Abstract
A 72-year-old Caucasian man incurring a prostate hypertrophy presented with a right forearm nodule, the growth of which appeared to parallel the rise in his blood prostate-specific antigen (PSA) level. Echographic examination was consistent with a median-nerve schwannoma, and was confirmed upon magnetic resonance imaging (MRI). Excision of the nodule was readily performed without significant neural damage, and its schwannoma nature was confirmed upon immunohistochemistry analysis. Importantly, blood PSA dropped abruptly from ≈13 to ≈5 ng/ml within 2 months postschwannoma resection, a swift drastic reduction unachievable with oral dutasteride alone. However, 6 weeks later, a new nodule became apparent on the back of the left knee and was identified as a second schwannoma, thereby suggesting that its growth could have been stimulated by the resection of the first schwannoma, as previously described for vestibular schwannomas. The second schwannoma was in fact two: the bigger one was in the common fibular nerve and the smaller one in the tibial nerve. Both echography and MRI results were confirmed upon surgical resection of the bigger knee schwannoma. Although the third schwannoma has not yet been resected and formally characterized, we face a schwannomatosis case with an unexpected potential exosome-mediated stimulating effect on PSA secretion (PSA immunohistochemistry was negative on both schwannomas). On the other hand, preliminary genomic analysis showed a deficient balance for chromosome 22, the very chromosome carrying the three main genes involved in schwannomatosis. This age-related schwannomatosis case is thus discussed in light of the following: age-related DNA repair deficiency culminating in loss of chromosome/heterozygosity; CpG methylation/demethylation-based epigenetic aging; age-related functional decline of the immune system responsible for inefficient elimination of abnormal cells and subsequent tumorigenic cell turn-over; exosome-mediated pathologic intercellular communications; and prostate-invading brain neural progenitors as pathologic peripheral nervous system (PNS) cells.
Keywords: age-related schwannomatosis, chromosome 22 somatic loss, pathologic exosome-mediated intercellular communications, prostate hyperplasia
Introduction
Human schwannoma cells have been shown to efficiently shed and incorporate exosomes,1–4 thereby enabling extended bidirectional interactions with distant target cells via blood, urine, and other body fluids, as initially described with other cell types.5–7 Such interactions culminate with infectious prion exosomes,8 and with experimental invasive vehicles for blood-brain-barrier-free delivery of therapeutic RNAs/proteins,4,9–12 thereby paralleling new generation nanoparticles such as emerging lentivirus-like bionanoparticles.13 Exosomes are well-established mediators of Schwann cell communications in the peripheral nervous system (PNS), acting mainly on cell proliferation, senescence, and apoptosis,14–20 and could shift to abnormal cell networking in pathologic schwannoma cells, as suggested by the present age-related schwannomatosis case report.
Case report: age-related schwannomatosis and increased blood PSA
Identification, resection, and characterization of a right-forearm schwannoma
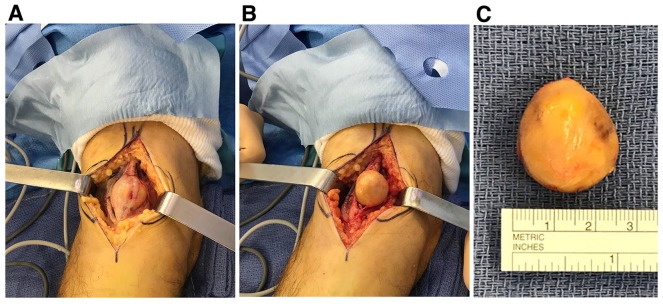
A 72-year-old Caucasian man incurring a prostate hypertrophy presented with a right forearm nodule, the growth of which appeared to parallel the rise in his blood prostate-specific antigen (PSA) level. The nodule began to be palpable about 3 years earlier, when blood PSA was shown to have increased above the critical 4 ng/ml value during a routine blood control. There was no history of pain or paresthesias in the right hand and forearm, even when pressure was applied to the nodule, thereby suggesting it was a lipoma. On ultrasound examination, the nodule was shown to be associated with the median nerve, and was subsequently identified as a schwannoma upon magnetic resonance imaging (MRI; not shown). Although painless, this median-nerve schwannoma was large enough (≈3 cm) to soon become a medical problem, and was thus readily resected without significant neural damage (Figure 1).
Figure 1.
Intraoperative view of the right-forearm schwannoma. Full enucleation of the schwannoma from the median nerve was readily achieved: the nerve/tumor complex was isolated from the surrounding structures (a), a well-defined cleavage plane was demonstrated and the tumor was meticulously dissected from the nerve fascicles (b), and then completely removed (c). The nodule was a ≈3 cm beige ball weighing ≈7 g.
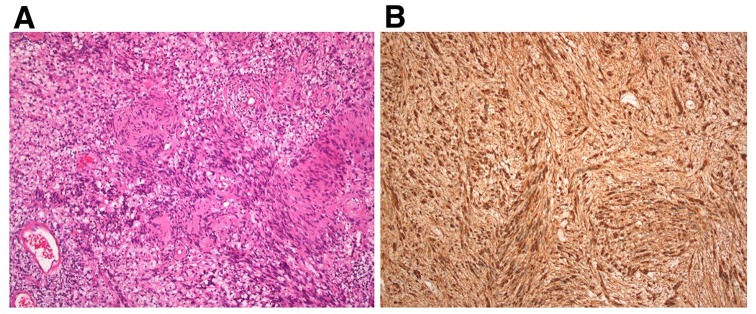
Histological examination of the surgical specimen established the diagnosis of schwannoma. As shown in Figure 2a, the excised tumor consisted in spindle-shaped cells with poorly defined eosinophilic cytoplasm, without atypia, and with nuclear palisading. The nodule comprised compact areas (Antoni A), foci of palisaded nuclei (Verocay bodies), and a less frequent loose-textured component with round nuclei and loose-knit processes (Antoni B). Focal thick-walled, hyalinized blood vessels, lymphocytic infiltrate, foamy histiocytes, and degenerative tumoral spaces were also present. In addition, strong and uniform immunoreaction for S100 protein (polyclonal, Ventana, prediluted) was seen in all tumor cells (Figure 2b). Importantly enough, in contrast with S100 protein, immunohistochemistry with human PSA antibodies was completely negative (ER-PR8 clone, Dako, 1/100 dilution).
