Abstract
A one-pot three-component tandem reaction involving a key Pictet–Spengler-like annulation step has been developed, providing an efficient method for the synthesis of 3,4-dihydroquinazolines in moderate to good yields from amides, aldehydes, and amines. The multicomponent triflic anhydride mediated reaction tolerates the installation of numerous functional groups, affording extensive diversity about the heterocyclic scaffold.
Introduction
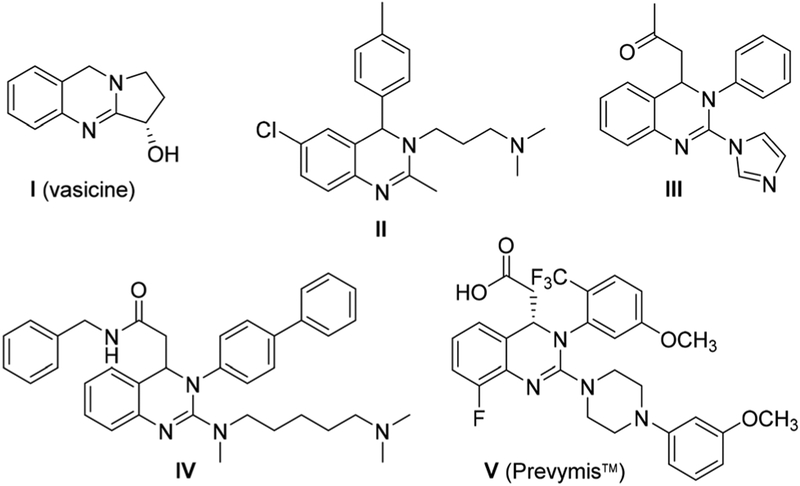
The 3,4-dihydroquinazoline scaffold is an important structural feature common to natural products and synthetic compounds with known pharmacological properties. Naturally occurring vasicine (I, Fig. 1), for example, is an alkaloid found in Adhatoda vasica and Peganum harmala plants that has been used medicinally for centuries to treat respiratory ailments.1 Biological properties exhibited by members of this compound class include antiparasitic (e.g. II),2 antifungal (e.g. III),3 antitumor (e.g. IV),4 and antiviral (e.g. V)5 activities, among others (Fig. 1).6 Interest in this heterocyclic scaffold has led to the development of numerous synthetic methods for its construction. Common methods of 3,4-dihydroquinazoline synthesis include reduction of quinazoline7 or quinazolinone8 compounds, condensation-type reactions of ortho-amino benzylamines with carbonyl derivatives,9 and ring formation via addition of nucleophiles to carbodiimide intermediates,10 as well as unique multicomponent one-pot methods.11 Despite numerous available methodologies for the synthesis of 3,4-dihydroquinazolines, many suffer from common drawbacks including costly or lengthy syntheses, issues which have hampered exploration of diversity among members of this compound class.
Fig. 1.
Biologically active 3,4-dihydroquinazolines.
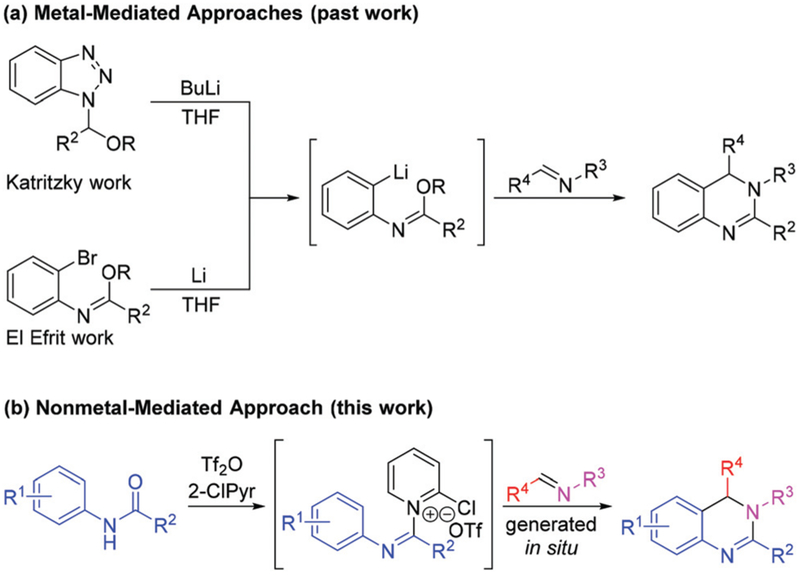
A much less frequently encountered approach to 3,4-dihydroquinazoline synthesis involves construction of the scaffold’s heterocyclic ring through an intramolecular Pictet–Spengler-like annulation reaction.12 The Pictet–Spengler reaction is a well-established method of carbon–nitrogen bond formation that involves intramolecular attack of an arene to a tethered iminium species.13 The reaction is commonly used for the installation of a cyclic amine present in partially saturated heterocyclic ring systems including tetrahydroisoquino-lines and tetrahydro-β-carbolines. An analogous Pictet–Spengler-like synthesis of the partially saturated 3,4-dihydroquinazoline heterocyclic ring, which contains an amidine rather than an amine, requires the generation and subsequent intramolecular annular capture of a N-amidinyliminium species. Such an approach has previously been investigated by the Katritzky12a and El Efrit12b groups, whose studies involved in situ generation of aryllithium imidates followed by treatment with imines (Scheme 1a). While the lithiated intermediates successfully capture imines, the potential molecular diversity about the resultant heterocyclic scaffold is limited by the incompatibility of the strongly nucleophilic intermediates and the reagents used to generate them with many organic functional groups. Therefore, a Pictet–Spengler-type approach to the synthesis of this scaffold which obviates the use of metals would permit access to increasingly diverse members of this compound class. Herein we report a metal-free Pictet–Spengler-like synthesis of 3,4-dihydroquinazolines using commercially or readily available N-aryl amides, amines, and aldehydes in one-pot Tf2O-mediated tandem reaction (Scheme 1b).
