Abstract
Narcolepsy type 1 (NT1) is a chronic orphan disorder, caused by the selective and irreversible loss of hypocretin/orexin (ORX) neurons, by a probable autoimmune process. Little is known about NT2 etiology and prevalence, sharing with NT1 excessive daytime sleepiness (EDS) and dysregulation of rapid eye movement (REM) sleep, but without cataplexy and loss of ORX neurons. Despite major advances in our understanding of the neurobiological basis of NT1, management remains nowadays only symptomatic. The main and most disabling symptom, EDS, is managed with psychostimulants, as modafinil/armodafinil, methylphenidate, or amphetamines as a third-line therapy. Narcolepsy is an active area for drug development, and new wake-promoting agents have been developed over the past years. Pitolisant, a selective histamine H3 receptor inverse agonist, has been recently approved to treat patients with NT1 and NT2. Solriamfetol, a phenylalanine derivative with dopaminergic and noradrenergic activity will be soon a new therapeutic option to treat EDS in NT1 and NT2. Sodium oxybate, used for decades in adult patients with narcolepsy, was recently shown to be effective and safe in childhood narcolepsy. The discovery of ORX deficiency in NT1 opened new therapeutic options oriented towards ORX-based therapies, especially nonpeptide ORX receptor agonists that are currently under development. In addition, immune-based therapies administered as early as possible after disease onset could theoretically slow down or stop the destruction of ORX neurons in some selected patients. Further well-designed controlled trials are required to determine if they could really impact on the natural history of the disease. Given the different clinical, biological and genetic profiles, narcolepsy may provide a nice example for developing personalized medicine in orphan diseases, that could ultimately aid in similar research and clinical efforts for other conditions.
Keywords: hypocretin/orexin, narcolepsy type 1, narcolepsy type 2, sleepiness, cataplexy, immune-based therapies
Introduction
Narcolepsy was formerly divided into narcolepsy with and without cataplexy. In the revised International Classification of Sleep Disorders, they were renamed narcolepsy type 1 (NT1) and narcolepsy type 2 (NT2).
NT1 is a chronic and disabling sleep disorder with excessive daytime sleepiness (EDS) and cataplexy, sudden loss of muscle tone triggered by positive emotions, being the main symptoms of the disorder.1,2 Patients may also exhibit sleep paralysis, hypnagogic and hypnopompic hallucinations, and disturbed nocturnal sleep. This rare disease affects 0.026–0.05% of the population.3 It often starts in childhood or adolescence and requires a lifelong treatment. Patients often present several comorbid conditions including cardiometabolic comorbidities (e.g. obesity, type 2 diabetes, sleep apnea, cardiovascular diseases),4,5 neuropsychiatric comorbidities (e.g. mood disorders, anxiety, eating disorders and attention-deficit hyperactivity disorder), and other sleep disorders such as restless legs syndrome,6 periodic leg movements,7 rapid eye movement (REM) and non-REM (NREM) sleep parasomnias. NT1 has a unique pathophysiology: it is due to the irreversible destruction of hypocretin/orexin (ORX) neurons with an absence or low levels of ORX in the cerebrospinal fluid (CSF), probably by an autoimmune mechanism, in genetically predisposed individuals [carrying the allele human leukocyte antigen (HLA) DQB1*06:02].8
NT2 shares all symptoms of NT1, except cataplexy. Diagnosis requires night-time and daytime polysomnography, with a sleep latency under or equal 8 min and at least two sleep onsets in REM sleep on multiple sleep latency tests. The prevalence and pathophysiology of NT2 remain mostly unclear, likely due to the heterogeneity of the condition and the often-imprecise diagnosis and variability of the phenotype. NT2 may be caused by a moderate loss of ORX neurons, or by a different unknown process. Patients with low CSF ORX levels (<110 pg/ml) are relabeled NT1, even those without cataplexy (<20% of cases). All patients with narcolepsy with normal CSF ORX are categorized as NT2.9 In contrast to NT1, the clinical course of NT2 remains unclear. Some patients may later develop cataplexy and be reclassified as NT1, other patients may have a chronic stable condition, and in 25–50% of patients with NT2, EDS may improve spontaneously, without the persistent neurophysiologic hallmarks of narcolepsy.10
Narcolepsy is associated with impaired cognitive ability, poor quality of life, and risk of work, home, and car accidents.11–13 Patients with narcolepsy require first nonpharmacological treatment with behavioral modifications such as a regular night-time sleep schedule, avoiding sleep deprivation, short scheduled naps, diet, work and school accommodations, and sometimes, psychotherapy. Such approaches should, however, be studied specifically in clinical trials. In most patients with narcolepsy, pharmacological symptomatic treatments are mandatory to manage EDS, and, when present and severe, cataplexy and other comorbidities (e.g. psychiatric, metabolic, and cardiovascular comorbidities). In the first part of this review, we detailed, in brief, drugs recommended and currently used in narcolepsy. New drugs developed in recent years with potential for extension of indication in children are detailed in the second part. Finally, we reported on therapeutic perspectives, ORX therapies, and immune-based therapies.
Drugs currently prescribed
The destruction of ORX neurons in NT1 is irreversible. Current recommended therapies in narcolepsy are limited to symptomatic treatment, targeting the main symptoms of the disease, depending on their severity.1,14–16 The different treatments act on several neurotransmitters with effects being dose-dependent and with large individual variabilities in drug responses.
Current drugs for narcolepsy are listed in Table 1. EDS is usually managed with psychostimulants. Modafinil and armodafinil, its R-enantiomer, increase the extracellular concentration of dopamine by inhibiting its transporter; however, their exact mechanism of action is complex and still unclear.17 Methylphenidate blocks the reuptake of monoamines, mainly dopamine and norepinephrine, but does not inhibit the vesicular monoamine transporter. Pitolisant acts via the histaminergic pathway (see the following paragraph for more details).
