Abstract
Context
Previous studies have shown that the endocannabinoid system plays a major role in energy metabolism through the actions of its main mediators, 2-arachidonoyl-sn-glycerol (2-AG) and anandamide (AEA).
Objective
We examined serum levels of major endocannabinoid mediators and their association with clinical parameters in patients with end-stage renal disease (ESRD).
Design and Setting
Serum concentrations of 2-AG and AEA were measured in patients on maintenance hemodialysis (MHD) and controls, and correlations with various clinical and laboratory indices were examined. 2-AG was also measured in age and sex-matched healthy subjects for comparison of levels in patients undergoing MHD.
Main Outcome Measure
Serum 2-AG.
Results
Serum 2-AG levels were significantly elevated in patients with ESRD compared with healthy controls. Higher levels of 2-AG were found in patients on MHD compared to healthy subjects, and similar findings were seen in a second set of subjects in independent analyses. Among 96 patients on MHD, 2-AG levels correlated significantly and positively with serum triglycerides (ρ = 0.43; P < 0.0001), body mass index (ρ = 0.40; P < 0.0001), and body anthropometric measures and negatively with serum high-density lipoprotein cholesterol (ρ = −0.33; P = 0.001) following adjustment for demographic and clinical variables.
Conclusions
In patients on MHD, levels of serum 2-AG, a major endocannabinoid mediator, were increased. In addition, increasing serum 2-AG levels correlated with increased serum triglycerides and markers of body mass. Future studies will need to evaluate the potential mechanisms responsible for these findings.
Keywords: end-stage renal disease, maintenance hemodialysis, cachexia, endocannabinoid system, body mass index, 2-arachidonoylglycerol
The prevalence of chronic kidney disease (CKD) in the United States and worldwide has increased dramatically over the past few decades [1, 2]. It is well known that patients with advanced CKD and end-stage renal disease (ESRD) have a significantly increased risk of all-cause and cardiovascular mortality, and in spite of many recent improvements, patients with ESRD on maintenance hemodialysis (MHD) continue to experience an annual mortality rate of approximately 17% per year [3]. The factors responsible for this disproportionately elevated risk of death have not been fully identified. In fact, traditional risk factors such as obesity and hypertriglyceridemia cannot explain the magnitude of the risk observed in these patients, given that they are paradoxically associated with better survival in observational studies [4, 5]. Conversely, there is accumulating evidence that cachexia (protein energy wasting) and impaired energy metabolism may play a more prominent role in the higher risk of mortality in patients with ESRD [6, 7].
Although recent research has shown that the endocannabinoid system may counteract the effects of cachexia [8], its role in patients with ESRD has not yet been examined. This system is composed of endogenous, bioactive, lipid-derived mediators, the endocannabinoids, which exert their effects through specific G protein‒coupled receptors: cannabinoid (CB)-1 and CB2. The most extensively studied endocannabinoids are anandamide (AEA) and 2-arachidonoyl-sn-glycerol (2-AG) [8, 9]. The endocannabinoid system plays important roles in many different physiologic processes, especially energy metabolism, by overseeing energy requirements and expenditure. Activation of the endocannabinoid system leads to increased intake of energy-rich foods and decreased energy expenditure via promotion of white adipogenesis and inhibition of brown adipose tissue activation [8, 10–12]. In addition, endocannabinoid system activation stimulates molecular pathways involved in energy storage, including fatty acid synthesis and lipogenesis. Therefore, it is not surprising that overactivity of this system has been associated with obesity, hypertriglyceridemia, and metabolic syndrome in animals and humans [8, 11, 12]. In addition, recent studies in patients with obesity have found elevated serum levels of endocannabinoids, and extensive work has demonstrated a causative relationship between abnormal endocannabinoid system activity and development of metabolic syndrome [13–15].
Given that ESRD treated with MHD is associated with abnormal energy metabolism and cachexia, we hypothesized that serum AEA and/or 2-AG levels may be altered in this patient population. We sought to evaluate how alterations in serum AEA and/or 2-AG levels in MHD patients correlate with laboratory and clinical parameters. We measured and compared concentrations of serum AEA and 2-AG in MHD patients and healthy controls and tested our findings from the MHD cohort in a second set of independent analyses in another laboratory using liquid chromatography/mass spectrometry techniques.
1. Materials and Methods
A. Study Population
The study population consisted of healthy controls and MHD patients. Healthy subjects (without hypertension, diabetes, other major cardiovascular comorbidities, or medication use) were recruited for this study by the University of California (UC), Irvine Institute for Clinical and Translational Science. MHD patients were randomly selected from a subcohort of MHD patients who were enrolled in the initial phase of the Malnutrition, Diet, and Racial Disparities in Chronic Kidney Disease (MADRAD) study (ClinicalTrials.gov NCT01415570) and had an available laboratory measurement collected between April and July 2014. MHD patients were matched to healthy controls according to age (±10 years) and sex. MADRAD is a prospective cohort study examining differences in dietary factors and nutritional status across racial/ethnic groups of MHD patients recruited from outpatient dialysis facilities in the South Bay‒Los Angeles, California area. The enrollment criteria of MADRAD have been previously described [16].
