbers of macrophages per a standard square unit of lung section were calculated.
Results
Topical p38 MAPK inhibitor and TXA significantly attenuated mtDNA release. Both TXA and the topical p38 MAPK inhibitor reduced lung inflammation as represented by decreased macrophage infiltration. Circulating syndecan-1 levels showed no difference between the untreated burn group and either treatment group.
Conclusion
Both p38 MAPK inhibitor and TXA demonstrated the ability to attenuate burn induced DAMP release and lung inflammation. Beyond its role as an anti-fibrinolytic, TXA may have significant anti-inflammatory effects pertinent to burn resuscitation. Further study is required; however, TXA may be a useful adjunct in burn resuscitation and other non-hemorrhagic shock states.
Keywords: Tranexamic acid, DAMPs, mitochondrial DNA, Burn injury, SIRS, p38 MAPK inhibitor
Background
Despite significant improvements in burn care over the past 60 years, severe burns that are refractory to current fluid resuscitation practices continue to challenge burn care providers, and remain a common cause of burn related mortality and morbidity. This is primarily because of inflammatory and infectious complications resulting from inhalation injury and/or an overstimulated and dysfunctional immune response.1 The loss of microvascular integrity is central to burn pathophysiology and leads to large fluid volume resuscitation requirements, ultimately precipitating widespread tissue edema and end organ dysfunction.2 The vascular endothelial tight junctions & glycocalyx brush border have been studied in trauma and are postulated to contribute to as much as 70% of vascular luminal integrity.3 Furthermore, traumatic injury is known to significantly alter glycocalyx structure and endothelial cell tight junctions through systemic inflammatory cell signaling, resulting in hyper-permeability, increased capillary leak, tissue edema and organ failure.3,4 Pre-clinical data has demonstrated that endovascular integrity is preserved with plasma-based resuscitations when compared to crystalloid in hemorrhagic shock models.4,5 In large clinical trials, plasma based resuscitation has been shown to decrease vascular permeability after hemorrhage resulting from injury, resulting in improved outcomes.6 The preponderant evidence clearly demonstrates that blood product resuscitation is superior to crystalloid and colloid based resuscitations. This phenomenon, however, has not been studied to any extent in burn injury. Despite the well-documented inferiority of crystalloid and colloid fluid solutions in trauma, they remain the mainstay of fluid resuscitation in the setting of burn injury. Given the deleterious effects on endovascular integrity caused by salt-based fluid resuscitation, adjuncts that may reduce these negative effects are desirable.
Recent literature has shown that early systemic inflammatory signaling after injury is induced by a large group of circulating molecules collectively referred to as damage associated molecular patterns (DAMPs) and is responsible for the initial propagation of SIRS and the endotheliopathy of trauma.7,8 Previous work in a rodent model has shown that application of a topical inhibitor of p38 MAPK applied directly to the burn wound decreases systemic cytokine expression and reduces pulmonary inflammation and edema.9 Recent evidence also demonstrates that tranexamic acid (TXA) - in addition to its use as an anti-fibrinolytic – has anti-inflammatory effects in vivo.10 Furthermore, our group has characterized important links between damage associated molecular patterns (DAMPs) and endothelial cell tight junctions as well as leukocyte inflammatory signaling. The results of our preliminary work - which are in agreement with recently published results by Diebel et. al. (11) - show that TXA preserved tight junction integrity and protected endothelial monolayer in vitro (unpublished). Therefore, we hypothesized that administration of TXA after burn injury would attenuate DAMP release and reduce lung inflammation in a rodent burn model. As the anti-inflammatory effects of TXA and the topical p38 MAPK inhibitor are well established, we focused our investigation on elucidating their effects on early (prior to cytokine release from inflammatory cells) damage molecule release.9,12 Specifically, we studied the effects of TXA upon the plasma levels of mtDNA and lung inflammation after severe burn and compared them with the effects of a topical p38 MAPK inhibitor.
Methods
Experimental Animals
All experiments were performed in accordance with the guidelines set forth by the National Institutes of Health for care and use of animals. Approval for the experimental protocol was obtained from the Maine Medical Center Research Institute’s Institutional Animal Care and Use Committee (IACUC). Age and weight matched male C57/BL6 mice (Charles-River Laboratories) weighing 22–25 g were allowed to acclimate for 1 week before being used in any experiment. Animals had unrestricted access to standard chow and water ad libitum throughout the course of the study.
