Abstract
Most physicians understand venous thromboembolism (VTE) to be an acute and time-limited disease. However, pathophysiological and epidemiological data suggest that in most patients VTE recurrence risk is not resolved after the first 6 months of anticoagulation. Recurrence rates are high and potentially life-threatening. In these cases, it would make sense to prolong anticoagulation for an undetermined length of time. However, what about the bleeding rates, induced by prolonged anticoagulation? Would they not outweigh the benefit of reducing the VTE recurrent risk? How long should anticoagulation be continued, and should all patients suffering from VTE be provided with extended anticoagulation? This review will address the most recent data concerning extended anticoagulation in VTE secondary prophylaxis.
The reviews of this paper are available via the supplementary material section.
Keywords: deep vein thrombosis, direct oral anticoagulants, extended anticoagulation, provoked, pulmonary embolism, treatment, unprovoked, venous thromboembolism
Introduction
Venous thromboembolism (VTE) is a highly prevalent and potentially fatal disease. VTE is the third most common cause of cardiovascular death, following acute coronary artery disease and stroke, and is responsible for more than 3 million deaths/year worldwide.1,2 Since the 1960s, it has been known that anticoagulation favorably impacts the outcome of VTE,3,4 and it is well established that this is the basic step in the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE),5 the two most common VTE clinical presentations. However, even when adequately treated, VTE mortality is surprisingly high. Data from the Global Anticoagulant Registry in the FIELD, Venous Thromboembolism (GARFIELD-VTE) registry demonstrated that, in a cohort of 10,315 VTE patients, from 419 centers and 28 countries, overall mortality was 4.5% in 6 months.6 Thus, a VTE episode of any sort should not be taken lightly.
Until recently, VTE has been understood to be a time-limited acute disease. Therefore, withdrawal of anticoagulant drugs after a limited period of VTE treatment, typically 3–6 months, was the standard of care.7 However, some patients present several recurrent VTE events, once anticoagulation is stopped. Besides the well-known classical transient VTE risk factors, such as estrogen use, smoking, cancer, surgery, and immobility, several persistent VTE risk factors have been described, such as thrombophilia-inducing genetic mutations.8 In addition, recent data suggest that VTE is related to a chronic and systemic inflammatory state, associated with persistent elevated inflammatory serum markers, and is much more than a mere consequence of acute local risk factors.9 In these cases, it would make sense to prolong anticoagulation for an undetermined period of time, as suggested by the most recent guidelines.10
However, extended anticoagulation does not come without risks, increased bleeding rates are a risk and could lead to unfavorable risk–benefit ratios. How much this ratio is influenced by the duration of anticoagulation will determine how long the therapy should be maintained and for who. These are the main points that will be addressed in this review in an attempt to consider suitable recommendations based on the most recent data published in the field.
Is VTE recurrence really a problem?
Data from large and now classic cohorts of VTE patients demonstrate the magnitude of VTE recurrence. Prandoni and colleagues followed 1626 consecutive, first episode VTE patients who had anticoagulation discontinued.11 After 1 year, 11% of all the patients had presented another VTE episode. After 10 years, surprisingly, 39.9% of patients showed VTE recurrence. When the VTE recurrence rate was compared between patients with a provoked index event (such as hormone use, severe clinical illness, trauma, or surgery, primarily orthopedic) and patients with unprovoked VTE, a higher 10 years recurrence rate was observed in the unprovoked group (52.6%; 95% CI 45.6–59.5 versus 22.5%; 95% CI 17.2–27.8). A shorter duration of anticoagulation and increasing age were also associated with higher recurrence rates. In addition, Baglin and colleagues followed 781 consecutive, first episode VTE patients, who had discontinued anticoagulation.12 The study divided the patients into three groups: surgery-related VTE, with no recurrence for 2 years of follow up; unprovoked VTE (the highest recurrence rate, 19.4%); and nonsurgical, transient VTE risk factors (8%). Of interest, recurrence rates did not differ between patients that had thrombophilia identified and those that did not. Antiphospholipid syndrome and active cancer patients were excluded from both cohorts.
These data lead to the conclusions: VTE recurrence is a highly significant problem, even after the first episode; there is a population at high risk of VTE recurrence with unprovoked VTE and it is possible that this high-risk population would benefit the most from extended anticoagulation; and provoked VTE comprising a broad spectrum of situations, with different recurrence rates. Surgical-related VTE is associated with a low recurrence rate and probably gains no benefit from extended anticoagulation therapy in this context.
Should extend anticoagulation be provided for unprovoked VTE?
Once it was defined that the unprovoked VTE patients were the ones at the highest risk of recurrence and, therefore, a potential target for an extended period of anticoagulation, the efficacy and safety of this approach needed to be demonstrated. Until the 2010s long-term oral anticoagulation for VTE treatment was carried out primarily using vitamin K antagonists (VKA), so these were the drugs initially evaluated.
In 1999 the first randomized controlled trial on this topic was published. A total of 162 unprovoked VTE patients, treated for 3 months, were randomized to receive warfarin or placebo for an additional 24 months.13 The time in therapeutic range [(TTR), the percentage of INR that remains in the target zone] achieved in the study was 64% (guidelines recommend that, ideally, TTR should be above 60%). Extended anticoagulation promoted a 95% reduction in VTE recurrence. Warfarin extended patients had 1.3% of cumulative recurrence at 24 months, and placebo patients had 27.4% (p < 0.001). However, extended warfarin patients had a 3.8% occurrence of major bleeding versus 0 in the placebo group (p = 0.09).