Scheme 1.
New Pictet–Spengler-like approach for 3,4-dihydroquinazoline synthesis.
Results and discussion
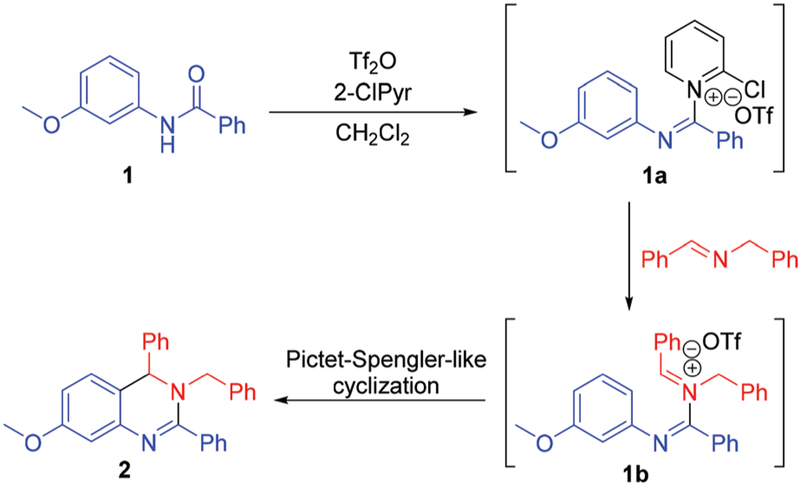
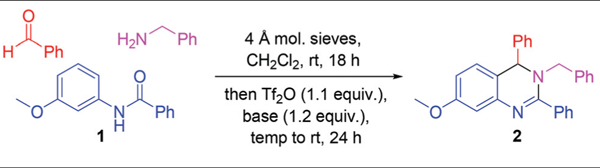
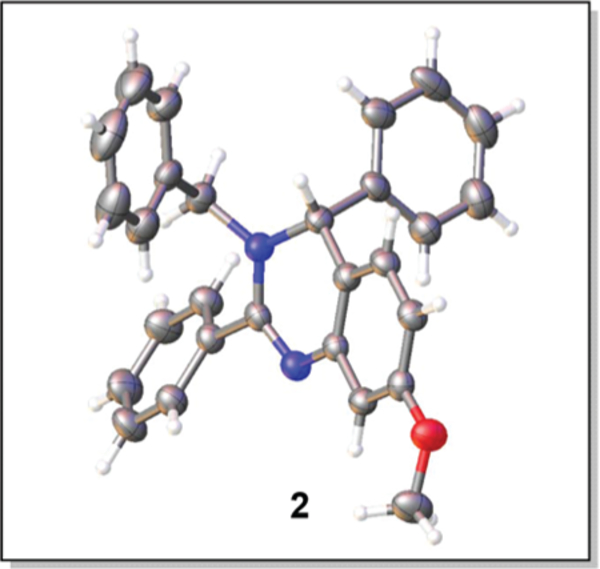
The synthesis of 3,4-dihydroquinazolines via a metal-free Pictet–Spengler-like annulation required a reliable method for the generation of the N-amidinyliminium annulation precursor (e.g. 1b, Scheme 2). We investigated the synthesis of this precursor, and the ensuing annulation, via established Tf2O-mediated N-aryl amide dehydration14 to generate 1a followed by imine addition (Scheme 2). Indeed, treatment of amide 1 with Tf2O and 2-chloropyridine (2-ClPyr) followed by N-benzylidenebenzylamine afforded 3,4-dihydroquinazoline 2 in 36% yield (Table 1, entry 1), the structure of which was confirmed by single crystal X-ray analysis. The reaction yield was improved through addition of 4 Å molecular sieves (Table 1, entry 2), conditions which reduced amide recovery resulting from hydrolysis of water-sensitive intermediates 1a and 1b. Next, molecular sieve-promoted in situ imine generation15 was pursued under similar conditions, whereby the required imine was to be formed from the corresponding amine and aldehyde prior to amide dehydration. True to design, stirring a mixture of benzylamine, benzaldehyde, and amide 1 in CH2Cl2 with 4 Å molecular sieves prior to treatment with Tf2O and 2-ClPyr afforded 2 in 61% yield (Table 1, entry 3). Additional screening efforts were then performed to reveal optimal conditions for our one-pot multicomponent reaction. Desired 3,4-dihydroquinazoline 2 was obtained in the presence and absence of a variety of pyridine bases (Table 1, entries 3–8), with the use of 2-ClPyr (entry 3) providing the greatest product yield. The temperature at which 2-ClPyr and Tf2O were added was found to be important, as optimal yields were observed when addition occurred at −41 °C (Table 1, entry 9). Further modifications to reaction conditions, such as heating to reflux instead of room temperature after addition of Tf2O (Table 1, entry 11) or allowing the reaction to proceed longer (Table 1, entry 12) did not provide substantial increases in product yield.
