Abstract
Background:
Stroke is common in patients with end-stage renal disease (ESRD) treated with hemodialysis (HD) and associated with high mortality rate. In the general population, atrial fibrillation (AF) is a major risk factor for stroke and therapeutic anticoagulation is associated with risk reduction, whereas in ESRD the relationship is less clear.
Objective:
The purpose of this study is to demonstrate the influence of AF on stroke rates and probability in those on HD following competing risk analyses.
Design:
A national record linkage cohort study.
Setting:
All renal and stroke units in Scotland, UK.
Patients:
All patients with ESRD receiving HD within Scotland from 2005 to 2013 (follow-up to 2015).
Measurements:
Demographic, clinical, and laboratory data were linked between the Scottish Renal Registry, Scottish Stroke Care Audit, and hospital discharge data. Stroke was defined as a fatal or nonfatal event and mortality derived from national records.
Methods:
Associations for stroke were determined using competing risk models: the cause-specific hazards model and the Fine and Gray subdistribution hazards model accounting for the competing risk of death in models of all stroke, ischemic stroke, and first-ever stroke.
Results:
Of 5502 patients treated with HD with 12 348.6-year follow-up, 363 (6.6%) experienced stroke. The stroke incidence rate was 26.7 per 1000 patient-years. Multivariable regression on the cause-specific hazard for stroke demonstrated age, hazard ratio (HR) (95% confidence interval [CI]) = 1.04 (1.03-1.05); AF, HR (95% CI) = 1.88 (1.25-2.83); prior stroke, HR (95% CI) = 2.29 (1.48-3.54), and diabetes, HR (95% CI) = 1.92 (1.45-2.53); serum phosphate, HR (95% CI) = 2.15 (1.56-2.99); lower body weight, HR (95% CI) = 0.99 (0.98-1.00); lower hemoglobin, HR (95% CI) = 0.88 (0.77-0.99); and systolic blood pressure (BP), HR (95% CI) = 1.01 (1.00-1.02), to be associated with an increased stroke rate. In contrast, the subdistribution HRs obtained following Fine and Gray regression demonstrated that AF, weight, and hemoglobin were not associated with stroke risk. In both models, AF was significantly associated with nonstroke death.
Limitations:
Our analyses derive from retrospective data sets and thus can only describe association not causation. Data on anticoagulant use are not available.
Conclusions:
The incidence of stroke in HD patients is high. The competing risk of “prestroke” mortality affects the relationship between AF and risk of future stroke. Trial designs for interventions to reduce stroke risk in HD patients, such as anticoagulation for AF, should take account of competing risks affecting associations between risk factors and outcomes.
Keywords: stroke, hemodialysis, atrial fibrillation, competing risk, mortality
Abrégé
Contexte:
Les accidents vasculaires cérébraux (AVC) sont fréquents chez les patients atteints d’insuffisance rénale terminale (IRT) traités en hémodialyse (HD), et sont associés à des taux de mortalité élevés. Dans la population générale, la fibrillation auriculaire (FA) est un important facteur de risque de subir un AVC. L’administration d’anticoagulants est associée à une réduction du risque, mais cette relation demeure incertaine en contexte d’IRT.
Objectif:
L’étude vise à démontrer l’influence de la FA sur la probabilité et les taux d’AVC chez les patients hémodialysés atteints de FA, à la suite d’analyses des risques concurrents.
Type d’étude:
Une étude de cohorte par jumelage des registres nationaux.
Cadre:
Toutes les unités de néphrologie et de soins spécialisés en AVC de l’Écosse (Royaume-Uni).
Sujets:
Tous les patients atteints d’IRT et traités par hémodialyse entre 2005 et 2013 en Écosse. Les patients ont été suivis jusqu’en 2015.
Mesures:
Les données démographiques, cliniques et de laboratoire ont été jumelées aux données du Scottish Renal Registry, du Scottish Stroke Care Audit et aux notes inscrites dans les dossiers médicaux à la sortie de l’hôpital. Les AVC ont été classés comme événement fatal ou non fatal, et la mortalité a été dérivée des registres nationaux.
Méthodologie:
Les associations de l’AVC ont été établies à l’aide de modèles de risques concurrents, soit un modèle de risque lié à la cause et l’approche de Fine et Gray, tenant compte du risque concurrent de mortalité dans les modèles incluant tous les types d’AVC, les AVC ischémiques et les premiers AVC.
Résultats:
Des 5 502 patients hémodialysés, suivis sur un total de 12 348,6 ans, 363 (6,6 %) ont subi un AVC. Le taux d’incidence d’un AVC était de 26,7 pour 1000 années-patient. La régression multivariée sur le risque lié à la cause pour les AVC a démontré que l’âge (RR: 1,04 [IC 95 %] [1,03-1,05]), la FA (RR: 1,88 [1,25-2,83]), les antécédents d’AVC (RR 2,29 [1,48-3,54]), le diabète (RR: 1,92 [1,45-2,53]), le taux de phosphate sérique (RR: 2,15 [1,56-2,99]), un faible poids corporel (RR: 0,99 [0,98-1,00]), un faible taux d’hémoglobine (RR: 0,88 [0,77-0,99]) et la pression systolique (RR: 1,01 [1,00-1,02]) étaient associés à un plus grand risque de subir un AVC. En revanche, les rapports de risque de sous-distribution obtenus par l’approche Fine et Gray ont démontré que la FA, le poids et le taux d’hémoglobine n’étaient pas associés à un risque d’AVC. Les deux modèles ont associé la FA à la mortalité non liée à un AVC de façon significative.
Limites:
Nos analyses dérivent d’ensembles de données rétrospectives, et par conséquent, ne peuvent que décrire une association et non la causalité. Les informations sur l’anticoagulant prescrit n’étaient pas disponibles.
Conclusion:
L’incidence des AVC chez les patients hémodialysés est élevée. Le risque concurrent de mortalité « pré-AVC » affecte le lien entre la FA et le risque de subir un AVC dans le futur. La conception d’essais cliniques sur les interventions visant à réduire les risques d’AVC chez les patients hémodialysés, notamment le traitement de la FA par les anticoagulants, devrait tenir compte des risques concurrents qui affectent les associations entre les facteurs de risques et les résultats.
What was known before
The incidence of, and mortality following, stroke in patients on hemodialysis (HD) for end-stage renal disease (ESRD) is higher than in the general population. In the general population, atrial fibrillation (AF) is associated with a 5-fold increase in stroke risk, a risk factor mitigated by use of anticoagulation. The influence of this key modifiable risk factor and thus the benefits from treatment are less clear in ESRD.
What this adds
This study provides a descriptive insight into the use of competing risk models in renal epidemiology for stroke after adjusting for the competing risk of death. We demonstrate that the presence of AF is significantly associated with an increase in stroke rates in surviving HD recipients, whereas we did not detect a significant association between AF and stroke probability in competing risk analyses. We propose that the high mortality rate in HD and its association with AF may explain the apparent lack of association of AF with stroke.
Introduction
The assumption that risk factors for disease in the general population will also predict outcome in those with end-stage renal disease (ESRD) can be false and could lead to ineffective and potentially harmful treatment. For example, there is an established association between rising blood pressure (BP)1 or serum cholesterol2 and survival in the general population, but “reverse epidemiology” has been observed in those on maintenance dialysis.3 In the absence of specific trials in people with ESRD, this makes establishing the best preventive care in ESRD problematic. For example, the absence of benefit from statin use in hypercholesterolemia in people with ESRD was only appreciated following 3 randomized control trials in maintenance dialysis patients4-6 and a meta-analysis of low-density lipoprotein (LDL) cholesterol–lowering therapy across a range of renal functions.7 Therefore, until well-designed trials advance our knowledge, we must make the best use of available observational data, to assess both efficacy and safety of treatments.