Extended anticoagulation appeared to work, but bleeding rates were still a matter of concern. In 2003 the PREVENT (long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism) study proposed a different approach for extended anticoagulation: a low-intensity warfarin scheme, which aimed to keep INR between 1.5 and 1.9.14 The study enrolled 508 unprovoked VTE patients that had received full dosage anticoagulation for a median of 6.5 months who were then randomized to low-intensity warfarin (target INR 1.5–2.0) or placebo and followed for up to 4.3 years (mean 2.1). The low-intensity warfarin group had fewer episodes of VTE recurrence (2.6% versus 7.2%), a risk reduction of 64% (95% CI 0.19–0.67). Two patients in the placebo group and five in the low-intensity warfarin group had major bleeding (p = 0.25). Reduction in the intensity of anticoagulation for long-term use was an appealing idea. In the same year; however, the ELATE15 (comparison of low-intensity warfarin therapy with conventional intensity warfarin therapy for the long-term prevention of recurrent venous thromboembolism) study evaluated low-intensity warfarin compared with conventional warfarin for extended anticoagulation, enrolling 738 unprovoked VTE patients who had completed at least 3 months of warfarin therapy. Patients were randomized to receive standard or low-intensity warfarin, for 2.4 years of follow up, on average. The standard warfarin group had fewer VTE episodes [0.7 versus 1.9%, hazard ratio (HR) 2.8; 95% CI 1.1–7.0] and the same number of major bleeding events (0.9 versus 1.1%, HR 1.2; 95% CI 0.4–3.0) compared with the low-intensity warfarin group. Conventional intensity warfarin therapy proved to be more effective than low-intensity warfarin for the long-term prevention of recurrent VTE. The low-intensity warfarin regimen did not reduce the risk of bleeding. While extended anticoagulation with VKA proved its efficacy over time, the data on low-intensity anticoagulation with warfarin was not encouraging, and this practice was abandoned. However, the idea would re-emerge using different drugs.
How long should extended anticoagulation be maintained?
With the role of extended anticoagulation for secondary prophylaxis of unprovoked VTE defined, how long should it be maintained? This issue was addressed in the Prolonged Anticoagulation During 18 months versus Placebo after Initial 6 month treatment for the First Episode of Idiopathic Pulmonary Embolism (PADIS-PE) trial.16 It was a randomized, double-blind trial, carried out in two stages. In stage 1, 371 first episode, unprovoked PE patients, from 14 French centers, that were treated for at least 6 months with a vitamin K antagonist were randomized to receive warfarin or placebo for more than 18 months. After 18 months, warfarin and placebo were withdrawn, and the patients were followed up for more than 24 months (stage 2). The aim was to establish if an initial prolonged anticoagulation period would be enough, or if this anticoagulation would be needed for a period longer than 18 months. The TTR of PADIS-PE was 69.8%. During Stage 1 (extended anticoagulation), recurrent VTE occurred in 3 patients in the warfarin group and 25 patients in the placebo group (HR 0.15; 95% CI 0.05–0.43). Major bleeding occurred in four patients in the warfarin group and in one patient in the placebo group (HR 0.75; 95% CI 0.47–1.18). When analyzing the primary outcome of the study (recurrent VTE plus major bleeding, considering that morbidity of VTE and bleeding are similar), extended anticoagulation was beneficial, warfarin patients had fewer events (3.3%) than the placebo (13.5%; HR 0.22; 95% CI 0.09–0.55). However, the most striking result of PADIS-PE came from the Stage 2 analysis. Once extended anticoagulation was withdrawn, the event rate increased sharply, and at the end of the 42 months of follow-up, previously treated warfarin patient events matched the placebo group in terms of cumulative event rate (20.8% of events in the warfarin group versus 24% in the placebo, HR 0.75; 95% CI 0.47–1.18). The benefit that extended anticoagulation with warfarin provided after the first, unprovoked PE, was not maintained after discontinuation. Therefore, there is no legacy effect of extended anticoagulation, once it is withdrawn. The long-term risk of VTE recurrence is similar if the patient did not receive any extended anticoagulation and is quite high (20% in 4 years). For the prolonged benefit, anticoagulation should be provided for more extended periods of time, possibly with an undefined termination. Recently, a meta-analysis on this topic concluded that longer (mean 18.6 months) compared with shorter anticoagulation therapy (mean 7.5 months) significantly reduced all-cause mortality in patients with VTE at intermediate risk of recurrence [(RR) 0.47, 95% CI 0.29–0.75].17
Can we identify higher-risk patients for VTE recurrence?
The use of biomarkers to identify an individual with a higher risk of VTE recurrence has always been a tempting possibility. Between several potential molecules, the most promising and studied in this setting is the D-dimer measurement. Over the last decade, some studies evaluated the hypothesis that D-dimer assays could play a role in gauging the appropriate duration of anticoagulation in first episode VTE patients. In the PROLONG (D-dimer testing to determine the duration of anticoagulation study)18 608 first episode, unprovoked VTE patients who had received a VKA for at least three months were evaluated. Anticoagulation was then stopped, and 30 days later D-dimer blood levels were measured. The D-dimer assay was abnormal in 36.7% of patients. These positive D-dimer patients were then randomized to resume VKA therapy or maintain without anticoagulation. VTE recurrence occurred in 15% of positive D-dimer patients without anticoagulation and in 2.9% of the positive D-dimer patients receiving VKA therapy (HR 4.26, 95% CI 1.23–14.6, p = 0.02). However, VTE recurred in 6.2% of the negative D-dimer patients. This result impacted negatively on the clinical use of D-dimers as the sole determinant of long-term anticoagulation therapy after VTE, because the negative value is not enough to warrant a low VTE recurrence rate in the absence of anticoagulation.
The same conclusion was obtained from a prospective cohort of 319 patients presenting the first episode of unprovoked VTE that had D-dimer levels evaluated after suspension of anticoagulation. It observed an overall incidence rate of recurrent VTE of 6.9% per year in patients with two negative D-dimer results, except in young women on estrogen containing therapy, where no recurrent VTE was reported.19 In addition, a meta-analysis was used to evaluate PROLONG and other studies to determine the isolated role of a D-dimer when assessing the risk after unprovoked VTE and more importantly, its role in the suspension of anticoagulation in patients with low risk for VTE recurrence.20 In this meta-analysis, 1818 first episode, unprovoked VTE patients from 7 studies were evaluated. The HR for D-dimer status (positive versus negative) was 2.59 (95% CI 1.90–3.52), reinforcing the higher risk for recurrence in positive D-dimer patients after interruption of anticoagulation, regardless of the timing of post anticoagulation D-dimer testing or patient age. No study or assay-specific D-dimer was found and reassessing the analysis according to specific quantitative D-dimer cutoff points (500 and 250 µg/l) did not change the results. Again, the risk of recurrence in negative D-dimer patients was relatively high (3.7 per 100 patient-years, 95% CI 3.2–4.3), hindering the use of the D-dimer assay as the standalone test for withdrawing the anticoagulation for VTE patients. However, it is theoretically possible to use a D-dimer test in combination with other clinical variables, increasing accuracy and possibly the negative predictive value of this assessment and, therefore, improving safety if the suspension of anticoagulation is considered.21
Other risk factors for VTE recurrence, beyond the provoked or unprovoked condition and D-dimer levels, are relevant when considering the extension of anticoagulation or not. Men, for example, appear to have a risk of VTE recurrence 1.5 times higher than women,22–24 and similarly, obesity25 and non-O blood type26 are also independent predictors of recurrent VTE. Could these risk factors be considered and evaluated in combination, to identify which patient should receive prolonged anticoagulation, and which should not? This approach has been adopted, and some multiparametric models to predict VTE recurrence have been developed. The Vienna Prediction Model27 prospectively evaluated a cohort of 929 patients, considering mainly sex, location of first VTE, and D-dimer level after anticoagulation. Through a nomogram, the risk of recurrence was calculated for individual patients. Patients at the low-risk level in the Vienna model presented a VTE recurrence rate of 4.4%. In the D-dimer, Age, Sex, and Hormone (DASH) study,28 1818 VTE patients were evaluated, considering abnormal D-dimer after stopping anticoagulation, age below 50 years, male sex, and VTE not associated with the use of hormonal therapy. If the patient had a score of 0 or 1, the VTE recurrence rate at this low-risk level was 3.1%. Despite being promising, these scores still lack in negative predictive value, and their usefulness in clinical practice needs to be evaluated.