Burn procedure
C57/BL6 male mice were anesthetized via intraperitoneal injection (IP) of a ketamine/xylazine mixture. The dorsal hairs were clipped, and animals were placed in an insulating mold device with an opening calculated to expose 40% total body surface area. After immersion in 80°C water for 30 seconds (to insure a full thickness injury), the burn area was scrub debrided with dry sterile gauze. Animals were resuscitated by IP injection with lactated Ringer’s solution according to the following formula: 4cc × (%TBSA burn) × weight (kg). Each animal received 0.3 mg/kg buprenorphine (Buprenex; Reckitt & Coleman Pharmaceuticals) at 1-hour post injury by subcutaneous injection. Animals were housed by experimental group with no more than five per cage. Sham animals were subjected to identical procedure, resuscitation, and buprenorphine injection, but immersed in room temperature water.
For topical inhibition of p38 MAPK, the inhibitor used was 1×10−3 M SB202190 (Tocris, Minneapolis, MN). Topical inhibitor was applied directly to the burn wound in 2% solution of Hypromellose (Sigma, St. Louis, MO) in PBS. Dose-response studies were performed in a previous topical inhibitor study.9 For TXA treatment, mice received IP injection of a 25 mg/ml solution of tranexamic acid (Mylan, Rockford, IL) in PBS to a final dose of 100 mg per kg body weight. PBS was used as the sham treatment vehicle for topical treatments. Each experimental group consisted of 4–5 animals.
Plasma preparation
Mice were sacrificed by isoflurane inhalation to effect. Blood was obtained through heart puncture and transferred to Eppendorf tubes containing 100 μl of 3.8% sodium citrate. Platelet-free plasma was prepared by two cycles of centrifugation each at 2000g × 10 min.
Determination of mitochondrial DNA content in plasma
mtDNA was isolated from plasma using a Qiamp DNA Blood Mini Kit (51106). Manufacturer’s instructions were followed using 150μl of PBS added to 50μl of plasma sample. 50μl of mtDNA was eluded from the columns. Plasma levels of mtDNA were determined using quantitative PCR. 5μl of the isolated plasmid DNA was analyzed using Biorad iQ Sybr Green Supermix and primers: sense (5’-CCCAGCTACTACCATCATTCAAGT) as (5’-GATGGTTTGGGAGATTGGTTGATGT) and cycled 40 times on a Biorad CFX real time machine. Absolute numbers were calculated from isolated mouse mtDNA internal standard curve.
Determination of syndecan 1 content in plasma
Plasma samples of 40 to 50μl were analyzed according to manufacturer’s instructions by Mouse Syndecan-1/SDC1 Picokine ELISA Kit (Boster, Pleasanton, CA) or Murine CD138 ELISA Set (MyBioSource, San Diego, CA).
Study of macrophage content in lungs
Mouse lungs were fixed overnight in 10% neutral formalin, then transferred for 24h to 70% ethanol and after that passed to the Histopathology core of MMCRI for histochemical staining. In brief, paraffin sections were immunoperoxidase stained for macrophages using the rabbit anti-Mac1 antibodies (Abcam, Cambridge, MA) and counterstained with hematoxylin. Photographs of five random sections of lungs per each animal were taken using 40x objective. Image J program was used to analyze the abundance of macrophages in lung sections (excluding cell-free areas, such as alveoli) that was expressed as mean macrophage number per standard square unit.
Statistical analysis
The experiments were triplicated with consistent results and the results of representative experiments are shown. Quantitative data were analyzed, and graphs were produced using the GraphPad Prism program. Mean values of analyzed parameters and standard deviations (SD) are presented in the graphs. Of 3 standard experimental groups we always performed two comparisons: burnt to control and burnt + treatment to burnt. To adjust for these two comparisons a Bonferroni adjustment was performed where p-values were required to be less than 0.05/2 or 0.025. Only p-values <0.025 are reported as significant. When an additional treatment group was added, the third comparison, between two burnt groups with different treatment types (TXA and TXA + p38 MAPK inhibitor) was made, and the required p-value was adjusted to 0.05/3 or 0.017. We used the unpaired t-test to determine the significance of differences between compared values. When values exhibited unequal variances, we applied the unpaired t-test with Welch correction.