Serum levels of 2-AG and AEA were measured in 50 MHD patients and 21 age- and sex-matched healthy controls. Additional MHD patients were added for comparisons of serum 2-AG in 96 total MHD patients and 21 healthy controls (Supplemental Fig. 1 in an online repository [17]). Then, to test the robustness of our findings, we repeated these analyses with a second set of subjects in another laboratory with expertise in extraction and analysis of endocannabinoids. The second set of subjects included 10 healthy controls and 37 age-, sex-, race-, and ethnicity-matched MHD patients with serum AEA and 2-AG measurements. The 2-AG and AEA levels were measured in serum samples obtained from all patients before hemodialysis treatments at baseline. Serum was collected during the same time as routine blood tests conducted at dialysis clinics and was frozen at −80°C until analyses were performed.
The study was approved by the institutional review committees of the Los Angeles Biomedical Research Institute at Harbor-UCLA, Torrance, California, and the UC Irvine Medical Center, Orange, California. Patients were included in the study if they provided written/signed informed consent.
B. Lipid Extraction and Analysis
In the first set of analyses, methanol (1.5 mL) containing internal standards [2H4]AEA (1 pmol) and [2H8]2AG (250 pmol) was added to serum (0.75 mL). Lipids were extracted using chloroform (3 mL) and 0.1 M sodium chloride (1 mL). The organic phases were dried under N2, reconstituted in chloroform (2 mL), and applied to open-bed silica gel columns to fractionate lipid groups on the basis of polarity. Eluted fractions containing AEA and arachidonoylglycerol (chloroform/methanol, 9:1, v/v) were dried under N2, and the residue was reconstituted in a 60-µL solvent mixture of chloroform and methanol (1:3, v/v) for liquid chromatography/mass spectrometry and liquid chromatography with tandem mass spectrometry analyses (for additional details on lipid extraction and analysis, see the supplemental material [17]).
The verification analyses were performed in a separate independent laboratory (Dr. Nicholas DiPatrizio) with expertise in endocannabinoid extraction and analysis in human samples [18–20]. Methanol (1 mL) containing internal standards [2H4]AEA (1 pmol) and [2H5]2AG (250 pmol) was added to serum (0.5 mL). Lipids were extracted using chloroform (2 mL) and 0.1 M sodium chloride (0.5 mL). The organic phases were dried under N2, reconstituted in chloroform (2 mL), and applied to open-bed silica gel columns to fractionate lipid groups on the basis of polarity. Eluted fractions containing AEA and 2-AG (chloroform/methanol, 9:1, v/v) were dried under N2, and the residue was reconstituted in a 100-µL solvent mixture of chloroform and methanol (1:1, v/v) for ultra performance liquid chromatography with tandem mass spectrometry analyses (for additional details on lipid extraction and analysis, see the supplemental material [17]).
C. Demographic, Clinical, and Laboratory Characteristics of MHD Patients
Baseline demographic and clinical data (including age, sex, race, and ethnicity) were obtained by MADRAD study coordinators. Diabetes as a preexisting comorbid condition was ascertained by MADRAD study coordinators according to patient self-reported history and obtained via International Classification of Diseases, Ninth Revision codes at the time of study entry. Dialysis vintage for MHD patients was calculated as the interval of time between the date of the patient’s first dialysis treatment and the date of serum AG or AEA measurement. Data on body mass index (BMI) using postdialysis weight were also obtained from electronic records of the dialysis facilities. In addition, patient body composition surrogates were measured by MADRAD study coordinators during treatment visits. Further details about the MADRAD study ascertainment of body anthropometry have been previously reported [16].
Routine laboratory measurements including lipid panels were obtained from electronic records of the dialysis facilities. Blood samples were drawn using standardized techniques and measured using automated and standardized methods at a central laboratory in Deland, Florida, typically within 24 hours. An extended serum lipid panel was measured at the UC Irvine Medical Center laboratory. Very-low-density lipoprotein concentrations were measured and not calculated. Serum concentrations of IL-6 were determined using ELISA (Waltham, MA) assay kits from R&D Systems (Minneapolis, MN) and Affymetrix Thermo Fisher Scientific (Waltham, MA) per manufacturer’s protocol.
D. Beck Depression Index Score and Quality of Life Measures
To assess depression and the severity of its symptoms over the past 2 weeks prior to assessment, patients completed the Beck Depression Inventory-II questionnaire. The Beck Depression Inventory-II score is the sum of the responses to 21 questions, each ranked on a scale from 0 to 3. Patients also completed the 36-Item Short Form Health Survey quality of life questionnaire. The responses to individual questions were scored and then calculated to assess patient physical and mental health domains, as well as eight dimensions of health: physical functioning, role limitations due to physical health, role limitations due to personal or emotional problems, energy/fatigue, emotional well-being, social functioning, bodily pain, and general health [21].
In all analyses, laboratory tests and health questionnaire scores collected closest to the date of serum 2-AG or AEA measurement were used.
E. Statistical Analyses
The distribution of serum 2-AG was presented as a histogram (Supplemental Fig. 2 [17]), and the normality of serum 2-AG and AEA was evaluated with Shapiro-Wilk tests. In all analyses, serum 2-AG and AEA levels were described using medians [interquartile range (IQR)]. Baseline patient characteristics were summarized with means ± SDs, medians (IQR), or proportions where appropriate. Comparisons of serum 2-AG and AEA levels between MHD patients and healthy controls were conducted using the nonparametric Wilcoxon‒Mann-Whitney U test. Furthermore, serum 2-AG and AEA levels were compared between strata of demographic and clinical characteristics in MHD patients using similar methods.
Spearman rank correlation coefficients (ρ) were calculated to describe the relationship between serum 2-AG and AEA levels with clinical and laboratory markers and additionally with body anthropometric measures, quality of life, and depression scores. Correlation analyses were adjusted for age, sex, race, ethnicity, diabetes, and dialysis vintage covariates.