Figure 2.
Microscopic characterization of the right-forearm schwannoma. (a) HES stain, ×100 magnification. (b) S100 protein immunostaining, ×100 magnification.
HES, Hematoxylin-eosin-safran.
Subsequent drastic drop in blood PSA
Importantly enough, after an initial small reduction induced by dutasteride medication, blood PSA dropped abruptly from ≈13 to ≈5 ng/ml within 3 months (2 months post-schwannoma resection), a swift drastic reduction unachievable with oral dutasteride alone. Such a reduction in blood PSA level by approximately 62% in 3 months (≈13 to ≈5 ng/ml) suggested that, in addition to the action of oral dutasteride, a synergistic effect resulted from schwannoma excision itself. Of note, oral dutasteride (0.5 mg Avodart, once daily) was initiated as a medicine for benign prostatic hyperplasia (BPH, prostate MRI compatibility) 56 days before schwannoma resection, and resulted in reduced blood PSA level by 12% (from a ≈14.8 ng/ml PSA baseline to ≈13 ng/ml) in about 1 month (Table 1).
Table 1.
Case report synopsis.
| Avodart 21 March 2018 |
S1 exeresis 15 May 2018 |
Knee S2 S3 1 July 2018 |
S2 exeresis 10 October 2018 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Date | 27 November 2017 | 10 January 2018 | 16 February 2018 | 14 March 2018 | 10 April 2018 | 18 June 2018 | 18 July 2018 | 21 August 2018 | 24 September 2018 | 24 October 2018 | 14 November 2018 | 14 December 2018 | 14 January 2019 | 25 February 2019 | 27 March 2019 |
| PSA ng/ml |
12.76 | 14.07 | 14.32 | 14.79 | 13.04 | 7.50 | 4.93 | 7.12 | 4.86 | 12.09 | 5.67 | 5.41 | 4.82 | 4.21 | 3.37 |
| PSA free/total |
0.12 | 0.12 | 0.12 | 0.11 | 0.16 | 0.13 | ND | 0.22 | 0.17 | 0.10 | ND | 0.18 | 0.15 | 0.16 | 0.18 |
| Prostate MRI |
6 February 2018 | 19 June 2018 | 27 November 2018 |
March 2018: Beginning of dutasteride medication.
May 2018: First schwannoma resection (S1, right forearm, median nerve).
July 2018: Second schwannoma apparition (left knee, double schwannoma S2, S3).
October 2018: Second schwannoma resection (S2, common fibular nerve).
MRI, magnetic resonance imaging; PSA, prostate-specific antigen.
Interestingly, Table 1 shows that, starting from 14.79 ng/ml (100% baseline) on 14 March, blood PSA level dropped to 4.93 ng/ml (33% baseline) within 5 months, but then increased to 7.12 ng/ml (48% baseline) on 21 August, that is, about 2 months after the appearance of a new schwannoma-like nodule on the back of the left knee, thereby establishing a potential correlation between the recurrence of PSA level increase and the emergence of a sizeable new schwannoma, therefore providing some additional support to the idea of a potential stimulating effect of schwannoma tumors on blood PSA level.
Growth, resection, and characterization of a second schwannoma (left knee)
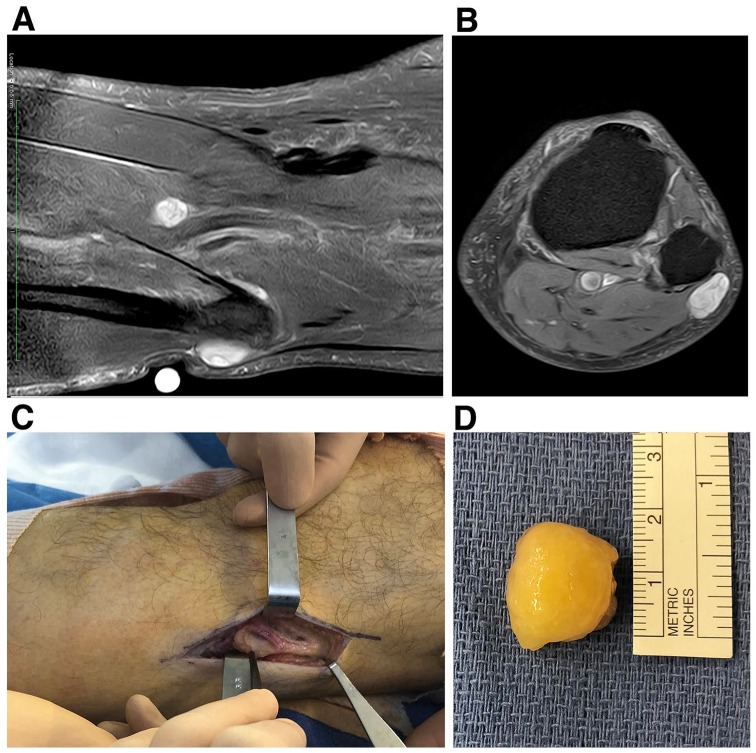
As expected according to percussion-induced Tinel’s like paresthesias along the sciatic nerve side of the leg, the left-knee nodule was readily identified as a double schwannoma, in which the protruding larger one was in the common fibular nerve, and the smaller one deeper in the tibial nerve [echographic (not shown) and MRI evaluations, Figure 3a,b]. Both echographic and MRI results were confirmed upon surgical resection of the larger knee schwannoma (Figure 3c,d).
Figure 3.