Table 1.
Current drugs available for the treatment of narcolepsy.
| Drug | Usual daily doses for adults | Indication | Class of evidence for use in childhood narcolepsy | Approval |
|---|---|---|---|---|
| Modafinil | 100–400 mg | Sleepiness | No clinical trial | FDA, EMA |
| Armodafinil | 100–250 mg | Sleepiness | No clinical trial | FDA |
| Methylphenidate | 10–60 mg | Sleepiness | No clinical trial | FDA, EMA |
| Sodium oxybate | 4.5–9 g | Sleepiness, cataplexy, disturbed nighttime sleep | Recent trial with class I evidence30 | FDA, EMA |
| Pitolisant | 9–36 mg | Sleepiness, cataplexy | Ongoing international clinical trial | EMA; FDA (sleepiness) |
| Solriamfetol | 75–150 mg | Sleepiness | NA | FDA; under review by the EMA |
| D-amphetamines | 5–60 mg | Sleepiness | No clinical trial | FDA |
| Serotonin and norepinephrine-reuptake inhibitors: venlafaxine | 37.5–300 mg | Cataplexy | No clinical trial | – |
EMA, European Medicines Agency; FDA, US Food and Drug Administration; NA, not available.
For cataplexy and other symptoms related to deregulation of REM sleep such as hallucinations and sleep paralysis, sodium oxybate and antidepressant agents are really effective. Sodium oxybate, the sodium salt of gamma hydroxybutyrate (GHB), is a gamma-aminobutyric acid receptor B agonist. The mechanism explaining its efficacy in treating narcolepsy remains unclear; however, it may inhibit the activity of the noradrenergic neurons from the locus coeruleus during nocturnal sleep with a rebound effect of these neurons during daytime that promote wakefulness. Sodium oxybate is effective for EDS, cataplexy and for disturbed night-time sleep, with maximal effects reached after 3 months.18–20 Serotonin and norepinephrine-reuptake inhibitors (venlafaxine, but also duloxetine) are often prescribed to manage cataplexy, while selective serotonin-reuptake inhibitors [e.g. fluoxetine, citalopram, dopamine and norepinephrine-reuptake inhibitors (e.g. reboxetine, atomoxetine)], and low doses of tricyclic antidepressants (clomipramine) are often used as second line. In contrast to sodium oxybate, an abrupt withdrawal of antidepressant may cause a cataplexy rebound.
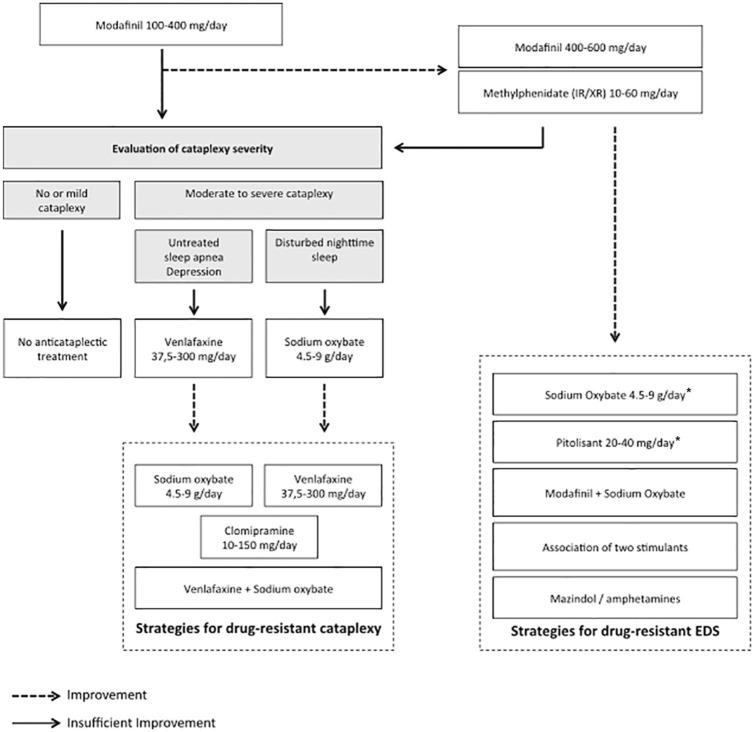
The efficacy of most of these drugs (i.e. psychostimulants and sodium oxybate) was proven by class I evidence-based studies in NT1 and NT2, whereas antidepressants are widely used, but based on expert consensus only. Several recent reviews provide recommended management strategies in narcolepsy.15 We proposed a decision tree for adapting the therapy for sleepiness and cataplexy (Figure 1).15 Psychostimulants such as modafinil, armodafinil, pitolisant or sodium oxybate are first-line strategies. In second line, methylphenidate can be used. Amphetamine mixed salts or D-amphetamine that promote monoamine (dopamine at low dose, norepinephrine at high dose, and also serotonin) release through multiple mechanisms may be used in third line only, due to potential cardiovascular and psychiatric side effects. Indeed, we recently reported that patients with NT1 treated with psychostimulants have higher diastolic blood pressure and heart rate than untreated patients, suggesting an increased long-term risk of cardiovascular diseases that requires careful follow up and management.21 Sodium oxybate is still the first-line medication recommended for cataplexy, but a recent randomized, placebo-controlled trial showed that pitolisant is also effective in that indication. Finally, mazindol, a tricyclic imidazolidine derivative, blocking the reuptake of dopamine and norepinephrine with some similarities with amphetamine, was also proposed as a third-line treatment for sleepiness and cataplexy in patients with narcolepsy; however, this drug has been withdrawn from the market, for the moment.
Figure 1.
Decision tree for managing sleepiness and cataplexy in narcolepsy type 1.
