Abstract
Background:
Hyponatremia in cancer patients is often caused by the syndrome of inappropriate antidiuretic hormone secretion (SIADH). The aim of this observational multicenter study was to analyze the medical and economic implications of SIADH in this setting.
Methods:
This study included 90 oncological patients from 28 Italian institutions that developed SIADH between January 2010 and September 2015. Data on clinical–pathological characteristics, anticancer therapies, hyponatremia, and related treatments were statistically analyzed.
Results:
The majority were lung cancer patients (73%) with metastatic disease at the onset of hyponatremia (83%). A total of 76 patients (84%) were hospitalized because of SIADH and less than half (41%) received tolvaptan for SIADH treatment. The duration of hospitalization was significantly longer in patients who did not receive tolvaptan and in those who do not reach sodium normalization during hospitalization. Patients who experienced a second episode of hyponatremia following tolvaptan dose modification/discontinuation presented a significantly lower serum sodium value at the time of hospitalization and minimum sodium value during hospitalization compared with patients who had not experienced another episode. The severity of hyponatremia, defined as minimum sodium value during hospitalization with a cut-off value of 110 mmol/l, and not obtaining sodium correction during hospitalization significantly correlated with overall survival rate.
Conclusions:
Hyponatremia due to SIADH could result in longer hospitalization and in a decreased overall survival when not adequately treated, and tolvaptan represents an effective treatment with a potential effect of both improving overall survival and decreasing duration of hospitalization.
Keywords: hyponatremia, prognosis, SIADH, study, tolvaptan
Introduction
Hyponatremia is defined as a serum sodium level lower than 135 mmol/l and it can be classified according to the severity (mild 130–134 mmol/l, moderate 125–129 mmol/l, severe <125 mmol/l) or the development (acute, decrease within 48 h, or chronic, decrease in more than 48 h) of the disease.1
Although there are differences in serum sodium cut-off points and in clinical settings, hyponatremia represents the most common electrolyte disorder in cancer patients,2,3 particularly in those who are hospitalized with a frequency up to 40%.4
The syndrome of inappropriate antidiuretic hormone secretion (SIADH) represents the main cause of hyponatremia in cancer patients. Other causes include heart failure, nephritic syndrome, extracellular volume depletion, pulmonary disorders, central nervous system disturbances (including stroke and hemorrhage,) and drugs including tricyclic antidepressants, selective serotonin reuptake inhibitors, opioids, chemotherapeutic agents (especially platinum-based regimens), and, more recently, targeted agents and immunotherapy.3,5–7
SIADH may result from many different disease processes that disrupt the normal mechanisms that regulate arginine vasopressin (AVP) secretion. In cancer patients, this can be due to the ectopic release of antidiuretic hormone or to secondary AVP elevations as in paraneoplastic syndrome.8 The diagnostic criteria for SIADH were originally defined by Bartter and Schwartz in 1967 (Table 1) and they remain unchanged today.9–11
Table 1.
Diagnostic criteria for SIADH.
| Criteria for diagnosing SIADH |
|---|
| Essential criteria |
| • Clinical euvolemia, as defined by the absence of signs of
hypovolemia (orthostasis, tachycardia, decreased skin turgor,
dry mucous membranes) or hypervolemia (subcutaneous edema,
ascites) • Decreased effective osmolality of the extracellular fluid (Posm <275 mOsm/kg H2O) • Inappropriate urinary concentration (Uosm >100 mOsm/kg H2O with normal renal function) at some level of plasma hypo-osmolality • Elevated urinary sodium excretion (>20–30 mmol/l) while on normal salt and water intake • Absence of other potential causes of euvolemic hypoosmolality: hypothyroidism, hypocortisolism (Addison’s disease or secondary adrenal insufficiency) and diuretic use |
| Supplementary criteria |
| • Abnormal water load test (inability to excrete at least 90% of
a 20 ml/kg water load in 4 h and/or failure to dilute Uosm to
<100 mOsm/kg H2O) • Plasma AVP level inappropriately elevated relative to plasma osmolality • No significant correction of serum [Na+] with volume expansion but improvement after fluid restriction |
AVP, arginine vasopressin; SIADH, syndrome of inappropriate antidiuretic hormone secretion
The management of hyponatremia secondary to SIADH is dependent on the presence of related symptoms, the severity, and the duration of hyponatremia taking into account that incorrect management could adversely affect the patient’s outcome.1 When possible, correction of the underlying cause represents the most suitable therapeutic choice. Therapeutic options for the treatment of hyponatremia secondary to SIADH in cancer patients are the same as for other causes of SIADH including fluid restriction, sodium administration, or the use of selective vasopressin receptor antagonists. Other drugs such as urea and demeclocycline can be used for the treatment of euvolemic hyponatremia due to SIADH. However, the use of these drugs is limited owing to constraints on their availability in different countries and their tolerability.12,13
In the management of hyponatremia, it is important to monitor the rate of correction because a rapid increase in sodium levels could cause the development of osmotic demyelination, an irreversible condition that can lead to death. Therefore, the correction rate should not exceed 10 mmol/l/24 h with a goal of 6–8 mmol/l/24 h and serum sodium levels have to be monitored particularly in the first 24 h.12
Hyponatremia represents an indicator of poor prognosis in malignancies and increases mortality14,15 and morbidity leading to increased length of hospital stay with a considerable effect on costs.4,16–18
In the literature, a limited amount of data are available on SIADH in cancer patients. This observational multicenter study aimed to analyze treatments, outcomes, and the medical and economic implications of SIADH in a real-life setting of cancer patients reflecting a national perspective.