Data on BMI were sourced primarily from large dialysis organization electronic records or imputed with available BMI levels collected by MADRAD study coordinators for those missing BMI (14%). All patients had complete data on all covariates. Two-sided P values <0.05 were considered statistically significant. For correlation analyses in the primary cohort, we used a stricter definition of P value <0.0001 to be considered significant to account for multiple comparisons. Analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC).
2. Results
A. Cohort Characteristics
The primary cohort consisted of 96 MHD patients. Baseline characteristics are presented in Table 1. The mean (±SD) age of the cohort was 52 ± 12 years; 64% were female, and 52% had diabetes.
Table 1.
Baseline Characteristics of 96 Patients Undergoing Maintenance Hemodialysis
| Variable | Total |
|---|---|
| N | 96 |
| Age ± SD, y | 52 ± 12 |
| Female, % | 64 |
| Race, % | |
| White | 83 |
| Asian | 17 |
| Hispanic ethnicity, % | 53 |
| Diabetes, % | 52 |
| Access type, % | |
| AV fistula | 69 |
| AV graft | 6 |
| Central venous catheter | 25 |
| Body mass index, kg/m2 | 27.9 ± 6.3 |
| Laboratory tests | |
| Albumin, g/dL | 4.0 ± 0.3 |
| Creatinine, mg/dL | 9.3 ± 3.1 |
| Ferritin, ng/mL | 619 (387–886) |
| TIBC, mg/dL | 231.2 ± 38.2 |
| PTH, pg/mL | 380 (265–576) |
| Lipid panel | |
| VLDL-C, mg/dL | 12 (6–28) |
| Triglycerides, mg/dL | 126 (92–213) |
| Cholesterol, mg/dL | 143.1 ± 38.8 |
| HDL-C, mg/dL | 42.2 ± 20.2 |
| LDL-C, mg/dL | 77.3 ± 28.4 |
| LPA-C, mg/dL | 2 (1–4) |
| NHDL, mg/dL | 100.8 ± 39.2 |
| IL-6, pg/mL | 2 (1–5) |
| Vintage, d | 1363 (683–2270) |
Data are presented as percentages, mean ± SD, or median (interquartile range) where appropriate.
Abbreviations: AV, arteriovenous; HDL-C, high-density lipoprotein cholesterol; IL-6, interleukin-6; LDL-C, low-density lipoprotein cholesterol; LPA-C, lipoprotein(a) cholesterol; NHDL, non‒high-density lipoprotein; PTH, parathyroid hormone; TIBC, total iron-binding capacity; VLDL-C, very-low-density lipoprotein cholesterol.
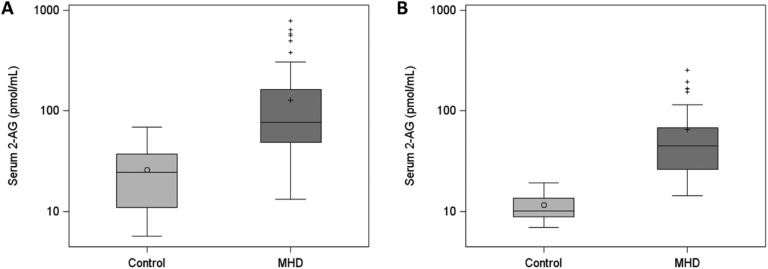
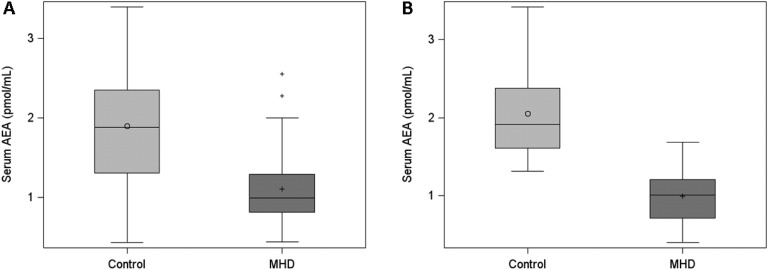
In examination of serum endocannabinoid levels, we found that median (IQR) concentrations of serum 2-AG were higher in 96 MHD patients [76 (49 to 163) pmol/mL] than in 21 controls [25 (11 to 37) pmol/mL] (Fig. 1A). Similar results for serum 2-AG were found in the confirmatory analysis of 10 controls and 37 age-, sex-, race-, and ethnicity-matched MHD patients. Levels of serum 2-AG were significantly higher in MHD patients than in controls [median (IQR), 45 (26 to 68) pmol/mL and 10 (9 to 13) pmol/mL, respectively] (Fig. 1B). In contrast, serum AEA concentrations were lower in 50 MHD patients than in 21 controls: median (IQR), 1.00 (0.82 to 1.29) pmol/mL and 1.88 (1.31 to 2.35) pmol/mL, respectively (Fig. 2A). These findings were similarly observed in a second independent laboratory using a separate set of 10 controls and 37 age-, sex-, race-, and ethnicity-matched unique MHD patients with the following AEA measurements: median (IQR), 1.91 (1.61 to 2.38) pmol/mL and 1.01 (0.71 to 1.21) pmol/mL, respectively (Fig. 2B). We also examined serum 2-AG and AEA levels according to demographic and clinical characteristics among MHD patients but found no differences in 2-AG or AEA concentrations within each group (Table 2).
Figure 1.