Stroke is common in the ESRD population and the incidence is up to 10-fold greater than in the general population.8 It is associated with a high mortality rate,9 physical frailty,10 cognitive dysfunction,11 and dialysis withdrawal.12 Understanding the risk factors for stroke in ESRD is needed to optimize prevention. Atrial fibrillation (AF)13 is an important risk factor for stroke14 and risk is mitigated by use of anticoagulant drugs.14 However, the bleeding risk associated with anticoagulation is much higher in the ESRD population than that observed in patients without ESRD15 and the less frequently acknowledged complications of warfarin are of greater significance in patients with ESRD, specifically accelerated vascular calcification,16 and calciphylaxis.17 Reports on the effects of AF are inconsistent - potentially because despite the increasing number of publications examining the factors associated with stroke,18-33 most fail to address an important issue of survival analysis—the concept of a competing risk. Briefly, a competing risk is an event that occurs during follow-up which forever prevents the event of interest occurring. For instance, in the situation of time-to-stroke analyses, death would act as a competing risk, forever preventing future stroke. Simply censoring death could lead to misinterpretation of an effect. Such risk must be acknowledged in the statistical models chosen.
We performed a record linkage analysis of national data in Scotland to examine stroke in ESRD in those receiving hemodialysis (HD), with focus on the following: stroke incidence rate, mortality following stroke, and factors associated with stroke occurrence. We hypothesized that stroke incidence rate and mortality following stroke would be high and that analyzing our data adjusting for the competing risk of death would demonstrate the differential impact of risk factors on the rates and probability of stroke and death.
Methods
Using a National Health Service Scotland Safe Haven workspace, we linked the Scottish Renal Registry, Scottish Stroke Care Audit (SSCA), Scottish Morbidity Records 01, and National Records Scotland Death Records over the period of January 1, 2005 to December 31, 2013. This created a data set which allowed determination of the following: (1) stroke incidence rates in HD, (2) factors associated with stroke in HD using a competing risk approach, and (3) case fatality following stroke.
Data sets
The Scottish Renal Registry (SRR) is a nationwide data set, contributed to by all 9 adult renal units. Data are collected on multiple variables including baseline demographics, primary renal diagnoses, and annual census lab and clinical variables.34 All patients receiving renal replacement therapy (RRT) for ESRD are included in the renal registry. Detailed data on cause and date of death are captured by the SRR allowing the inclusion of stroke cases diagnosed at, but not before, death.
The SSCA35 was established in 2002 to monitor the performance of stroke care against guideline-based clinical standards throughout Scotland. Data are collected on patient demographics, stroke subtype, and outcomes. This data set had provided a complete coverage of all hospitals managing acute stroke since 2005.
The Scottish Morbidity Records 01 (SMR01) collects data on all nonobstetric, nonpsychiatric hospital discharges since 1968.36 Since 1989, SMR01 has been used to plan financial management of hospitals to ensure a high completion rate. Internal audit of these data supports overall 89% accuracy for Main Condition diagnosis.37
The National Records of Scotland is a nonministerial department of the Scottish Government, established in 2011 following the merger of the General Register Office for Scotland and the National Archives of Scotland. They are responsible for registration of life events including deaths. Stroke as a cause of death was identified using enhanced mortality data available within the SRR’s Scottish Mortality Audit in Renal Replacement Therapy (SMARRT). However, prescription data are not available and therefore the potential influence of anticoagulation use on cause of death is not reported.
Definitions
HD Recipients
All patients commencing RRT for ESRD in Scotland are recorded within the SRR. End-stage renal disease is a diagnosis determined by the clinician initiating RRT. We included only adults (those ≥16 years at study inception) who were receiving HD. The study inception date was January 1, 2005 in those receiving HD at the start of the study period or the date of commencing HD as the first modality of RRT for ESRD occurring thereafter. The end date was recorded as the date of stroke, change in RRT modality, death, or end of the study.
Stroke
All nonfatal and fatal strokes were included in our analyses. From the SSCA, we extracted the date and subtype of the first stroke during the study period. To ensure complete capture of stroke, SMR01 was interrogated for the presence and dates of International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes pertaining to stroke episodes (I60, I61, I62.9, I63, and I64) excluding subdural and extradural hemorrhages and transient ischemic attack (TIA). Using SMARRT data within the SRR, all cases where “Cerebrovascular accident, ERA-EDTA code 22” was listed as the primary cause of death were extracted.
Baseline Characteristics
The SMR01 was interrogated from 1981 until December 31, 2013 for the presence of ICD-10 codes relating to atrial fibrillation/flutter (I48), ischemic heart disease (I21, 24, 24.8, I24.9, I25, I25.1, I25.2, I25.5, and I25.8), diabetes mellitus (E10 and E11), hypercholesterolemia (E78), obesity (E66), smoking (F17), and hypertension (I10 and I15). To prevent the overlap of diagnoses, ICD codes for prior stroke (I60, I61, I62.9, I63, and I64) were pulled from 1981 until the date of first stroke in those who experience stroke, or until December 31, 2013 in those who do not.
The Scottish Government provides online calculators allowing use of patient postcode to generate an urban-rural classification (http://www.isdscotland.org/Products-and-Services/GPD-Support/Geography/Urban-Rural-Classification/) and divisions of socioeconomic deprivation, the Scottish Index of Multiple Deprivation (SIMD; http://www.gov.scot/Topics/Statistics/SIMD). Using patient postcode at commencing RRT in those on RRT or postcode at stroke for the general population, deprivation quintiles were calculated and categorized into the most deprived (quintiles 1 and 2) or least deprived (quintiles 3-5). Rurality classification is based on population size and accessibility to an urban area. The 6-fold classification was used and subdivided into urban (population > 3000) and rural (population < 3000).
Clinical and Lab Values
The annual census collects data on BP, weight, use of erythropoietin-stimulating agents, and blood results, namely, blood hemoglobin, serum albumin, phosphate, adjusted calcium, and urea reduction ratio. Data were collected and have been provided as a median (interquartile range [IQR]) of all results over the entire study period or until date of stroke, whichever comes first.
