Abstract
Background: Exercise has been shown to reduce adverse outcomes related to breast cancer. However, the rate of adherence to physical exercise is very low among breast cancer survivors (BCS). This study investigated the effects of high supervision ratio resistance training (RT), once a week for 8 weeks, on changes in body composition and muscular strength in BCS. Methods: Twenty-five female BCS undergoing hormone therapy were randomized into resistance training group (TG, n = 12) or control (CG, n = 13) group. The TG performed 8 weeks of supervised RT, with 1 trainer per volunteer, once a week. Body composition was evaluated by dual-energy X-ray absorptiometry, and muscle strength was evaluated by 10 repetition maximum (10 RM) for leg press (45°) and bench press exercises. A 1-way analysis of variance was used to compare within-group effects at pre- and post-intervention. An analysis of covariance test was used to compare post-intervention values, using pre-intervention measures as covariates. The effect size (ES) was calculated by Cohen’s d. Results: The TG improved muscle strength in 10 RM leg press (45°; Δ 33.75 ± 11.51 kg, P = .02; ES = 0.96) and bench press (Δ 4.08 ± 1.83 kg, P = .01; ES = 1.15). Adherence to training was more than 99%. Changes in body composition were not detected. There were no changes in the CG for any assessment. Conclusion: Once-weekly supervised RT could be an alternative to increase the adherence to exercise and improve muscular strength in BCS.
Keywords: strength training, breast cancer, exercise, muscle strength
Introduction
Breast cancer (BC) is the second most common cancer in the world and the most frequent in women.1 BC is the fifth cause of death among all types of cancer and the leading cause of cancer death in women in developing countries.2 Unfortunately, the high prevalence of a sedentary lifestyle in BC survivors (BCS) contributes to lower muscular strength and higher adiposity,3 further impairing health and might increase mortality rates.3
The presence of obesity in BCS may increase the risk of relapse and death.4 In addition, excessive body fat is associated with inflammatory status, increased androgens in estrogen aromatization, and oxidative stress,5 all of which are considered key elements for tumor growth and proliferation.6 Thus, control of body fat appears to be crucial for BCS. Furthermore, improved muscle fitness could help lower cancer-related death rates, because muscular strength was associated with lower cancer mortality.7 In this context, physical exercise has emerged as an effective tool due to its capability to increase muscular strength and lean mass in women diagnosed with BC.8
Studies involving resistance training (RT) in BCS have increased over the years. Authors of a systematic review identified a few chronic studies that utilized RT in BCS, reporting increased muscular strength after RT, with 2 or 3 sessions per week.9 This weekly frequency is recommended by the American College of Sports Medicine for RT in BCS10 and the elderly.11 However, only 24% of BCS adhere to these guidelines.12 Specifically, adherence to RT programs decreases when supervision is discontinued, going from 92% to 66%, for supervised and unsupervised training, respectively.13 Such a low adherence rate diminishes the impact of RT.14 It has been suggested that supervision ratio is an important element related to RT outcomes.15 Ramírez-Campillo et al16 compared the changes in muscular strength in untrained older women performing RT under different supervision ratios (high [1 coach per 1 volunteer] vs low supervision [1 coach per 10 volunteers]) and found that lower body strength gains were greater in participants training under higher supervision ratios.
Time commitment is often cited as a barrier to initiate an exercise program. To address this, reducing training frequency has been proposed as a way to increase exercise adherence.17 This strategy has been shown to elicit positive training adaptations in older individuals.18 An RT program of minimal sessions per week coupled with high supervision could increase participation and improve muscular strength. Therefore, the purpose of the present study was to investigate the effects of 1 supervised (1 trainer per trainee) RT session per week on body composition and muscular performance in BCS undergoing hormone therapy. We hypothesized that supervised RT once per week will provide similar muscle strength gains to multiple session per week over 8 weeks in BSC.
Methods
Experimental Approach to the Problem
This study was a randomized controlled clinical trial lasting 8 weeks. BCS women undergoing hormonal therapy (a convenience sample) were randomized to a resistance training group (TG) or control group (CG). The randomization was performed via a website (www.randomization.com) with one-to-one allocation. The BCS were contacted via phone calls and face-to-face interactions at the Mastology and Oncology Ambulatory of the University Hospital of the Federal University of Goiás. The TG patients participated in a highly supervised RT program (1:1 coach to patient ratio) for one 35-minute full-body session once per week for 8 weeks. The CG group did not perform any kind of structured exercise and were requested not to change their habitual physical activity habits. Better comprehension of the effects of the minimal dose of RT program on changes in muscle strength and body composition could be helpful for BCS during hormone therapy, and for health professionals and coaches in prescribing a resistance exercise program.