Results
Topical application of a p38 MAPK inhibitor suppresses the release of circulating mtDNA in mice after severe burn
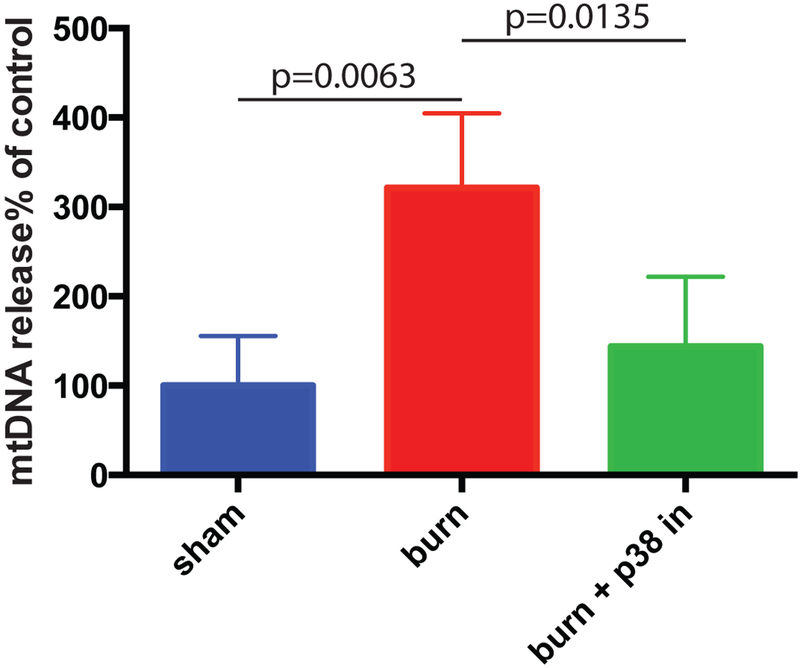
Our previous study (9) has shown that topical p38 MAPK inhibitor (SB 202190) attenuated burn-induced SIRS in mice. DAMP release is postulated to be the earliest event in the immune response to injury.7 Thus, we verified whether p38 MAPK inhibition could also suppress burn-induced systemic release of DAMPs. We used circulating mitochondrial DNA (mtDNA) as a surrogate for generalized systemic DAMP release. SB 202190 was topically applied as 1mM solution immediately after mice underwent the burn procedure as in our previous study. Mice were sacrificed 5 hours after burn. mtDNA content in plasma was determined using Q-PCR. The topical application of the p38 MAPK inhibitor resulted in a statistically significant reduction in plasma mtDNA levels (Figure 1).
Figure 1. p38 MAPK inhibitor suppresses the release of mtDNA to mouse bloodstream induced by severe burn.
p38 MAPK inhibitor SB 202190 was topically applied immediately after mice underwent burning procedure. Mean and SD of mtDNA plasma content are shown. The mean of the control (sham) group was taken as a reference (100%) and used to divide all the means by. The statistical significance of differences between sham (N=4) and burn (N=4), and burn and burn + p38 MAPK inhibitor (N=7) groups was determined using t-test with Welch correction.
TXA suppresses the release of circulating mtDNA in mice after severe burn
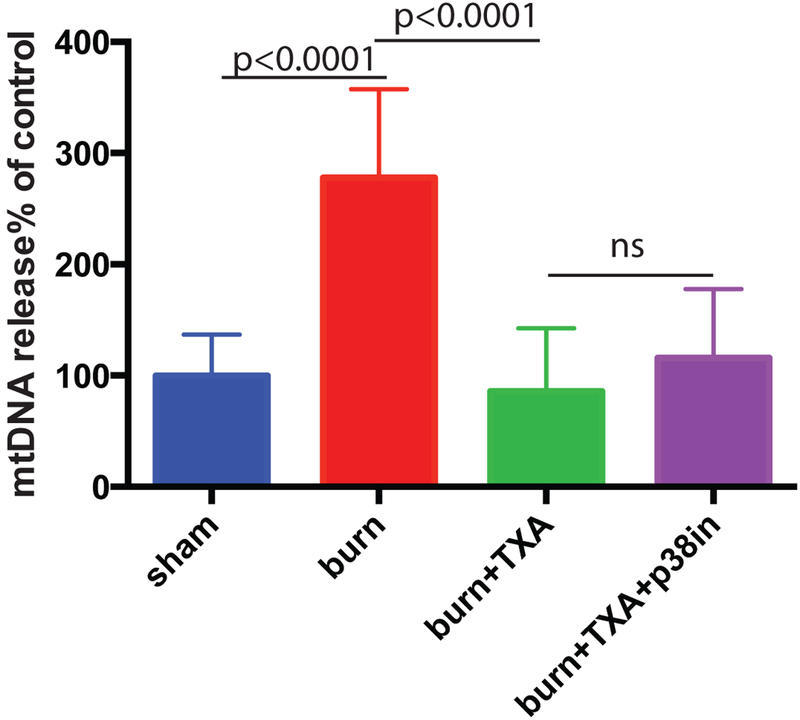
Previous research has established that TXA has anti-inflammatory effects similar to p38 MAPK inhibitor including suppression of pro-inflammatory cytokines.12 Mice received intraperitoneal injection of TXA in the dose of 100 mg per 1 kg body weight immediately after undergoing the burn procedure. A group of mice received both TXA injection and topical application of a p38 MAPK inhibitor. Mice were sacrificed at 5 hours after burn. mtDNA content in plasma was determined using Q-PCR. TXA demonstrated a statistically significant reduction in plasma mtDNA levels (Figure 2). The suppression was similar when topical p38 MAPK inhibitor was applied simultaneously with TXA (Figure 2) and no further decrease of mtDNA plasma levels was observed.