Statistical Analyses
Stroke incidence rates were calculated using events as the numerator and follow-up time in years as the denominator and expressed as per 1000/patient-years. Data are presented as median (IQR) for continuous data or total number (%) for categorical data. Demographics are compared using Mann-Whitney U or chi-square test as appropriate. Using predetermined clinical factors relating to stroke or cardiovascular outcome (age, sex, past medical history of AF, stroke, diabetes and ischemic heart disease, predialysis systolic BP, predialysis weight, blood hemoglobin, and serum phosphate), we performed 2 competing risk analyses. Firstly, to analyze the effect of covariates on the rate at which events occur, we performed multivariable cause-specific Cox proportional hazards analyses to calculate the cause-specific hazard (CSH) ratios for stroke and nonstroke death, presented as CSH ratios with 95% confidence intervals (CIs). We ensured that there was no significant multicollinearity between variables by calculating the variance inflation factor, using a cut-off of 5. Secondly, to determine which variables affect the probability of each event occurring over time (incidence), we used the Fine and Gray regression model, calculating the subdistribution hazard ratio (SHR) for the occurrence of stroke and also prestroke death. Analyses were repeated to assess 3 distinct groups: (1) all stroke cases, (2) ischemic stroke only by omitting all cases of hemorrhagic stroke, and (3) first-ever stroke by removing all cases with a prior history of stroke. Finally, we used both the Kaplan-Meier (KM) estimator and the cumulative incidence competing risk (CICR) method to calculate the cumulative incidence of stroke and prestroke death based on the presence or absence of AF. We did not perform imputation for missing data as missing data were not missing at random. Data linkage was performed using SAS v9.4 (SAS Institute Inc, Cary, North Carolina), and analyses, including competing risk analyses, were performed using Stata 14.1 (StataCorp, College Station, Texas). We used the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) cohort checklist when writing our report.38
Ethical Approval
The data sets used in this article work within the “NHS Code of Practice on Protecting Patient Confidentiality” which incorporates the requirements of statute and common law including the Data Protection Act, the Human Rights Act, and the Adults with Incapacity (Scotland) Act. Access and use of the data for the purpose of this study were approved following a National Services for Scotland (NSS) proportionate governance review by the Privacy Advisory Committee of ISD, NHS Scotland Reference 55/14.
Results
Characteristics of the Study Population
A total of 5502 adult patients were receiving or commenced HD for ESRD between January 1, 2005 and December 31, 2013 in Scotland. The median age (IQR) at commencing RRT was 65.1 (23.3) years, and 2271 (41.3%) were female. Median duration of follow-up was 665 (1098) days. Baseline characteristics of the cohort are shown in Table 1 with demographics of subjects with and without AF (as a variable of interest) in Table 2.
Table 1.
Baseline Demographics of HD Patients—No Stroke Versus All Stroke.
| No stroke | Stroke | All | P value | |
|---|---|---|---|---|
| n | 5139 | 363 | 5502 | |
| Median age (IQR), years | 64.8 [24.0] | 69.9 [15.4] | 65.1 [23.3] | <.0001 |
| Female (%) | 2105 [41.0] | 166 [45.7] | 2271 [41.3] | .08 |
| Primary renal diagnosis (%) | ||||
| Glomerulonephritis | 748 [14.6] | 44 [12.1] | 792 [14.4] | .22 |
| Interstitial disease | 1093 [21.3] | 64 [17.6] | 1157 [21.0] | .11 |
| Multisystem | 1262 [24.6] | 94 [25.9] | 1356 [24.7] | .57 |
| Diabetes | 1085 [21.1] | 98 [27.0] | 1183 [21.5] | .01 |
| Other | 941 [18.3] | 63 [17.4] | 1004 [18.3] | .73 |
| Missing | 10 [0.2] | 10 [0.2] | ||
| Urban rurality status (%) | ||||
| Urban | 773 [15.0] | 54 [14.9] | 827 [15.0] | |
| Rural | 4366 [85.0] | 309 [85.1] | 4675 [85.0] | 1.00 |
| Deprivation status (%) | ||||
| Least (SIMD quintiles 3-5) | 3722 [72.4] | 259 [71.4] | 3981 [72.4] | |
| Most (SIMD quintiles 1 and 2) | 1417 [27.6] | 104 [28.7] | 1521 [27.6] | .67 |
| Past medical history (%) | ||||
| Atrial fibrillation | 384 [7.5] | 45 [12.4] | 429 [7.8] | <.01 |
| Ischemic heart disease | 1774 [34.5] | 138 [38.0] | 1912 [34.8] | .19 |
| Stroke | 197 [3.8] | 36 [9.9] | 233 [4.2] | <.0001 |
| Diabetes | 1772 [34.5] | 148 [40.8] | 1920 [34.9] | .02 |
| Hypercholesterolemia | 679 [13.2] | 66 [18.2] | 745 [13.5] | .01 |
| Obesity | 352 [6.9] | 25 [6.9] | 377 [6.9] | 1.00 |
| Smoking | 317 [6.2] | 14 [3.9] | 331 [6.0] | .09 |
| Hypertension | 3649 [71.0] | 271 [74.7] | 3920 [71.3] | .17 |
| Clinical variables, median (IQR) | ||||
| SBP, mm Hg | 138 [29.0] | 142 [30.0] | 138.5 [29.0] | <.01 |
| DBP, mm Hg | 71 [18.5] | 69 [20.0] | 71 [18.5] | .25 |
| Weight, kg | 72.8 [23.5] | 70.25 [25.6] | 72.6 [23.6] | <.01 |
| Use of ESA | 3791 [73.8] | 251 [69.2] | 4042 [73.5] | .056 |
| Laboratory variables, median (IQR) | ||||
| Hemoglobin, g/dL | 11.4 [1.8] | 11.3 [1.6] | 11.35 [1.8] | .29 |
| Serum albumin, g/L | 37 [7.0] | 36.5 [7.0] | 37 [7.0] | .53 |
| Serum phosphate, mmol/L | 1.46 [0.56] | 1.56 [0.58] | 1.47 [0.56] | <.01 |
| Serum adjusted calcium, mmol/L | 2.36 [0.19] | 2.33 [0.20] | 2.36 [0.20] | <.01 |
| Urea reduction ratio | 71.5 [8.5] | 71 [10] | 71.5 [8.5] | .99 |
| Death at follow-up | 3152 [61.3] | 328 [90.4] | 3480 [63.3] | <.0001 |
Note. IQR = interquartile range; SIMD = Scottish Index of Multiple Deprivation; SBP = systolic blood pressure; DBP = diastolic blood pressure; ESA = erythropoietin-stimulating agent; HD = hemodialysis.
Table 2.
Baseline Demographics of HD Patients Split by the Presence of Atrial Fibrillation.