First and confirmatory analyses of serum 2-AG concentrations in patients receiving MHD compared with control subjects. (A) Serum 2-AG concentrations in the first analysis of 96 MHD patients and 21 control subjects. (B) Serum 2-AG concentrations in the confirmatory analysis of 37 MHD patients and 10 control subjects. Serum 2-AG levels are presented on a logarithmic scale for visual purposes only. P < 0.0001 for both, Wilcoxon‒Mann-Whitney U test. Horizontal lines in boxplots represent the median, circle and cross in boxplots represent the mean in the control and MHD groups, respectively, error bars represent the lower (25th percentile - 1.5*IQR) and upper (75th percentile + 1.5*IQR) boundaries, and crosses outside boxplots represent outliers.
Figure 2.
First and confirmatory analyses of serum AEA concentrations in patients receiving MHD compared with control subjects. (A) Serum AEA concentrations in the first analysis of 50 MHD patients and 21 control subjects. (B) Serum AEA concentrations in the confirmatory analysis of 37 MHD patients and 10 control subjects. P < 0.0001 for both, Wilcoxon‒Mann-Whitney U test. Horizontal lines in boxplots represent the median, circle and cross in boxplots represent the mean in the control and MHD groups, respectively, error bars represent the lower (25th percentile - 1.5*IQR) and upper (75th percentile + 1.5*IQR) boundaries, and crosses outside boxplots represent outliers.
Table 2.
Serum AEA and 2-AG Levels in Patients Undergoing MHD Stratified by Demographic and Clinical Characteristics
| Subgroup | Endocannabinoids | |||
|---|---|---|---|---|
| Serum AEA (pmol/mL) n = 50 |
Serum 2-AG (pmol/mL) n = 96 | |||
| Median (IQR) | P Value | Median (IQR) | P Value | |
| Age, y | ||||
| <50 | 0.98 (0.82–1.16) | 0.37 | 73 (51–161) | 0.73 |
| ≥50 | 1.05 (0.82–1.29) | 78 (48–165) | ||
| Sex | ||||
| Female | 0.96 (0.76–1.29) | 0.19 | 81 (53–177) | 0.25 |
| Male | 1.05 (0.95–1.43) | 75 (44–127) | ||
| Race | ||||
| White | 1.00 (0.89–1.33) | 0.38 | 80 (48–172) | 0.19 |
| Asian | 0.88 (0.76–1.29) | 67 (51–101) | ||
| Ethnicity | ||||
| Hispanic | 0.98 (0.89–1.26) | 0.78 | 83 (52–216) | 0.13 |
| Non-Hispanic | 1.05 (0.76–1.35) | 73 (48–127) | ||
| Dialysis vintage, d | ||||
| <1095 | 0.99 (0.82–1.29) | 0.62 | 96 (51–172) | 0.42 |
| ≥1095 | 1.02 (0.83–1.33) | 72 (48–144) | ||
| Presence of diabetes | ||||
| Yes | 0.98 (0.88–1.53) | 0.43 | 78 (48–174) | 0.63 |
| No | 1.01 (0.77–1.17) | 75 (49–142) | ||
Data are presented as median (IQR).
B. Correlations of Serum 2-AG and AEA With Clinical and Laboratory Indices
Serum 2-AG positively correlated with BMI, midarm circumference, biceps and triceps skin folds, serum triglyceride (TG) levels, and very-low-density lipoprotein cholesterol (VLDL-C) after adjustment for age, sex, race, ethnicity, diabetes, and dialysis vintage covariates (Table 3). Conversely, serum 2-AG negatively correlated with serum high-density lipoprotein cholesterol (HDL-C; ρ = −0.33; P = 0.001). After consideration for multiple comparisons, Spearman correlation coefficients only for VLDL-C, TG, and BMI with 2-AG remained statistically significant. Unadjusted and adjusted correlation coefficients of 2-AG with additional laboratory tests, quality of life, and depression data are presented in Supplemental Table 1 [17].
Table 3.
Unadjusted and Adjusted Spearman Rank Correlation Coefficients (ρ) of Serum 2-AG With Relevant Laboratory and Body Anthropometric Data in 96 Patients Undergoing Maintenance Hemodialysis
| Variable | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| ρ | P Value | ρ | P Value | |
| Laboratory tests | ||||
| Albumin, g/dL | 0.05 | 0.62 | 0.08 | 0.47 |
| Creatinine, mg/dL | 0.02 | 0.86 | 0.03 | 0.83 |
| Ferritin, ng/mL | 0.03 | 0.78 | 0.07 | 0.56 |
| TIBC, mg/dL | 0.28 | 0.01 | 0.32 | 0.004 |
| PTH, pg/mL | −0.02 | 0.87 | −0.002 | 0.98 |
| Lipid panel | ||||
| VLDL-C, mg/dL | 0.44 | <0.0001 | 0.42 | <0.0001 |
| Triglycerides, mg/dL | 0.47 | <0.0001 | 0.43 | <0.0001 |
| Cholesterol, mg/dL | 0.28 | 0.005 | 0.23 | 0.03 |
| HDL-C, mg/dL | −0.31 | 0.002 | −0.33 | 0.001 |
| LDL-C, mg/dL | 0.18 | 0.09 | 0.13 | 0.21 |
| LPA-C, mg/dL | 0.20 | 0.05 | 0.21 | 0.05 |
| NHDL, mg/dL | 0.37 | 0.0003 | 0.33 | 0.001 |
| IL-6, pg/mL | −0.05 | 0.65 | 0.009 | 0.94 |
| Body mass index, kg/m2 | 0.43 | <0.0001 | 0.40 | <0.0001 |
| Body anthropometry | ||||
| Biceps skin fold, mm | 0.34 | 0.0008 | 0.32 | 0.002 |
| Triceps skin fold, mm | 0.33 | 0.001 | 0.32 | 0.002 |
| Midarm muscle circ, mm | 0.09 | 0.40 | 0.12 | 0.29 |
| Midarm circ, mm | 0.34 | 0.0009 | 0.33 | 0.002 |
| NIR body fat % | 0.31 | 0.002 | 0.31 | 0.004 |
Adjusted model includes age, sex, race, ethnicity, diabetes, and dialysis vintage as covariates.