Preoperative MRI evaluation (a,b) and intraoperative view (c,d) of the left-knee nodule. Two well-circumscribed lesions involving the common fibular nerve and the tibial nerve, respectively, were identified (a,b). While they both exhibited an heterogenous T2 hypersignal, they showed a weak and strong gadolinium-mediated enhancement (not shown), respectively. The common fibular nerve schwannoma was larger (24 × 14 mm) than the tibial nerve one (15 × 9 mm) and was readily enucleated without any neural damage (c,d). The common fibular nerve was carefully handled and its upper part with its lateral sural cutaneous branch was surgery free (c).
MRI, magnetic resonance imaging.
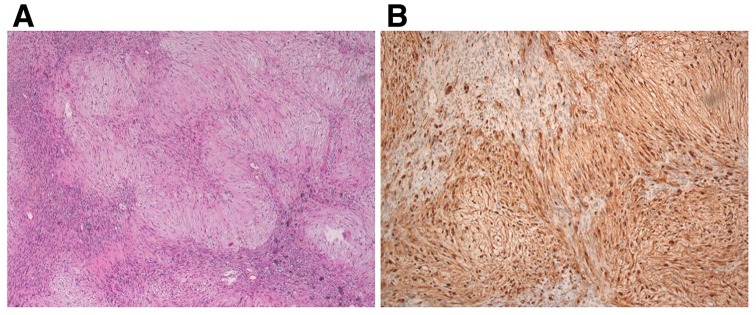
Although truly a schwannoma, this 24 × 15 mm oval-shaped nodule looked quite different from the forearm one (Figure 3d). It was surrounded by a thick translucid envelope, suggesting it could have been an intraneural lipoma. However, consistent with the MRI diagnosis, histologic and immunohistochemistry features were shown to be schwannoma-specific (Figure 4). Hematoxylin-eosin-safran (HES) stain disclosed alternating Antoni A and Antoni B patterns, hyalinized and thickened blood vessels, and cystic degenerative areas (Figure 4a). In addition, as in the aforementioned forearm nodule, strong and uniform immunoreaction was seen in all tumor cells for S100 protein (Figure 4b) but was absent for PSA (not shown).
Figure 4.
Microscopic characterization of the larger left-knee schwannoma (common fibular nerve nodule). (a) HES stain, ×50 magnification. (b) S100 protein immunostaining, ×100 magnification.
HES, Hematoxylin-eosin-safran.
Of note, due to its small size and deeper tibial location, the second left-knee schwannoma has not yet been resected. It has been the subject of a careful 1-year follow-up aimed at monitoring both its potential boosted growth and stimulating effect on blood PSA level. However, unlike its resected cognates, it has kept its original small size (15 × 9 mm, 5 July 2018 MRI) as shown on a new left-knee MRI (27 June 2019). Consistent with its small size and absence of growth, it is associated with a low blood PSA level (2.88 ng/ml, 25 June 2019).
Fluctuations in blood PSA level and reduction of prostate hyperplasia
As pinpointed above, 2 months post-first-schwannoma resection, blood PSA level dropped to 33% baseline value (4.93 ng/ml), then increased up to 48% baseline value (7.12 ng/ml) due to a potential correlation with increased knee double-schwannoma growth, and appeared to fluctuate up to the resection of the larger knee-schwannoma. Subsequent to light knee surgery, a drastic blood PSA increase (82% baseline value; Table 1) was noted, most likely resulting from a side effect of peridural anesthesia (acute urinary retention and catheter-based urine draining). At 1 month post-knee-surgery, blood PSA dropped to 38% of baseline value (5.67 ng/ml; Table 1) and continued to decrease during the following months. By the end of March 2019, blood PSA had dropped below the critical 4ng/ml value (3.37 ng/ml, 23% baseline value; Table 1) and stayed at around that level up to the end of June 2019 (2.88 ng/ml, 19% baseline value, 25 June 2019).
Importantly, blood PSA levels correlated with pathological prostatic volumes, thereby highlighting the potential synergistic therapeutic effect of oral dutasteride and schwannoma resections. Interestingly, after both schwannoma resections, a significant prostatic volume regression was noticed at 1 month postsurgery: 22% (70 ml) and 28% (65 ml) of initial volume (90 ml), respectively. Of note, the data gained with a new prostate MRI (16 May 2019) were identical to the previous ones (27 November 2018), thereby showing that regression of prostate volume following dustateride medication and both large schwannoma resections stabilized at 65 ml around 1 month after excision of the second large schwannoma (10 October 2018) and 8 months after the beginning of dustasteride medication (21 March 2018). MRI follow up clearly showed that pathologic prostate volumes resulted from transitional zone hyperplasia, mainly the right median lobe, and highlighted the absence of target area both in the transitional and peripheral zones, thereby diagnosing a perfect compatibility with a BPH case as confirmed by late May MRI (16 May 2019).
Second left-knee schwannoma and age-related schwannomatosis
The second left-knee nodule is the third schwannoma identified so far (Figure 3a,b), thereby indicating that, even though it has not yet been resected and formally characterized, we are facing an age-related schwannomatosis or neurofibromatosis type 2 (NF2) case. The fact that we have more than two non-intradermal schwannomas, with pathological confirmation for two of them, and that we are free of bilateral vestibular tumors, is consistent with a schwannomatosis diagnosis,21–23 that is, an age-related schwannomatosis that appeared on a 70-year-old patient. Importantly, this case seems to present two potential unusual features: a potential stimulating effect both on blood PSA level, and, upon resection of the larger schwannoma, on cognate schwannoma growth.
As shown above, both unusual features are associated with the first schwannoma (median nerve, right forearm), and appear to miss to the nongrowing third schwannoma (tibial nerve, left knee), which was restricted to its initial small size and linked to a low blood PSA level (2.88 ng/ml, 25/06/2019). The second schwannoma (common fibular nerve, left knee) stands in between: its stimulating action on PSA production is inferred by the fluctuating increase in blood PSA level during its pre-resection growth phase (Table 1), and by the gradual, but drastic, drop in PSA following its resection and culminating at a 19% baseline value (2.88 ng/ml) 9 months post-resection. Importantly, an extended follow up aimed at identifying a potential growth recurrence of the third schwannoma, or the occurrence of new schwannomas is in progress, and could soon benefit from emerging whole-body MRI.