Are there any other therapeutic options for secondary VTE prophylaxis?
Aspirin
Since the 1980s aspirin has been considered for secondary VTE prophylaxis.29 Low cost, favorable side-effect profile, comfortable posology, and relatively low bleeding risk made aspirin an attractive alternative.
This possibility was evaluated systematically in the Aspirin for the Prevention of Recurrent Venous Thromboembolism (the Warfarin and Aspirin, WARFASA) study.30 A total of 402 first unprovoked VTE patients, that completed 6–18 months of anticoagulation were randomized to receive aspirin 100 mg once daily or placebo and followed for 2 years. Recurrent VTE events were less frequent in the aspirin group (6.6% versus 11.2% per year; HR 0.58; 95% CI 0.36–0.93). One patient in each group had a major bleeding episode. Even with an unacceptably high VTE recurrence rate/year (6.6%), aspirin was demonstrated to have a role in secondary prophylaxis, with no apparent increase in major bleeding.
In the same year, the Aspirin to Prevent Recurrent Venous Thromboembolism (ASPIRE) study evaluated 822 first unprovoked VTE patients that completed anticoagulation and were randomized to receive aspirin 100 mg once daily or placebo and followed up to 4 years.31 The aspirin group patients had a recurrent VTE rate of 4.8%, compared with 6.5% in the placebo group (HR 0.74; 95% CI 0.52–1.05). However, aspirin did promote a reduction in major vascular events (VTE, myocardial infarction, stroke, or cardiovascular death) of 34% compared with placebo (p = 0.01), without adding significantly in the major bleeding risk.
Individual patients from the WARFASA and ASPIRE trials were evaluated in the International Collaboration of Aspirin Trials for Recurrent Venous Thromboembolism (INSPIRE) study.32 In this collaborative re-evaluation, aspirin reduced recurrent VTE per year (5.1% versus 7.5%; HR 0.68; 95% CI 0.51–0.90; p = 0.008) and major vascular events (5.7% versus 8.7%; HR 0.66; 95% CI 0.50–0.86; p = 0.002), without increasing the major bleeding rate (0.4% for placebo and 0.5% for aspirin, per year). These results demonstrated that aspirin might play a role in the secondary prophylaxis of VTE. However, when compared with other therapeutic options for this objective, aspirin efficacy results are quite disappointing. In the EINSTEIN-CHOICE trial,33 rivaroxaban achieved a reduction in the VTE recurrence of 70%, when compared with aspirin, with similar bleeding rates. In this study, patients receiving aspirin presented a rate of 5% of VTE recurrence after 1 year. Therefore, since its efficacy is statistically proven, the role of aspirin in the long-term secondary prophylaxis for VTE is restricted mainly to patients with absolute contraindications to other therapeutic modalities, such as the direct oral anticoagulants (DOACs).
Sulodexide
Sulodexide is a natural glycosaminoglycan with antithrombotic and profibrinolytic properties, which can be administered orally and impairs normal hemostasis to a lesser extent than heparin, and has a very low risk of bleeding. In 2015 the Sulodexide in Secondary Prevention of Recurrent DVT (SURVET) trial evaluated 615 unprovoked VTE patients, treated with VKA for 3–12 months. Patients were randomized to receive sulodexide 2 × 250 lipasemic units capsules twice daily or placebo, for 2 years.34 Recurrence of VTE occurred in 15 of the 307 patients who received sulodexide (4.9%, 95% CI 2.9–8.1%) when compared with 30 of the 308 patients who received placebo (9.7%, 95% CI 6.8–13.7% - HR 0.49, 95% CI 0.27–0.92; p = 0.02). No major bleeding occurred in either group.
Despite the favorable results, the SURVET trial presented some methodological issues. The proportion of PE patients was low (7.6%). A significant proportion of patients entered the study with major protocol violations (some patients had short or no anticoagulant treatment prior to trial inclusion, others had long untreated intervals prior to randomization and some had longer anticoagulation treatment). Furthermore, 5% of the patients interrupted the study prematurely without having reached the endpoint. These limitations led some authors to suggest caution when applying SURVET data to the overall population.35 More recently, a meta-analysis re-evaluated sulodexide data for secondary VTE prevention, considering SURVET and three other trials, involving a total of 1461 patients, and concluded that sulodexide reduced the recurrence (RR) of VTE (RR 0.51, 95% CI 0.35–0.74, p = 0.0004), with a bleeding rate of 0.28%.36
Direct oral anticoagulants
In 2009 DOACs began to be used for VTE treatment. These new drugs, one direct thrombin inhibitor (dabigatran) and three Xa antagonists (rivaroxaban, apixaban, and edoxaban) proved to be, in large randomized controlled trials, at least as effective as the conventional VKA anticoagulant strategy for the short-term prevention of VTE recurrence in a general population. Their great advantage was in safety, bleeding rates were significantly reduced with DOACs use.37,38 These data led to the indication of DOACs as the first choice for anticoagulation for VTE treatment not related to cancer.10 Could these drugs be utilized for extended anticoagulation and secondary prophylaxis of VTE recurrence?