Abbreviations: circ, circumference; HDL-C, high-density lipoprotein cholesterol; IL-6, interleukin-6; LDL-C, low-density lipoprotein cholesterol; LPA-C, lipoprotein(a) cholesterol; NHDL, non‒high-density lipoprotein; NIR, near-infrared; PTH, parathyroid hormone; TIBC, total iron-binding capacity; VLDL-C, very-low-density lipoprotein cholesterol.
There were no significant correlations between serum AEA and seven lipid panels (VLDL-C, TG, cholesterol, HDL-C, low-density lipoprotein cholesterol, lipoprotein(a)-cholesterol, and non‒high-density lipoprotein; Supplemental Table 2 [17]). The correlations between serum AEA and normalized protein catabolic rate and unsaturated iron-binding capacity were modest but significant (ρ = −0.43; P = 0.004 and ρ = 0.32; P = 0.03, respectively) following adjustment for age, sex, race, ethnicity, diabetes, and dialysis vintage covariates.
3. Discussion
ESRD is associated with a disproportionately elevated risk of death. Traditional risk factors for mortality such as obesity and hypertriglyceridemia do not consistently explain the mortality risk observed in these patients and can be associated with improved outcomes [4, 5]. Alternatively, cachexia is a common complication of ESRD and plays a prominent role in the morbidity and mortality associated with this disease [6]. Therefore, there has been a focus on identifying cachexia-related risk factors, which can better explain ESRD-associated mortality and provide new potential targets for therapy [22].
In this regard, data on the association of endocannabinoids with clinical and laboratory markers are very limited in patients with ESRD. The only available report is a study by Friedman et al. [23], which did not find any differences in plasma (EDTA-preserved tubing) endocannabinoid levels between healthy controls and patients with ESRD. However, it should be noted that the small sample size (n = 20), significant sex differences between the control and ESRD groups, and use of plasma (vs our samples which were serum) in their study make interpretation and comparison with our study difficult [23]. Our study evaluated serum endocannabinoid levels in a large cohort of patients with ESRD who were compared with age- and sex-matched healthy controls. We found significantly increased serum concentrations of 2-AG in patients with ESRD undergoing MHD, as measured in two different laboratories and two separate sets of patients and controls. In MHD patients, serum concentrations of 2-AG positively correlated with serum TG and TG-rich lipoprotein levels. In addition, serum 2-AG levels correlated positively with BMI and indices of increased body fat and midarm circumference on anthropometric measurements. These findings are in line with available literature indicating that higher serum endocannabinoid levels can be associated with increased body fat content [11]. Furthermore, serum 2-AG levels negatively correlated with serum HDL-C concentrations, which is consistent with previously published data regarding the role of the endocannabinoid system in HDL metabolism [24–26].
It is also interesting to note that although activation of the endocannabinoid system may be partly responsible for the associations observed in this study, it may not be the only mechanism at play, given that our findings are unique to 2-AG and that serum AEA levels did not have similar correlations with clinical and laboratory markers. In this regard, differential changes and correlations observed for 2-AG and AEA in our study were also observed in other studies [27, 28]. The mechanisms responsible for and implications of these findings are less clear. However, in a study by Côté et al. [29], it was argued that 2-AG, rather than AEA, was the more selective and effective activator of the EC system, pointing out that AEA has also been reported to bind to several other targets. Therefore, it was argued that differential changes in these two endocannabinoid mediators during physiologic or pathologic conditions can be observed, given that they may be mediating different effects [29]. However, it is also important to recognize that clear mechanisms that can account for these differential changes need to be examined in future studies in order to delineate the possible causes of these observations. In addition, future studies will need to address the potential differences between serum and plasma levels of these lipid-derived mediators, given the distinctions noted in our studies compared with findings of the other investigation [23].
There is accumulating evidence that the endocannabinoid system plays a key role in increasing energy intake and enhancing energy preservation via several different mechanisms, including inhibition of brown adipose tissue formation and thermogenesis [8, 11, 12]. For instance, it has been shown that reduced levels of 2-AG in the forebrain of mice lead to increased brown adipose tissue thermogenesis and a lean body phenotype [30], whereas CB1 receptor activation in white adipose tissue prevents the transdifferentiation of white adipocytes into the thermogenic brown fat phenotype characterized by increased UCP-1, resulting in an obese phenotype [31]. Furthermore, other clinical investigations also found a positive correlation between circulating 2-AG levels and markers of body mass, including body fat content [29]. Therefore, it is not surprising that increasing serum levels of 2-AG positively correlate with indices of increased body mass in patients undergoing hemodialysis.