Preliminary genomic data
Microarray-based comparative genomic hybridization (aCGH; SurePrint G3 Human CGH Microarray, Agilent Technologies, Santa Clara, CA, USA) performed on DNA extracted from a formalin-fixed paraffin-embedded (FFPE) sample of the common fibular nerve schwannoma disclosed a simple chromosome imbalance comprising one chromosome 18 gain (log2 ratio = 0.15) and one chromosome 22 loss (log2 ratio = –0.30). Such a quantitative genomic analysis pinpoints the somatic loss of one entire chromosome 22, the very chromosome carrying the three main genes involved in schwannomatosis (see Discussion), thereby warranting complementary analysis on the median nerve schwannoma, and, possibly, on the third schwannoma identified so far.
Stabilisation of prostatic hyperplasia and schwannomatosis, and subsequent holmium laser enucleation of the prostate
By the end of March 2019, blood PSA had dropped below the critical 4 ng/ml value (see above and Table 1) and remained at that level up to the end of June 2019 (2.88 ng/ml, 25 June 2019). Such a stable low level of blood PSA correlated with extended plateauing of prostate hyperplasia regression (65 ml) and the small size (15 × 9 mm) of the growth-less third schwannoma, as evidenced by prostate and left-knee MRIs performed on 16 May 2019 and 27 June 2019, respectively.
Such an absence of schwannoma emergency, and the stabilisation of a putative BPH case at 65 ml volume, stood thus as a very strong incentive for holmium laser enucleation of the prostate (HoLEP), that is, efficient dustateride-independent long-lasting transurethral surgical treatment of BPH.
Directed at the enucleation of the transitional zone, HoLEP was performed on 1 July 2019 using a 120-W holmium laser (Lumenis, Yokneam, Israel). Pathological analysis demonstrated a specimen weight of 50 g of BPH, consistent with negative digital rectal examination and careful extended prostate MRI follow up (Table 1 and 16/05/2019 MRI). However, it included a 2 mm wide low-risk adenocarcinoma: Gleason score 6 (3 + 3), grade group 1, stage pT1aNXMK.
As expected, an uneventful postoperative recovery was associated with a drastic drop in blood PSA level (0.04 ng/ml, 29 July 2019), thereby demonstrating the absence of significant ectopic PSA production in our patient (dutasteride was discontinued on 1 July 2019), and supporting the hypothesis of the direct prostate-stimulating ability of the resected schwannomas.
Discussion: age-related schwannomatosis and potential exosome-mediated pathologic connexions
Schwannomatosis and potential exosome-mediated connexions with distant cells
The present age-related schwannomatosis case appears to be restricted to the most current peripheral nerve locations of schwannomas, that is, the arms and the legs, involving mainly the median and sciatic/fibular nerves, respectively,22,24,25 and stands thus as a rather classic situation. However, this schwannomatosis case exhibits two potential important unusual/undescribed features: a potential stimulating effect on PSA secretion, and a potential growth-stimulation effect on a cognate schwannoma tumor when a large schwannoma tumor is resected. Of note, in both cases, we are apparently dealing with interactions between distant cells/organs (involving schwannomas, prostate/ectopic PSA-producing tissues and, most likely, normal Schwann cells), for which the main connecting paths appear to be blood and other body fluids, thereby suggesting that we are dealing with exosome-mediated cell interactions, as previously shown in vitro for vestibular schwannoma specific-toxicity on cochlear cells.1
As emphasized in the Introduction, exosomes are well-established mediators of Schwann cell communications in the PNS, acting mainly on cell proliferation, senescence, and apoptosis.14–20 They could thus shift to an abnormal cell networking in pathologic schwannoma cells, as evidenced here by the potential growth stimulation of knee schwannoma cells and downregulation of PSA-secreting cells after forearm schwannoma resection, and fluctuating upregulation and gradual drastic downregulation of PSA-secreting cells during the pre-resection growth phase and the post-resection follow up of the large knee schwannoma, respectively.
Potential schwannoma growth stimulation upon resection of a cognate schwannoma
Growth stimulation of a schwannoma after resection of a large cognate is unusual but has been previously described for bilateral vestibular schwannomas.26,27 The main potential mechanisms for contralateral tumor-growth induction were hypothesized to be either the release from a space restriction problem or the paracrine action of a growth factor upon surgical excision of the cognate large tumor.26 For the induction by a growth factor, they envisioned its diffusion through the cerebrospinal fluid, and restricted its action to an autocrine/paracrine mechanism because it did not act on concurrent meningiomas (8 out of 11 patients presented with meningiomas in addition to bilateral vestibular schwannomas). One potential broadening interpretation deals with target cell specificity, which could rely on exosome delivery instead of direct growth-factor diffusion. Exosomes are all the more attractive as they appear to be instrumental in the mediation of Schwann cell communications in the PNS (as emphasized in the Introduction), and could thus be involved directly in the growth stimulation of the contralateral schwannomas26,27 and of the large knee-schwannoma in our case report. The hypothesis is that, because the growth stimulation is subsequent to the surgical resection of the large schwannoma, it could originate from tumor-adjacent resident Schwann cells as an exosome-mediated regenerative reaction to the sensing of tumor enucleation as a major wound process, and could thus reach distant schwannoma cells from contralateral and remaining cognate tumors, respectively. Such a growth stimulation should be transient, and could act as a proliferative induction on the relevant distant schwannoma, thereby resulting in a short-term (5–10 months)27 or long-term (several years)26 increase in tumor size. In the present case report, we need an extended follow up in order to evaluate the situation for the currently indolent nongrowing third schwannoma. Interestingly, in the two cases reported by von Eckardstein and colleagues, the initial short-term growth phase of the contralateral schwannomas preceded a dramatic spontaneous long-term tumor regression.27
Potential balance between blood PSA downregulation and converse schwannoma growth stimulation
In the growth induction process described in our case report, surgery-free remaining schwannomas stand thus as potential pathologic targets of the regenerative ability of normal Schwann cells, a major feature of PNS preservation.28 Conversely, in the absence of ectopic PSA production by both resected schwannomas, their pathological networking appeared to involve a potential connection with PSA-secreting cells, possibly a proliferative stimulation contributing to prostatic hyperplasia of the patient. Such a connection between distant schwannoma and target prostate cells might thus rely on exosome delivery of a growth factor (protein or relevant RNAs) such as brain derived neurotrophic factor (BDNF). BDNF is well expressed in a series of schwannomas,29 and has been recently shown to be a potential urine quantitative-marker for BPH.30 The systematic association of BPH with significant high levels of urine BDNF suggests that BDNF overexpression is indeed involved in BPH. Consistent with this hypothesis, the main BDNF receptor (tropomyosin-related kinase receptor B, TrkB) has been shown to be overexpressed in BPH prostatic cells (and cancer cells31). Thus, BDNF produced by schwannoma cells could have a local autocrine/paracrine proliferative action (schwannoma tumor growth29) and a distant exosome-mediated target-specific effect on prostatic cells.