Subjects
Twenty-six female BCS participated in the study. The eligibility criteria were the following: confirmed BC stages I to III; ages 40 to 65 years; being in menopause, according to the World Health Organization guidelines19; not involved in any regular exercise program in the past 6 months; had completed cancer-related therapies including surgery, chemotherapy, and/or radiotherapy at least 6 months prior to enrolling; currently undergoing hormone therapy (tamoxifen or aromatase inhibitor); and received medical clearance for physical exercise. Patients were excluded from the study if they had neurological or musculoskeletal limitations that could compromise exercise performance and/or any uncontrolled chronic disease that could represent a risk to their health.
The study was approved by Research Ethics Committee of the Federal University of Goias (CAAE: 50717115.4.0000.5083) and by the Research Ethics Committee of the Clinical Hospital of the Federal University of Goias (CAAE: 50717115.4.3001.5078), and registered with the Registro Brasileiro de Ensaios Clínicos (ReBEC) as RBR-5bqfyt. All participants provided written consent.
Procedures
Experimental Design
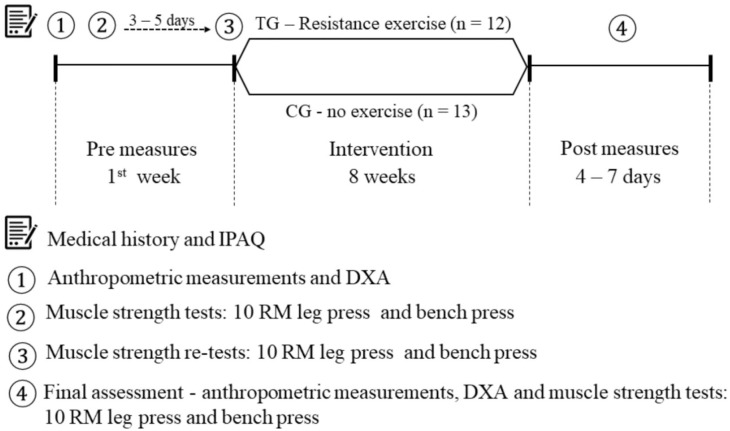
The first visit involved medical history, filling out the International Physical Activity Questionnaire,20 and anthropometric and body composition measurements. Visit 2 involved familiarization with muscular strength assessments. Visit 3 included the retest of the muscular strength assessments. Patients rested 3 to 5 days between visits 2 and 3. Retests were conducted in order to improve reliability of assessment and provide familiarization to reduce learning effects giving false improvement in strength. After these tests, participants from TG took part in the intervention for 8 weeks, while the CG remained with their usual activities. All patients in the TG were reassessed 4 to 7 days after the last training session. The procedures are further depicted in Figure 1.
Figure 1.
Experimental design the study. IPAQ, International Physical Activity Questionnaire; DXA, dual-energy X-ray absorptiometry; RM, repetition maximum; TG, training group; CG, control group.
Anthropometric and Body Composition Assessments
Body mass index (BMI) was calculated based on body mass and height (BMI = weight [kg]/height squared [m2]). Fat and lean mass were assessed using dual-energy X-ray absorptiometry (DXA; General Electric Healthcare Model, Madison, WI). Data were analyzed using GE Medical Systems Lunar software. A professional performed the assessments of DXA. DXA scans were performed in the morning. During the DXA, volunteers remained in a supine position with their lower limbs relaxed, and the upper limbs were positioned along the body with forearms pronated. The imaging device was calibrated and tested as recommended by the manufacturer. After analysis of the entire body area, the DXA provided information on total mass, lean mass, and fat mass.
Muscular Strength Assessments
Muscular strength was assessed using the 10 repetition maximum (10 RM) test on the leg press (45°) and bench press exercises. The participants had three to five 10 RM attempts for each exercise. The warm-up was one set of 10 repetitions with 50% of the estimated 10 RM load. In the attempts, if the participant performed 11 repetitions, the load was increased by 5% to 10%. The test load of 10 RM for each participant was determined so they could complete the 10th repetition and not be able to perform the 11th repetition. The rest interval between the attempts was 3 minutes. The cadence was not controlled, but participants were requested to control the eccentric phase and maximum speed to concentric movement. Leg press and bench press exercise techniques followed the recommendation of the National Strength and Conditioning Association.21 In both exercises, 2 experienced strength-training professionals supervised the assessments, initially with the leg press followed by the bench press. The retest was performed 3 to 5 days later using the maximum load achieved on the first day.22
The 10 RM tests presented values from moderate to excellent reliability according to the criteria of Koo and Li.23 The intraclass correlation coefficient (ICC) was 0.94 (95% confidence interval [CI] = 0.81-0.98); typical error of measurement 8.55 kg; coefficients of variation (CV) 7.28% (95% CI = 2.94-11.62) and 0.85% (95% CI = 0.52-0.94); typical error of measurement 1.82 kg; and CV 8.93% (95% CI = 3.76-14.10) for the 10 RM tests for leg press (45°) and bench press, respectively.