Figure 2. TXA suppresses the release of mtDNA to mouse bloodstream induced by severe burn.
Mice received intraperitoneal injection of TXA immediately after undergoing burning procedure. A group of mice received both TXA injection and topical p38 MAPK inhibitor. Mean and SD of mtDNA plasma content are shown. The mean of the control (sham) group was taken as a reference (100%) and used to divide all the means by. The statistical significance of differences between sham (N=8) and burn (N=9), burn and burn + TXA (N=10), and burn + TXA and burn +TXA + p38 MAPK inhibitor (N=10) groups was determined using t-test with Welch correction.
TXA and Topical p38 MAPK inhibitor suppress macrophage infiltration in mouse lungs
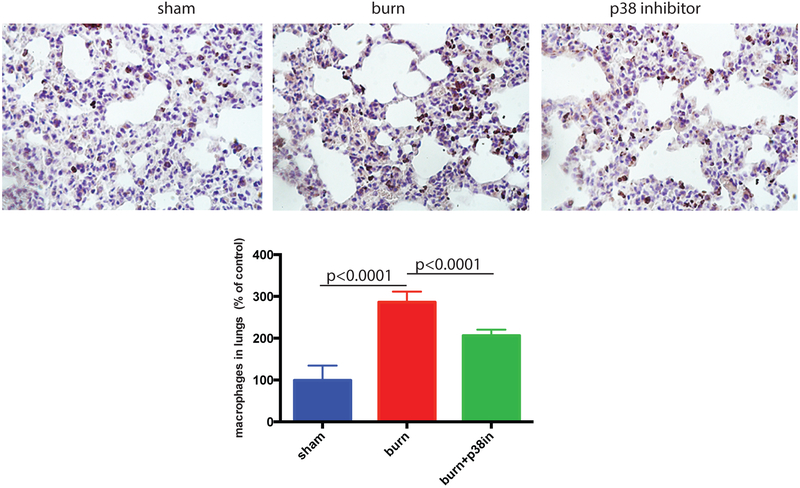
The topical p38 MAPK inhibitor was applied immediately after mice underwent the burn procedure. After sacrifice, mouse lungs were formalin fixed; paraffin sections were prepared, and stained for macrophage marker Mac1 (brown) using immunoperoxidase method and counterstained with hematoxylin. The abundance of macrophages per tissue section area was calculated using Image J program. Topical p38 MAPK inhibitor significantly reduced lung inflammation as represented by decreased macrophage infiltration (Figure 3). Representative images further support these findings.
Figure 3. p38 MAPK inhibitor suppresses the burn-induced increase of macrophage presence in mouse lungs.
p38 MAPK inhibitor (SB 202190) was topically applied immediately after mice underwent burning procedure. Mouse lungs were formalin fixed, paraffin sectioned and stained for macrophage marker Mac1 (brown). The abundance of macrophages per tissue section area was calculated using Image J program. Mean and SD of macrophage abundance are shown. The mean of the control (sham) group was taken as a reference (100%) and used to divide all the means by. The statistical significance of differences between sham (N=3) and burn (N=4), and burn and burn + p38 MAPK inhibitor (N=4) groups was determined using t-test with Welch correction. Representative micrograph images are displayed.