Scheme 2.
Tandem assembly of 3,4-dihydroquinazolines.
Table 1.
Optimization of reaction conditionsa
 | |||
|---|---|---|---|
| Entry | Base | Temp. (°C) | Yieldb (%) |
| 1c,d | 2-Chloropyridine | −10 | 36 |
| 2d | 2-Chloropyridine | −10 | 56 |
| 3 | 2-Chloropyridine | −10 | 61 |
| 4 | 2-Fluoropyridine | −10 | 45 |
| 5 | Pentafluoropyridine | −10 | 38 |
| 6 | 2-Methoxypyridine | −10 | 0 |
| 7 | Pyridine | −10 | 21 |
| 8 | None | −10 | 34 |
| 9 | 2-Chloropyridine | −41 | 84 |
| 10 | 2-Chloropyridine | −78 | 70 |
| 11e | 2-Chloropyridine | −41 | 64 |
| 12f | 2-Chloropyridine | −41 | 85 |
Conditions: 1 (1.0 mmol), benzylamine (1.1 mmol), benzaldehyde(1.1 mmol), 4 Å mol sieves (1.0 g), CH2Cl2 (10.0 mL), rt, 18 h; then base (1.2 mmol), Tf2O (1.1 mmol), temperature; then rt, 24 h.
Isolated yield.
4 Å mol sieves were not added.
N-Benzylidenebenzylamine (1.1 mmol) was used instead of benzylamine and benzaldehyde.
Reflux during final step.
48 h for final step.
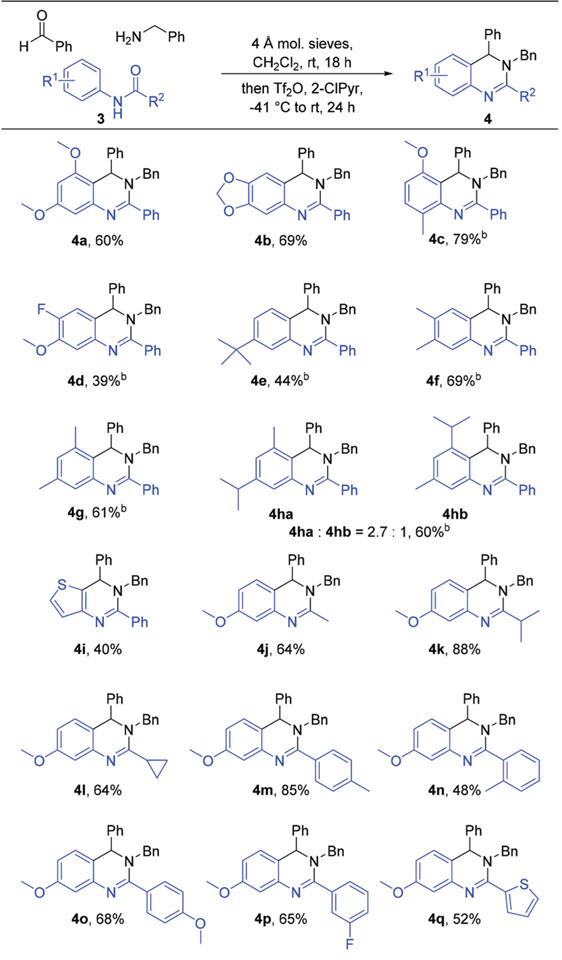
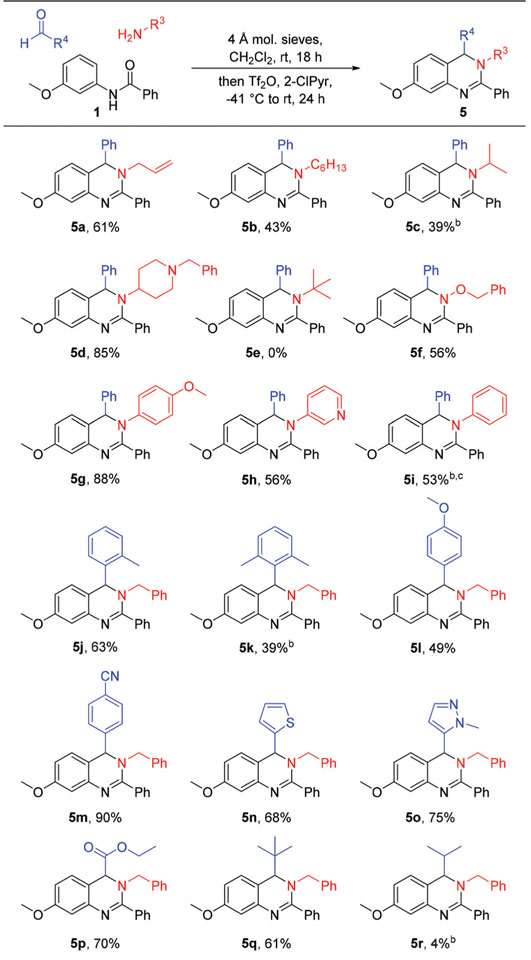
With optimal conditions identified, exploration of the reaction scope initiated with examination of the amide component (Table 2). Concerning N-arene substitution, the general trend observed was that the use of amides bearing electron rich N-arenes led to higher product yields when compared to the use of electron poor N-arenes, a result of enhanced nucleophilicity of the N-arene ring leading to an increase in the rate of the Pictet–Spengler-like annulation step. Alkoxy derivatives 4a–4c were prepared in moderate to good yields, whereas the yield of fluoro alkoxy derivative 4d was markedly lower, even when the reaction proceeded for an additional 24 hours after the addition of Tf2O. The use of amides bearing alkyl substituted N-arenes also led to moderate yields of desired 3,4-dihydroquinazoline products, with products of monoalkylated N-aryl amides being formed in lower yields than those from the dialkylated counterparts (4e vs. 4f–4h). A single regioisomer product was routinely isolated in these reactions even though the formation of an additional regioisomer product in most reactions was possible, an outcome that was likely driven by steric factors. In order to assess the effect of steric interactions on reaction regioselectivity, N-aryl amide 3h bearing two different substituents (methyl and isopropyl) meta to the amide nitrogen was prepared. When submitted to reaction conditions, the amide was converted to a mixture of regioisomers 4ha and 4hb (2.7 : 1 ratio),16 in which the major product resulted from annulation from the less sterically congested carbon ortho to the nitrogen on the amide N-arene. Lastly, an amide bearing a nucleophilic heterocycle rather than a substituted benzene was used to install a core thiophene