Resistance Training Protocol
The TG patients underwent a highly supervised RT program (1:1 coach to patient ratio), once per week for 8 weeks, while the CG group did not perform any kind of structured exercise. Experienced sports science professionals supervised the training. Two familiarization sessions were conducted in the week prior to the beginning of the exercise program. After the familiarization, the patients participated in 8 weeks of RT.
The RT program included traditional resistance exercises in the following order: leg press (45°), stiff-legged dead lift, barbell bench press, supinated lat pull down, and sit-ups. The participants were instructed to perform all exercises until volitional failure, with the exception of the stiff-legged dead lift and abdominal exercises. Each exercise was performed with 3 sets of 8 to 12 repetitions. The load obtained at the10 RM test was used for the leg press (45°) and the bench press. For the lat pull down, the load was determined to allow the patient to achieve 8 to 12 repetitions until volitional failure. Since the stiff-legged dead lift is an exercise that demands proper technique, a low load of 20% to 30% of body mass was chosen in order to complete 8 to 12 repetitions.
Exercise intensity was adjusted, if necessary, for each set to maintain the proposed number of repetitions. Recovery between sets was 2 minutes, as recommended by Vieira et al.24 Abdominal exercises were performed with 3 sets of 20 repetitions and 1 minute of rest between sets. The patients were instructed to control the eccentric phase of the movement for approximately 2 seconds and to perform rapid concentric muscular action for approximately 1 second. This cadence was not measured or performed with assistance of a metronome. The exercises were performed without transitions or pause between the movement cycles.
Before each session, a warmup was performed involving leg press and bench press (1 × 10 repetitions with 50% of 10 RM). The sports science professionals maintained a patient training diary containing all the information related to the exercises (sets, repetitions, and absolute load per exercise), as well as a section to report possible intercurrences throughout the exercise program. Each training session lasted approximately 35 minutes.
Statistical Analysis
Power calculations were performed using G*Power 3.1.9.2 software for F tests (analysis of covariance [ANCOVA]), comparing the difference between post-intervention means, with significance level set at .05 for the 2 groups, one covariate (baseline muscle strength). Calculations indicated that the intervention was sufficiently powered with a total sample of 25 subjects. For the 10 RM leg press, an effect size (ES) of 2.05 was determined, which resulted in a power test (1 − β) of > 0.9999. For the 10 RM bench press, an ES of 1.12 was determined, which resulted in a power test (1 − β) of 0.9996.
Descriptive statistics were calculated as mean and standard deviation (SD). The normality of the data was evaluated by the Shapiro-Wilk normality test. For qualitative data, Fisher’s exact test was utilized using the Monte Carlo procedure with 50 000 randomizations. Ordinal variables were evaluated by the Wilcoxon (rank sum) test. Independent sample t tests were used to compare characteristics of the sample for normally distributed data. For nonnormally distributed data, the Wilcoxon (rank sum) test was used. Baseline and post-intervention means were compared by 1-way analysis of variance. In cases of nonnormally distributed data, the Kruskal-Wallis test was used. ANCOVA was used to compare post-intervention values, using baseline values as covariates. Unadjusted means ± SDs are shown in the tables and text. The ICC was performed to verify the reliability of the 10 RM test-retest. The ICC form used was a 2-way mixed effects, mean of k measurements, and absolute agreement.
The ES was performed according to Cohen d,25 classified as small (0.20), medium (0.50), or large (>0.80) effect. Values below 0.20 were classified as trivial.
The level of significance for all data was set at P < .05. The mentioned analyses were performed using R (version 3.4.2) and SPSS (version 22).