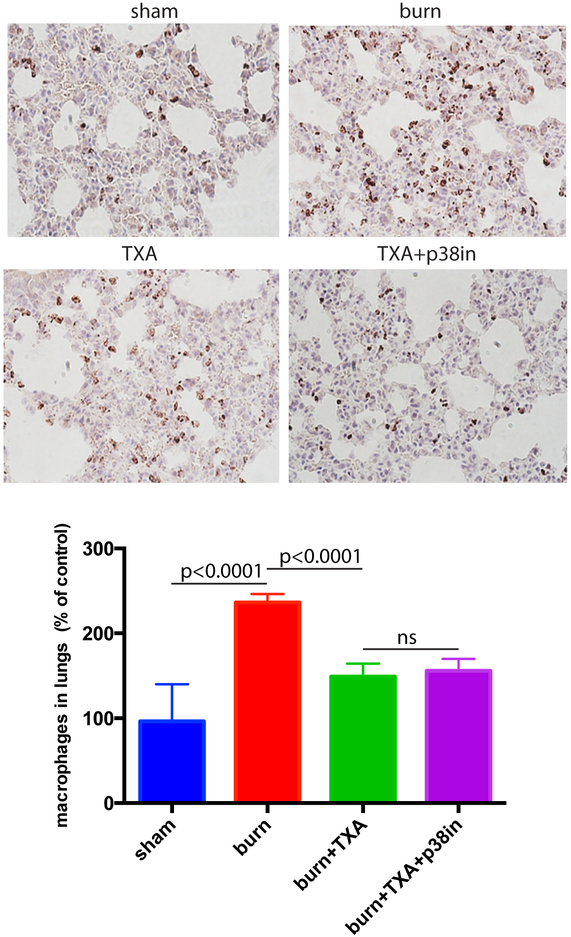
Mice received intraperitoneal injection of TXA immediately after undergoing burning procedure. A group of mice received both TXA injection and topical inhibition. Mice were sacrificed 5h after burn, and histochemical staining of lungs for macrophages was performed as described above. TXA significantly reduced lung inflammation as represented by decreased macrophage infiltration (Figure 4). Representative images further support these findings. Combined treatment of burned mice with TXA and p38 MAPK inhibitor did not show any further decrease of macrophage infiltration (Figure 4).
Figure 4. TXA inhibitor suppresses the burn-induced increase of macrophage presence in mouse lungs.
Mice received intraperitoneal injection of TXA immediately after undergoing burning procedure. A group of mice received both TXA injection and the topical p38 MAPK inhibitor. Mouse lungs were formalin fixed, paraffin sectioned, and stained for macrophage marker Mac1 (brown). The abundance of macrophages per tissue section area was calculated using Image J program. Mean and SD of macrophage abundance are shown. The mean of the control (sham) group was taken as a reference (100%) and used to divide all the means by. The statistical significance of differences between sham (N=4) and burn (N=4), burn and burn + TXA (N=4), and burn + TXA and burn + TXA + p38 MAPK inhibitor (N=4) groups was determined using t-test with Welch correction. Representative micrographs are displayed.
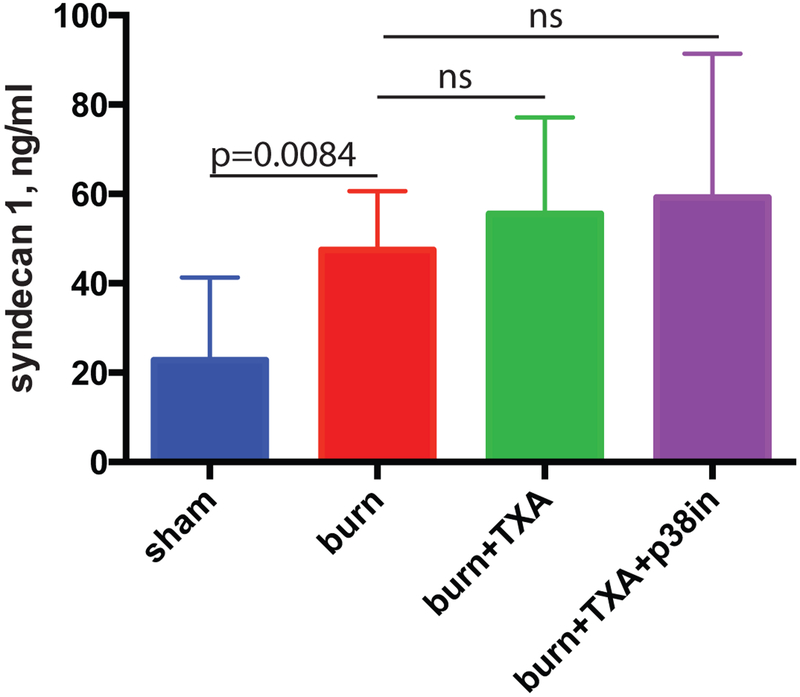
TXA alone or in combination with p38 MAPK inhibitor does not suppress the shedding of syndecan-1 induced by severe burns
Mice received intraperitoneal injection of TXA immediately after burn injury. A group of mice received both TXA injection and the topical inhibitor. After sacrifice, blood was obtained, plasma prepared and shed syndecan-1, which is a marker of endothelial glycocalyx loss, was determined using ELISA. TXA did not suppress the shedding of syndecan-1 from endothelial cells (Figure 5). Combined treatment of mice with TXA and topical p38 MAPK inhibitor similarly demonstrated no decrease in circulating syndecan levels (Figure 5).