unit in 4i. Diversification at the C2 position (e.g. R2) was also investigated through variation of the amide acyl group. Nearly all tested amides, including those bearing alkyl, aryl, and heteroaryl acyl groups, were successfully integrated into the product scaffolds in moderate to good yields without issue (e.g. 4j–4q). However, incorporation of very bulky groups at this position proved to be challenging. Comparison of 4m and 4n revealed the more sterically demanding o-toluyl substituent was installed in a lower yield than the p-toluyl group. Furthermore, the use of amides bearing the larger mesityl or tert-butyl groups in the same position provided no observable products.
Table 2.
Synthesis of 3,4-dihydroquinazolines through variation of the starting amidea
Conditions: 3 (1.0 mmol), benzylamine (1.1 mmol), benzaldehyde (1.1 mmol), 4 Å mol sieves (1.0 g), CH2Cl2 (10.0 mL), rt, 18 h; then 2-ClPyr (1.2 mmol), Tf2O (1.1 mmol), −41 °C; then rt, 24 h. Isolated yield.
48 h for final step.
Structural diversity at the N3 position was explored through the use of various amines (Table 3). Linear and branched alkyl groups were readily introduced at the N3 position through the addition of alkanamines (5a–5d). However, similar to at the C2 position, the incorporation of the large tert-butyl group was unsuccessful (e.g. 5e). These results demonstrate that the approach of the imine towards the intermediate generated from amide dehydration (e.g. 1a) is sensitive to steric interactions and becomes unfavorable when attempting to insert bulky groups at the C2 and N3 positions. An alkoxyamine was also installed at the N3 position (e.g. 5f) through the use of O-benzylhydroxylamine. Lastly, aromatic and heteroaromatic substituents were incorporated from the use of corresponding amines (5g–5i). The insertion of the 4-anisyl and the 3-pyridyl groups from the parent amines proceeded smoothly (5g and 5h, respectively). However, the use of aniline did not provide any product under the optimized reaction conditions. Modification of reaction conditions to include the use of 2-fluoropyridine (2-FPyr) instead of 2-ClPyr was thus investigated as a means of generating the highly electrophilic nitrilium intermediate during amide dehydration.17 Under the modified conditions with 2-FPyr, aniline was successfully installed to yield desired 5i. The use of 2-FPyr was also investigated in reactions wherein products were unable to be synthesized due to steric interactions (e.g. 5e), but those attempts proved to be unsuccessful.
Table 3.
Variation of the amine and aldehyde componentsa
Conditions: 1 (1.0 mmol), amine (1.1 mmol), aldehyde (1.1 mmol), 4 Å mol sieves (1.0 g), CH2Cl2 (10.0 mL), rt, 18 h; then 2-ClPyr (1.2 mmol), Tf2O (1.1 mmol), −41 °C; then rt, 24 h. Isolated yield.