Results
Participants
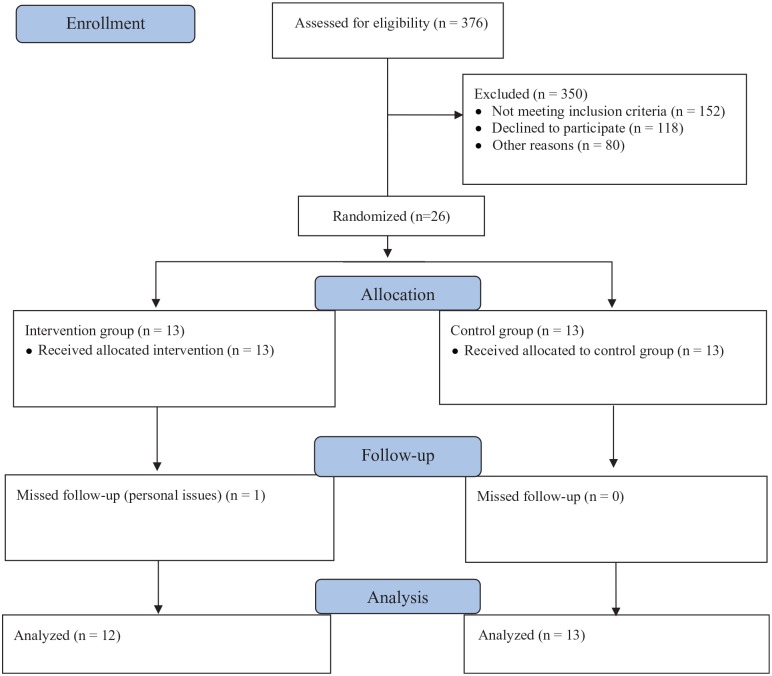
Three hundred seventy-six women were identified as potential volunteers for the study; however, 350 women did not qualify or refused to participate in the study. Most volunteers were not included due to difficulty regarding transportation, age range, or were not currently on hormone therapy. Thus, 26 women were randomized to either TG (n = 13) or CG (n = 13). A diagram of participation and follow-up of the study is provided in Figure 2.
Figure 2.
Participant flow throughout trial.
Baseline Characteristics
The baseline characteristics of patients are shown in Table 1. No differences were found between TG and CG, although in the initial screening of subjects, 3 TG volunteers reported shoulder pain in the ipsilateral limb to the surgery. After the intervention, these patients did not report an increase or worsening of this pain.
Table 1.
Baseline Characteristics of Study Population.
| Characteristics | CG (n = 13) | TG (n = 12) | P |
|---|---|---|---|
| Age (year), mean (SD) | 54.3(5.2) | 55.0 (5.8) | .76 |
| Education, n (%) | |||
| <8 years of the study | 7 (54) | 7 (58) | .85 |
| >8 years of the study | 6 (46) | 5 (42) | |
| Self-reported race, n (%) | |||
| Caucasian | 8 (62) | 7 (58.3) | 1.00 |
| Non Caucasian | 5 (38) | 5 (41.7) | |
| Occupation, n (%) | |||
| Teacher | — | 1 (8.3) | .05 |
| Homemaker or cleaner | 3 (23) | 3 (25) | |
| Unemployed | 8 (62) | 4 (33.3) | |
| Sales | 2 (15) | — | |
| Retired | — | 4 (33.3) | |
| Marital status, n (%) | |||
| Single | 4 (31) | 2 (16.7) | .85 |
| Married | 7 (54) | 7 (58.3) | |
| Divorced | 1 (8) | 1 (8.3) | |
| Widow | 1 (8) | 2 (16.7) | |
| Arterial hypertension, n (%) | 4 (31) | 3 (23) | 1.00 |
| Diabetes, n (%) | 2 (15) | 1 (8) | 1.00 |
| Months since cancer diagnosis, mean (SD) | 40 (13.3) | 43.6 (19.9) | .92 |
| Cancer stage, n (%) | |||
| I | 3 (23) | 5 (42) | .43 |
| II | 9 (69) | 6 (50) | |
| III | 1 (8) | 1 (8) | |
| Chemotherapy, n (%) | 12 (92) | 9 (75) | .32 |
| Adjuvant | 7 (54) | 4 (44.4) | .67 |
| Neoadjuvant | 5 (38) | 5 (55.6) | |
| Radiotherapy, n (%) | 11 (85) | 11 (92) | 1.00 |
| Hormone therapy, n (%) | 1.00 | ||
| Tamoxifen | 11 (85) | 11 (92) | 1.00 |
| Aromatase inhibitors | 2 (15) | 1 (8) | |
| Self-reported lymphedema, n (%) | 7 (54) | 3 (25) | .22 |
| Level physical activity (MET-h/week), mean (SD) | 30.7 (32.1) | 22.2 (23.4) | .65 |
Abbreviations: CG, control group; TG, resistance training group; MET, metabolic equivalent of task.
Body Composition
No differences were observed between groups before the training period (baseline). In addition, the training program did not change body composition in either group (Table 2). Although the ES was trivial (ES = −0.05), BMI was significantly different for CG versus TG after the training protocol (ANCOVA test, P = .03; Table 2).
Table 2.