Figure 5. TXA alone or in combination with p38 MAPK inhibitor does not suppress the shedding of syndecan-1 induced by severe burn.
Mice received intraperitoneal injection of TXA immediately after undergoing burning procedure. A group of mice received both TXA injection and topical p38 MAPK inhibitor. Plasma concentration of shed syndecan-1 was determined using ELISA. Mean and SD of syndecan-1 plasma content (ng/ml) are shown. The statistical significance of differences between sham (N=8) and burn (N=9), burn and burn + TXA (N=10), and burn and burn + TXA + p38 MAPK inhibitor (N=10) groups was determined using t-test with Welch correction.
Discussion
Fluid resuscitation of the critically injured burn patient can be particularly challenging for burn care providers. Adjunct treatments that could decrease fluid volume requirements may lead to reduced morbidity and mortality in this trauma population. Whereas plasma resuscitation is often used as a ‘rescue’ treatment in burns, its widespread use is neither practical nor without risk.13 Crystalloid and colloid (usually albumin) fluid resuscitation remain the standard in burns despite the growing body of evidence of its deleterious effects on glycocalyx integrity in trauma.14 Currently, no viable alternative exists. These fluids exacerbate the capillary leak and tissue edema associated with burn induced SIRS and worsen organ dysfunction. The role of early damage molecule (DAMP) signaling in the propagation of this SIRS response is now well established in the trauma literature.7,8,15 Thus, treatment strategies aimed at reducing or blocking the effectiveness of these early inflammation signals are promising therapeutic options to improve outcomes from burn-induced SIRS related complications.
We have demonstrated that topical inhibition of a key inflammatory signaling regulator - p38 MAPK – can effectively reduce the levels of an important circulating mitochondrial DAMP. Similarly, we have shown that TXA decreases circulating mtDNA, though the mechanism is unclear. The recent in vivo study by Teng et. al. (12) demonstrated that TXA suppresses NF-κB pathway signaling. In addition to mitochondrial DAMPs, we believe that downregulation of inflammatory non-mitochondrial DAMPs with either treatment is also likely. Our data also show that suppression of mtDNA release is associated with a reduction in lung inflammation as demonstrated by the relative extent of inflammatory cell infiltration. As pulmonary complications are the leading cause of death after severe burns, the apparent effectiveness of these adjunct treatments is encouraging for their ability to reduce lung inflammation.