Patients and methods
Study population and data collection
The study population included adult patients with a histologically or cytologically confirmed diagnosis of solid tumors from 28 Italian institutions that have developed SIADH between January 2010 and September 2015. SIADH was confirmed by evaluating volemic status, the measurement of serum sodium concentration and serum osmolality, urine sodium concentration and urine osmolality, thyroid function tests, and serum cortisol. Hypovolemic and hypervolemic hyponatremia were excluded as well as hyponatremia due to other endocrine causes including adrenal insufficiency based on adrenal gland metastasis or hypothyroidism. The diagnosis of SIADH was based on the Bartter criteria that were all required for inclusion in this study.9
Retrospective, anonymized data on clinical–pathological characteristics, anticancer treatments, hyponatremia, and related signs/symptoms, SIADH treatments and their efficacy/toxicity were accessed and statistically analyzed. Data about hospitalization (length of stay and costs) were recorded for the patients who were hospitalized. According to Italian law (resolution 1 March 2012, Gazzetta Ufficiale n.72 of 26 March 2012), ethics approval and informed consent were not required for this study owing to its retrospective nature, the use of anonymous data, and the fact that it was not associated with any change in patient’s management. All patients gave their written informed consent to all the diagnostic–therapeutic procedures. This study has been realized with University funding.
Statistical analysis
Clinical data were collected from medical chart reviews and electronic records. Overall survival (OS) was defined as the interval between the date of SIADH diagnosis to the last follow up or death, irrespective of cause. For hospitalized patients, the authors also estimated OS from the date of hospital admission. Survival distribution was estimated by the Kaplan–Meyer method with Rothman’s 95% confidence intervals and compared across the groups using the log-rank test.
Cox proportional hazards models were applied to investigate the patient characteristics predictors of survival in univariate and multivariable analysis. Variables not fitting at univariate analysis were excluded from the multivariate model. The nonmulticollinearity of the grouped covariates was also analyzed. The variables were investigated using visual (histogram and probability plots) methods to determine whether they were normally distributed. Descriptive analyses were presented using the mean for normally distributed variables, but median, minimum, and maximum were used for those that were nonnormally distributed. The paired Student t test was used for normally distributed related variables. The Wilcoxon test was used for the related nonnormally distributed variables. The significance level in the univariate model for inclusion in the multivariate final model was more freely set at a 0.2 level. The likelihood ratio test was conducted to evaluate the improvement in prediction performance gained by backward elimination of variables from the prognostic model. All other significance levels were set at a 0.05 value and all p values were two-sided. Statistical analyses were performed using MedCalc version 11.4.4.0 (MedCalc Software, Mariakerke, Belgium).
Economic analysis
The cost of hospitalization was calculated by summing the direct medical costs (including all the interventions adopted to prevent, diagnose and treat a specific pathology and relative to goods and services moved because of the pathology but not directly connected to its medical management), indirect costs (including the effect that the pathology has on the social sphere of the patient, for example, loss of working days by the patient or by their caregiver) and common business costs (including the repercussions that direct costs generate on healthcare system, other welfare institutions, companies and organizations in which the patient and their caregivers operate, and the community as a whole). Quantification of the estimated daily costs considered the mean of daily direct medical costs, daily indirect costs and daily common business folded in each Institution included.19 Costs were obtained by multiplying the estimated daily cost with the number of days of hospitalization for every patient at any specific hospital. This could potentially introduce weighting errors but making an estimate was unavoidable because the specific costs for every recovery were not available and there was variability between the centers involved.
Results
A total of 90 cancer patients who experienced SIADH between January 2010 and September 2015 in 28 Italian Cancer Centers were included in the study.
The patient characteristics are summarized in Table 2. The male/female ratio was 58/32 (64/36%) and median age at diagnosis was 67 years (range 30–83 years). Histotypes and primary tumor sites were numerous (as reported in Table 2) representing a number of small subgroups with a low number of samples, and, for this reason, not easily comparable. In addition, this study included a high number of lung cancer patients (73%) and patients with metastatic disease at the onset of hyponatremia (83%).