Reprinted from Barateau et al.15
EDS, excessive daytime sleepiness; IR, immediate release; XR, extended release.
*Sodium oxybate and pitolisant can also be used as first-line therapies.
Dose adjustment, switching to another drug, and prescription of multiple medications is often required when managing narcolepsy in the long term.22 Treatment of comorbidities is unfortunately often neglected but can be really important for some patients. Finally, measuring the severity of the narcolepsy symptoms and treatment efficacy became a major outcome in management optimization. Accordingly, the narcolepsy severity scale (NSS), a self-reported questionnaire, has been developed and validated to assess symptom frequency, severity, consequences, and changes over time after treatment to further provide guidance on whether treatment goals are met.23 With this questionnaire, the patient should become an active participant in the assessment/quantification of the main symptomatic complaints and in treatment decisions.
Drugs recently developed
Newer classes of wake-promoting compounds have been developed in recent years, and others are expected to emerge within the next few years.
Pitolisant is a new wake-promoting agent, a selective histamine H3-receptor inverse agonist, that acts presynaptically and activates histamine neurons. According to a class I evidence-based study, pitolisant is an effective treatment for EDS, superior to placebo, with results close to modafinil, although noninferiority has not been demonstrated.24 This drug, currently only available in Europe, was recently approved by the European Medicines Agency to treat narcolepsy with or without cataplexy. Pitolisant is taken in a morning single dose (9–36 mg/day), and has a good tolerance profile even after 1 year of follow up.25 To manage cataplexy, sodium oxybate and antidepressants remain the first-line strategy; however, pitolisant was also reported effective in cataplexy in a recent randomized, placebo-controlled trial.26 This compound can also be used as first line, and is of particular interest in patients with cardiovascular comorbidities, with cardiovascular side effects occurred with psychostimulants (such as modafinil or methylphenidate) or in a context of comorbid psychiatric condition. Pitolisant is generally well tolerated, with only few adverse events that include headache, irritability, anxiety, nausea, and insomnia.
Solriamfetol (JZP-110) is a new high-potency wake-promoting agent, a selective dopamine and norepinephrine-reuptake inhibitor without promoting the release of monoamines. An international, double-blind, randomized, placebo-controlled trial recently showed its efficacy on both objective and subjective sleepiness, and its safety in narcolepsy with and without cataplexy.27 Patients had a major increase in the mean sleep latency on the maintenance of wakefulness test, and a significant reduction in the Epworth Sleepiness Scale score. This new drug was also shown to be effective in reducing EDS in obstructive sleep apnea.28 Adverse events reported for solriamfetol included headache, nausea, decreased appetite, dry mouth, anxiety, and small increases in blood pressure and heart rate.
Other molecules are currently being tested. The combination of modafinil with connexin-30 inhibitors to potentiate its effect (by modifying the gap junctional coupling of astroglial networks),17,29 a new formulation of sodium oxybate with less sodium, and a longer-acting form of sodium oxybate, to improve patients’ compliance, could emerge as alternative therapeutic options in the next few years.
Clinical evidence shows that medications approved for adults also seem effective in children, and drugs for adults have been commonly used in childhood narcolepsy. However, until recently, class I evidence-based studies were still lacking. An international randomized placebo-controlled trial published this year eventually showed the efficacy and safety of sodium oxybate in pediatric narcolepsy.30 Another multicentric clinical trial assessing both efficacy and safety of pitolisant is currently ongoing in pediatric narcolepsy. Further clinical trials are also needed in other groups with special needs, such as pregnant women, the elderly population, the perioperative period,31 and in the context of metabolic, cardiovascular, and psychiatric disorders.
Drugs in development
Orexin-based therapy
In contrast to the almost unknown pathophysiology of NT2, the mechanism underlying NT1 is ORX deficiency, thus ORX-based treatments are highly promising in NT1.
ORX-A and ORX-B are neurotransmitters, cleaved from a single precursor (prepro-ORX) peptide, that were discovered 20 years ago. Across species, especially in mice and dogs, disrupted ORX signaling leads to a narcoleptic phenotype with EDS and cataplexy. ORX-1 binds to ORX-1 receptor (ORX1R) selectively, whereas both ORX-1 and ORX-2 bind to ORX-2 receptor (ORX2R) nonselectively. As narcolepsy-like phenotypes such as wakefulness fragmentation were observed in ORX2R knockout mice, but not in ORX1R knockout mice, activation of OX2R could be expected to promote wakefulness. Considering this unique pathophysiology, ORX-based treatments, and particularly agonists acting at ORX2R level may provide better effectiveness for treatment of narcolepsy in addressing the full extent and spectrum of symptoms. Accordingly, several approaches were recently considered in different ways: intranasal administration of ORX peptides, development of ORX-receptor agonists (i.e. particularly on receptor 2), ORX neuronal transplantation, transforming stem cells into ORX neurons, and ORX gene therapy (Table 2).
Table 2.
List of orexin-based therapies tested in animal models and in human narcolepsy.