48 h for final step. c 2-FPyr used instead of 2-ClPyr.
Variation of the aldehyde component was then investigated as a means of synthesizing 3,4-dihydroquinazolines differing at the C4 position (Table 3). The use of substituted benzaldehydes readily provided products with substituted phenyl groups at this position. While alkyl groups about the phenyl group were tolerated, increases in steric bulk at the ortho position of the C4 phenyl substituent provided lower product yields than the phenyl substituent itself (compare 2 to 5j and 5k). However, the observed reduction in yield was likely not due to steric factors alone, as modulation of the electronics at this position revealed that reaction yields are decreased by the use of electron rich aldehydes compared to electron deficient aldehydes (e.g. 5l vs. 5m). These results are in line with a Pictet–Spengler mechanism of ring formation, in which the electronic character installed in the cyclization precursor intermediate (e.g. 1b, Scheme 2) from the aldehyde component modulates the rate of capture by the N-arene nucleophile. Incorporation of electron deficient aldehydes enhances the electrophilicity of the cyclization precursors, thereby resulting in higher reaction yields. Additional substituents were also installed at the C4 position including heteroaromatic groups (e.g. 5n and 5o) and an ester (5p). Finally, the use of aliphatic aldehydes in the reaction was pursued. The use of pivaldehyde provided C4-alkyl product 5q in moderate yields, whereas the use of isobutyraldehyde provided very little of desired product 5r. Similarly low yields were observed when additional aldehydes bearing an alpha hydrogen, such as hexanal, hydrocinnamaldehyde, and isovaleraldehyde were tested in the reaction. We postulate that the drop in yields when using these aldehydes is likely due to deprotonation of the alpha hydrogen of the iminium intermediates under the basic reaction conditions to generate enamines,18 which would lack the requisite reactivity to undergo annulation.
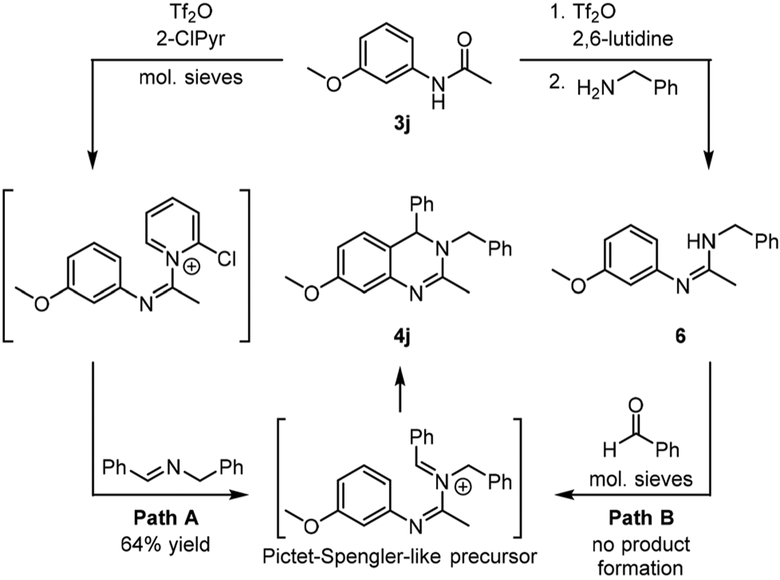
Generation and subsequent intramolecular capture of a Pictet–Spengler-like precursor is required for successful synthesis of 3,4-dihydroquinazolines under our studied reaction conditions. Our results demonstrate installation of an imine subsequent to amide dehydration as an efficient method of achieving this transformation (e.g. Scheme 3, Path A). We also sought to determine whether the Pictet–Spengler-like annulation pathway might be occurring through an amidine intermediate. Throughout our studies, amidines were identified in many reaction mixtures following aqueous workup. The ami-dines were presumed to have formed from attack of the amide dehydration intermediate by an amine19 rather than an imine due to incomplete imine formation prior to treatment with Tf2O or through hydrolysis of unreacted Pictet–Spengler-like annulation precursor present at the time of workup. The presence of amidines in the reaction mixtures also presented an alternative reactive pathway for product formation involving initial generation of an amidine followed by reaction with an aldehyde to generate the annulation precursor (Scheme 3, PathB). To test this alternative pathway, amidine 6 was prepared from amide 3j through treatment with Tf2O and 2,6-lutidine followed by benzylamine (Scheme 3).20 Isolated 6 was then treated with benzaldehyde, 2-ClPyr, and 4 Å molecular sieves in CH2Cl2 to mimic optimized reaction conditions, but no desired product was observed. Furthermore, additional reaction conditions were evaluated, including variations in base (including base-free and acidic conditions), solvent, and temperature. Under none of the tested conditions was any 3,4-dihydroquinazoline 4j ever observed, indicating that contributions to reaction yields from a reaction pathway involving an amidine intermediate is unlikely under our multicomponent reaction conditions.
Scheme 3.
Investigation of reaction pathway.