The first study to evaluate a DOAC in this setting was the EINSTEIN-extension study.39 In this study, 1196 VTE patients (70% of them unprovoked) that had been anticoagulated for at least 3 months were randomized to receive 20 mg of rivaroxaban once daily or placebo, for an additional 6 or 12 months. Rivaroxaban promoted an 82% reduction in the VTE recurrence rate (1.3 versus 7.1%; HR 0.18; 95% CI 0.009–0.39; p < 0.001). Major bleeding was present in 0.7% of the rivaroxaban group and did not occur in the placebo group. The EINSTEIN-extension study was the first study to demonstrate that full anticoagulation dosage of a DOAC could be used for long-term, secondary VTE prophylaxis, with low bleeding rates.
In addition, the RE-SONATE study evaluated dabigatran 150 mg twice daily or placebo, for 6 months, for 1353 VTE patients that have received at least 6 months of anticoagulation.40 Dabigatran patients had fewer VTE recurrence events than placebo (0.4 versus 5.6%; HR 0.08; 95% CI 0.02–0.25). Major bleeding occurred in 0.3% of patients in the dabigatran group and none in placebo. Similar to the PADIS-PE study, once extended anticoagulation was withdrawn, the VTE recurrence rate increased sharply. However, unlike PADIS-PE, the benefit of extended treatment with dabigatran during the 12 months of follow-up after discontinuation of dabigatran was partially maintained. Extended secondary treatment with dabigatran was also compared with conventional warfarin intensity in the RE-MEDY study,40 which included 2856 VTE patients, anticoagulated at least for 3 months. Patients were then randomized and followed for at least 6 months. Dabigatran was noninferior to warfarin in VTE recurrence (1.8 versus 1.3%; HR 1.44; 95% CI 0.27–1.02; p = 0.01 for noninferiority), and induced less clinically relevant bleeding (5.6 versus 10.2%; HR 0.54; 95% CI 0.41–0.71). Therefore, DOACs were superior to placebo in extended VTE treatment, and at least as effective as warfarin, but safer, with lower bleeding rates.
DOACs have been successfully used for primary VTE prophylaxis in orthopedic patients over the last decade.41,42 Considering the low-intensity warfarin studies and the favorable results of DOACs in this field, a question arises, could the DOAC prophylactic dose and not only the full anticoagulation dose, be sufficient for long-term secondary VTE prophylaxis?
This alternative was evaluated in the apixaban for the Initial Management of PE and DVT as a First-Line Therapy Extension (AMPLIFY-EXT) study.43 A total of 2486 VTE patients (90% unprovoked) who had completed 6–12 months of anticoagulation were randomized between three groups: placebo, apixaban 2.5 mg twice daily (prophylactic dose), and apixaban 5 mg twice daily (anticoagulation dose) and were followed for 12 months. Recurrent VTE occurred in 8.8% of the placebo, 1.7% in the apixaban prophylactic dose and 1.7% in the apixaban anticoagulation dose (p < 0.001 for both comparisons with placebo). Major bleeding occurred in 0.5% in the placebo group, 0.2% in the apixaban prophylactic dose and 0.1% in the apixaban anticoagulation dose. Therefore, DOACs could be used not only in full treatment but also in prophylactic treatment, theoretically safer than conventional anticoagulation. In this context, would there still be a role for aspirin in secondary, long-term VTE prophylaxis?
This comparison was made in the EINSTEIN-CHOICE trial.33 A total of 3396 VTE patients previously anticoagulated for at least 6 months (40% unprovoked) were randomized into three groups: aspirin 100 mg once daily, rivaroxaban 10 mg once daily (prophylactic dose), and rivaroxaban 20 mg once daily (anticoagulation dose). VTE recurrence occurred in 4.4% of the aspirin group, in 1.2% of the rivaroxaban prophylactic dose, and in 1.5% of the rivaroxaban anticoagulation dose (p < 0.001 for both comparisons with aspirin). Major bleeding was similar in the three groups: 0.3% in the aspirin group, 0.4% in the rivaroxaban prophylactic group, and 0.5% in the rivaroxaban anticoagulation groups. VTE recurrence in the aspirin group is unacceptably high in 1 year (4.4%), and the rivaroxaban prophylactic and anticoagulation doses performed similarly, both in efficacy and safety. The major trials evaluating extended anticoagulation after the first episode of VTE are summarized in Table 1.
Table 1.
Major randomized trials in extended anticoagulation after the first episode of VTE.