The complete issue of our reported schwannomatosis case is still pending because the remaining small tibial nerve schwannoma (the third one) is ‘growthless’ to date, and we do not know if it will exit from its presumed indolent state or if other new schwannomas will appear. However, an extended follow up of blood PSA level after resection of the second large schwannoma (common fibular nerve) showed that a balance between blood PSA downregulation and converse schwannoma growth stimulation most likely exists for the two large schwannomas. As shown above, excision of the first schwannoma (median nerve) was first associated with a swift drastic PSA downregulation, that was unachievable with oral dutasteride alone, and then blood PSA level increased and fluctuated, subsequent to the appearance and growth of the second large schwannoma (common fibular nerve). PSA downregulation resumed after resection of this second schwannoma, and was fully established after recovery from peridural anesthesia side-effects, culminating at a 19% PSA baseline value (2.88 ng/ml) on 25 June 2019. Importantly, although low, this blood PSA level did drastically drop to 0.04 ng/ml 1 month after the aforementioned HoLEP therapy, thereby demonstrating the absence of significant ectopic PSA production in our patient (dutasteride was discontinued on the day of surgery), and supporting the aforementioned hypothesis of the direct prostate stimulating ability of cells from the resected schwannomas.
Age-related schwannomatosis: genomics, epigenomics, and immune system decline
The aforementioned schwannomatosis diagnosis is a clinical one in which the last criteria requiring MRI demonstration of the absence of bilateral vestibular schwannomas21–23 is not yet available. Both schwannomatosis and neurofibromatosis type 2 (NF2) diseases are characterized by the onset of multiple schwannomas, and have often diagnostic overlaps even after basic molecular diagnosis, thereby necessitating complementary refined genomic and epigenomic characterization in order to clarify the cases.22,32,33 As recently reviewed,23,34 both schwannomatosis and NF2 are autosomal dominant syndromes caused by the constitutional mutation of one tumor suppressor gene (NF2, SMARCB1 or LZTR1) that predispose the development of multiple schwannomas through somatic inactivation of the relevant remaining wild type allele either alone (NF2) or in combination with the somatic biallelic inactivation of NF2 (SMARCB1- or LZTR1-associated schwannomatosis). The NF2 gene is thus involved in both cases either alone as the constitutional mutant (NF2) or as the somatic biallelic mutant associated with either the SMARCB1 or LZTR1 mutant gene (schwannomatosis). Therefore, in theory, NF2 disease should be specified through the identification of a constitutional NF2 mutation in non-schwannoma control cells. However, from a practical point of view, constitutional NF2 mutants comprise mosaic type cases in which the mutation is restricted to a few non-schwannoma control cells, thereby requiring a more complex molecular analysis involving two independent tumors from the relevant patient (Updated Manchester criteria).22,23,33
The propensity of schwannomatosis patients to incur the development of multiple schwannomas is eased by the fact that all three tumor suppressor genes are located within an ≈8.7 Mb on the same chromosome 22,34 thereby enabling the somatic elimination of one copy of all three tumor suppressor genes through a single loss-of-heterozygosity (LOH) event, a major step in the somatic biallelic inactivation of the NF2 gene and of the wild type SMARCB1 or LZTR1 allele (four hits/three steps mechanism).34,35 Of note, preliminary genomic results gained in our case on the second resected schwannoma have shown a deficient balance for chromosome 22, thereby providing the first direct genomic support to the schwannomatosis diagnosis and to an age-related pathology. Regarding the relevant genes, the fact that LOH could culminate in whole chromosome loss has led speculation on the potential involvement of other tumor suppressor genes (located on chromosome 22) in order to cover the 60% and 14% sporadic and familial schwannomatosis cases, respectively, that are not associated with the SMARCB1 or LZTR1 gene.34 In addition, the somatic loss of such unidentified genes could also possibly promote schwannoma cases with monoallelic somatic inactivation of the NF2 gene.34
The fact that the first (associated to the median nerve) and the second (associated to the common fibular nerve) schwannoma appeared when the patient was ≈70 years old suggests that they are part of an age-related disorder. And indeed, preliminary schwannoma genomic data disclosed a deficient balance for chromosome 22 (and the gain of one chromosome 18), thereby pinpointing a major feature of the genomic instability hallmark of aging.36 Such aging genomic hallmarks include nuclear and mitochondrial DNA damage culminating in chromosome missegregation/loss,37 and could be synergized both by the telomere attrition (genomic impact) and epigenetic alteration (gene silencing) hallmarks of aging in order to complete the aforementioned four hits/three steps inactivation of the relevant tumor suppressor genes.34,36,38–40 Importantly, stem cell exhaustion is another hallmark of aging,36 and, among other effects, translates into immunosenescence,36,41–43 thereby hampering immunosurveillance against premalignant cells and compromising antitumor immunity in elderly people.41,44 The late outbreak of schwannomas in our schwannomatosis case report may thus result, at least in part, from such an age-related immune downregulation, characterized by low amounts of naïve T cells, ‘exhaustion’ of potentially tumor-specific memory T cells, and higher amounts of suppressive cells.45 Therefore, the schwannoma tumor cells from our elderly case-report most likely derive from aged schwann cells (regenerative myelin and Remak cells) in which inactivation of relevant tumor suppressor genes and freedom from anti-tumor immunity result from the chronologic aging process incurred by the schwannomatosis patient,46 that is, a patient carrying a mono-allelic constitutional mutation on an autosomal tumor suppressor gene located on chr 22 (see above).