Furthermore, activation of the endocannabinoid system via 2-AG may also play a causative role in elevated TG levels in ESRD. Dyslipidemia of CKD and ESRD is characterized by increased levels of serum TGs and TG-rich lipoproteins [32]. One proposed mechanism underlying these findings is activation of the nuclear transcription factor sterol regulatory element binding protein-1c (SREBP-1c) in the liver and adipose tissue [33, 34]. In addition, there is downregulation of the machinery involved in fatty acid β-oxidation, including carnitine palmitoyltransferase-1. Together, these alterations lead to increased TG production, tissue content, and serum levels. It is interesting to note that activation of CB1 receptors (i.e., via increased 2-AG levels) has been also shown to stimulate SREBP-1c and decrease carnitine palmitoyltransferase-1 activity and mRNA expression [35]. These effects have also been shown to cause increased serum and hepatic TG content. Therefore, significantly increased serum 2-AG levels, which may indicate overactivity of the endocannabinoid system, may also be partly causing the hypertriglyceridemia observed in ESRD.
Several limitations of this study need to be mentioned. Although the findings described here are thought-provoking, they remain observational in nature, and mechanistic studies are needed to verify a causal link between serum 2-AG levels and obesity/hypertriglyceridemia. Similarly, the functional relationship between 2-AG and AEA, as well as the potential roles of AEA and other fatty acid ethanolamides in ESRD, if any, remains to be determined. Furthermore, although we adjusted for multiple variables, the possibility of residual confounding cannot be excluded. Although 2-AG is a potent activator of the endocannabinoid system, the role of circulating endocannabinoids in the activation of the endocannabinoid system has not been fully elucidated. Another potential limitation is the use of nonfasting serum in the analysis of lipid-derived mediators. Although many previous studies of serum endocannabinoid levels used fasting serum or plasma samples [23], it should be noted that Gatta-Cherifi et al. [36] showed that serum levels of 2-AG were not affected by feeding. In addition, we used nonfasting serum across all of the groups in this study, including our healthy controls; therefore, the likelihood of alterations in serum AEA and/or 2-AG levels due to timing of food intake is less likely. Finally, our studies do not indicate the source of 2-AG in serum, and this will need to be investigated in future studies. Potential mechanisms responsible for increased serum 2-AG levels include increased production by the gastrointestinal tract [12], oxidative stress‒related inhibition of monoacylglycerol lipase, the main enzyme responsible for 2-AG breakdown, and activation of platelets and platelet activating factor in hemodialysis. This latter mechanism can also potentially explain the differences in levels of 2-AG found in our study, given that we used serum compared with Friedman et al. [23] who used plasma [37–39]. These pathways will need to be examined in future mechanistic studies to determine the underlying cause of increased serum 2-AG in MHD patients.
In conclusion, serum concentrations of the endocannabinoid mediator 2-AG were increased in MHD patients and significantly and positively correlated with BMI, markers of increased body mass, and serum TG concentrations. Future studies are needed to determine the mechanisms responsible for these findings and the association of serum endocannabinoid levels with ESRD-related outcomes.
Acknowledgments
The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through grant no. UL1 TR001414. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We are also grateful to UC Irvine Institute for Clinical and Translational Science for their support. The opinions expressed in this article are those of the authors and do not represent the views of the US Department of Veterans Affairs or the US Government.
Financial Support: H.M. is supported by a career development award from the Office of Research and Development of the Department of Veterans Affairs (1 IK CX 001043-01A2). E.S. is supported by a career development award from the Office of Research and Development of the Department of Veterans Affairs (IK2-CX001266-01). K.K.-Z. is supported by NIH [National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)] grant no. K24-DK091419 and philanthropic grants from Mr. Harold Simmons, Mr. Louis Chang, Joseph Lee, and AVEO, Inc. C.M.R. is supported by NIH (NIDDK) grant no. K23-DK102903. N.V.D. and D.A.A. are supported by NIH grants no. DK119498, DA034009, and DK114978. H.M., E.S., and K.K.-Z. are employees of the US Department of Veterans Affairs.