| Orexin-based therapy | Administration, methods | Animal models/human narcoleptic patients | Effects on narcoleptic symptoms | Notes/limitations | Reference |
|---|---|---|---|---|---|
| ORX-A replacement | Intravenous and intrathecal ORX-A | ORX-ligand-deficient narcoleptic dog | Intravenous administration: transient reduction of cataplexy, no effect on sleep; intrathecal: no effect | Very high doses administered intravenously | Fujiki et al.32 |
| Intracerebroventricular ORX-A | ORX-neuron-ablated mice | Reduction of cataplexy and sleepiness | – | Mieda et al.33 | |
| Intranasal ORX-A | Sleep-deprived rhesus monkeys | NA | Reduction of the effects of sleep deprivation on cognitive performances | Deadwyler et al.34 | |
| Intranasal ORX-A | Patients with NT1* | No effect on cataplexy Reduction of REM sleep quantity, stabilization of REM sleep (reduced direct wake-to-REM transitions) | n = 8 patients | Baier et al.35* | |
| Intranasal ORX-A | Patients with NT1* | No effect on cataplexy Reduction of REM sleep duration, stabilization of REM sleep (less wake–REM sleep transitions) | n = 14 patients | Weinhold et al.36* | |
| Nonpeptide selective ORX-B-receptor agonist | Intracerebroventricular and intraperitoneal (YNT-18537)** | Models of narcoleptic mice | Reduction of sleepiness and cataplexy | Also promotes wakefulness in wild type mice (intravenously administered) | Irukayama-Tomobe et al.38** |
| ORX cell transplantation | Implantation of ORX neurons in the lateral hypothalamus | Neurotoxin-ablated ORX neuron rats | Reduction of sleepiness | – | Arias-Carrión39 |
| ORX gene therapy | Overexpression of prepro-ORX transgene | Models of narcoleptic mice | Reduction of cataplexy, stabilization of REM sleep, slight effect on sleepiness | – | Mieda et al.;33 Blanco-Centurion et al.;40 Kantor et al.41 |
| Transient expression of ligand in the lateral hypothalamus with herpes simplex vector | ORX–KO mice | Reduction of cataplexy | Increase of REM sleep at night | Liu et al.42 | |
| Delivery of the ORX gene into brain areas using recombinant adeno-associated viral vectors | Models of narcoleptic mice | Reduction of cataplexy | – | Liu et al.43,44 |
The only two studies performed in humans.
Promising therapy possibly used in humans in the near future.
KO, knockout; NT1, narcolepsy type 1; REM, rapid eye movement; ORX, hypocretin/orexin.
ORX replacement therapy remains ineffective nowadays, as this neuropeptide does not cross the blood–brain barrier. Per os and intravenous ORX administration in animal models of NT1 were almost unsuccessful because of this impermeability. However, intraventricular administration of ORX suppressed the symptoms of narcolepsy in ORX/ataxin-3 neuron-ablated mice.33 A recent study showed that slow infusion of orexin delivered via a chronically implanted intrathecal catheter at the upper lumbar level in ORX knockout mice decreased cataplexy and sleep-onset REM sleep with unchanged sleep/wake states both quantitatively and qualitatively.45 This study supports the concept of chronic intrathecal ORX delivery through an implantable pump as a potential therapy for refractory patients with NT1. A noninvasive method via intranasal administration of ORX (a method targeting drugs to the brain along the olfactory and trigeminal neural pathways) could also be of interest, with few preclinical and clinical data.34,36
In the near future, nonpeptide ORX-receptor agonists, currently under development, may be promising candidates for treating narcolepsy. A first study reported that systemic administration of a high dose of selective ORX-receptor-2 agonist, YNT-185, improved symptoms in mice models of narcolepsy, with the suppression of cataplexy-like episodes and the promotion of wakefulness. These results provided a proof of concept for a mechanistic therapy of NT1 by ORX2R agonists.38 However, YNT-185, with limited in vivo efficacy, appears not suitable for further clinical development. A second ORX2R-selective agonist, TAK-925, when injected intravenously showed robust wake-promoting effects in wild-type mice and nonhuman primates (marmosets and cynomolgus monkeys), and increased wakefulness time and completely recovered from wakefulness fragmentation and cataplexy-likeness in ORX/ataxin-3 transgenic mice, a narcolepsy mouse model with ORX deficiency.46,47 Preliminary data also showed that TAK-925 attenuated the body weight gain in orexin/ataxin-3 mice without changing the daily food intake.48 These results persisted after 14 days of subchronic systemic administration, favoring that, if results are confirmed and the drug is safe in humans, TAK-925 may treat a broad range of narcolepsy symptoms without causing ORX2R desensitization. New promising nonpeptide ORX-receptor agonists with per os administration are also currently under development. In the future, use of ORXR2 agonists as efficient stimulants could also be of interest in decreasing EDS in patients with NT2 and idiopathic hypersomnia, conditions associated with normal CSF ORX levels.
Other perspectives, such as cell replacement technique using ORX neurons derived from pluripotent stem cells, were also tested with success in rodents;49 it could become a final option for very severe and drug-resistant narcoleptic patients.50 Finally, ORX gene therapy may become a reality in future, as it improved symptoms in narcoleptic mice (Table 2).