Conclusion
We present an age-related schwannomatosis case, most likely a sporadic one since there is no known family background on either parental side. The fact that the first schwannoma appeared when the patient was ≈70 years-old suggests that he had just been through a critical aging step, culminating in a stem cell exhaustion hallmark phase,36 hitting most likely both normal schwann cells and immune stem cells, thereby synergizing an outbreak of Schwann cells-derived benign PNS tumors (three nodules). Although benign, this early schwannoma outbreak is a major health hazard and will hopefully be very soon stopped by one of the current approaches aimed at ‘rejuvenating’ aged stem cells,42,47 and improving healthy aging.48–51 Among them, the restoration of NAD+ homeostasis in different resident stem cell populations could be instrumental because it should re-establish efficient DNA repair into most stem cell types,52–54 and could possibly be achieved by a safe hit-and-run epigenetic protocol,55–57 possibly synergized by dietary complementation.57 NAD+ downregulation being a general aging feature, ‘rejuvenation’ should be broad enough to include the immune system in addition to the Schwann cells and prostatic stem cells reported in our case.
Interestingly, we have identified potential pathologic cell interactions between the schwannoma tumor cells and both distant cognate schwannoma tumor and prostate cells, thereby illustrating another basic hallmark of aging: the altered intercellular communication one with its prominent ‘inflammaging’ phenotype.36,58,59 Such potential interactions between schwannoma tumor cells and distant tumor/normal stem cells are very important both from a fundamental and clinical points of view because they are consistent with in vitro data gained on human vestibular schwannoma cells and their exosomes-mediated cochlear cell-specific toxicity,1 and emphasize the existence of a potential pathologic counterpart to the well-established schwann cell-specific exosome-mediated regenerative network driven by aged resident schwann cells and their schwannoma derivatives (see above).14–20 As detailed above, pathologic interactions in the present report include a potential stimulation of PSA-secreting cells by forearm and knee schwannoma cells (contributing, possibly, to prostate hyperplasia), and a potential growth stimulation of knee schwannoma cells (subsequent to forearm schwannoma resection) by resident forearm aged schwann cells. Such potential newly evidenced exosome-mediated pathologic intercellular communications are all the more important as brain neural progenitor cells have been shown to migrate into normal and pathologic prostate tissues and appear to contribute both to BPH and adenocarcinoma progressions,60,61 thereby suggesting that these new PNS-based cells could be pathologic targets of normal/aged schwann cells and schwannoma derivatives.
The aforementioned pathologic interactions possibly include specific connexions with the low grade and most-likely indolent incidental prostate cancer (iPCa)62 identified after HoLEP surgery. Thus, they deserve a careful extended follow up on our case-report patient both for BPH/iPCa long-term recovery and schwannomatosis indolent stability, and warrant complementary detailed biochemical and molecular biology analyses, focused primarily on HoLEP and schwannoma FFPE samples, thereby culminating possibly in new developments of the emerging exosomes/extracellular vesicles-mediated therapeutic/prophylactic armamentarium.12,63
Acknowledgments
We thank Professor Paul Hofman and Professor Florence Pedeutour, Heads of the Laboratory of Clinical and Experimental Pathology, and of the Laboratory of Solid Tumor Genetics, respectively, University Hospital of Nice-Côte d’Azur University, and Dr. Florence Dupré, Head of the Department of Pathology, Princess Grace Hospital, Monaco, for their expert interest in the presented case. Measurements of blood PSA were performed by Cerballiance Laboratory, Saint-Laurent du Var (France). Bérengère Chignon-Sicard, Daniel Chevallier and Roger Bertolotti are senior co-authors.
Footnotes
Consent for publication: Written informed consent was provided by the patient.
Funding: The author(s) received no financial support for the research, authorship, and publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Daniel Chevallier  https://orcid.org/0000-0002-0236-6458
https://orcid.org/0000-0002-0236-6458
Contributor Information
Bérengère Chignon-Sicard, Department of Plastic and Reconstructive Surgery, Pasteur 2 University Hospital, Côte d’Azur University, Nice, France.
Véronique Hofman, Laboratory of Clinical and Experimental Pathology, Pasteur 2 University Hospital, Côte d’Azur University, Nice, France.
Daniel Chevallier, Department of Urology and Kidney Transplantation, Pasteur 2 University Hospital, Côte d’Azur University, 06001 Nice Cedex 1, France.
Jean-Michel Cucchi, Diagnostic Imaging Department, Princess Grace Hospital, Monaco.
Marius Ilié, Laboratory of Clinical and Experimental Pathology, Pasteur 2 University Hospital, Côte d’Azur University, Nice, France.
Bérengère Dadone-Montaudié, Laboratory of Solid Tumors Genetics, Nice University Hospital, Côte d’Azur University, Nice, France.
Florence Paul, Private Medical Imaging Center “777”, Saint-Laurent du Var, France.
Xavier Carpentier, Department of Urology, Princess Grace Hospital, Monaco.
Hervé Quintens, Department of Urology, Princess Grace Hospital, Monaco.