Conclusion
In conclusion, we have developed an efficient one-pot procedure for the synthesis of diverse 3,4-dihydroquinazolines via a Tf2O-mediated tandem assembly of amides, amines, and aldehydes. The reaction involves a key Pictet–Spengler-like annulation step under metal-free conditions for the formation of the partially saturated heterocyclic ring. Further studies concerning the scope and synthetic applications of this chemistry, as well as biological properties of 3,4-dihydroquinazoles, are underway in our laboratory.
Supplementary Material
Acknowledgements
We thank Grace Hubbell and Travis Quevillon for assistance with the synthesis of starting materials. We also thank Richard Staples of Michigan State University for assistance recording X-ray data and Judy Westrick, director of the Lumigen Instrumentation Center at Wayne State University, for assistance recording HRMS data. This work was supported by funding from the National Institutes of Health (1R03AI126233–01) and the National Science Foundation (MRI award 1626523).
Footnotes
Conflicts of interest
There are no conflicts to declare.
Electronic supplementary information (ESI) available. CCDC 1862436. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ob01596e
Notes and references
- 1.(a) Claeson UP, Malmfors T, Wikman G and Bruhn JG,J. Ethnopharmacol, 2000, 72, 1–20; [DOI] [PubMed] [Google Scholar]; (b) Nepali K, Sharma S,Ojha R and Dhar KL, Med. Chem. Res, 2013, 22, 1–15; [Google Scholar]; (c) Liu W, Wang Y, He D-D, Li S-P, Zhu Y-D, Jiang B,Cheng X-M, Wang Z-T and Wang C-H, Phytomedicine, 2015, 22, 1088–1095. [DOI] [PubMed] [Google Scholar]
- 2.Patterson S, Alphey MS, Jones DC, Shanks EJ,Street IP, Frearson JA, Wyatt PG, Gilbert IH and Fairlamb AH, J. Med. Chem, 2011, 54, 6514–6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W-J, Li Q, Liu D-L and Ding M-W, J. Agric. Food Chem, 2013, 61, 1419–1426. [DOI] [PubMed] [Google Scholar]
- 4.(a) Lee JY, Park SJ, Park SJ, Lee MJ, Rhim H,Seo SH and Kim K-S, Bioorg. Med. Chem. Lett, 2006, 16, 5014–5017; [DOI] [PubMed] [Google Scholar]; (b) Heo JH, Seo HN, Choe YJ, Kim S,Oh CR, Kim YD, Rhim H, Choo DJ, Kim J and Lee JY, Bioorg. Med. Chem. Lett, 2008, 18, 3899–3901; [DOI] [PubMed] [Google Scholar]; (c) Al-Obaid AM, Abdel-Hamide SG, El-Kashef HA, Abdel-Aziz AA-M, El-Azab AS, Al-Khamee HA and El-Subbagh HI, Eur. J. Med. Chem, 2009, 44, 2379–2391; [DOI] [PubMed] [Google Scholar]; (d) Jung SY, Lee SH, Kang HB, Park HA,Chang SK, Kim J, Choo DJ, Oh CR, Kim YD,Seo JH, Lee K-T and Lee JY, Bioorg. Med. Chem. Lett, 2010, 20, 6633–6636; [DOI] [PubMed] [Google Scholar]; (e) Kang HB, Rim H-K, Park JY,Choi HW, Choi DL, Seo J-H, Chung K-S, Huh G,Kim J, Choo DL, Lee K-T and Lee JY, Bioorg. Med. Chem. Lett, 2012, 22, 1198–1201; [DOI] [PubMed] [Google Scholar]; (f) Rim H-K, Lee H-W,Choi IS, Park JY, Choi HW, Choi J-H, Cho Y-W,Lee JY and Lee K-T, Bioorg. Med. Chem. Lett, 2012, 22, 7123–7126; [DOI] [PubMed] [Google Scholar]; (g) Jang SJ, Choi HW, Choi DL, Cho S,Rim H-K, Choi H-E, Kim K-S, Huang M, Rhim H,Lee K-T and Lee JY, Bioorg. Med. Chem. Lett, 2013, 23, 6656–6662. [DOI] [PubMed] [Google Scholar]