Immune-based therapy
Background for the use of immune-based therapy
The main symptoms of NT1 are related to ORX deficiency, due to the selective destruction of this small population of hypothalamic ORX neurons, suspected to be mediated by an autoimmune process.8 A growing body of evidence supports this hypothesis, with many epidemiological, biological, and clinical findings: rare family cases and frequent discordance in monozygotic twins, young, and bimodal age at onset, association with the 2009 H1N1 influenza pandemic and its vaccine Pandemrix®, and with streptococcal infections. Genetic background is of major importance in narcolepsy, as more than 98% of patients with NT1 carry the HLA class II HLA-DQB1*06:02 allele (versus only 25% of the general population). More recently, the role of HLA class I and other immune gene variants (purinergic receptor P2RY11, T-cell receptor alpha locus TRA) was shown.14 However, no specific antibodies against ORX neurons have yet been discovered. An explanation could be the very small number of antibodies, with a damaged cerebral area very restricted, or the immune activation of T cells specifically. Another explanation could be the time course of the disease. When a patient is symptomatic, ORX neurons could be already irreversibly destroyed, and the delay between onset of symptoms and diagnosis is very long, 8–10 years on average.51 A recent breakthrough paper using a highly sensitive method to detect rare T-cell populations reported the presence of polyclonal autoreactive CD4+ T lymphocytes that recognize epitopes of the entire prepro-ORX peptide in NT1, but not in healthy control individuals.52
Based on the hypothesis of the immune-mediated destruction of ORX neurons, the idea of using immune-based therapies close to disease onset to prevent their destruction previously emerged, with a first case report published 15 years ago. Other attempts to use such therapies in narcoleptic patients were since reported, but they are all uncontrolled case studies, with very small numbers of patients. We reported and summarized those studies and their results in a recent review.22 A list of immune-based therapies that were tested in human narcolepsy is presented in Table 3. Those treatments, if effective to slow down or stop the autoimmune process, are intended to modify the natural history and the long-term disease outcomes in highly selected patients, particularly if administered very closed to disease onset. For example, corticosteroids, intravenous immunoglobulins (IVIgs), plasmapheresis, rituximab, and alemtuzumab have been tested, but with variable efficacy, if any. IVIgs were more often evaluated, probably because of their good efficacy and tolerability in many autoimmune diseases. Some NT1 patients improved on EDS and cataplexy while others did not; however, no well-designed controlled trial has been performed so far. Only one adult patient received IVIg treatment 15 days after disease onset, that completely reversed the clinical symptoms (EDS and cataplexy) and normalized CSF ORX levels, that were initially undetectable.53 The difficulty is to administer the treatment very early and close to disease onset, when the process targeting ORX neurons is not too advanced and could still be reversed. That could explain why results thus far have been contradictory, maybe due to varying degrees, types, and advanced processes of hypothalamic damage. Despite some encouraging results, there is no current evidence to guide those immunomodulatory approaches. Further well-designed controlled trials close to disease onset are required to determine if they could affect the natural history or clinical symptom burden in narcolepsy.
Table 3.
List of immune-based therapies tested in human narcolepsy.
| Immune-based therapy | Number of patients [age(s), years] | Delay between onset of first symptoms and therapy | Effect on narcoleptic symptoms | Effect on ORX-A levels | References |
|---|---|---|---|---|---|
| Corticosteroids: prednisolone | 1 (8) | 2 months | No effect on MSLT (but no cataplexy at time of therapy) | No effect: undetectable before and after therapy | Hecht et al.54 |
| IVIgs | 1 (10) | 5 months | Transient improvement of EDS and cataplexy subjectively assessed | No effect: undetectable before and after therapy | Lecendreux et al.55 |
| 4 (10, 21, 12, 52) | 4 months, 2 months, 8 months, 9 years | Improvement of cataplexy, no clear effect on EDS | Slight increase in one patient | Dauvilliers et al.56
and follow up: Dauvilliers57 |
|
| 4 (9, 9, 13, 6) | 11 months, 12 months, 9 months, 4 months | Objective and persistent improvement for one patient; no effect for the others | NA | Plazzi et al.58 | |
| 4 (43, 59, 45, 53) | 4 months, 17 years, 4 years, 11 months | Transient improvement of EDS and cataplexy for two patients; no effect for the two others | NA | Valko et al.59 | |
| 1 (28) | 2 weeks | Clear effect on cataplexy; moderate effect on EDS
assessed by MSLT; reoccurrence at follow up |
Normalization 1 month after the third IVIg perfusion | Dauvilliers et al.53 | |
| 1 (22) | <1 month | Improvement of cataplexy; no objective effect | No effect: undetectable before and after the therapy | Knudsen et al.60 | |
| 22 (mean 9.7 years; SD 2.6 years) | Median: 0.7 years (minimum: 0.01 years; maximum: 2.4 years) | No improvement of symptoms compared with standard care alone (all patients also received psychostimulants or anticataplectic agents) | NA | Lecendreux et al.61* | |
| 1 (55) | 7 years | Transient improvement cataplexy; similar response when repeated treatment; no significant difference between placebo and IVIgs | NA | Fronczek et al.62 | |
| Plasmapheresis then azathioprine then IVIgs | 1 (60) | 2 months | Very transient benefit of plasmapheresis on cataplexy; no effect of IVIgs | NA | Chen et al.63 |
| Alemtuzumab | 1 (79) | 62 years | Complete resolution of cataplectic attacks; no effect on EDS |
NA | Donjacour et al.64 |
| Rituximab | 1 (12) | 2 years | Subjective and transient improvement of EDS and cataplexy | NA | Sarkanen et al.65 |
Nonrandomized, open-label, controlled, longitudinal, observational retrospective study.
EDS, excessive daytime sleepiness; IV, intravenous; Ig, immunoglobulin; MSLT, multiple sleep latency test; MWT, maintenance of wakefulness test; NA, not available; ORX, orexin; SD, standard deviation.
Perspectives on immune-based therapy
In a recent review on the topic, innovative immune-based treatments in NT1 were proposed: natalizumab, fingolimod, abatacept, monoclonal antibodies targeting T or B cells, tumor necrosis factor alpha blockers, anakinra, antigen-specific therapies, or cyclophosphamide.22 However, we must keep in mind that those medications have major risks of serious side effects. Furthermore, in case reports and series where immunotherapy has been successful in narcolepsy, it is difficult to state whether the improvement of the symptoms can be attributed to the drug, the placebo effect, or the spontaneous clinical course of the disease. In future trials, immune-based drugs should be given to highly selected narcoleptic patients. Those potentially responsive patients would have an ‘inflammatory’ or the ‘autoimmune’ process ongoing. The natural history of the loss of ORX neurons and CSF ORX levels in NT1 remains unknown, and the gradient and slope of the destruction of ORX neurons could be variable among narcoleptic patients. Indeed, some patients develop severe narcolepsy with cataplexy in few days, while others develop a progressive disease with first EDS and then cataplexy months or years later.66 Moreover, a progressive decrease in CSF ORX-A levels over time was reported in some patients with NT1, who had a second lumbar puncture.67,68 Recent animal models of narcolepsy also favor the idea that underlying immune pathogeny may result from a chronic multistep process, with aggravation of the narcolepsy phenotype upon repeated injections of cytotoxic CD8+ T cells.69
Future research should focus on the discovery of new reliable biomarkers, to identify the best patient responders to immunomodulators. Of course, the precise understanding of the immune mechanisms destroying ORX neurons is required above all, to propose rational immune-targeting therapies.