Changes in Body Composition and Muscular Strength After 8 Weeks of RT.
| CG (n = 13), Mean ± SD |
TG (n = 12), Mean ± SD |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Baseline | Post | Δ | P a | Baseline | Post | Δ | P a | P b |
| Muscular strength | |||||||||
| Leg press 10 RM (kg)c | 83.08 ± 27.95 | 81.54 ± 30.64 | −1.54 ± 6.58 | .89 | 73.33 ± 33.05 | 107.08 ± 37.26 | 33.75 ± 11.51 | .02d | <.0001e |
| Bench press 10 RM (kg)c | 17.00 ± 4.55 | 17.38 ± 5.12 | 0.38 ± 1.61 | .84 | 16.33 ± 3.39 | 20.42 ± 3.70 | 4.08 ± 1.83 | .01d | <.0001e |
| Anthropometric and body composition | |||||||||
| Body mass (kg)f | 67.80 ± 9.45 | 67.38 ± 8.96 | −0.42 ± 1.74 | .95 | 67.06 ± 12.89 | 67.71 ± 12.96 | 0.65 ± 0.01 | .86 | .07 |
| BMIf | 26.78 ± 4.04 | 26.60 ± 3.74 | −0.18 ± 0.69 | .89 | 27.97 ± 5.01 | 28.25 ± 5.07 | 0.28 ± 0.38 | .79 | .03g |
| Body fat (%)c | 44.67 ± 6.05 | 44.46 ± 5.84 | −0.21 ± 1.82 | .92 | 46.38 ± 6.18 | 46.23 ± 5.67 | −0.15 ± 1.26 | .95 | .73 |
| Fat mass (kg)f | 29.58 ± 7.52 | 29.25 ± 7.24 | −0.34 ± 1.33 | .97 | 30.52 ± 9.14 | 30.67 ± 8.83 | 0.15 ± 0.82 | .81 | .23 |
| Lean mass (kg)c | 35.97 ± 4.06 | 35.87 ± 3.69 | −0.11 ± 1.69 | .94 | 34.24 ± 5.24 | 34.84 ± 5.52 | 0.50 ± 1.02 | .82 | .37 |
Abbreviations: RT, resistance training; CG, control group; SD, standard deviation; TG, resistance training group; Δ, posttest minus baseline; RM, maximal repetition; BMI, body mass index.
Comparison between baseline to posttest.
Refers to the analysis of covariance test, comparison between TG versus CG in post-intervention.
Refers to the analysis of variance test.
Significant main effect for time.
Significant main effect for TG versus CG.
Refers to the Kruskal-Wallis test in comparison between baseline to posttest.
Significant main effect for CG versus TG.
Muscular Strength
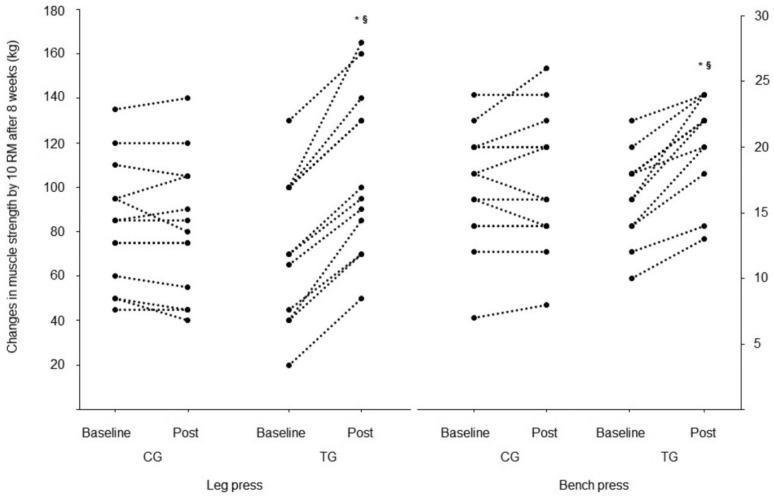
No differences were observed between groups at baseline. Muscular strength was significantly higher post-intervention in the TG for leg press (34 ± 13%, P < .02) and bench press (20 ± 8%, P < .01), compared with pretraining. A large ES was observed for muscle strength in the TG (leg press 10 RM = 0.96, bench press 10 RM = 1.15). There were no changes (P > .05) in the CG for leg press or bench press (−4 ± 10% and 2 ± 9%, respectively). The ANCOVA analysis revealed higher 10 RM scores for the TG compared with the CG (P < .0001) for both the leg press and bench press (Table 2 and Figure 3).
Figure 3.
Changes in muscular strength by 10 RM. CG, control group; TG, resistance training group. *Significant main effect from baseline. §Significant main effect for TG versus CG.
Adherence and Adverse Events
Adherence to the training was 99.04%. There was only one session missed by 1 volunteer. Lymphedema symptoms of upper limb pain was not reported by any of the patients. One patient had urinary incontinence twice during the training session and at the final 10 RM test at leg press (45°).