| No AF | AF | All | P value | |
|---|---|---|---|---|
| n | 5073 | 429 | 5502 | |
| Median age (IQR), years | 64.6 [23.9] | 70.9 [15.8] | 65.1 [23.3] | <.0001 |
| Female (%) | 2114 [41.67] | 157 [36.6] | 2271 [41.3] | .04 |
| Primary renal diagnosis (%) | ||||
| Glomerulonephritis | 729 [14.4] | 63 [14.7] | 792 [14.4] | .83 |
| Interstitial disease | 1081 [21.3] | 76 [17.7] | 1157 [21.0] | .08 |
| Multisystem | 1245 [24.5] | 111 [25.9] | 1356 [24.7] | .56 |
| Diabetes | 1104 [21.8] | 79 [18.4] | 1183 [21.5] | .11 |
| Other | 907 [17.9] | 97 [22.6] | 1004 [18.3] | .02 |
| Missing | 7 [0.1] | 3 [0.7] | 10 [0.2] | |
| Urban rurality status (%) | ||||
| Urban | 770 [15.2] | 57 [13.3] | 827 [15.0] | |
| Rural | 4303 [84.8] | 372 [86.7] | 4675 [85.0] | .32 |
| Deprivation status (%) | ||||
| Least (SIMD quintiles 3-5) | 3672 [72.4] | 309 [72.0] | 3981 [72.4] | |
| Most (SIMD quintiles 1 and 2) | 1401 [27.6] | 120 [28.0] | 1521 [27.6] | .87 |
| Past medical history (%) | ||||
| AF | 0 | 429 [100] | 429 [7.8] | <.0001 |
| Ischemic heart disease | 1678 [33.1] | 234 [54.6] | 1912 [34.8] | <.0001 |
| Stroke | 193 [3.8] | 40 [9.3] | 233 [4.2] | <.0001 |
| Diabetes | 1773 [35.0] | 147 [34.3] | 1920 [34.9] | .79 |
| Hypercholesterolemia | 679 [13.4] | 66 [15.4] | 745 [13.5] | .27 |
| Obesity | 339 [6.7] | 38 [8.9] | 377 [6.9] | .07 |
| Smoking | 314 [6.2] | 17 [4.0] | 331 [6.0] | .07 |
| Hypertension | 3575 [70.5] | 345 [80.4] | 3920 [71.3] | <.0001 |
| Clinical variables, median (IQR) | ||||
| SBP, mm Hg | 139.0 [29.0] | 132.3 [31.8] | 138.5 [29.0] | <.0001 |
| DBP, mm Hg | 71.0 [19.0] | 68 [18.3] | 71 [18.5] | <.01 |
| Weight, kg | 72.6 [23.8] | 72.8 [22.0] | 72.6 [23.6] | .92 |
| Use of ESA | 3765 [74.2] | 277 [64.6] | 4042 [73.5] | <.0001 |
| Laboratory variables, median (IQR) | ||||
| Hemoglobin, g/dL | 11.4 [1.8] | 11.5 [1.8] | 11.4 [1.8] | .15 |
| Serum albumin, g/L | 37.0 [7.0] | 36.5 [7.0] | 37.0 [7.0] | .05 |
| Serum phosphate, mmol/L | 1.47 [0.56] | 1.50 [0.54] | 1.47 [0.56] | .02 |
| Serum adjusted calcium, mmol/L | 2.35 [0.19] | 2.36 [0.21] | 2.36 [0.20] | .71 |
| Urea reduction ratio | 71.5 [9.0] | 70 [9] | 71.5 [8.5] | .13 |
| Stroke cases | 318 [6.3] | 45 [10.5] | 363 [6.6] | <.01 |
| Death at follow-up | 3087 [60.9] | 393 [91.6] | 3480 [63.3] | <.0001 |
Note. Mann-Whitney U or chi-square test is applied, when comparing HD with transplant and PD with transplant. AF = atrial fibrillation; IQR = interquartile range; SIMD = Scottish Index of Multiple Deprivation; SBP = systolic blood pressure; DBP = diastolic blood pressure; ESA = erythropoietin-stimulating agent; HD = hemodialysis.
There were 363 strokes during the follow-up period accounting for 6.6% of all patients; 45 (12.4%) were hemorrhagic, and the remainder were ischemic or unspecified (including those listed as primary cause of death). Cumulative follow-up was 12 348.6 years. Unadjusted stroke incidence rate for all ESRD patients is 26.7 strokes per 1000 patient-years. The incidence rate of stroke in the incident population (≤90 days of RRT) was 36.4 per 1000 patient-years.
Demographics demonstrate that those who experience stroke were more likely to be older and have diabetic nephropathy, a past medical history of AF, prior stroke, diabetes, and hypercholesterolemia. Clinical variables revealed higher median systolic BP, lower body weight, higher median serum phosphate, and lower median serum calcium in those who experience stroke.
Survival Analyses: Cause-Specific Hazards and Subdistribution Hazards Model Regressions
The rate of event occurrence was assessed by multivariable regression on the CSH for stroke. This demonstrated significance for conventional stroke risk factors, namely, age (hazard ratio [HR] [95% CI] = 1.04 [1.03-1.05]), presence of AF (HR [95% CI] = 1.88 [1.25-2.83]), prior stroke (HR [95% CI] = 2.29 [1.48-3.54]), and diabetes (HR [95% CI] = 1.92 [1.45-2.53]). Higher median serum phosphate (HR [95% CI] = 2.15 [1.56-2.99]) was associated with a greater stroke rate, as was lower body weight (HR [95% CI] = 0.99 [0.98-1.00]), lower median hemoglobin (HR [95% CI] = 0.88 [0.77-0.99]), and higher predialysis systolic BP (HR [95% CI] = 1.01 [1.00-1.02]). The Fine and Gray subdistribution hazards model was used to determine factors which influence the probability of stroke occurrence over time. This demonstrated an increase in stroke risk from increasing age, prior stroke, diabetes, serum phosphate, and systolic BP. There was no significant effect of AF, weight, or hemoglobin on stroke risk. In both competing risk models, AF was significantly associated with mortality (see Table 3).
Table 3.
Regression Analyses Using Competing Risk Techniques for All Stroke and Prestroke Death in Hemodialysis Patients.
| Cause-specific hazards model | Subdistribution hazards model | |||||||
|---|---|---|---|---|---|---|---|---|
| Stroke | Prestroke death | Stroke | Prestroke death | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | SHR (95% CI) | P value | SHR (95% CI) | P value | |
| Demographics | ||||||||
| Age (years) | 1.04 (1.03-1.05) | <.001 | 1.04 (1.04-1.05) | <.001 | 1.02 (1.01-1.04) | <.001 | 1.03 (1.03-1.04) | <.001 |
| Female | 0.96 (0.72-1.28) | .80 | 0.72 (0.64-0.80) | <.001 | 1.12 (0.86-1.46) | .40 | 0.75 (0.67-0.84) | <.001 |
| Past medical history | ||||||||
| Atrial fibrillation | 1.88 (1.25-2.83) | <.01 | 1.60 (1.36-1.88) | <.001 | 1.53 (1.00-2.32) | .05 | 1.40 (1.18-1.67) | <.001 |
| Prior stroke | 2.29 (1.48-3.54) | <.001 | 1.10 (0.87-1.38) | .44 | 2.30 (1.49-3.54) | <.001 | 1.03 (0.80-1.32) | .83 |
| Diabetes | 1.92 (1.45-2.53) | <.001 | 1.63 (1.64-1.82) | <.001 | 1.54 (1.17-2.02) | <.01 | 1.45 (1.30-1.62) | <.001 |
| Ischemic heart disease | 0.89 (0.68-1.56) | .38 | 0.98 (0.88-1.08) | .69 | 0.96 (0.74-1.25) | .76 | 1.04 (0.93-1.15) | .51 |
| Clinical variable | ||||||||
| Predialysis SBP | 1.01 (1.00-1.02) | <.01 | 1.00 (0.99-1.00) | <.001 | 1.01 (1.00-1.02) | <.01 | 0.99 (0.99-1.00) | <.001 |
| Predialysis weight | 0.99 (0.98-1.00) | <.01 | 0.99 (0.98-0.99) | <.001 | 0.99 (0.98-1.00) | .09 | 0.99 (0.99-0.99) | <.001 |
| Hemoglobin | 0.88 (0.77-0.99) | .04 | 0.75 (0.71-0.78) | <.001 | 1.00 (0.91-1.1) | .93 | 0.76 (0.72-0.81) | <.001 |
| Serum phosphate | 2.15 (1.56-2.99) | <.001 | 2.05 (1.80-2.34) | <.001 | 1.54 (1.1-2.14) | .01 | 1.52 (1.24-1.85) | <.001 |
Note. Initially, a cause-specific Cox proportional hazards regression examines multivariable models for stroke and prestroke death. The competing risk model presents multivariable regression for stroke or prestroke death using the Fine and Gray model of subdistribution hazards. HR = hazard ratio; CI = confidence interval; SHR = subdistribution hazard ratio; SBP = systolic blood pressure.
The models were repeated for those with ischemic stroke (Table 4) and those with first-ever stroke (Table 5). Variables consistently associated with stroke were age, diabetes, prior stroke (where applicable), and higher serum phosphate.