Coraline Bence-Gauchiez, Department of Pathology, Princess Grace Hospital, Monaco.
Didier Caselles, Private Medical Office, Nice, France.
Jacqueline Rossant, Private Office of Medical Expertises, Nice, France.
Matthieu Durand, Department of Urology and Kidney Transplantation, Pasteur 2 University Hospital, Côte d’Azur University, Nice, France.
Roger Bertolotti, Gene Therapy and Regulation, Faculty of Medicine, Côte d’Azur University, Nice, France.
References
- 1. Soares VY, Atai NA, Fujita T, et al. Extracellular vesicles derived from human vestibular schwannomas associated with poor hearing damage cochlear cells. Neuro Oncol 2016; 18: 1498–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Provenzano L, Ryan Y, Hilton DA, et al. Cellular prion protein (PrPC) in the development of Merlin-deficient tumours. Oncogene 2017; 36: 6132–6142. [DOI] [PubMed] [Google Scholar]
- 3. Mizrak A, Bolukbasi MF, Ozdener GB, et al. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol Ther 2013; 21: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hall J, Prabhakar S, Balaj L, et al. Delivery of therapeutic proteins via extracellular vesicles: review and potential treatments for Parkinson’s disease, glioma, and schwannoma. Cell Mol Neurobiol 2016; 36: 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hawari FI, Rouhani FN, Cui X, et al. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc Natl Acad Sci U S A 2004; 101: 1297–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 2004; 101: 13368–13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caby MP, Lankar D, Vincendeau-Scherrer C, et al. Exosomal-like vesicles are present in human blood plasma. Int Immunol 2005; 17: 879–887. [DOI] [PubMed] [Google Scholar]
- 8. Fevrier B, Vilette D, Archer F, et al. Cells release prions in association with exosomes. Proc Natl Acad Sci U S A 2004; 101: 9683–9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alvarez-Erviti L, Seow Y, Yin H, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 2011; 29: 341–345. [DOI] [PubMed] [Google Scholar]
- 10. Mendt M, Kamerkar S, Sugimoto H, et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018; 3: pii: 99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haraszti RA, Miller R, Stoppato M, et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol Ther 2018; 26: 2838–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoo KW, Li N, Makani V, et al. Large-scale preparation of extracellular vesicles enriched with specific microRNA. Tissue Eng Part C Methods 2018; 24: 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu B, Javidi-Parsijani P, Makani V, et al. Delivering SaCas9 mRNA by lentivirus-like bionanoparticles for transient expression and efficient genome editing. Nucleic Acids 2019; 47(8):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lopez-Verrilli MA, Court FA. Transfer of vesicles from schwann cells to axons: a novel mechanism of communication in the peripheral nervous system. Front Physiol 2012; 3: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia 2013; 61: 1795–1806. [DOI] [PubMed] [Google Scholar]
- 16. Ching RC, Kingham PJ. The role of exosomes in peripheral nerve regeneration. Neural Regen Res 2015; 10: 743–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lopez-Leal R, Court FA. Schwann cell exosomes mediate neuron-glia communication and enhance axonal regeneration. Cell Mol Neurobiol 2016; 36: 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ching RC, Wiberg M, Kingham PJ. Schwann cell-like differentiated adipose stem cells promote neurite outgrowth via secreted exosomes and RNA transfer. Stem Cell Res Ther 2018; 9: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qing L, Chen H, Tang J, et al. Exosomes and their microRNA cargo: new players in peripheral nerve regeneration. Neurorehabil Neural Repair 2018; 32: 765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou M, Hu M, He S, et al. Effects of RSC96 schwann cell-derived exosomes on proliferation, senescence, and apoptosis of dorsal root ganglion cells in vitro. Med Sci Monit 2018; 24: 7841–7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Plotkin SR, Albers AC, Babovic-Vuksanovic D, et al. Update from the 2013 international neurofibromatosis conference. Am J Med Genet A 2014; 164A: 2969–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kehrer-Sawatzki H, Kluwe L, Friedrich RE, et al. Phenotypic and genotypic overlap between mosaic NF2 and schwannomatosis in patients with multiple non-intradermal schwannomas. Hum Genet 2018; 137: 543–552. [DOI] [PubMed] [Google Scholar]
- 23. Evans DG, Bowers NL, Tobi S, et al. Schwannomatosis: a genetic and epidemiological study. J Neurol Neurosurg Psychiatry 2018; 89: 1215–1219. [DOI] [PubMed] [Google Scholar]
- 24. Peyre M, Kalamarides M. Les Journées Neurofibromatoses 2017: Schwannomatoses, https://www.youtube.com/watch?v=nYQfaMtSl7Y (2017, accessed 7 December 2018).