The endothelial glycocalyx is a ‘brush border’ between the stream of circulating blood and the endothelial cell monolayer.14 This structure lines all blood vessels throughout the body and within organs and is composed principally of glycosaminoglycans, proteoglycans, heparin sulfates and chondroitin sulfates. The glycocalyx serves a barrier function and is essential to regulation of fluid and cellular extravasation into the interstitium. The glycocalyx accounts for most of the vascular luminal integrity of visceral capillary beds and is thus critical to mitigating tissue edema.14 Syndecans are a unique endothelial cell transmembrane bound protein, considered the ‘root and stem’ of the glycocalyx structure. After injury, these transmembrane proteins are cleaved and shed as a result of leukocyte mediated enzymatic breakdown - compromising the structure of the barrier that leads to capillary leak.14 Syndecan-1 is frequently used as a circulating marker of glycocalyx shedding. Our results showed no significant difference in the quantity of syndecan-1 released into the circulation (Figure 5) between the untreated burn animals and either treatment group. In fact, syndecan-1 levels trended slightly higher in both treatment groups when compared to the burn. Our hypothesis regarding syndecan-1 shedding was not supported by these experiments. This finding would seem to contradict the reduction in early inflammatory DAMP release and notable decrease in lung inflammation. This may simply mean that shedding of syndecan-1is a gross marker of systemic inflammation and endothelial injury. Interestingly, Deibel et. al. (11) has demonstrated that TXA reduces syndecan-1 shedding in an in vitro microfluidics model using HUVEC’s. While their findings contradict our study, we feel the in vivo nature of our work is a more complex biochemical interaction than the in vitro model. Furthermore, our time point of sacrifice at 5 hours may not have captured differences in earlier glycocalyx shedding events as was evaluated in the Diebel et. al. study. While one would anticipate the treatment groups to have lower circulating syndecan-1 levels, we feel this finding can be potentially explained in a few ways. First, animals were sacrificed at 5 hours after injury and thus differences may not have had enough post-injury time to develop. Similarly, distinguishing events may occur much earlier on the order of 1–2 hours after injury. Second, shedding of syndecan-1 is a factor associated with systemic inflammation in general (regardless of etiology).16 The levels of syndecan in circulation do not necessarily correlate with the ‘brush border’ integrity as new endothelial glycocalyx is reconstituted. Furthermore, glycocalyx re-population after injury is poorly understood.14,17 The circulating levels therefore do not fully describe what is happening on the vessel wall. Third, other cell types can produce syndecan-1 and release it into circulation.18 Shed syndecan-1 may also have a role as an acute phase reactant, however, this is speculation. Nonetheless, the clear evidence of decreased DAMP release and reduced lung inflammation in the treatment groups is reassuring. Future work will focus on imaging of the glycocalyx under experimental conditions.
This study has several important limitations. First, we used a brief course experimental model with analyses of DAMP release and syndecan-1 shedding in 5 hours after initial injury. This may have missed latent DAMP release events in a longer-term experiment. In addition, we did not evaluate any parameter of organ function in our experiments, although we did observe that both TXA and p38 MAPK treated mice demonstrated better activity during recovery when compared with untreated burned animals. Most importantly, a murine model does not fully represent human immune response; particularly as it relates to burn trauma.9 In future experiments, we plan to use a swine burn model where we can evaluate these treatments in a clinically relevant fluid resuscitation event. Furthermore, the porcine model will allow for evaluation of organ function with more fidelity and applicability to human burn injury.
Interestingly, while TXA and the topical p38 MAPK inhibitor produced similar results, combined treatment did not show any synergistic effects. Given these findings, TXA – with its more practical clinical application – may be effective as a stand-alone agent. Since it may be administered intravenously, it can integrate seamlessly with standard acute burn care practices. TXA also has a well-established track record of use in humans with a well-defined and acceptable side effect profile. Furthermore, tranexamic acid may have a role in mitigating the negative effects of fluid resuscitation in other non-hemorrhagic shock conditions such as sepsis. Pre-clinical research into a potential role for TXA in septic shock resuscitation is warranted and future work is planned; however, this remains speculation.
Both TXA and topical p38 MAPK inhibitor demonstrated the potential to attenuate burn induced DAMP release and SIRS, while reducing lung inflammation. Beyond its role as an anti-fibrinolytic, TXA may have significant anti-inflammatory effects pertinent to burn resuscitation. Further study is required; however, TXA may be a useful adjunct treatment in burn resuscitation and other non-hemorrhagic shock conditions.