- 5.(a) Goldner T, Hewlett G, Ettischer N, Ruebsamen-Schaeff H, Zimmermann H and Lischka P, J. Virol, 2011, 85, 10884–10893; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Marschall M, Stamminger T, Urban A,Wildum S, Ruebsamen-Schaeff H, Zimmermann H and Lischka P, Antimicrob. Agents Chemother, 2012, 56, 1135–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Grosso JA, Nichols DE, Nichols MB and Yim GKW, J. Med. Chem, 1980, 23, 1261–1264; [DOI] [PubMed] [Google Scholar]; (b) Decker M, Krauth F and Lehmann J, Bioorg. Med. Chem, 2006, 14, 1966–1977; [DOI] [PubMed] [Google Scholar]; (c) Baxter EW, Conway KA,Kennis L, Bischoff F, Mercken MH, De Winter HL,Reynolds CH, Tounge BA, Luo C, Scott MK,Huang Y, Braeken M, Pieters SMA, Berthelot DJC,Masure S, Bruinzeel WD, Jordan AD, Parker MH,Boyd RE, Qu J, Alexander RS, Brenneman DE and Reitz AB, J. Med. Chem, 2007, 50, 4261–4264; [DOI] [PubMed] [Google Scholar]; (d) Peters J-U, Luebbers T, Alanine A, Kolczewski S,Blasco F and Steward L, Bioorg. Med. Chem. Lett, 2008, 18, 256–261; [DOI] [PubMed] [Google Scholar]; (e) Peters J-U, Luebbers T, Alanine A,Kolczewski S, Blasco F and Steward L, Bioorg. Med. Chem. Lett, 2008, 18, 262–266; [DOI] [PubMed] [Google Scholar]; (f) Park B, Nam JH, Kim JH,Kim HJ, Onnis V, Balboni G, Lee K-T, Park JH,Catto M, Carotti A and Lee JY, Bioorg. Med. Chem. Lett, 2017, 27, 1179–1185; [DOI] [PubMed] [Google Scholar]; (g) Kim JH, Jeong HR, Jung DW,Yoon HB, Kim SY, Kim HJ, Lee K-T, Gadotti VM,Huang J, Zhang F-X, Zamponi GW and Lee JY, Bioorg. Med. Chem, 2017, 25, 4656–4664. [DOI] [PubMed] [Google Scholar]
- 7.(a) Smith RF, Briggs PC, Kent RA, Albright JA and Walsh EJ, Heterocycl J. Chem., 1965, 2, 157–161; [Google Scholar]; (b) Smith JG, Sheepy JM and Levi EM, J. Org. Chem, 1976, 41, 497–501; [Google Scholar]; (c) Kita Y, Higashida K, Yamaji K,Iimuro A and Mashima K, Chem. Commun, 2015, 51, 4380–4382. [DOI] [PubMed] [Google Scholar]
- 8.(a) Jaen JC, Gregor VE, Lee C, Davis R and Emmerling M, Bioorg. Med. Chem. Lett, 1996, 6, 737–742; [Google Scholar]; (b) Dukat M, Alix K, Worsham J, Khatri S and Schulte MK, Bioorg. Med. Chem. Lett, 2013, 23, 5945–5948. [DOI] [PubMed] [Google Scholar]
- 9.(a) Ishikawa F, Watanabe Y and Saegusa J, Chem. Pharm. Bull, 1980, 28, 1357–1364; [DOI] [PubMed] [Google Scholar]; (b) Zhang JF, Barker J, Lou B and Saneii H, Tetrahedron Lett, 2001, 42, 8405–8408; [Google Scholar]; (c) Wang X, Song A, Dixon S, Kurth MJ and Lam KS, Tetrahedron Lett, 2005, 46, 427–430; [Google Scholar]; (d) Kumar RA,Saidulu G, Prasad KR, Kumar GS, Sridhar B and Reddy KR, Adv. Synth. Catal, 2012, 354, 2985–2991; [Google Scholar]; (e) Ghosh AK, Pandey S, Gangarajula S, Kulkarni S,Xu X, Rao KV, Huang X and Tang J, Bioorg. Med. Chem. Lett, 2012, 22, 5460–5465; [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Mercan D, Cetinkaya E and Sahin E, Inorg. Chim. Acta, 2013, 400, 74–81; [Google Scholar]; (g) Kumar RA, Saidulu G, Sridhar B, Liu ST and Reddy KR, J. Org. Chem, 2013, 78, 10240–10250; [DOI] [PubMed] [Google Scholar]; (h) Saidulu G, Kumar RA, Anitha T, Srinivasulu A,Sridhar B, Liu ST and Reddy KR, RSC Adv, 2014, 4, 55884–55888; [Google Scholar]; (i) Li C, An S, Zhu Y, Zhang J, Kang Y,Liu P, Wang Y and Li J, RSC Adv, 2014, 4, 49888–49891. [Google Scholar]
- 10.(a) Saito T, Tsuda K and Saito Y, Tetrahedron Lett, 1996, 37, 209–212; [Google Scholar]; (b) Wang FJ and Hauske JR, Tetrahedron Lett, 1997, 38, 8651–8654; [Google Scholar]; (c) Molina P, Aller E and Lorenzo A, Synthesis, 1998, 283–287; [Google Scholar]; (d) Tarraga A,Molina P, Curiel D, Lopez JL and Velasco MD, Tetrahedron, 1999, 55, 14701–14718; [Google Scholar]; (e) Lee BH, Lee JY,Chung BY and Lee YS, Heterocycles, 2004, 63, 95–105; [Google Scholar]; (f) He P, Wu J, Nie Y-B and Ding M-W, Eur. J. Org. Chem, 2010, 1088–1095. [Google Scholar]