Conclusion
Narcolepsy is a rare disease, but its main and most disabling symptom, EDS, is a frequent condition. This could explain why narcolepsy is such an active area for drug development, and that in recent years, several new wake-promoting agents have been developed as symptomatic medications. Considering NT1 unique pathophysiology, the selective destruction of ORX neurons by a probable autoimmune process, other approaches are being explored to alternatively manage the disease. Immune-based therapies administered close to disease onset may be promising, with some successful but rare attempts to slow down or stop the autoimmune process. However, results remain controversial so far and there is a real need for future research to better understand the immune process targeting ORX neurons, to identify targeted populations of patients with an active immune process ongoing, to further select better responders to effective therapies in well-designed clinical trials, close to disease onset. Among ORX-based therapies, ORX-receptor-2 agonists seem the most promising option in the near future. Given the different clinical, biological, and genetic profiles, we hope to propose a personalized treatment to narcoleptic patients in the next few years involving several targets and several drugs for a precise medical approach.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: Dr Barateau declares no conflicts of interest in preparing this article. Pr Dauvilliers has received funds for speaking, board engagements, and travel to conferences with UCB pharma, Jazz, Theranexus, Avadel, Takeda, Harmony Biosciences, Idorsia, and Bioprojet.
Contributor Information
Lucie Barateau, Service de Neurologie, Gui-de-Chauliac Hospital, Montpellier, France; Sleep-Wake Disorders Center, Gui-de-Chauliac Hospital, CHU Montpellier, France; National Reference Network for Narcolepsy, Montpellier, France; Inserm U1061, Montpellier, France.
Yves Dauvilliers, Service de Neurologie, Gui-de-Chauliac Hospital, CHU Montpellier, 80 avenue Augustin Fliche, 34295 Montpellier Cedex 5, France.
References
- 1. Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet 2007; 369: 499–511. [DOI] [PubMed] [Google Scholar]
- 2. Scammell TE. Narcolepsy. N Engl J Med 2015; 373: 2654–2662. [DOI] [PubMed] [Google Scholar]
- 3. Ohayon MM, Priest RG, Zulley J, et al. Prevalence of narcolepsy symptomatology and diagnosis in the European general population. Neurology 2002; 58: 1826–1833. [DOI] [PubMed] [Google Scholar]
- 4. Kok SW, Overeem S, Visscher TLS, et al. Hypocretin deficiency in narcoleptic humans is associated with abdominal obesity. Obes Res 2003; 11: 1147–1154. [DOI] [PubMed] [Google Scholar]
- 5. Dauvilliers Y, Jaussent I, Krams B, et al. Non-dipping blood pressure profile in narcolepsy with cataplexy. PloS One 2012;7:e38977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Plazzi G, Ferri R, Antelmi E, et al. Restless legs syndrome is frequent in narcolepsy with cataplexy patients. Sleep 2010; 33: 689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dauvilliers Y, Pennestri M-H, Petit D, et al. Periodic leg movements during sleep and wakefulness in narcolepsy. J Sleep Res 2007; 16: 333–339. [DOI] [PubMed] [Google Scholar]
- 8. Bassetti C, Adamantidis A, Burdakov D, et al. Narcolepsy – clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat Rev Neurol 2019; in press [DOI] [PubMed] [Google Scholar]
- 9. AASM: American Academy of Sleep Medicine. ICSD-3: International Classification of Sleep Disorders, 3rd ed. Darien, Illinois American Academy of Sleep Medicine; 2014. [Google Scholar]
- 10. Lopez R, Doukkali A, Barateau L, et al. Test–retest reliability of the multiple sleep latency test in central disorders of hypersomnolence. Sleep 2017; 40: zsx164. [DOI] [PubMed] [Google Scholar]
- 11. Pizza F, Jaussent I, Lopez R, et al. Car crashes and central disorders of hypersomnolence: a French study. PloS One 2015; 10: e0129386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bayard S, Croisier Langenier M, Cochen De, Cock V, et al. Executive control of attention in narcolepsy. PloS One 2012; 7: e33525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dauvilliers Y, Paquereau J, Bastuji H, et al. Psychological health in central hypersomnias: the French Harmony study. J Neurol Neurosurg Psychiatry 2009; 80: 636–641. [DOI] [PubMed] [Google Scholar]
- 14. Kornum BR, Knudsen S, Ollila HM, et al. Narcolepsy. Nat Rev Dis Primer 2017; 3: 16100. [DOI] [PubMed] [Google Scholar]
- 15. Barateau L, Lopez R, Dauvilliers Y. Treatment options for narcolepsy. CNS Drugs 2016; 30: 369–379. [DOI] [PubMed] [Google Scholar]
- 16. Thorpy M, Zhao CG, Dauvilliers Y. Management of narcolepsy during pregnancy. Sleep Med 2013; 14: 367–376. [DOI] [PubMed] [Google Scholar]
- 17. Duchêne A, Perier M, Zhao Y, et al. Impact of astroglial connexins on modafinil pharmacological properties. Sleep 2016; 39: 1283–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dauvilliers Y, Roth T, Guinta D, et al. Effect of sodium oxybate, modafinil, and their combination on disrupted nighttime sleep in narcolepsy. Sleep Med 2017; 40: 53–57. [DOI] [PubMed] [Google Scholar]