Acknowledgements
We used the services of MMCRI Molecular Phenotyping Core, and Histopathology and Histomorphometry Core, both supported by NIH CTR grant U54GM115516. In addition, Histopathology Core is also supported by NIH COBRE grant P20GM121301. We would like to thank the staff at the Research institute for their assistance in completing this project. Chloe Kumpel and Tee Soul were intern students of the University of Southern Maine, and Kathleen Pyburn was an intern student of the Southern Maine Community College
Disclosure
Funding: This project was funded by the American Association for the Surgery of Trauma (AAST) Research & Education scholarship. Awarded June 2016.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Wolf SE, Kauvar DS, Wade CE, Cancio LC, Renz EP, Horvath EE, White CE, Park MS, Wanek S, Albrecht MA, Blackbourne LH, Barillo DJ, Holcomb JB. Comparison between civilian burns and combat burns from Operation Iraqi Freedom and Operation Enduring Freedom. Annals of Surgery. 2006. June; 243(6): 786–92; discussion 792–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cumming J, Purdue GF, Hunt JL, O’Keefe GE. Objective estimates of the incidence and consequences of multiple organ dysfunction and sepsis after burn trauma. Journal Trauma & Acute Care Surgery. 2001; 50(3): 510–5. [DOI] [PubMed] [Google Scholar]
- 3.Rahbar E, Cardenas JC, Baimukanova G, Usadi B, Bruhn R, Pati S, Ostrowski SR, Johansson PI, Holcomb JB, Wade CE. Endothelial Glycocalyx Shedding and Vascular Permeability in severely injured trauma patients. Journal of Translational Medicine. 2013; 13(117). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng Z, Pati S, Potter D, Brown R, Holcomb JB, Grill R, Wataha K, Park PW, Xue H, Kozar RA. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyper-permeability after hemorrhagic shock and is associated with loss of syndecan-1. Shock. 2013; 40(3): 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kozar RA, Peng Z, Zhang R, Holcomb JB, Pati S, Park P, Ko TC, Paredes A. Plasma Restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesthesia & Analgesia. 2011; 112(6): 1289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The PROPPR Study Group. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. Journal of the American Medical Association. 2015. February 3; 313(5):471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating Mitochondrial DAMP’s Cause Inflammatory Responses to Injury. Nature. 2010. March; 464(7285): 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmons JD, Lee YL, Mulekar S, Kuck JL, Brevard SB, Gonzalez RP, Gillespie MN, Richards WO. Elevated Levels of Plasma Mitochondrial DNA DAMP’s Are Linked to Clinical Outcome in Severely Injured Human Subjects. Annals of Surgery. 2013. October; 258(4): 591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter D, Warsen A, Hocking A, Cuschieri J, Arbabi SA. Delayed Topical p38 MAPK Inhibition Attenuates Full Thickness Burn Wound Inflammatory Signaling. Journal of Burn Care & Research. 2014. Mar-Apr; 35(2): e83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu X, Dubick MA, Schwacha MG, Cap AP, Darlington DN. TranexamicAcid Attenuates The Loss of Lung Barrier Function in a Rat Model of Polytrauma And Hemorrhage With Resuscitation. Shock. 2017. April; 47(4):500–505. [DOI] [PubMed] [Google Scholar]
- 11.Diebel ME, Martin JV, Liberati DM, Diebel LN. The temporal response and mechanism of action of tranexamic acid in endothelial glycocalyx degradation. Journal Trauma Acute Care Surgery. 2018. January; 84(1):75–80. [DOI] [PubMed] [Google Scholar]
- 12.Teng Y, Feng C, Liu Y, Jin H, Gao Y, Li T. Anti-inflammatory effect of tranexamic acid against trauma-hemorrhagic shock-induced acute lung injury in rats. Experimental Animals. 2018. July 30; 67(3): 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones LM, Deluga N, Bhatti P, Scrape SR, Bailey JK, Coffey RA. TRALI following fresh frozen plasma resuscitation from burn shock. Burns. 2017. March; 43(2): 397–402. [DOI] [PubMed] [Google Scholar]
- 14.Chignalia AZ, Yetimakman F, Christiaans SC, Unal S, Bayrakci B, Wagener BM, Russell RT, Kerby JD, Pittet JF, Dull RO. The Glycocalyx and Trauma: a review. Shock. 2016. April; 45 (4):338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao X, Wigginton J. Estrogen-provided cardiac protection following burn trauma is mediated through a reduction in mitochondria-derived DAMPs. American Journal of Physiology & Heart Circulatory Physiology. 2014; 306: H882–H894. [DOI] [PubMed] [Google Scholar]
- 16.Naumann DN, Hazeldine J, Dinsdale RJ, Bishop JR, Midwinter MJ, Harrison P, Hutchings SD, Lord JM. Endotheliopathy is associated with higher levels of cell-free DNA following major trauma: A prospective observational study. PLoS One. 2017. December 19; 12 (12): e0189870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Wang Z, Liu J, Zhang Z, Chen Y. Suppressing Syndecan-1 Shedding Ameliorates Intestinal Epithelial Inflammation through Inhibiting NF-κB Pathway and TNF-α. Gastroenterology Research & Practice. 2016; (64). [DOI] [PMC free article] [PubMed] [Google Scholar]