Table 4.
Regression Analyses, Using Competing Risk Techniques for Ischemic Stroke and Prestroke Death in Hemodialysis Patients.
| Cause-specific hazards model | Subdistribution hazards model | |||||||
|---|---|---|---|---|---|---|---|---|
| Stroke | Prestroke death | Stroke | Prestroke death | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | SHR (95% CI) | P value | SHR (95% CI) | P value | |
| Demographics | ||||||||
| Age (years) | 1.04 (1.03-1.05) | <.001 | 1.04 (1.04-1.05) | <.001 | 1.03 (1.02-1.05) | <.001 | 1.04 (1.03-1.04) | <.001 |
| Female | 1.08 (0.80-1.45) | .61 | 0.72 (0.64-0.80) | <.001 | 1.26 (0.95-1.66) | .11 | 0.72 (0.64-0.81) | <.001 |
| Past medical history | ||||||||
| Atrial fibrillation | 1.88 (1.22-2.90) | <.01 | 1.60 (1.36-1.88) | <.001 | 1.52 (0.97-2.37) | .07 | 1.38 (1.15-1.65) | <.001 |
| Prior stroke | 2.46 (1.57-3.86) | <.001 | 1.10 (0.87-1.38) | .43 | 2.47 (1.58-3.86) | <.001 | 1.01 (0.78-1.30) | .96 |
| Diabetes | 2.07 (1.54-2.78) | <.001 | 1.63 (1.46-1.82) | <.001 | 1.65 (1.24-2.20) | <.01 | 1.48 (1.32-1.65) | <.001 |
| Ischemic heart disease | 0.91 (0.69-1.20) | .50 | 0.98 (0.88-1.08) | .63 | 0.98 (0.74-1.29) | .88 | 1.01 (0.91-1.12) | .83 |
| Clinical variable | ||||||||
| Predialysis SBP | 1.01 (1.00-1.01) | .03 | 1.00 (0.99-1.00) | <.001 | 1.01 (1.00-1.01) | .1 | 0.99 (0.99-1.00) | <.001 |
| Predialysis weight | 0.99 (0.98-1.00) | .12 | 0.99 (0.98-0.99) | <.001 | 0.99 (0.98-1.00) | .20 | 0.99 (0.99-0.99) | <.001 |
| Hemoglobin | 0.89 (0.78-1.02) | .09 | 0.74 (0.71-0.78) | <.001 | 1.02 (0.91-1.13) | .78 | 0.77 (0.73-0.81) | <.001 |
| Serum phosphate | 2.00 (1.39-2.87) | <.001 | 2.04 (1.79-2.33) | <.001 | 1.45 (1.09-1.93) | .01 | 1.81 (1.55-2.11) | <.001 |
Note. Initially, a cause-specific Cox proportional hazards regression examines multivariable models for stroke and prestroke death. The competing risk model presents multivariable regression for stroke or prestroke death using the Fine and Gray model of subdistribution hazards. HR = hazard ratio; CI = confidence interval; SHR = subdistribution hazard ratio; SBP = systolic blood pressure.
Table 5.
Regression Analyses Using Competing Risk Techniques for First-Ever Stroke and Prestroke Death in Hemodialysis Patients.
| Cause-specific hazards model | Subdistribution hazards model | |||||||
|---|---|---|---|---|---|---|---|---|
| Stroke | Prestroke death | Stroke | Prestroke death | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | SHR (95% CI) | P value | SHR (95% CI) | P value | |
| Demographics | ||||||||
| Age (years) | 1.04 (1.03-1.05) | <.001 | 1.04 (1.04-1.05) | <.001 | 1.03 (1.02-1.04) | <.001 | 1.04 (1.03-1.04) | <.001 |
| Female | 0.95 (0.71-1.27) | .71 | 0.70 (0.63-0.79) | <.001 | 1.13 (0.85-1.49) | .41 | 0.74 (0.66-0.83) | <.001 |
| Past medical history | ||||||||
| Atrial fibrillation | 1.81 (1.16-2.81) | <.01 | 1.60 (1.35-1.89) | <.001 | 1.47 (0.93-2.32) | .10 | 1.41 (1.18-1.69) | <.001 |
| Prior stroke | — | — | — | — | — | — | — | — |
| Diabetes | 2.06 (1.54-2.76) | <.001 | 1.63 (1.46-1.83) | <.001 | 1.66 (1.25-2.20) | <.001 | 1.44 (1.28-1.62) | <.001 |
| Ischemic heart disease | 0.86 (0.65-1.14) | .29 | 0.97 (0.88-1.08) | .61 | 0.94 (0.71-1.25) | .68 | 1.04 (0.93-1.15) | .49 |
| Clinical variable | ||||||||
| Predialysis SBP | 1.01 (1.00-1.01) | .02 | 1.00 (0.99-1.00) | <.001 | 1.01 (1.00-1.01) | .01 | 0.99 (0.99-1.00) | <.001 |
| Predialysis weight | 0.99 (0.98-1.00) | <.01 | 0.99 (0.98-0.99) | <.001 | 0.99 (0.98-1.00) | .09 | 0.99 (0.99-0.99) | <.001 |
| Hemoglobin | 0.85 (0.75-0.97) | .02 | 0.75 (0.71-0.78) | <.001 | 0.98 (0.88-1.09) | .71 | 0.77 (0.73-0.81) | <.001 |
| Serum phosphate | 2.22 (1.56-3.11) | <.001 | 2.13 (1.86-2.43) | <.001 | 1.56 (1.11-2.19) | .01 | 1.54 (1.25-1.89) | <.001 |
Note. Initially, a cause-specific Cox proportional hazards regression examines multivariable models for stroke and prestroke death. The competing risk model presents multivariable regression for stroke or prestroke death using the Fine and Gray model of subdistribution hazards. HR = hazard ratio; CI = confidence interval; SHR = subdistribution hazard ratio; SBP = systolic blood pressure.
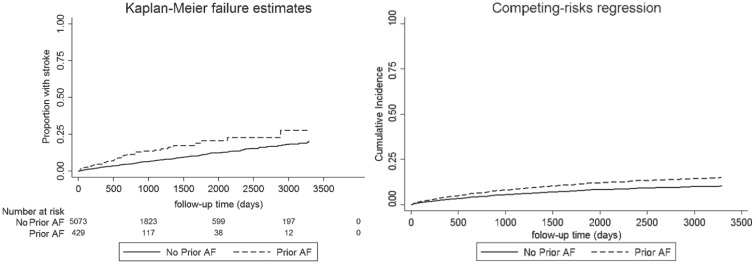
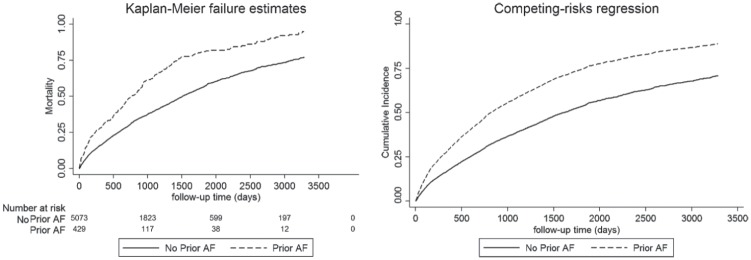
Both KM estimates and CICR curves show a positive influence of AF on stroke and nonstroke death. However, the overestimation of this risk by the KM estimate due to the presence of a competing risk is observed (see Figures 1 and 2).