Discussion
The aim of this study was to investigate the effects of one supervised RT session per week on body composition and muscular performance in BCS compared with controls, as this RT frequency had not been investigated in BCS. Confirming our hypothesis, the supervised RT once per week significantly improved muscular strength in BCS. However, body composition did not change over the 8 weeks of supervised RT once per week. To our knowledge, this study is the first to demonstrate that 8 sessions of supervised RT, using a one-on-one approach, performed once per week might be useful to increase the muscle strength and to present excellent attendance in BCS.
BCS studies that investigated RT reported muscular strength gains for lower body ranging from a 17% to 39% increase.13,26-28 In studies with concurrent and combined training, ranging from 8 to 24 weeks, gains of 32% to 50% were reported for muscular strength in the lower limbs.29-31 In the present study, a 58% increase was observed, which was higher than previous studies. The gains for upper body strength (26%) were similar than those reported in the BCS literature (23% to 39%).13,27,28 However, it is noteworthy that participants in the present study took part in only one session per week, totaling 8 RT sessions. This is in contrast to previous studies where greater training frequencies were utilized (2-3 times per week). Our study intervention lasted 8 weeks compared with 12 weeks,28 16 weeks,26 6 months,13 and 12 months,13,27 for other studies in BCS. Therefore, our results showed that 8 sessions of RT with high supervision approach (1:1) increased strength in a similar amount to multiple sessions.
One possible explanation for the muscular strength results found in this study could be due to the strict control of the protocol. Previous studies in different populations suggest that a high supervision rate might be important to guarantee high degrees of effort put forth during RT.15,32 Schmitz et al13 used a training protocol consisting of 3 sets of 10 repetitions. For upper limbs, the load was progressive and started with 0.25 kg, and for lower limbs, load 10 maximal repetitions were used, with a small group supervision ratio (1 professional for 4 patients) during the first 13 weeks. The duration of the session was approximately 60 minutes, 2 times a week for 6 months or 12 months.13 Schmitz et al33 used a training protocol consisting of 3 sets of 10 repetitions with small group supervision for 13 weeks. The duration of the session was 90 minutes performed twice a week for 12 months. Hagstrom et al26 performed 2 to 3 sets of 8 to 10 repetitions with 8 RM load and small group supervision (1:1-5). Training was performed 3 times a week for 17 weeks, and the session lasted approximately 60 minutes. Madzima et al28 used the superset design: they performed 3 sets of 10 repetitions at 65% 1 RM, the last set performed to fatigue. The similarity of our results for muscle strength could be explained by high supervision, because a high supervision rate (1:1) in RT may help promote better neuromuscular adaptations. Our findings are unique because our volume, training frequency, and duration of the sessions (~35 minutes) were significantly lower than the previous studies mentioned in BCS.13,27 This raises questions about the real need of greater weekly volume and frequency for muscle strength gains in BCS, especially if we consider that low time commitment might increase exercise adherence. However, this interesting approach, 1 RT session per week, needs to be investigated in the long term.
Body composition (lean and fat body mass) did not change for any group, as previously reported in BCS studies with 2 and 3 weekly sessions.26,27,33 Only 2 studies in BCS found a significant increase in lean body mass (+0.88 kg).13,28 The lack of change in fat and lean body mass might be explained by the absence of diet control, although RT alone might be not able to change the resting metabolic rate in postmenopausal BCS.34 In addition, RT performed once a week may not generate a significant change in daily energy expenditure. Another factor may be the use of DXA as the only method of assessing body composition.
DXA is a reliable and valid method for assessing total body composition. However, it may be limited to local measures of comparison between lean and fat tissues due to its inability to differentiate types of fat (visceral, subcutaneous, and intramuscular) and lean soft tissue (muscles and organs).35 Previous studies found significant increases in vastus lateralis muscle thickness, as measured by ultrasound and computed tomography, without changes in body composition, as assessed by DXA, in older individuals36 and postmenopausal BCS.34 Scanlon et al36 found improvements in muscle quality, increased muscular strength relative to cross-sectional area of the thigh, and muscle quality determined by ultrasound echo intensity, without observing changes in body composition when assessed by DXA. Similarly, Serra et al34 also did not find changes in body composition after RT was performed 3 times per week for 16 weeks as assessed by DXA, but they found a significant change in muscular area at the mid-thigh (10%) tested by computed tomography in BCS. More studies are needed to explore these changes in muscle quality in the oncological population.
The limitations of this study were the lack of nutritional control to analyze the absence of change in body composition, and lack of applying different muscle strength tests such as isometric or functional tests to assess muscle performance in a nonspecific task. These factors could explain the lack of changes in body fat mass and muscle strength gain in different tasks that were not part of training. Future studies with RT in BCS during hormone therapy need to focus on nutritional control and to compare different RT frequency with dynamic and isometric muscle strength tests on changes in body composition and muscle strength.