| Trial | Year | n | Drugs evaluated | Major findings |
|---|---|---|---|---|
| Kearon et al.13 | 1999 | 162 (Unprovoked) |
Warfarin versus placebo for 24 months | Reduction in VTE recurrence of 95% (p < 0.001). VTE recurrence warfarin 1.3% versus 27.4% placebo. Major bleeding 3.8% versus 0 placebo (p = 0.09) |
| PREVENT14 | 2003 | 508 (Unprovoked) | Low-intensity warfarin (INR 1.5–1.9) versus
placebo. Mean of follow up 2.1 years |
Reduction in VTE recurrence of 64% (p < 0.05). VTE recurrence, low-intensity warfarin 2.6% versus 7.2% placebo. Major bleeding 5 patients versus 2 placebo (p = 0.25) |
| ELATE15 | 2003 | 738 (Unprovoked) | Low-intensity warfarin (INR 1.5–1.9) versus
Conventional warfarin Mean of follow up 2.4 years |
Conventional warfarin with fewer VTE events (0.7% versus 1.9% p < 0.05) and the same major bleeding rates (0.9% versus 1.1%) |
| EINSTEIN-EXT39 | 2010 | 1196 (70% unprovoked) | Rivaroxaban versus placebo followed for 6 or 12 months | Reduction in VTE recurrence of 82% (p < 0.001). VTE recurrence, rivaroxaban 1.3% versus 7.1% placebo. Major bleeding, 0.7% versus 0 placebo |
| WARFASA30 | 2012 | 402 (Unprovoked) | Aspirin versus placebo followed for 2 years | VTE recurrence 6.6% aspirin versus 11.2% placebo per year. HR 0.58; 95% CI 0.36–0.93. No difference in major bleeding |
| ASPIRE31 | 2012 | 822 (Unprovoked) | Aspirin versus placebo followed for 4 years | VTE recurrence 4.8% aspirin versus 6.5% placebo per year. HR 0.74; 95% CI 0.52–1.05. No difference in major bleeding |
| RE-SONATE40 | 2013 | 1353 | Dabigatran versus placebo followed for 6 or 12 months | Reduction in VTE recurrence of 92% with dabigatran (0.4 versus 5.6%; HR 0.08; 95% CI 0.02–0.25). Major bleeding 0.3% versus 0 placebo |
| RE-MEDY41 | 2013 | 2856 | Dabigatran versus warfarin followed for 6 months | Dabigatran was noninferior to warfarin in VTE recurrence (1.8 versus 1.3%; HR 1.44; 95% CI 0.27–1.02; p = 0.01 for noninferiority), and induced less clinically relevant bleeding (5.6 versus 10.2%; HR 0.54; 95% CI 0.41–0.71) |
| AMPLIFY-EXT43 | 2013 | 2486 (90% unprovoked) | Apixaban 2.5 mg 2 × d (prophylactic dosage) versus Apixaban 5 mg 2 × d (anticoagulation dosage) versus placebo followed for 12 months | Recurrent VTE occurred in 8.8% placebo, 1.7% apixaban prophylactic, 1.7% in the apixaban anticoagulation (p < 0.001). Major bleeding 0.5% placebo, 0.2% apixaban prophylactic, 0.1% apixaban anticoagulation |
| SURVET34 | 2015 | 615 (Unprovoked) | Sulodexide 50 mg 2 × d versus placebo followed for 2 years | VTE recurrence 4.9% sulodexide, 9.7% placebo (p = 0.025). No major bleeding in both groups |
| EINSTEIN CHOICE33 | 2017 | 3396 (40% Unprovoked) | Aspirin 100 mg versus rivaroxaban 10 mg 1 × d (prophylactic dosage) versus rivaroxaban 20 mg 1 × d (anticoagulation dosage) followed for 12 months | VTE recurrence 4.4% aspirin, 1.2% rivaroxaban prophylactic 1.5% rivaroxaban anticoagulation (p < 0.001). Major bleeding 0.3% aspirin, 0.4% rivaroxaban prophylactic, 0.5% rivaroxaban anticoagulation |
VTE, venous thromboembolism.
Edoxaban did not have a specific trial evaluating extended anticoagulation in VTE, but HOKUSAI-VTE,44 the phase III clinical trial that evaluated edoxaban against warfarin for short and long-term anticoagulation in VTE therapy lasted 12 months (instead of 6 months usually recommended by guidelines). The study demonstrated the noninferiority of edoxaban in efficacy, with a safer profile, concerning bleeding. Therefore, it is additional evidence of extended anticoagulation for VTE secondary prophylaxis.
Should we extend anticoagulation in provoked VTE?
While abundant data suggest an indication of extended anticoagulation in unprovoked VTE, in provoked VTE the scenario is less clear. Should a 20% rate of VTE recurrence in 10 years11 warrant mandatory long-term anticoagulation? What about bleeding, do DOACs emergence with their favorable safety profile, interfere with this balance? Is DOACs prophylactic dose enough? Is anticoagulation provided by aspirin enough?
Some of the answers come from the EINSTEIN-CHOICE trial analysis.33 In this study, 60% of VTE patients had a provoked VTE. In this population, the recurrent VTE rate in 1 year of follow up was 3.6% in aspirin-treated patients, compared with 0.9% in the rivaroxaban 10 mg group and 1.4% in the rivaroxaban 20 mg. The magnitude of VTE recurrence reduction was the same in the unprovoked and in the provoked VTE population, 70%, the difference is the higher absolute risk reduction in the unprovoked group. This evidence suggests that the recurrence rate in provoked VTE is not low (3.6% in 1 year, even using aspirin), therefore, making it reasonable to extend anticoagulation for secondary VTE prophylaxis also in the provoked population.
In an attempt to better understand how the provoked VTE population behaved with secondary prophylaxis, a recent study re-evaluated the data of the EINSTEIN-EXT and EINSTEIN-CHOICE studies.45 Determinant factors that defined the provoked status of VTE were divided between major and minor, persistent, and transitory (Table 2).
Table 2.
Risk factors stratification of provoked VTE and VTE recurrence after 1 year of follow up (modified from Becattini et al.30).
| Provoked VTE risk factors | Major | Minor |
|---|---|---|
| Persistent | Active cancer (excluding basal cell or squamous cell skin cancer) | Inflammatory bowel disease, lower extremity paralysis, congestive heart failure, body mass index >30 kg/m2 creatinine clearance <50 ml/min, family history of VTE, known thrombophilia |
| Drug used for extended prophylaxis (n), VTE recurrence after 1 year of treatment | Rivaroxaban (n = 82) 0 | Rivaroxaban (n = 1184) 2.4% |
| Aspirin (n = 39) 5.1% | Aspirin (n = 468) 4.5% | |
| Placebo (n = 26) 3.8% | Placebo (n = 248) 10.7% | |
| Transient | Major surgery, trauma, cesarean section |
Immobilization, travel >8 h, pregnancy, puerperium, estrogen use, lower limb trauma with transient impairment or mobility |
| Drug used for extended prophylaxis (n), VTE recurrence after 1 year of treatment | Rivaroxaban (n = 125) 0 | Rivaroxaban (n = 268) 0.4% |
| Aspirin (n = 37) 0 | Aspirin (n = 121) 4.2% | |
| Placebo (n = 17) 0 | Placebo (n = 56) 7.1% |
VTE, venous thromboembolism.
In this analysis, no recurrence occurred in patients with VTE provoked by major transient risk factors. In VTE provoked by minor persistent risk factors, rates of recurrence where 2.4% in the 1184 rivaroxaban-treated patients, 4.5% in the 468 aspirin-treated patients and 10.7% in the 248 placebo patients. For patients with minor transient risk factors, recurrence rates were 0.4% in the 268 patients given rivaroxaban, 4.2% in the 121 patients given aspirin, and 7.1% in the 56 patients given the placebo. However, in the unprovoked VTE patients, the rates of recurrence in the 1173 patients given rivaroxaban, the 468 patients given aspirin, and the 243 patients given placebo were 2.0%, 5.9%, and 10.0%, respectively. Therefore, recurrence rates in patients with VTE provoked by minor persistent or minor transient risk factors were not significantly lower than those with unprovoked VTE (HR 0.81; 95% CI 0.56–1.16, and HR 0.68; 95% CI 0.32–1.30, respectively). Therefore, except for major surgery, trauma, and cesarean section induced VTE, all other provoked VTEs may benefit from extended anticoagulation therapy in the same way that the unprovoked population does.