- 25. Merker VL, Esparza S, Smith MJ, et al. Clinical features of schwannomatosis: a retrospective analysis of 87 patients. Oncologist 2012; 17: 1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peyre M, Goutagny S, Imbeaud S, et al. Increased growth rate of vestibular schwannoma after resection of contralateral tumor in neurofibromatosis type 2. Neuro Oncol 2011; 13: 1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Von Eckardstein KL, Beatty CW, Driscoll CL, et al. Spontaneous regression of vestibular schwannomas after resection of contralateral tumor in neurofibromatosis Type 2. J Neurosurg 2010; 112: 158–162. [DOI] [PubMed] [Google Scholar]
- 28. Jessen KR, Mirsky R, Lloyd AC. Schwann cells: development and role in nerve repair. Cold Spring Harb Perspect Biol 2015; 7: a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kramer F, Stöver T, Warnecke A, et al. BDNF mRNA expression is significantly upregulated in vestibular schwannomas and correlates with proliferative activity. J Neurooncol 2010; 98: 31–39. [DOI] [PubMed] [Google Scholar]
- 30. Wang LW, Li JL, Yu Y, et al. Association of increased urine brain derived neurotrophic factor with lower urinary tract symptoms in men with benign prostatic hyperplasia. J Huazhong Univ Sci Technolog Med Sci 2017; 37: 531–535. [DOI] [PubMed] [Google Scholar]
- 31. Bronzetti E, Artico M, Forte F, et al. A possible role of BDNF in prostate cancer detection. Oncol Rep 2008; 19: 969–974. [DOI] [PubMed] [Google Scholar]
- 32. Fisher MJ, Belzberg AJ, de Blank P, et al. 2016 Children’s tumor foundation conference on neurofibromatosis type 1, neurofibromatosis type 2, and schwannomatosis. Am J Med Genet A 2018; 176: 1258–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Louvrier C, Pasmant E, Briand-Suleau A, et al. Targeted next-generation sequencing for differential diagnosis of neurofibromatosis type 2, schwannomatosis, and meningiomatosis. Neuro Oncol 2018; 20: 917–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kehrer-Sawatzki H, Farschtschi S, Mautner VF, et al. The molecular pathogenesis of schwannomatosis, a paradigm for the co-involvement of multiple tumour suppressor genes in tumorigenesis. Hum Genet 2017; 136: 129–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sestini R, Bacci C, Provenzano A, et al. Evidence of a four-hit mechanism involving SMARCB1 and NF2 in schwannomatosis-associated schwannomas. Hum Mutat 2008; 29: 227–231. [DOI] [PubMed] [Google Scholar]
- 36. López-Otín C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell 2013; 153: 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baker DJ, Dawlaty MM, Wijshake T, et al. Increased expression of BubR1 protects against aneuploidy and cancer and extends healthy lifespan. Nat Cell Biol 2013; 15: 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, tetrahymena and yeast to human cancer and aging. Nat Med 2006; 12: 1133–1138. [DOI] [PubMed] [Google Scholar]
- 39. Booth LN, Brunet A. The aging epigenome. Mol Cell 2016; 62: 728–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sen P, Shah PP, Nativio R, et al. Epigenetic mechanisms of longevity and aging. Cell 2016; 166: 822–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yanes RE, Gustafson CE, Weyand CM, et al. Lymphocyte generation and population homeostasis throughout life. Semin Hematol 2017; 54: 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leins H, Mulaw M, Eiwen K, et al. Aged murine hematopoietic stem cells drive aging-associated immune remodeling. Blood 2018; 132: 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pawelec G. Age and immunity: what is ‘immunosenescence’? Exp Gerontol 2018; 105: 4–9. [DOI] [PubMed] [Google Scholar]
- 44. Senovilla L, Vitale I, Martins I, et al. An immunosurveillance mechanism controls cancer cell ploidy. Science 2012; 337: 1678–1684. [DOI] [PubMed] [Google Scholar]
- 45. Pawelec G. Does patient age influence anti-cancer immunity? Semin Immunopathol 2019; 41: 125–131. [DOI] [PubMed] [Google Scholar]
- 46. Arthur-Farraj PJ, Latouche M, Wilton DK, et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 2012; 75: 633–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ryu D, Zhang H, Ropelle ER, et al. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci Transl Med 2016; 8: 361ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Conboy IM, Conboy MJ, Wagers AJ, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005; 433: 760–764. [DOI] [PubMed] [Google Scholar]
- 49. Ocampo A, Reddy P, Martinez-Redondo P, et al. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell 2016; 167: 1719–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. López-Otín C, Galluzzi L, Freije JMP, et al. Metabolic control of longevity. Cell 2016; 166: 802–821. [DOI] [PubMed] [Google Scholar]
- 51. Mahmoudi S, Xu L, Brunet A. Turning back time with emerging rejuvenation strategies. Nat Cell Biol 2019; 21: 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Verdin E. NAD⁺ in aging, metabolism, and neurodegeneration. Science 2015; 350: 1208–1213. [DOI] [PubMed] [Google Scholar]
- 53. Guarente L. Cell Metabolism. The resurgence of NAD⁺. Science 2016; 352: 1396–1397. [DOI] [PubMed] [Google Scholar]
- 54. Katsyuba E, Auwerx J. Modulating NAD+ metabolism, from bench to bedside. EMBO J 2017; 36: 2670–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bertolotti R. Translational perspectives — transient epigenetic gene therapy: hazard-free cell reprogramming approach and rising arm of a universal stem cell gene therapy platform. Gene Ther Regul 2009; 4: 11–39. [Google Scholar]
- 56. Bertolotti R. Transient epigenetic gene therapy as a hit-and-run targeted epigenome editing approach driven by nuclease-null CRISPR-dCas9 for age-related disorders and degenerative diseases. Mol Ther 2018; 26(5 Suppl. 1): abstract 232–233. [Google Scholar]
- 57. Bertolotti R. Delivery of non-coding RNAs by mesenchymal stem cell-derived exosomes as an in-vivo hit-and-run targeted epigenome remodeling approach for age-related disorders and degenerative diseases. Mol Ther 2019; 27(4 Suppl. 1): abstract 361–362. [Google Scholar]
- 58. Salminen A, Kaarniranta K, Kauppinen A. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging (Albany NY) 2012; 4: 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000; 908: 244–254. [DOI] [PubMed] [Google Scholar]
- 60. Schafer ST, Gage FH. Nerve cells from the brain invade prostate tumours. Nature 2019; 569: 637–638. [DOI] [PubMed] [Google Scholar]
- 61. Mauffrey P, Tchitchek N, Barroca V, et al. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 2019; 569: 672–678. [DOI] [PubMed] [Google Scholar]
- 62. Capogrosso P, Capitanio U, Vertosick EA, et al. Temporal trend in incidental prostate cancer detection at surgery for benign prostatic hyperplasia. Urology 2018; 122: 152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Peak TC, Praharaj PP, Panigrahi GK, et al. Exosomes secreted by placental stem cells selectively inhibit growth of aggressive prostate cancer cells. Biochem Biophys Res Commun 2018; 499: 1004–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]