Figure 1.
Effect of prior AF on stroke incidence using the KM estimator and the cumulative incidence function curve.
Note. Although both images demonstrate a significant univariable association of AF with stroke (P < .01), this comparison graphically demonstrates the overestimation of cumulative incidence from the KM estimator when the competing risk of prestroke death is not considered. AF = atrial fibrillation; KM = Kaplan-Meier.
Figure 2.
Effect of prior AF on the incidence of prestroke death using the KM estimator and the cumulative incidence function curve.
Note. Both images demonstrate a significant univariable association of AF with prestroke mortality (P < .01) and demonstrate the overestimation of cumulative incidence from the KM estimator when the competing risk of stroke is not considered. AF = atrial fibrillation; KM = Kaplan-Meier.
Outcome Following Stroke
Case fatality (death within 7 days) for all stroke cases was 22.6% (n = 82) and 328 of 363 (90.4%) died during follow-up. Fatality was 34.7% at 28 days and 61.7% at 1 year. Fatality was higher in those with hemorrhagic stroke with respective 7-, 28-, and 365-day fatality of 33.3%, 51.1%, and 82.2% compared with 21.1%, 32.4, and 58.8%.
Discussion
Following the linkage of national data sets, we have gained further insight into the incidence, associations, and outcomes of stroke in a UK-based HD population. We have highlighted the high incidence of stroke in HD patients and a notable increase in stroke incidence in the first 90 days after starting dialysis. This highlights both the importance of this condition and also a period of greater risk for our patients. Furthermore, we have described the fatality following stroke, with alarming results—such that less than 50% are alive 1 year later. Recognizing this underpins the critical need to accurately identify treatable risk factors for stroke—targets for preventive strategies in dialysis patients. Finally, we have used our data to highlight an often-ignored consideration in survival analysis, that is, competing risks.
Stroke Incidence and Associations
Our stroke incidence and case fatality rates are comparable to previously published literature,8,39-42 and the findings of advancing age, prior stroke, diabetes, low body weight, and low blood hemoglobin are recognized.43 Unique to our study, high serum phosphate was found to be significantly associated with stroke, persisting throughout all our analyses. The effect of AF on the development of stroke in ESRD remains unclear. In this study, AF, modeling on the CSH, demonstrated an increase in rates of stroke and prestroke death. However, our mortality rate is high—63% of all patients deceased at follow-up; thus, assessing the probability of stroke occurrence in this context requires complementary data from the subdistribution hazard. In doing so, we discovered that AF failed to demonstrate a significant effect on stroke risk. We explore the reasons behind this.
Competing Risk Analyses in Clinical Practice
As described, a competing risk is an event arising during a study which can interfere with the event of interest occurring.44 In our study, where stroke is our event of interest, death is an important competing risk as it will forever prevent this event of interest occurring. Bluntly, it is impossible to present with stroke after death.
The survival analyses by the KM estimator and Cox regression models are frequently used to demonstrate the influence of variables on outcome incidence (ie, the probability of occurrence) or hazard (ie, the rate of occurrence in those still at risk), respectively. Both methods are capable of handling data with incomplete follow-up time. They do so by censoring patients who cannot be followed up to their event of interest, for whatever reason, or at study completion. A key assumption of survival analyses states that censoring should be independent,45 that is, a random event such that those censored out of the study have the same future risk of the event of interest as those remaining in the study.
In our study, if we fail to consider death as a competing risk and simply censor such patients, this would leave such cases in the KM estimator, remaining “at risk” for the duration of the study, which is clearly impossible. In situations where death rates are high or follow-up prolonged, this can lead to significant overestimations in stroke incidence. In Cox regression, a competing risk will inflate the relative differences between groups, resulting in biased HRs and misinterpretations of the variable effect.
No statistical test can detect the presence of a competing risk; thus, when considering any event of interest, the researcher must carefully consider if any censored outcomes could interfere with the likelihood of the event of interest.
Two techniques are available via common statistical packages: modeling on the CSH and modeling on the subdistribution hazard.46 The CSH is the instantaneous rate of the event of interest in subjects free of any event and thus “specific” to the event of interest, that is, only those capable of having the event of interest remain in the risk set. The subdistribution hazard is the instantaneous rate of the event of interest in those who have not had the event of interest and includes those who may have had the competing risk. Therefore, SHR is a ratio in a nonexistent population and as such should not be interpreted as an HR. In simple terms, a subdistribution hazard > 1 means that the cumulative incidence of the event is higher in subjects with this variable at the start of the study than those without. Relating this to our study, the CSH for stroke describes the instantaneous rate of stroke in those who have not yet experienced stroke and are still alive, whereas the subdistribution hazard for stroke is the instantaneous rate of stroke in a group who have not yet experienced stroke and includes those who died prior to stroke. Presenting both methods provides the reader with complementary information; the CSH is better suited to describe the cause of disease, whereas the subdistribution hazard is useful in prognosis and treatment planning. Therefore, our data suggest that although AF associates with stroke in those on HD, its presence is more likely to predict death prior to stroke occurrence.
An important message to conclude this section is that the effect of a competing risk on survival analysis is greater if the competing risk is frequent. An absolute competing risk event of >10% merits serious consideration.45 Therefore, it is imperative that in renal epidemiology we consider death as a competing risk. The lack of clarity on the influence of AF on stroke risk is perhaps a little more understandable when the literature is reviewed.18-33 We found only 1 study from this century considering death as a competing risk (see Table 6).
Table 6.
Publications Examining Risk Factors for Stroke in Dialysis Patients Since 2000 Where Prior AF Was Included in the Analysis Variables.
| Author | Year | Country | Year of study | Study design | N | Stroke incidence | AF rate (%) | CRR | AF: stroke risk | AF: mortality |
|---|---|---|---|---|---|---|---|---|---|---|
| Wiesholzer | 2001 | Austria | 1975-1997 | R.cohort | 430 | 3.78 | 14.2 | No | No effect | + |
| Vazquez | 2003 | Spain | 1998-2002 | Uncertain | 173 | 10.5 | 13.6 | No | +a | + |
| Vazquez | 2006 | Spain | 1998-2004 | P.cohort | 164 | 15 | 12.2 | No | +a | No effectb |
| To | 2007 | Australia/New Zealand | 2003-2005 | R.cohort | 155 | 3.04 | 25.8 | No | No effectc | No effectc |
| Genovesi | 2008 | Italy | 2003-2006 | P.cohort | 476 | NA | 26.7 | No | No effect | + |
| Vazquez | 2009 | Spain | 2003-2007 | P.cohort | 256 | 1.35 | 12.1 | No | + | + |
| Wizemann | 2010 | DOPPSd | 1998-2000 | P.cohort | 17 513 | 3.4e | 12.5 | No | + | + |
| Sanches-Perales | 2010 | Spain | 1999-2005 | Uncertain | 449 | 2.41 | 7.3 | No | + | NA |
| Wetmore | 2013 | USA | 2000-2005 | R.cohort | 56 734 | 2.28 | 9.9 | No | + | NA |
| Findlay | 2015 | UK | 2007-2012 | R.cohort | 1382 | 4.15-5.01 | 21.2 | No | No effect | + |
| Shih | 2015 | Taiwan | 1998-2011 | R.cohort | 6772 | 3.35e | 8.7 | Yes | No effect | + |
| Toida | 2016 | Japan | 2009-2012 | P.cohort | 1551 | 2.15 | 10.2 | No | + | NA |
| Hasegawa | 2016 | Japan | 1999-2011 | P.cohort | 7002 | 6.3-6.6 | 5.7 | No | No effect | + |
| Airy | 2017 | USA | 2006-2011 | R. cohort | 85 377 | 3.55 | 14.3 | No | + | + |
| Mitsuma | 2018 | Japan | 2011-2015 | R.cohort | 380 | 2.05-3.63 | 14.5 | No | No effect | + |
| Abuhasira | 2018 | Israel | 2002-2015 | R.cohort | 1130 | 13.4-16.4 | 26.9 | No | No effect | + |
Note. Notably, despite 11 publications that noted a significant association between AF and death, only 1 study performed a competing risk analysis. Stroke incidence rates are presented as episodes per 100 patient-years. AF = atrial fibrillation; CRR = competing risk regression; R = retrospective; P = prospective; NA = not available, that is, not studied or presented; TIA = transient ischemic attack.