It is also interesting to note similarities between survival rates that both provoked and unprovoked VTE presents. Data from the Framingham Heart Study demonstrated that in a prospective cohort of 9754 patients, long-term mortality of provoked and unprovoked VTE were remarkably similar.46 If they are so similar in outcome and therapy, should this distinction between provoked and unprovoked VTE be continued? In contrast, because provoked VTE comprises a wide range of clinical situations and the only provocative factors unquestionably related to the low recurrence rates are trauma and the perioperative setting, should we abandon the provoked/unprovoked dichotomy and instead, adopt a trauma/surgery associated VTE or not, clarifying the necessity of long-term anticoagulation?47 This answer is yet to be determined.
Should long-term anticoagulation be provided for cancer-associated thrombosis?
Cancer-associated thrombosis (CAT) is a significant issue, both for medical specialists that deal with VTE and for oncologists.48,49 It is believed that approximately 20% of VTE patients have or will have cancer over a short period of time and that 20% of all cancer patients will present VTE during their clinical course.50 Furthermore, cancer is a significant cause of death in VTE patients and vice versa. CAT presents several peculiarities that distinguish it from other VTEs and is the target of intense interest in the recent medical literature.51 Specifics about this topic are beyond the scope of this review. However, of note, CAT patients carry a high risk of VTE recurrence and also major bleeding, when treated.52 Treatment of CAT requires a different approach to ordinary VTE cases, and, until recently, low-molecular-weight-heparins (LMWHs) were the first treatment choice, for at least 3 months after the acute VTE event.10,53 Guidelines also suggest the maintenance of anticoagulation while the oncologic risk factors for VTE recurrence remain, such as metastatic disease or chemotherapy. However, despite the guideline recommendations, long-term anticoagulation with subcutaneous LMWH commonly leads to low adherence rates in CAT patients and unsatisfactory rates of both VTE recurrence and bleeding.54 Recently, two DOACs (edoxaban and rivaroxaban) proved to viable choices for CAT therapy,55,56 and now are accepted treatment alternatives both for initial and for extended anticoagulation treatment in cancer patients.49,57
Conclusion
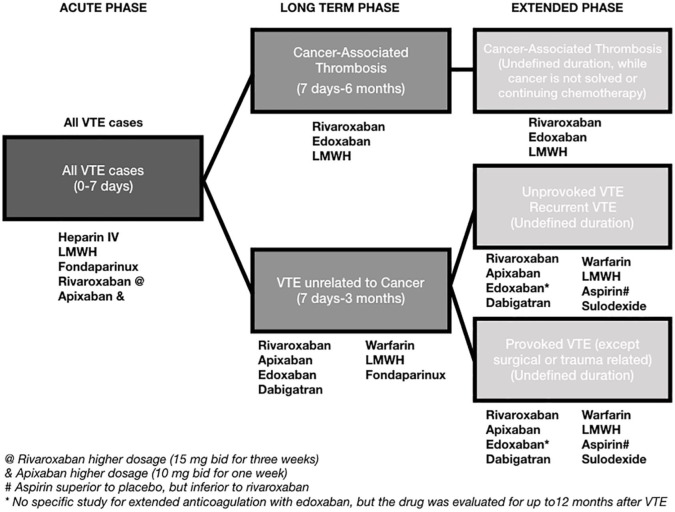
Recurrence rates after the first episode of VTE are high and potentially life-threatening. For optimization of clinical management and definition of anticoagulation duration, patients are usually characterized as having a provoked VTE (secondary to known VTE risk factors, such as immobilization, surgery, hormone use, and clinical illness) or an unprovoked VTE. Recent data suggest that, for surgically-related provoked VTE, anticoagulation may be suspended after the first 3 months of therapy. For unprovoked VTE or other causes of provoked VTE, data suggest that extended anticoagulation for 18 months is beneficial, with a possible impact on overall survival. Despite the theoretical benefit of more extended anticoagulation periods, to the best of the authors’ knowledge, no controlled data is currently available. Both warfarin and DOACs are possible therapeutic alternatives for secondary prophylaxis of VTE recurrence. Aspirin also has some beneficial effect, but the VTE recurrence rate is still high even with its use, so aspirin may be considered as an option for patients unable to use DOACs or warfarin. Rivaroxaban and apixaban are DOACs that may be used for extended anticoagulation in VTE both at their prophylactic or full anticoagulation dose. The summary of the recommendations is displayed in Figure 1.
Figure 1.
Summary of recommendations for extended anticoagulation after a first VTE event.
Take-home message
The recurrence rate of VTE is high and potentially life-threatening. Extended anticoagulation (longer than 3 months) is indicated for all causes of VTE, except for surgical or trauma-related VTE. For this purpose, both DOACs and warfarin may be employed.
Supplemental Material
Supplemental material, Author_response_to_reviewer_comments for Extended anticoagulation after venous thromboembolism: should it be done? by Caio J. Fernandes, Daniela Calderaro, Bruna Piloto, Susana Hoette, Carlos Vianna Poyares Jardim and Rogério Souza in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_1_v.1 for Extended anticoagulation after venous thromboembolism: should it be done? by Caio J. Fernandes, Daniela Calderaro, Bruna Piloto, Susana Hoette, Carlos Vianna Poyares Jardim and Rogério Souza in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_2_v.1 for Extended anticoagulation after venous thromboembolism: should it be done? by Caio J. Fernandes, Daniela Calderaro, Bruna Piloto, Susana Hoette, Carlos Vianna Poyares Jardim and Rogério Souza in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_2_v.2 for Extended anticoagulation after venous thromboembolism: should it be done? by Caio J. Fernandes, Daniela Calderaro, Bruna Piloto, Susana Hoette, Carlos Vianna Poyares Jardim and Rogério Souza in Therapeutic Advances in Respiratory Disease
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: Caio J. Fernandes received lecture fees and participated in the advisory boards of Bayer, Boehringer-Ingelheim, and Daiichi-Sankyo. Daniela Calderaro received lecture fees and participated in the advisory boards of Bayer, Pfizer, and Daiichi-Sankyo. Bruna Piloto and Susana Hoette declare no conflict of interest. Carlos Vianna Poyares Jardim received lecture fees and participated in the advisory boards of Bayer and Boehringer-Ingelheim. Rogério Souza received lecture fees and participated in the advisory boards of Bayer, and Pfizer.