- 19. Roth T, Dauvilliers Y, Guinta D, et al. Effect of sodium oxybate on disrupted nighttime sleep in patients with narcolepsy. J Sleep Res 2016; 26: 407–414. [DOI] [PubMed] [Google Scholar]
- 20. Bogan RK, Roth T, Schwartz J, et al. Time to response with sodium oxybate for the treatment of excessive daytime sleepiness and cataplexy in patients with narcolepsy. J Clin Sleep Med 2015; 11: 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bosco A, Lopez R, Barateau L, et al. Effect of psychostimulants on blood pressure profile and endothelial function in narcolepsy. Neurology 2018; 10–1212. [DOI] [PubMed] [Google Scholar]
- 22. Barateau L, Liblau R, Peyron C, et al. Narcolepsy type 1 as an autoimmune disorder: evidence, and implications for pharmacological treatment. CNS Drugs 2017; 31: 821–834. [DOI] [PubMed] [Google Scholar]
- 23. Dauvilliers Y, Beziat S, Pesenti C, et al. Measurement of narcolepsy symptoms: the Narcolepsy Severity Scale. Neurology 2017; 88: 1358–1365. [DOI] [PubMed] [Google Scholar]
- 24. Dauvilliers Y, Bassetti C, Lammers GJ, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol 2013; 12: 1068–1075. [DOI] [PubMed] [Google Scholar]
- 25. Dauvilliers Y, Arnulf I, Szakacs Z, et al. Long-term use of pitolisant to treat patients with narcolepsy: harmony III study. Sleep 2019; 42: zsz174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Szakacs Z, Dauvilliers Y, Mikhaylov V, et al. Safety and efficacy of pitolisant on cataplexy in patients with narcolepsy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2017;16: 200–207. [DOI] [PubMed] [Google Scholar]
- 27. Thorpy MJ, Shapiro C, Mayer G, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol 2019; 85: 359–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strollo PJ, Jr, Hedner J, Collop N, et al. Solriamfetol for the treatment of excessive sleepiness in OSA: a placebo-controlled randomized withdrawal study. Chest 2019; 155: 364–374. [DOI] [PubMed] [Google Scholar]
- 29. Liu X, Petit J-M, Ezan P, et al. The psychostimulant modafinil enhances gap junctional communication in cortical astrocytes. Neuropharmacology 2013; 75: 533–538. [DOI] [PubMed] [Google Scholar]
- 30. Plazzi G, Ruoff C, Lecendreux M, et al. Treatment of paediatric narcolepsy with sodium oxybate: a double-blind, placebo-controlled, randomised-withdrawal multicentre study and open-label investigation. Lancet Child Adolesc Health 2018; 2: 483–494. [DOI] [PubMed] [Google Scholar]
- 31. Hershner S, Dauvilliers Y, Chung F, et al. Knowledge gaps in the perioperative management of adults with narcolepsy: a call for further research. Anesth Analg 2019; 129: 204–211. [DOI] [PubMed] [Google Scholar]
- 32. Fujiki N, Yoshida Y, Ripley B, et al. Effects of IV and ICV hypocretin-1 (orexin A) in hypocretin receptor-2 gene mutated narcoleptic dogs and IV hypocretin-1 replacement therapy in a hypocretin-ligand-deficient narcoleptic dog. Sleep 2003; 26: 953–959. [DOI] [PubMed] [Google Scholar]
- 33. Mieda M, Willie JT, Hara J, et al. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc Natl Acad Sci USA 2004; 101: 4649–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deadwyler SA, Porrino L, Siegel JM, et al. Systemic and nasal delivery of orexin-A (hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J Neurosci 2007; 27: 14239–14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baier PC, Hallschmid M, Seeck-Hirschner M, et al. Effects of intranasal hypocretin-1 (orexin A) on sleep in narcolepsy with cataplexy. Sleep Med 2011; 12: 941–946. [DOI] [PubMed] [Google Scholar]
- 36. Weinhold SL, Seeck-Hirschner M, Nowak A, et al. The effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav Brain Res 2014; 262: 8–13. [DOI] [PubMed] [Google Scholar]
- 37. Nagahara T, Saitoh T, Kutsumura N, et al. Design and synthesis of non-peptide, selective orexin receptor 2 agonists. J Med Chem 2015; 58: 7931–7937. [DOI] [PubMed] [Google Scholar]
- 38. Irukayama-Tomobe Y, Ogawa Y, Tominaga H, et al. Nonpeptide orexin type-2 receptor agonist ameliorates narcolepsy-cataplexy symptoms in mouse models. Proc Natl Acad Sci USA 2017; 114: 5731–5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Arias-Carrión O, Murillo-Rodríguez E. Effects of hypocretin/orexin cell transplantation on narcoleptic-like sleep behavior in rats. PLoS One 2014; 9: e95342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blanco-Centurion C, Liu M, Konadhode R, et al. Effects of orexin gene transfer in the dorsolateral pons in orexin knockout mice. Sleep 2013; 36: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kantor S, Mochizuki T, Lops SN, et al. Orexin gene therapy restores the timing and maintenance of wakefulness in narcoleptic mice. Sleep 2013; 36: 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu M, Thankachan S, Kaur S, et al. Orexin (hypocretin) gene transfer diminishes narcoleptic sleep behavior in mice. Eur J Neurosci 2008; 28: 1382–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu M, Blanco-Centurion C, Konadhode R, et al. Orexin gene transfer into zona incerta neurons suppresses muscle paralysis in narcoleptic mice. J Neurosci 2011; 31: 6028–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu M, Blanco-Centurion C, Konadhode RR, et al. Orexin gene transfer into the amygdala suppresses both spontaneous and emotion-induced cataplexy in orexin-knockout mice. Eur J Neurosci 2016; 43: 681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kaushik MK, Aritake K, Imanishi A, et al. Continuous intrathecal orexin delivery inhibits cataplexy in a murine model of narcolepsy. Proc Natl Acad Sci USA 2018; 115: 6046–6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yukitake H, Ishikawa T, Suzuki A, et al. 0002 an orexin 2 receptor-selective agonist, TAK-925, shows robust wake-promoting effects in mice and non-human primates. Sleep 2018; 41: A1. [DOI] [PubMed] [Google Scholar]