Additional Information
Disclosure Summary: H.M. has received funding from the NIH, Department of Veterans Affairs Office of Research and Development, Amgen, and Novartis. K.K.-Z. has received honoraria and/or support from Abbott, AbbVie, Alexion, Amgen, the American Society of Nephrology, AstraZeneca, AVEO, Chugai, DaVita, Fresenius, Genentech, Haymarket Media, Hospira, Kabi, Keryx, NIH, National Kidney Foundation, Relypsa, Resverlogix, Sanofi, Shire, Vifor, and ZS Pharma. K.K.-Z., H.M., and D.P. declare the following conflicts of interest: They are inventors in a patent application filed by the University of California, Irvine that protects certain aspects of the work described in the present article. The remaining authors have nothing to disclose.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Glossary
Abbreviations:
- 2-AG
2-arachidonoyl-sn-glycerol
- AEA
anandamide
- BMI
body mass index
- CB
cannabinoid
- CKD
chronic kidney disease
- ESRD
end-stage renal disease
- HDL-C
high-density lipoprotein cholesterol
- IL-6
interleukin-6
- IQR
interquartile range
- MADRAD
Malnutrition
- Diet
and Racial Disparities in Chronic Kidney Disease
- MHD
maintenance hemodialysis
- PTH
parathyroid hormone
- IL-6
interleukin-6
- TG
triglyceride
- UC
University of California
- VLDL-C
very-low-density lipoprotein cholesterol
References and Notes
- 1. Kovesdy CP, Kalantar-Zadeh K. Enter the dragon: a Chinese epidemic of chronic kidney disease? Lancet. 2012;379(9818):783–785. [DOI] [PubMed] [Google Scholar]
- 2. Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, Chen M, He Q, Liao Y, Yu X, Chen N, Zhang JE, Hu Z, Liu F, Hong D, Ma L, Liu H, Zhou X, Chen J, Pan L, Chen W, Wang W, Li X, Wang H. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822. [DOI] [PubMed] [Google Scholar]
- 3. Vaziri ND, Navab K, Gollapudi P, Moradi H, Pahl MV, Barton CH, Fogelman AM, Navab M. Salutary effects of hemodialysis on low-density lipoprotein proinflammatory and high-density lipoprotein anti-inflammatory properties in patient with end-stage renal disease. J Natl Med Assoc. 2011;103(6):524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang TI, Streja E, Soohoo M, Kim TW, Rhee CM, Kovesdy CP, Kashyap ML, Vaziri ND, Kalantar-Zadeh K, Moradi H. Association of serum triglyceride to HDL cholesterol ratio with all-cause and cardiovascular mortality in incident hemodialysis patients. Clin J Am Soc Nephrol. 2017;12(4):591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doshi M, Streja E, Rhee CM, Park J, Ravel VA, Soohoo M, Moradi H, Lau WL, Mehrotra R, Kuttykrishnan S, Kovesdy CP, Kalantar-Zadeh K, Chen JL. Examining the robustness of the obesity paradox in maintenance hemodialysis patients: a marginal structural model analysis. Nephrol Dial Transplant. 2016;31(8):1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Obi Y, Qader H, Kovesdy CP, Kalantar-Zadeh K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr Opin Clin Nutr Metab Care. 2015;18(3):254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ikizler TA, Cano NJ, Franch H, Fouque D, Himmelfarb J, Kalantar-Zadeh K, Kuhlmann MK, Stenvinkel P, TerWee P, Teta D, Wang AY, Wanner C; International Society of Renal Nutrition and Metabolism. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013;84(6):1096–1107. [DOI] [PubMed] [Google Scholar]
- 8. DiPatrizio NV, Piomelli D. The thrifty lipids: endocannabinoids and the neural control of energy conservation. Trends Neurosci. 2012;35(7):403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Piomelli D. The endocannabinoid system: a drug discovery perspective. Curr Opin Investig Drugs. 2005;6(7):672–679. [PubMed] [Google Scholar]
- 10. Maccarrone M, Bab I, Bíró T, Cabral GA, Dey SK, Di Marzo V, Konje JC, Kunos G, Mechoulam R, Pacher P, Sharkey KA, Zimmer A. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci. 2015;36(5):277–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17(4):475–490. [DOI] [PubMed] [Google Scholar]
- 12. Gatta-Cherifi B, Cota D. New insights on the role of the endocannabinoid system in the regulation of energy balance. Int J Obes. 2016;40(2):210–219. [DOI] [PubMed] [Google Scholar]
- 13. Quercioli A, Pataky Z, Vincenti G, Makoundou V, Di Marzo V, Montecucco F, Carballo S, Thomas A, Staub C, Steffens S, Seimbille Y, Golay A, Ratib O, Harsch E, Mach F, Schindler TH. Elevated endocannabinoid plasma levels are associated with coronary circulatory dysfunction in obesity. Eur Heart J. 2011;32(11):1369–1378. [DOI] [PubMed] [Google Scholar]
- 14. Di Marzo V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia. 2008;51(8):1356–1367. [DOI] [PubMed] [Google Scholar]
- 15. Engeli S, Böhnke J, Feldpausch M, Gorzelniak K, Janke J, Bátkai S, Pacher P, Harvey-White J, Luft FC, Sharma AM, Jordan J. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54(10):2838–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rhee CM, Nguyen DV, Moradi H, Brunelli SM, Dukkipati R, Jing J, Nakata T, Kovesdy CP, Brent GA, Kalantar-Zadeh K. Association of adiponectin with body composition and mortality in hemodialysis patients. Am J Kidney Dis. 2015;66(2):313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moradi H, Park C, Igarashi M, Streja E, Argueta DA, Soohoo M, Daglian J, You AS, Rhee CM, Kashyap ML, DiPatrizio NV, Vaziri ND, Kalantar-Zadeh K, Piomelli D. Data from: Serum endocannabinoid levels in patients with end-stage renal disease. UC Irvine Dash 2019. Deposited 23 August 2019. 10.7280/D18W95. [DOI] [PMC free article] [PubMed]