ORCID iD: Caio J. Fernandes  https://orcid.org/0000-0002-4912-021X
https://orcid.org/0000-0002-4912-021X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Caio J. Fernandes, Cardiopulmonary Department, Heart Institute, University of Sao Paulo Medical School, 44, Av. Dr. Eneas de Carvalho Aguiar, Sao Paulo, 05403-000, Brazil; Cancer Institute, University of Sao Paulo Medical School, 251, Dr. Arnaldo Avenue, Sao Paulo, SP, Brazil; Sirio Libanes Hospital, 115, Adma Jafet St, Sao Paulo, SP, Brazil.
Daniela Calderaro, Cardiopulmonology Department, Heart Institute, University of Sao Paulo Medical School, Sao Paulo, SP, Brazil; Sirio Libanes Hospital, Sao Paulo, SP, Brazil.
Bruna Piloto, Cardiopulmonology Department, Heart Institute, University of Sao Paulo Medical School, Sao Paulo, SP, Brazil; Sirio Libanes Hospital, Sao Paulo, SP, Brazil.
Susana Hoette, Cardiopulmonology Department, Heart Institute, University of Sao Paulo Medical School, Sao Paulo, SP, Brazil.
Carlos Vianna Poyares Jardim, Cardiopulmonology Department, Heart Institute, University of Sao Paulo Medical School, Sao Paulo, SP, Brazil; Sirio Libanes Hospital, Sao Paulo, SP, Brazil.
Rogério Souza, Cardiopulmonology Department, Heart Institute, University of Sao Paulo Medical School, Sao Paulo, SP, Brazil; Sirio Libanes Hospital, Sao Paulo, SP, Brazil.
References
- 1. ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to global disease burden. Thromb Haemost 2014; 112: 843–852. [DOI] [PubMed] [Google Scholar]
- 2. Fernandes C, Jardim CVP, Alves JL, Jr, et al. Reperfusion in acute pulmonary thromboembolism. J Bras Pneumol 2018; 44: 180–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barritt DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism. A controlled trial. Lancet 1960; 1: 1309–1312. [DOI] [PubMed] [Google Scholar]
- 4. Kernohan RJ, Todd C. Heparin therapy in thromboembolic disease. Lancet 1966; 1: 621–623. [DOI] [PubMed] [Google Scholar]
- 5. Fernandes CJ, Luppino Assad AP, Alves-Jr JL, et al. Pulmonary embolism and gas exchange. Respiration 2019: 98: 253–262. [DOI] [PubMed] [Google Scholar]
- 6. Turpie AGG, Haas S, Weitz JI, et al. GARFIELD-VTE: 6-month outcomes. Res Pract Thromb Haemost 2017; 1: 1–15. [Google Scholar]
- 7. Volschan A, Caramelli B, Gottschall CA, et al. Guidelines for pulmonary embolism. Arq Bras Cardiol 2004; 83(Suppl. 1): 1–8. [DOI] [PubMed] [Google Scholar]
- 8. de Moerloose P, Alhenc-Gelas M, Boehlen F, et al. Deep venous thrombosis and thrombophilia: indications for testing and clinical implications. Semin Vasc Med 2001; 1: 89–96. [DOI] [PubMed] [Google Scholar]
- 9. Piazza G, Ridker PM. Is venous thromboembolism a chronic inflammatory disease? Clin Chem 2015; 61: 313–316. [DOI] [PubMed] [Google Scholar]
- 10. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149: 315–352. [DOI] [PubMed] [Google Scholar]
- 11. Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007; 92: 199–205. [DOI] [PubMed] [Google Scholar]
- 12. Baglin T, Luddington R, Brown K, et al. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet 2003; 362: 523–526. [DOI] [PubMed] [Google Scholar]
- 13. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999; 340: 901–907. [DOI] [PubMed] [Google Scholar]
- 14. Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003; 348: 1425–1434. [DOI] [PubMed] [Google Scholar]
- 15. Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003; 349: 631–639. [DOI] [PubMed] [Google Scholar]
- 16. Couturaud F, Sanchez O, Pernod G, et al. Six months vs extended oral anticoagulation after a first episode of pulmonary embolism: the PADIS-PE randomized clinical trial. JAMA 2015; 314: 31–40. [DOI] [PubMed] [Google Scholar]
- 17. Bova C, Bianco A, Mascaro V, et al. Extended anticoagulation and mortality in venous thromboembolism. A meta-analysis of six randomized trials. Thromb Res 2016; 139: 22–28. [DOI] [PubMed] [Google Scholar]
- 18. Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006; 355: 1780–1789. [DOI] [PubMed] [Google Scholar]
- 19. Kearon C, Spencer FA, O’Keeffe D, et al. D-dimer testing to select patients with a first unprovoked venous thromboembolism who can stop anticoagulant therapy: a cohort study. Ann Intern Med 2015; 162: 27–34. [DOI] [PubMed] [Google Scholar]
- 20. Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med 2010; 153: 523–531. [DOI] [PubMed] [Google Scholar]
- 21. Couturaud F. Guided duration of anticoagulation after unprovoked venous thromboembolism using D-dimer testing. Eur Respir J 2016; 47: 1313–1314. [DOI] [PubMed] [Google Scholar]
- 22. Kyrle PA, Minar E, Bialonczyk C, et al. The risk of recurrent venous thromboembolism in men and women. N Engl J Med 2004; 350: 2558–2563. [DOI] [PubMed] [Google Scholar]
- 23. McRae S, Tran H, Schulman S, et al. Effect of patient’s sex on risk of recurrent venous thromboembolism: a meta-analysis. Lancet 2006; 368: 371–378. [DOI] [PubMed] [Google Scholar]
- 24. Douketis J, Tosetto A, Marcucci M, et al. Risk of recurrence after venous thromboembolism in men and women: patient level meta-analysis. BMJ 2011; 342: d813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eichinger S, Hron G, Bialonczyk C, et al. Overweight, obesity, and the risk of recurrent venous thromboembolism. Arch Intern Med 2008; 168: 1678–1683. [DOI] [PubMed] [Google Scholar]
- 26. Gandara E, Kovacs MJ, Kahn SR, et al. Non-OO blood type influences the risk of recurrent venous thromboembolism. A cohort study. Thromb Haemost 2013; 110: 1172–1179. [DOI] [PubMed] [Google Scholar]
- 27. Eichinger S, Heinze G, Jandeck LM, et al. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121: 1630–1636. [DOI] [PubMed] [Google Scholar]
- 28. Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10: 1019–1025. [DOI] [PubMed] [Google Scholar]
- 29. Steele P. Trial of dipyridamole-aspirin in recurring venous thrombosis. Lancet 1980; 2: 1328–1329. [DOI] [PubMed] [Google Scholar]