Not just stroke “thromboembolic event”—including stroke, TIA, and systemic embolism.
Despite not being statistically significant, the mortality risk estimate was greater in those with AF.
Neither reached statistical significance, acknowledging the study was underpowered.
Consists of countries from Europe, Australia/New Zealand, North America, and Japan.
In those with AF only. + denotes that presence of AF has a positive effect (increases) the variable in each column (i.e. stroke risk or mortality).
AF and Stroke in ESRD
Our findings demonstrate, as others have,18,19,22-24,27,28,32,33 that AF is significantly associated with death. However, the effect of AF on stroke remains less clear. In this study, the presence of AF is associated with all-cause mortality; however, in our subdistribution hazards model, the influence of AF on stroke incidence lacks significance, a finding more obvious in ischemic or first-ever strokes. As discussed, the subdistribution hazard is useful for prognosis and treatment planning, and thus the interpretation of this message is important. Presently, the only licensed oral anticoagulation in use in the UK HD population is the vitamin K antagonist, warfarin, although direct-acting oral anticoagulants are used widely in the United States.47 Anticoagulation carries a higher risk of bleeding in ESRD and vitamin K antagonism is also associated with vascular calcification and calciphylaxis. The inherently high CHA2DS2-VASc score in the HD population would prompt anticoagulation prescription in the majority. We lack prescription data in our study to adjust for prescription of anticoagulation and so can only speculate on what degree of prestroke death associated with AF may be attributable, directly or indirectly, to complications such as major bleeding. In addition, the undeniable association of AF and stroke in the general population highlights the current need for a trial of anticoagulation in HD patients, an issue highlighted in a recent National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) report.48 However, such a trial must be powered taking into account that the chosen intervention is likely to have a meaningful effect on ischemic stroke in ESRD patients with AF, but the observed benefit may be influenced by the high background mortality rate.
Limitations
We present data from large national data sets and provide insight into competing risk analysis and its influence on prior analyses of stroke risk. However, we must acknowledge the following limitations. First, our data are retrospective and as such can only be used to describe association and not causation. Furthermore, our data use coded diagnoses to identify stroke; however, both sources are validated by stroke physicians (SSCA) or audit (SMR01) confirming an approximately 90% accuracy rate. We do not have access to prescription data to assess the effect of warfarin on AF. Although these data may have provided further information, it should be noted that no nationally held data set contains information on international normalised ratio, thus limiting its usefulness. Finally, it must be acknowledged that the prevalence of AF in this cohort is at the lower end of the range demonstrated in previous studies (5.7%-26.9%) and thus may underpower the detection of an association. This is highlighted in Tables 3 to 5 where the wide reported CIs for AF following competing risk adjustment prevent one from completely excluding AF as a risk factor for stroke, albeit a risk far lower than that imposed by AF in the general population.
Conclusions
This study has confirmed the high incidence of stroke in HD patients, with a disturbingly high mortality rate and highlighted the difficulties with assessing factors associated with stroke. We have attempted to explain the concept of competing risks as perhaps one reason for the lack of consistent reports regarding AF and stroke risk. We recommend all researchers consider competing risk in future studies.
Footnotes
Ethics Approval and Consent to Participate: The data sets used in this manuscript work within the ‘NHS Code of Practice on Protecting Patient Confidentiality’, which incorporates the requirements of statute and common law including the Data Protection Act, the Human Rights Act and the Adults with Incapacity (Scotland) Act. Access and use of the data for the purpose of this study were approved following a NSS proportionate governance review by the Privacy Advisory Committee of ISD, NHS Scotland, Reference 55/14.
Consent for Publication: All authors consent to the publication of this study.
Availability of Data and Materials: The data and materials are not available for this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: M.F. was funded by a Kidney Research UK Training Fellowship and a grant from Darlinda’s Charity for Renal Research.
ORCID iDs: Mark Findlay  https://orcid.org/0000-0002-1030-0766
https://orcid.org/0000-0002-1030-0766
Manish M. Sood  https://orcid.org/0000-0002-9146-2344
https://orcid.org/0000-0002-9146-2344
References
- 1. Franklin SS, Wong ND. Hypertension and cardiovascular disease: contributions of the Framingham Heart Study. Glob Heart. 2013;8:49-57. [DOI] [PubMed] [Google Scholar]
- 2. Lewington S, Whitlock G, Clarke R, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370(9602):1829-1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 3. Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63(3):793-808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 4. Fellström BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395-1407. [DOI] [PubMed] [Google Scholar]
- 5. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181-2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238-248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 7. Herrington WG, Emberson J, Mihaylova B, et al. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol. 2016;4(10):829-839. doi: 10.1016/S2213-8587(16)30156-5. [DOI] [PubMed] [Google Scholar]
- 8. Seliger S, Gillen DL, Longstreth WT, Kestenbaum B, Stehman-Breen CO. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003;64:603-609. [DOI] [PubMed] [Google Scholar]
- 9. Wetmore JB, Phadnis MA, Ellerbeck EF, Shireman TI, Rigler SK, Mahnken JD. Relationship between stroke and mortality in dialysis patients. Clin J Am Soc Nephrol. 2015;10:80-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960-2967. [DOI] [PubMed] [Google Scholar]
- 11. Sarnak MJ, Tighiouart H, Scott TM, et al. Frequency of and risk factors for poor cognitive performance in hemodialysis patients. Neurology. 2013;80(5):471-480. doi: 10.1212/WNL.0b013e31827f0f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Findlay MD, Donaldson K, Doyle A, et al. Factors influencing withdrawal from dialysis: a national registry study. Nephrol Dial Transplant. 2016;31(12):2041-2048. doi: 10.1093/ndt/gfw074. [DOI] [PubMed] [Google Scholar]