Table 2.
Patient’s demographics and clinical–pathological characteristics.
| Characteristics |
All patients
(n = 90) |
Tolvaptan
(n = 37) |
Other therapies
(n = 53) |
|---|---|---|---|
| Number of patients (n) (%) | |||
| Gender | |||
| Male | 58 (64) | 22 (59) | 36 (68) |
| Female | 32 (36) | 15 (41) | 17 (32) |
| Age | |||
| Median | 67 | 67 | 67 |
| Range | 30–83 | 49–80 | 30–83 |
| Cigarette smoking | |||
| Smokers | 36 (40) | 15 (40) | 21 (40) |
| Exsmokers | 25 (28) | 14 (38) | 11 (21) |
| Never smoked | 21 (23) | 7 (19) | 14 (2615) |
| ND | 8 (9) | 1 (3) | 7 (13) |
| Comorbidity | |||
| No | 15 (17) | 7 (19) | 8 (15) |
| Yes | 75 (83) | 30 (81) | 45 (85) |
| • Hypertension | 38 (42) | 11 (32) | 27 (51) |
| • Heart disease | 17 (19) | 9 (24) | 9 (17) |
| • COPD | 15 (17) | 6 (16) | 9 (17) |
| • Cerebrovascular disease | 4 (4) | 2 (5) | 2 (4) |
| • Diabetes mellitus | 11 (12) | 4 (11) | 7 (13) |
| • Liver disease | 2 (2) | 1 (3) | 1 (2) |
| • Clinical depression | 8 (9) | 3 (8) | 5 (9) |
| Primary tumor location | |||
| Lung | 66 (73) | 28 (74) | 38 (72) |
| Gastrointestinal tract | 8 (9) | 3 (8) | 5 (9) |
| Head and neck | 7 (8) | 1 (3) | 6 (11) |
| Ovary | 2 (2) | 1 (3) | 1 (2) |
| Liver or bile ducts | 1 (1) | 1 (3) | 0 |
| Prostate | 1(1) | 1 (3) | 0 |
| Kidney | 1(1) | 1 (3) | 0 |
| Breast | 1 (1) | 0 | 1 (2) |
| Skin | 1 (1) | 0 | 1 (2) |
| Uterus | 1 (1) | 0 | 1(2) |
| Other | 1 (1) | 1 (3) | 0 |
| Primary tumor histotype | |||
| Small cell carcinoma | 49 (54) | 20 (53) | 29 (54) |
| Adenocarcinoma | 19 (21) | 8 (22) | 11 (21) |
| Squamous carcinoma | 7 (8) | 3 (8) | 4 (7) |
| Sarcoma | 2 (2) | 0 | 2 (4) |
| NET | 2 (2) | 1 (3) | 1 (2) |
| Adenosquamous carcinoma | 1 (1) | 1 (3) | 0 |
| Serous carcinoma | 1 (1) | 1 (3) | 0 |
| Hepatocarcinoma | 1 (1) | 1 (3) | 0 |
| Ductal carcinoma | 1 (1) | 0 | 1 (2) |
| Cholangiocarcinoma | 1 (1) | 0 | 1 (2) |
| Melanoma | 1 (1) | 0 | 1 (2) |
| Other | 5 (6) | 2 (5) | 3 (6) |
| Stage at diagnosis | |||
| Stage I | 1 (1) | 0 | 1 (2) |
| Stage II | 3 (3) | 1 (3) | 2 (4) |
| Stage III | 15 (17) | 8 (22) | 7 (13) |
| Stage IV | 71 (79) | 28 (75) | 43 (81) |
| Stage at the onset of hyponatremia | |||
| Stage I | 0 | 0 | 0 |
| Stage II | 0 | 0 | 0 |
| Stage III | 15 (17) | 8 (22) | 7 (13) |
| Stage IV | 75 (83) | 30 (78) | 45 (87) |
| • Liver metastasis | 28 (31) | 13 (35) | 15 (28) |
| • Lung metastasis | 27 (30) | 10 (27) | 17 (32) |
| • Bone metastasis | 27 (30) | 13 (35) | 14 (26) |
| • Pleural metastasis | 16 (18) | 4 (11) | 12 (23) |
| • Brain metastasis | 18 (20) | 8 (22) | 10 (19) |
| • Adrenal gland metastasis | 16 (18) | 6 (16) | 10 (19) |
| Hospitalization for hyponatremia | |||
| No | 14 (16) | 0 (0) | 14 (26) |
| Yes | 76 (84) | 37 (100) | 39 (74) |
COPD, chronic obstructive pulmonary disease; ND, not determined; NET, neuroendocrine tumor.