- 30. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012; 366: 1959–1967. [DOI] [PubMed] [Google Scholar]
- 31. Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012; 367: 1979–1987. [DOI] [PubMed] [Google Scholar]
- 32. Simes J, Becattini C, Agnelli G, et al. Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation 2014; 130: 1062–1071. [DOI] [PubMed] [Google Scholar]
- 33. Weitz JI, Lensing AWA, Prins MH, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med 2017; 376: 1211–1222. [DOI] [PubMed] [Google Scholar]
- 34. Andreozzi GM, Bignamini AA, Davi G, et al. Sulodexide for the prevention of recurrent venous thromboembolism: the sulodexide in secondary prevention of recurrent deep vein thrombosis (SURVET) study: a multicenter, randomized, double-blind, placebo-controlled trial. Circulation 2015; 132: 1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blondon M, Bounameaux H. Secondary prevention of venous thromboembolism: one regimen may not fit all. Circulation 2015; 132: 1856–1859. [DOI] [PubMed] [Google Scholar]
- 36. Jiang QJ, Bai J, Jin J, et al. Sulodexide for secondary prevention of recurrent venous thromboembolism: a systematic review and meta-analysis. Front Pharmacol 2018; 9: 876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fernandes CJ, Alves Junior JL, Gavilanes F, et al. New anticoagulants for the treatment of venous thromboembolism. J Bras Pneumol 2016; 42: 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gavilanes-Oleas FA, Alves JL, Jr, Fernandes CJC, et al. Use of direct oral anticoagulants for chronic thromboembolic pulmonary hypertension. Clinics (Sao Paulo) 2018; 73: e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Investigators E, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363: 2499–2510. [DOI] [PubMed] [Google Scholar]
- 40. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368: 709–718. [DOI] [PubMed] [Google Scholar]
- 41. Venker BT, Ganti BR, Lin H, et al. Safety and efficacy of new anticoagulants for the prevention of venous thromboembolism after hip and knee arthroplasty: a meta-analysis. J Arthroplasty 2017; 32: 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cimminiello C, Prandoni P, Agnelli G, et al. Thromboprophylaxis with enoxaparin and direct oral anticoagulants in major orthopedic surgery and acutely ill medical patients: a meta-analysis. Intern Emerg Med 2017; 12: 1291–1305. [DOI] [PubMed] [Google Scholar]
- 43. Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013; 368: 699–708. [DOI] [PubMed] [Google Scholar]
- 44. Hokusai VTEI, Buller HR, Decousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369: 1406–1415. [DOI] [PubMed] [Google Scholar]
- 45. Prins MH, Lensing AWA, Prandoni P, et al. Risk of recurrent venous thromboembolism according to baseline risk factor profiles. Blood Adv 2018; 2: 788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Puurunen MK, Gona PN, Larson MG, et al. Epidemiology of venous thromboembolism in the framingham heart study. Thromb Res 2016; 145: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Albertsen IE, Piazza G, Goldhaber SZ. Let’s stop dichotomizing venous thromboembolism as provoked or unprovoked. Circulation 2018; 138: 2591–2593. [DOI] [PubMed] [Google Scholar]
- 48. Fernandes C. Evolution in the management of non-small cell lung cancer in Brazil. J Bras Pneumol 2017; 43: 403–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fernandes CJ, Morinaga LTK, Alves JL, Jr, et al. Cancer-associated thrombosis: the when, how and why. Eur Respir Rev 2019; 28. pii: 180119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Laporte S, Mismetti P, Decousus H, et al. Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) registry. Circulation 2008; 117: 1711–1716. [DOI] [PubMed] [Google Scholar]
- 51. Mukai M, Oka T. Mechanism and management of cancer-associated thrombosis. J Cardiol 2018; 72: 89–93. [DOI] [PubMed] [Google Scholar]
- 52. Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002; 100: 3484–3488. [DOI] [PubMed] [Google Scholar]
- 53. Lyman GH, Bohlke K, Khorana AA, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American society of clinical oncology clinical practice guideline update 2014. J Clin Oncol 2015; 33: 654–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Francis CW, Kessler CM, Goldhaber SZ, et al. Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: the DALTECAN study. J Thromb Haemost 2015; 13: 1028–1035. [DOI] [PubMed] [Google Scholar]
- 55. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med 2018; 378: 615–624. [DOI] [PubMed] [Google Scholar]
- 56. Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol 2018; 36: 2017–2023. [DOI] [PubMed] [Google Scholar]
- 57. Khorana AA, Noble S, Lee AYY, et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost 2018; 16: 1891–1894. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_response_to_reviewer_comments for Extended anticoagulation after venous thromboembolism: should it be done? by Caio J. Fernandes, Daniela Calderaro, Bruna Piloto, Susana Hoette, Carlos Vianna Poyares Jardim and Rogério Souza in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Extended anticoagulation after venous thromboembolism: should it be done? by Caio J. Fernandes, Daniela Calderaro, Bruna Piloto, Susana Hoette, Carlos Vianna Poyares Jardim and Rogério Souza in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Extended anticoagulation after venous thromboembolism: should it be done? by Caio J. Fernandes, Daniela Calderaro, Bruna Piloto, Susana Hoette, Carlos Vianna Poyares Jardim and Rogério Souza in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.2 for Extended anticoagulation after venous thromboembolism: should it be done? by Caio J. Fernandes, Daniela Calderaro, Bruna Piloto, Susana Hoette, Carlos Vianna Poyares Jardim and Rogério Souza in Therapeutic Advances in Respiratory Disease