- 47. Suzuki M, Yukitake H, Ishikawa T, et al. 0001 an orexin 2 receptor-selective agonist TAK-925 ameliorates narcolepsy-like symptoms in orexin/ataxin-3 mice. Sleep 2018; 41: A1. [Google Scholar]
- 48. Kimura H, Ishikawa T, Yukitake H, et al. An orexin 2 receptor-selective agonist, TAK-925, ameliorates narcolepsy-like symptoms and obesity in orexin/ataxin-3 transgenic mice. Sleep 2019; 42: A23. [Google Scholar]
- 49. Arias-Carrión O, Murillo-Rodríguez E. Effects of hypocretin/orexin cell transplantation on narcoleptic-like sleep behavior in rats. PLoS One 2014; 9: e95342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liblau RS, Vassalli A, Seifinejad A, et al. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol 2015; 14: 318–328. [DOI] [PubMed] [Google Scholar]
- 51. Luca G, Haba-Rubio J, Dauvilliers Y, et al. Clinical, polysomnographic and genome-wide association analyses of narcolepsy with cataplexy: a European Narcolepsy Network study. J Sleep Res 2013; 22: 482–495. [DOI] [PubMed] [Google Scholar]
- 52. Latorre D, Kallweit U, Armentani E, et al. T cells in patients with narcolepsy target self-antigens of hypocretin neurons. Nature 2018; 562: 63–68. [DOI] [PubMed] [Google Scholar]
- 53. Dauvilliers Y, Abril B, Mas E, et al. Normalization of hypocretin-1 in narcolepsy after intravenous immunoglobulin treatment. Neurology 2009; 73: 1333–1334. [DOI] [PubMed] [Google Scholar]
- 54. Hecht M, Lin L, Kushida CA, et al. Report of a case of immunosuppression with prednisone in an 8-year-old boy with an acute onset of hypocretin-deficiency narcolepsy. Sleep 2003; 26: 809–810. [DOI] [PubMed] [Google Scholar]
- 55. Lecendreux M, Maret S, Bassetti C, et al. Clinical efficacy of high-dose intravenous immunoglobulins near the onset of narcolepsy in a 10-year-old boy. J Sleep Res 2003; 12: 347–348. [DOI] [PubMed] [Google Scholar]
- 56. Dauvilliers Y, Carlander B, Rivier F, et al. Successful management of cataplexy with intravenous immunoglobulins at narcolepsy onset. Ann Neurol 2004; 56: 905–908. [DOI] [PubMed] [Google Scholar]
- 57. Dauvilliers Y. Follow-up of four narcolepsy patients treated with intravenous immunoglobulins. Ann Neurol 2006; 60: 153. [DOI] [PubMed] [Google Scholar]
- 58. Plazzi G, Poli F, Franceschini C, et al. Intravenous high-dose immunoglobulin treatment in recent onset childhood narcolepsy with cataplexy. J Neurol 2008; 255: 1549–1554. [DOI] [PubMed] [Google Scholar]
- 59. Valko PO, Khatami R, Baumann CR, et al. No persistent effect of intravenous immunoglobulins in patients with narcolepsy with cataplexy. J Neurol 2008; 255: 1900–1903. [DOI] [PubMed] [Google Scholar]
- 60. Knudsen S, Biering-Sørensen B, Kornum BR, et al. Early IVIg treatment has no effect on post-H1N1 narcolepsy phenotype or hypocretin deficiency. Neurology 2012; 79: 102–103. [DOI] [PubMed] [Google Scholar]
- 61. Lecendreux M, Berthier J, Corny J, et al. Intravenous immunoglobulin therapy in pediatric narcolepsy: a nonrandomized, open-label, controlled, longitudinal observational study. J Clin Sleep Med 2017; 13: 441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fronczek R, Verschuuren J, Lammers GJ. Response to intravenous immunoglobulins and placebo in a patient with narcolepsy with cataplexy. J Neurol 2007; 254: 1607–1608. [DOI] [PubMed] [Google Scholar]
- 63. Chen W, Black J, Call P, et al. Late-onset narcolepsy presenting as rapidly progressing muscle weakness: response to plasmapheresis. Ann Neurol 2005; 58: 489–490. [DOI] [PubMed] [Google Scholar]
- 64. Donjacour CEHM, Lammers GJ. A remarkable effect of alemtuzumab in a patient suffering from narcolepsy with cataplexy. J Sleep Res 2012; 21: 479–480. [DOI] [PubMed] [Google Scholar]
- 65. Sarkanen T, Alén R, Partinen M. Transient impact of rituximab in H1N1 vaccination-associated narcolepsy with severe psychiatric symptoms. Neurologist 2016; 21: 85–86. [DOI] [PubMed] [Google Scholar]
- 66. Dauvilliers Y, Montplaisir J, Molinari N, et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 2001; 57: 2029–2033. [DOI] [PubMed] [Google Scholar]
- 67. Lopez R, Barateau L, Evangelista E, et al. Temporal changes in the cerebrospinal fluid level of hypocretin-1 and histamine in narcolepsy. Sleep 2017; 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pizza F, Vandi S, Liguori R, et al. Primary progressive narcolepsy type 1: the other side of the coin. Neurology 2014; 83: 2189–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bernard-Valnet R, Yshii L, Quériault C, et al. CD8 T cell-mediated killing of orexinergic neurons induces a narcolepsy-like phenotype in mice. Proc Natl Acad Sci USA 2016; 113: 10956–10961. [DOI] [PMC free article] [PubMed] [Google Scholar]