- 13. Nochaiwong S, Ruengorn C, Awiphan R, Dandecha P, Noppakun K, Phrommintikul A. Efficacy and safety of warfarin in dialysis patients with atrial fibrillation: a systematic review and meta-analysis. Open Heart. 2016;3(1):e000441. doi: 10.1136/openhrt-2016-000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857-867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 15. Harel Z, Chertow GM, Shah PS, et al. Warfarin and the risk of stroke and bleeding in patients with atrial fibrillation receiving dialysis: a systematic review and meta-analysis. Can J Cardiol. 2017;33(6):737-746. doi: 10.1016/j.cjca.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 16. Han KH, O’Neill WC. Increased peripheral arterial calcification in patients receiving warfarin. J Am Heart Assoc. 2016;5(1):1-8. doi: 10.1161/JAHA.115.002665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nigwekar SU, Bloch DB, Nazarian RM, et al. Vitamin K–dependent carboxylation of matrix Gla protein influences the risk of calciphylaxis. J Am Soc Nephrol. 2017;28(6):1717-1722. doi: 10.1681/ASN.2016060651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wiesholzer M, Harm F, Tomasec G, Barbieri G, Putz D, Balcke P. Incidence of stroke among chronic hemodialysis patients with nonrheumatic atrial fibrillation. Am J Nephrol. 2001;21(1):35-39. doi: 10.1159/000046216. [DOI] [PubMed] [Google Scholar]
- 19. Vazquez E, Sanchez-Perales C, Lozano C, et al. Comparison of prognostic value of atrial fibrillation versus sinus rhythm in patients on long-term hemodialysis. Am J Cardiol. 2003;92(7):868-871. doi: 10.1016/s0002-9149(03)00904-4. [DOI] [PubMed] [Google Scholar]
- 20. Vázquez-Ruiz E, Sánchez-Perales C, Lozano-Cabezas C, et al. Incidence of atrial fibrillation in hemodialysis patients. A prospective long-term follow-up study. Rev Esp Cardiol. 2006;59:779-784. [PubMed] [Google Scholar]
- 21. To AC, Yehia M, Collins JF. Atrial fibrillation in haemodialysis patients: do the guidelines for anticoagulation apply. Nephrology (Carlton). 2007;12(5):441-447. doi: 10.1111/j.1440-1797.2007.00835.x. [DOI] [PubMed] [Google Scholar]
- 22. Genovesi S, Vincenti A, Rossi E, et al. Atrial fibrillation and morbidity and mortality in a cohort of long-term hemodialysis patients. Am J Kidney Dis. 2008;51(2):255-262. doi: 10.1053/j.ajkd.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 23. Vazquez E, Sanchez-Perales C, Garcia-Garcia F, et al. Atrial fibrillation in incident dialysis patients. Kidney Int. 2009;76:324-330. [DOI] [PubMed] [Google Scholar]
- 24. Wizemann V, Tong L, Satayathum S, et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int. 2010;77(12):1098-1106. doi: 10.1038/ki.2009.477. [DOI] [PubMed] [Google Scholar]
- 25. Sanchez-Perales C, Vazquez E, Garcia-Cortes MJ, et al. Ischaemic stroke in incident dialysis patients. Nephrol Dial Transplant. 2010;25(10):3343-3348. doi: 10.1093/ndt/gfq220. [DOI] [PubMed] [Google Scholar]
- 26. Wetmore JB, Ellerbeck EF, Mahnken JD, et al. Atrial fibrillation and risk of stroke in dialysis patients. Ann Epidemiol. 2013;23:112-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Findlay MD, Thomson PC, Fulton RL, et al. Risk factors of ischemic stroke and subsequent outcome in patients receiving hemodialysis. Stroke. 2015;46(9):2477-2481. doi: 10.1161/STROKEAHA.115.009095. [DOI] [PubMed] [Google Scholar]
- 28. Shih C-J, Ou S-M, Chao P-W, et al. Risks of death and stroke in patients undergoing hemodialysis with new-onset atrial fibrillation: a competing-risk analysis of a nationwide cohort. Circulation. 2016;133:265-272. doi: 10.1161/CIRCULATIONAHA.115.018294. [DOI] [PubMed] [Google Scholar]
- 29. Toida T, Sato Y, Nakagawa H, et al. Risk of cerebral infarction in Japanese hemodialysis patients: Miyazaki Dialysis Cohort Study (MID study). Kidney Blood Press Res. 2016;41(4):471-478. doi: 10.1159/000443448. [DOI] [PubMed] [Google Scholar]
- 30. Hasegawa J, Bieber B, Larkina M, et al. Cardiovascular and stroke risk in Japanese hemodialysis patients with atrial fibrillation. Ther Apher Dial. 2016;20(6):608-614. doi: 10.1111/1744-9987.12460. [DOI] [PubMed] [Google Scholar]
- 31. Airy M, Chang TI, Ding VY, et al. Risk profiles for acute health events after incident atrial fibrillation in patients with end-stage renal disease on hemodialysis. Nephrol Dial Transplant. 2017;33:1590-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mitsuma W, Matsubara T, Hatada K, et al. Atrial fibrillation had less impact on the risk of ischemic stroke in non-anticoagulated patients undergoing hemodialysis: insight from the RAKUEN study. Intern Med. 2018;57(16):2295-2300. doi: 10.2169/internalmedicine.0021-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abuhasira R, Mizrakli Y, Shimony A, Novack V, Shnaider A, Haviv YS. Atrial fibrillation characteristics in patients on haemodialysis vs. peritoneal dialysis. Sci Rep. 2018;8(1):2976. doi: 10.1038/s41598-018-21229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Information Divisions Scotland (ISD). The Scottish Renal Registry. http://www.srr.scot.nhs.uk/. Published March 10, 2017. Accessed September 11, 2019.
- 35. Information Divisions Scotland (ISD). Scottish Stroke Care Audit. http://www.strokeaudit.scot.nhs.uk/index.html. Published March 10, 2017. Accessed September 11, 2019.
- 36. Information Divisions Scotland (ISD). SMR Datasets—SMR01—General / Acute Inpatient and Day Case—ISD Scotland—Data Dictionary. http://www.ndc.scot.nhs.uk/Data-Dictionary/SMR-Datasets//SMR01-General-Acute-Inpatient-and-Day-Case/. Published March 10, 2017. Accessed September 11, 2019.
- 37. Information Services Division National Services Scotland. Assessment of SMR01 Data 2014–2015. https://www.isdscotland.org/Products-and-Services/Data-Quality/docs/Assessment-of-SMR01-Data-2014-15-report-180508.pdf. Accessed September 11, 2019.
- 38. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349. [DOI] [PubMed] [Google Scholar]
- 39. Seliger SL, Gillen D, Tirschwell D, Wasse H, Kestenbaum BR, Stehman-Breen CO. Risk factors for incident stroke among patients with end-stage renal disease. J Am Soc Nephrol. 2003;14:2623-2631. [DOI] [PubMed] [Google Scholar]
- 40. Sozio SM, Armstrong PA, Coresh J, et al. Cerebrovascular disease incidence, characteristics, and outcomes in patients initiating dialysis: the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) study. Am J Kidney Dis. 2009;54(3):468-477. doi: 10.1053/j.ajkd.2009.01.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Power A, Chan K, Singh SK, Taube D, Duncan N. Appraising stroke risk in maintenance hemodialysis patients: a large single-center cohort study. Am J Kidney Dis. 2012;59(2):249-257. doi: 10.1053/j.ajkd.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 42. Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20(10):2223-2233. doi: 10.1681/ASN.2009030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Herrington W, Haynes R, Staplin N, Emberson J, Baigent C, Landray M. Evidence for the prevention and treatment of stroke in dialysis patients. Semin Dial. 2014;28:35-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695-706. [DOI] [PubMed] [Google Scholar]
- 45. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601-609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stati Assoc. 1999;94:496-509. [Google Scholar]
- 47. Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138:1519-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bansal VK, Herzog CA, Sarnak MJ, et al. Oral anticoagulants to prevent stroke in nonvalvular atrial fibrillation in patients with CKD Stage 5D: an NKF-KDOQI controversies report. Am J Kidney Dis. 2017;70:859-868. [DOI] [PubMed] [Google Scholar]