- 11.(a) Wei W, Wen J, Yang D, Sun X, You J, Suo Y and Wang H, Tetrahedron, 2013, 69, 10747–10751; [Google Scholar]; (b) Bhuyan D, Sarma R, Dommaraju Y and Prajapati D, Green Chem, 2014, 16, 1158–1162; [Google Scholar]; (c) Xiong J, Wei X,Yan Y-M and Ding M-W, Tetrahedron, 2017, 73, 5720–5724; [Google Scholar]; (d) Wu C, Wang J, Zhang X-Y, Jia G-K, Cao Z, Tang Z, Yu X, Xu X and He W-M, Org. Biomol. Chem, 2018, 16, 5050–5054. [DOI] [PubMed] [Google Scholar]
- 12.(a) Katritzky AR, Zhang G, Jiang J and Steel PJ, J. Org. Chem, 1995, 60, 7625–7630; [Google Scholar]; (b) El Efrit ML, Hajjem B,Zantour H and Baccar B, Synth. Commun, 1996, 26, 3167–3173. [Google Scholar]
- 13.For reviews, see:; (a) Cox ED and Cook JM, Chem. Rev, 1995, 95, 1797–1842; [Google Scholar]; (b) Chrzanowska M and Rozwadowska MD, Chem. Rev, 2004, 104, 3341–3370; [DOI] [PubMed] [Google Scholar]; (c) Chrzanowska M, Grajewska A and Rozwadowska MD, Chem. Rev, 2016, 116, 12369–12465; [DOI] [PubMed] [Google Scholar]; (d) Stoeckigt J,Antonchick AP, Wu F and Waldmann H, Angew. Chem., Int. Ed, 2011, 50, 8538–8564. [DOI] [PubMed] [Google Scholar]
- 14.For reviews involving Tf2O amide activation, see:; (a) Baraznenok IL, Nenajdenko VG and Balenkova ES, Tetrahedron, 2000, 56, 3077–3119; [Google Scholar]; (b) Pace V, Holzer W and Olofsson B, Adv. Synth. Catal, 2014, 356, 3697–3736; [Google Scholar]; (c) Volkov A, Tinnis F, Slagbrand T, Trillo P and Adolfsson H, Chem. Soc. Rev, 2016, 45, 6685–6697; [DOI] [PubMed] [Google Scholar]; (d) Kaiser D, Bauer A, Lemmerer M and Maulide N, Chem. Soc. Rev, 2018, 47, 7899–7925; [DOI] [PubMed] [Google Scholar]; (e) Sato T,Yoritate M, Tajima H and Chida N, Org. Biomol. Chem, 2018, 16, 3864–3875. [DOI] [PubMed] [Google Scholar]; Also see:; (f) Movassaghi M and Hill MD, J. Am. Chem. Soc, 2006, 128, 14254–14255; [DOI] [PubMed] [Google Scholar]; (g) Movassaghi M, Hill MD and Ahmad OK, J. Am. Chem. Soc, 2007, 129, 10096–10097; [DOI] [PubMed] [Google Scholar]; (h) Movassaghi M and Hill MD, Nat. Protoc, 2007, 2, 2018–2023; [DOI] [PubMed] [Google Scholar]; (i) Hill MD and Movassaghi M, Synthesis, 2008, 823–827; [Google Scholar]; (j) Cui S-L,Wang J and Wang Y-G, J. Am. Chem. Soc, 2008, 130, 13526–13527; [DOI] [PubMed] [Google Scholar]; (k) Ahmad OK, Hill MD and Movassagh M, J. Org. Chem, 2009, 74, 8460–8463; [DOI] [PubMed] [Google Scholar]; (l) Xiao K-J, Wang A-E and Huang P-Q, Angew. Chem., Int. Ed, 2012, 51, 8314–8317; [DOI] [PubMed] [Google Scholar]; (m) Wezeman T, Zhong S,Nieger M and Braese S, Angew. Chem., Int. Ed, 2016, 55, 3823–3827; [DOI] [PubMed] [Google Scholar]; (n) Li L-H, Niu Z-J and Liang Y-M, Chem. – Eur. J, 2017, 23, 15300–15304; [DOI] [PubMed] [Google Scholar]; (o) Huang Y-H, Wang S-R,Wu D-P and Huang P-Q, Org. Lett, 2019, 21, 1681–1685. [DOI] [PubMed] [Google Scholar]
- 15.Baghbanzadeh M and Kappe CO, Aust. J. Chem, 2009, 62, 244–249. [Google Scholar]
- 16.The ratio of regioisomers was determined from crude NMR analysis. The regiochemical identities of the two isomers were determined by comparative NOESY analysis (see ESI†).
- 17.(a) Medley JW and Movassaghi M, J. Org. Chem, 2009,74, 1341–1344; [DOI] [PubMed] [Google Scholar]; (b) Ellsworth AA, Magyar CL,Hubbell GE, Theisen CC, Holmes D and Mosey RA, Tetrahedron, 2016, 72, 6380–6389. [Google Scholar]
- 18.Lemire A and Charette AB, Org. Lett, 2005, 7, 2747–2750. [DOI] [PubMed] [Google Scholar]
- 19.Charette AB and Grenon M, Tetrahedron Lett, 2000, 41, 1677–1680. [Google Scholar]
- 20.Baars H, Beyer A, Kohlhepp SV and Bolm C, Org. Lett, 2014, 16, 536–539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.