The present study demonstrated that a once-weekly RT session with a high supervision ratio produced high adherence and promoted gains in muscular strength in BCS. This is important for health professionals as it might help with expanding the number of BCS who receive exercise interventions in a time efficient manner to assist in survival.
Acknowledgments
We would like to thank the patients and professional staff for their effort and commitment to the research project.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: All experimental procedures were approved by the Research Ethics Committee of the Federal University of Goias (CAAE: 50717115.4.0000.5083) and by the Research Ethics Committee of the Clinical Hospital of the Federal University of Goias (CAAE: 50717115.4.3001.5078). Clinical Trial Registration: Registro Brasileiro de Ensaios Clínicos (RBR-5bqfyt).
ORCID iDs: Wanderson Divino Nilo dos Santos  https://orcid.org/0000-0002-4378-1041
https://orcid.org/0000-0002-4378-1041
Paulo Gentil  https://orcid.org/0000-0003-2459-4977
https://orcid.org/0000-0003-2459-4977
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Stewart BW, Wild CP. World Cancer Report 2014. Lyon, France: International Agency for Research on Cancer; 2014. [Google Scholar]
- 3. Kirkham AA, Bland KA, Sayyari S, Campbell KL, Davis MK. Clinically relevant physical benefits of exercise interventions in breast cancer survivors. Curr Oncol Rep. 2016;18:12. doi: 10.1007/s11912-015-0496-3 [DOI] [PubMed] [Google Scholar]
- 4. Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627-635. doi: 10.1007/s10549-010-0990-0 [DOI] [PubMed] [Google Scholar]
- 5. Gérard C, Brown KA. Obesity and breast cancer—role of estrogens and the molecular underpinnings of aromatase regulation in breast adipose tissue. Mol Cell Endocrinol. 2018;466:15-30. doi: 10.1016/j.mce.2017.09.014 [DOI] [PubMed] [Google Scholar]
- 6. Blücher C, Stadler SC. Obesity and breast cancer: current insights on the role of fatty acids and lipid metabolism in promoting breast cancer growth and progression. Front Endocrinol (Lausanne). 2017;8:293. doi: 10.3389/fendo.2017.00293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruiz JR, Sui X, Lobelo F, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ. 2008;337:a439. doi: 10.1136/bmj.a439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Battaglini CL, Mills RC, Phillips BL, et al. Twenty-five years of research on the effects of exercise training in breast cancer survivors: a systematic review of the literature. World J Clin Oncol. 2014;5:177-190. doi: 10.5306/wjco.v5.i2.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dos Santos WDN, Gentil P, de Moraes RF, et al. Chronic effects of resistance training in breast cancer survivors. Biomed Res Int. 2017;2017:8367803. doi: 10.1155/2017/8367803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmitz KH. American College of Sports Medicine Roundtable on exercise guidelines for cancer survivors: corrigendum. Med Sci Sports Exerc. 2011;43:195. doi: 10.1249/MSS.0b013e318202a02c [DOI] [PubMed] [Google Scholar]
- 11. American College of Sports Medicine; Chodzko-Zajko WJ, Proctor DN, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510-1530. doi: 10.1249/MSS.0b013e3181a0c95c [DOI] [PubMed] [Google Scholar]
- 12. Ottenbacher A, Yu M, Moser RP, Phillips SM, Alfano C, Perna FM. Population estimates of meeting strength training and aerobic guidelines, by gender and cancer survivorship status: findings from the Health Information National Trends Survey (HINTS). J Phys Act Health. 2015;12:675-679. doi: 10.1123/jpah.2014-0003 [DOI] [PubMed] [Google Scholar]
- 13. Schmitz KH, Ahmed RL, Hannan PJ, Yee D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev. 2005;14:1672-1680. doi: 10.1158/1055-9965.EPI-04-0736 [DOI] [PubMed] [Google Scholar]
- 14. Gentil P, Bottaro M. Effects of training attendance on muscle strength of young men after 11 weeks of resistance training. Asian J Sports Med. 2013;4:101-106. doi: 10.5812/asjsm.34489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gentil P, Bottaro M. Influence of supervision ratio on muscle adaptations to resistance training in nontrained subjects. J Strength Cond Res. 2010;24:639-643. doi: 10.1519/JSC.0b013e3181ad3373 [DOI] [PubMed] [Google Scholar]
- 16. Ramírez-Campillo R, Martínez C, de La Fuente CI, et al. High-speed resistance training in older women: the role of supervision. J Aging Phys Act. 2017;25:1-9. doi: 10.1123/japa.2015-0122 [DOI] [PubMed] [Google Scholar]