- 18. Angelini R, Argueta DA, Piomelli D, DiPatrizio NV. Identification of a widespread palmitoylethanolamide contamination in standard laboratory glassware. Cannabis Cannabinoid Res. 2017;2(1):123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Price CA, Argueta DA, Medici V, Bremer AA, Lee V, Nunez MV, Chen GX, Keim NL, Havel PJ, Stanhope KL, DiPatrizio NV. Plasma fatty acid ethanolamides are associated with postprandial triglycerides, ApoCIII, and ApoE in humans consuming a high-fructose corn syrup-sweetened beverage. Am J Physiol Endocrinol Metab. 2018;315(2):E141–E149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Little TJ, Cvijanovic N, DiPatrizio NV, Argueta DA, Rayner CK, Feinle-Bisset C, Young RL. Plasma endocannabinoid levels in lean, overweight, and obese humans: relationships to intestinal permeability markers, inflammation, and incretin secretion. Am J Physiol Endocrinol Metab. 2018;315(4):E489–E495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feroze U, Noori N, Kovesdy CP, Molnar MZ, Martin DJ, Reina-Patton A, Benner D, Bross R, Norris KC, Kopple JD, Kalantar-Zadeh K. Quality-of-life and mortality in hemodialysis patients: roles of race and nutritional status. Clin J Am Soc Nephrol. 2011;6(5):1100–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ebner N, Springer J, Kalantar-Zadeh K, Lainscak M, Doehner W, Anker SD, von Haehling S. Mechanism and novel therapeutic approaches to wasting in chronic disease. Maturitas. 2013;75(3):199–206. [DOI] [PubMed] [Google Scholar]
- 23. Friedman AN, Kim J, Kaiser S, Pedersen TL, Newman JW, Watkins BA. Association between plasma endocannabinoids and appetite in hemodialysis patients: a pilot study. Nutr Res. 2016;36(7):658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Després JP, Golay A, Sjöström L; Rimonabant in Obesity-Lipids Study Group. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353(20):2121–2134. [DOI] [PubMed] [Google Scholar]
- 25. Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J; RIO-North America Study Group. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295(7):761–775. [DOI] [PubMed] [Google Scholar]
- 26. Haas MJ, Mazza AD, Wong NC, Mooradian AD. Inhibition of apolipoprotein A-I gene expression by obesity-associated endocannabinoids. Obesity (Silver Spring). 2012;20(4):721–729. [DOI] [PubMed] [Google Scholar]
- 27. Caraceni P, Viola A, Piscitelli F, Giannone F, Berzigotti A, Cescon M, Domenicali M, Petrosino S, Giampalma E, Riili A, Grazi G, Golfieri R, Zoli M, Bernardi M, Di Marzo V. Circulating and hepatic endocannabinoids and endocannabinoid-related molecules in patients with cirrhosis. Liver Int. 2010;30(6):816–825. [DOI] [PubMed] [Google Scholar]
- 28. Zelber-Sagi S, Azar S, Nemirovski A, Webb M, Halpern Z, Shibolet O, Tam J. Serum levels of endocannabinoids are independently associated with nonalcoholic fatty liver disease. Obesity (Silver Spring). 2017;25(1):94–101. [DOI] [PubMed] [Google Scholar]
- 29. Côté M, Matias I, Lemieux I, Petrosino S, Alméras N, Després JP, Di Marzo V. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes. 2007;31(4):692–699. [DOI] [PubMed] [Google Scholar]
- 30. Jung KM, Clapper JR, Fu J, D’Agostino G, Guijarro A, Thongkham D, Avanesian A, Astarita G, DiPatrizio NV, Frontini A, Cinti S, Diano S, Piomelli D. 2-arachidonoylglycerol signaling in forebrain regulates systemic energy metabolism. Cell Metab. 2012;15(3):299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tedesco L, Valerio A, Dossena M, Cardile A, Ragni M, Pagano C, Pagotto U, Carruba MO, Vettor R, Nisoli E. Cannabinoid receptor stimulation impairs mitochondrial biogenesis in mouse white adipose tissue, muscle, and liver: the role of eNOS, p38 MAPK, and AMPK pathways. Diabetes. 2010;59(11):2826–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vaziri ND, Moradi H. Mechanisms of dyslipidemia of chronic renal failure. Hemodial Int. 2006;10(1):1–7. [DOI] [PubMed] [Google Scholar]
- 33. Korczynska J, Stelmanska E, Nogalska A, Szolkiewicz M, Goyke E, Swierczynski J, Rutkowski B. Upregulation of lipogenic enzymes genes expression in white adipose tissue of rats with chronic renal failure is associated with higher level of sterol regulatory element binding protein-1. Metabolism. 2004;53(8):1060–1065. [DOI] [PubMed] [Google Scholar]
- 34. Chapagain A, Caton PW, Kieswich J, Andrikopoulos P, Nayuni N, Long JH, Harwood SM, Webster SP, Raftery MJ, Thiemermann C, Walker BR, Seckl JR, Corder R, Yaqoob MM. Elevated hepatic 11β-hydroxysteroid dehydrogenase type 1 induces insulin resistance in uremia. Proc Natl Acad Sci USA. 2014;111(10):3817–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Bátkai S, Harvey-White J, Mackie K, Offertáler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115(5):1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gatta-Cherifi B, Matias I, Vallée M, Tabarin A, Marsicano G, Piazza PV, Cota D. Simultaneous postprandial deregulation of the orexigenic endocannabinoid anandamide and the anorexigenic peptide YY in obesity. Int J Obes. 2012;36(6):880–885. [DOI] [PubMed] [Google Scholar]
- 37. Tetta C, David S, Biancone L, Canino F, Cambi V, Camussi G. Role of platelet activating factor in hemodialysis. Kidney Int Suppl. 1993;39:S154–S157. [PubMed] [Google Scholar]
- 38. Berdyshev EV, Schmid PC, Krebsbach RJ, Schmid HH. Activation of PAF receptors results in enhanced synthesis of 2-arachidonoylglycerol (2-AG) in immune cells. FASEB J. 2001;15(12):2171–2178. [DOI] [PubMed] [Google Scholar]
- 39. Dotsey EY, Jung KM, Basit A, Wei D, Daglian J, Vacondio F, Armirotti A, Mor M, Piomelli D. Peroxide-dependent MGL sulfenylation regulates 2-AG-mediated endocannabinoid signaling in brain neurons. Chem Biol. 2015;22(5):619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]