- 17. Gentil P, Fischer B, Martorelli AS, Lima RM, Bottaro M. Effects of equal-volume resistance training performed one or two times a week in upper body muscle size and strength of untrained young men. J Sports Med Phys Fitness. 2015;55:144-149. [PubMed] [Google Scholar]
- 18. Barbalho MDSM, Gentil P, Izquierdo M, Fisher J, Steele J, Raiol RDA. There are no no-responders to low or high resistance training volumes among older women. Exp Gerontol. 2017;99:18-26. doi: 10.1016/j.exger.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization. Research on the Menopause in the 1990s: Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1996. doi: 10.1016/0378-5122(95)00967-1 [DOI] [PubMed] [Google Scholar]
- 20. Matsudo S, Araújo T, Matsudo V, et al. International Physical Activity Questionnaire (IPAQ): study of validity and reliability in Brazil. Rev Bras Atividade Física Saúde. 2001;6(2):5-18. doi: 10.12820/RBAFS.V.6N2P5-18 [DOI] [Google Scholar]
- 21. National Strength and Conditioning Association. Exercise Technique Manual for Resistance Training. 3rd ed. Champaign, IL: Human Kinetics; 2016. [Google Scholar]
- 22. Heyward VH, Gibson AL. Advanced Fitness Assessment and Exercise Prescription. 7th ed. Champaign, IL: Human Kinetics; 2014. [Google Scholar]
- 23. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155-163. doi: 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vieira CA, Battaglini CL, Ferreira-Junior JB, et al. Effects of rest interval on strength recovery in breast cancer survivors. Int J Sports Med. 2015;36:573-578. doi: 10.1055/s-0034-1398579 [DOI] [PubMed] [Google Scholar]
- 25. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 1st ed New York, NY: Academic Press; 1977. [Google Scholar]
- 26. Hagstrom AD, Marshall PWM, Lonsdale C, Cheema BS, Singh MAF, Green S. Resistance training improves fatigue and quality of life in previously sedentary breast cancer survivors: a randomised controlled trial. Eur J Cancer Care (Engl). 2015;25:784-794. doi: 10.1111/ecc.12422 [DOI] [PubMed] [Google Scholar]
- 27. Schmitz KH, Ahmed RL, Troxel A, et al. Weight lifting in women with breast-cancer–related lymphedema. N Engl J Med. 2009;361:664-673. doi: 10.1056/NEJMoa0810118 [DOI] [PubMed] [Google Scholar]
- 28. Madzima TA, Ormsbee MJ, Schleicher EA, Moffatt RJ, Panton LB. Effects of resistance training and protein supplementation in breast cancer survivors. Med Sci Sport Exerc. 2017;49:1283-1292. doi: 10.1249/MSS.0000000000001250 [DOI] [PubMed] [Google Scholar]
- 29. De Luca V, Minganti C, Borrione P, et al. Effects of concurrent aerobic and strength training on breast cancer survivors: a pilot study. Public Health. 2016;136:126-132. doi: 10.1016/j.puhe.2016.03.028 [DOI] [PubMed] [Google Scholar]
- 30. Cheema BS, Gaul CA. Full-body exercise training improves fitness and quality of life in survivors of breast cancer. J Strength Cond Res. 2006;20:14-21. [DOI] [PubMed] [Google Scholar]
- 31. Herrero F, Juan AFS, Fleck SJ, et al. Combined aerobic and resistance training in breast cancer survivors: a randomized, controlled pilot trial. Int J Sports Med. 2006;27:573-580. doi: 10.1055/s-2005-865848 [DOI] [PubMed] [Google Scholar]
- 32. Steele J, Raubold K, Kemmler W, Fisher J, Gentil P, Giessing J. The effects of 6 months of progressive high effort resistance training methods upon strength, body composition, function, and wellbeing of elderly adults. Biomed Res Int. 2017;2017:2541090. doi: 10.1155/2017/2541090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmitz KH, Ahmed RL, Troxel AB, et al. Weight lifting for women at risk for breast cancer-related lymphedema: a randomized trial. JAMA. 2010;304:2699-2705. doi: 10.1001/jama.2010.1837 [DOI] [PubMed] [Google Scholar]
- 34. Serra MC, Ryan AS, Ortmeyer HK, Addison O, Goldberg AP. Resistance training reduces inflammation and fatigue and improves physical function in older breast cancer survivors. Menopause. 2018;25:211-216. doi: 10.1097/GME.0000000000000969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prado CMM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr. 2014;38:940-953. doi: 10.1177/0148607114550189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scanlon TC, Fragala MS, Stout JR, et al. Muscle architecture and strength: adaptations to short-term resistance training in older adults. Muscle Nerve. 2014;49:584-592. doi: 10.1002/mus.23969 [DOI] [PubMed] [Google Scholar]