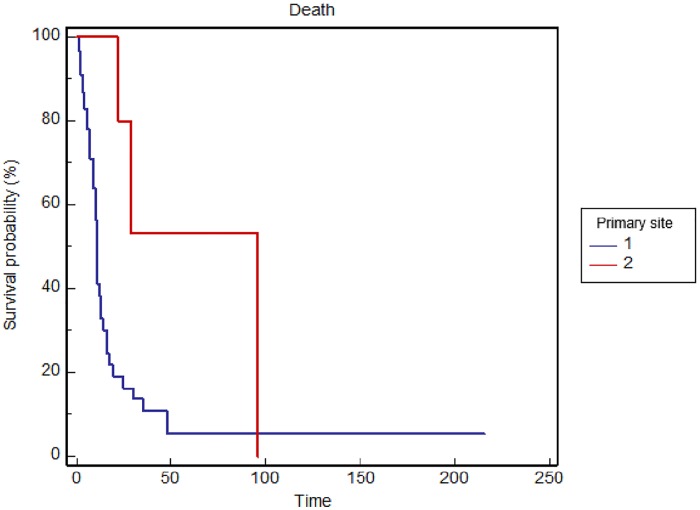
The site of the primary tumor (p = 0.0044) is significantly correlated with OS with shorter survival for lung neoplasms (Figure 1). Stratified by gender, median OS was 11 months in males and 24 months in females (p = 0.0016). Regarding stage at diagnosis, median OS was significantly lower in metastatic patients (11 versus 30 months, p = 0.0181). Therefore, univariate analysis demonstrated that with the lung as the primary site, male gender and tumor stage IV at diagnosis were significantly associated with worse OS, while comorbidities (p = 0.4643), liver (p = 0.5698), lung (p = 0.1209), or bone (p = 0.1891) metastases, and number of treatment lines before hyponatremia (p = 0.0808) did not reach statistical significance. A multivariate analysis, primary site (p = 0.0254) and gender (p = 0.0131) were predictors of OS, while a trend was observed in favor of the nonmetastatic stage at diagnosis (p = 0.0703).
Figure 1.
Overall survival stratified by primary tumor location (1 = lung versus 2 = other sites) in the general population.
A total of 76 patients (84%) were hospitalized because of SIADH (Table 3) and the median sodium level at admission was 120 mol/l (range 101–141 mmol/l). Eunatremic patients at time of admission experienced hyponatremia during hospitalization. The median duration of hospitalization was 12 days (range 2–100 days).
Table 3.
Hyponatremia’s characteristics in hospitalized patients.
| Characteristics |
All patients
(n = 76) |
Tolvaptan
(n = 37) |
Other therapies
(n = 39) |
|---|---|---|---|
| Number of patients (n) (%) | |||
| Days of hospitalization | |||
| Median | 12 | 10 | 15 |
| Range | 2–100 | 2–41 | 6–100 |
| Sodium value at the time of hospitalization (mEq/l) | |||
| Median | 120 | 118 | 122 |
| Range | 101–135 | 101–134 | 108–135 |
| Sodium correction during hospitalization | |||
| No | 17 (22) | 1 (3) | 16 (41) |
| Yes | 59 (78) | 36 (97) | 23 (59) |
A total of 37 patients (41%) received tolvaptan for SIADH treatment (group A) (Table 4). Other treatments for SIADH included hypertonic and saline solutions, diuretics, and fluid restriction (group B) (Table 5). Baseline characteristics were generally well balanced across all treatment groups, with the exception of gender and site of the primary tumor (Table 2).
Table 4.
Treatment characteristics in 37 patients treated with tolvaptan.
| Characteristics | Number of patients (n) (%) |
|---|---|
| Starting dose | |
| 30 mg/d.i.e. | 4 (13) |
| 15 mg/d.i.e. | 20 (54) |
| 7.5 mg/d.i.e. | 12 (30) |
| 7.5 mg on alternate days | 1 (3) |
| Dose modification | |
| No | 18 (49) |
| Yes | 19 (51) |
| Adverse events | |
| No | 31 (84) |
| Yes | 6 (16) |
| Other episodes of hyponatremia after tolvaptan dose modification/discontinuation | |
| No | 24 (65) |
| Yes | 13 (35) |
Table 5.
Hyponatremia’s treatments.
| Characteristics |
All patients
(n = 90) |
Tolvaptan
(n = 37) |
Other therapies
(n = 53) |
|---|---|---|---|
| Number of patients (n) (%) | |||
| Other treatment for hyponatremia except tolvaptan | |||
| No | 20 (22) | 20 (59) | 0 |
| Yes | 70 (78) | 17 (41) | 53 (100) |
| • Fluid restriction | 25 (28) | 5 (13) | 20 (38) |
| • Hypertonic saline (3% NaCl) | 38 (42) | 11 (30) | 27 (51) |
| • Isotonic saline | 18 (20) | 5 (13) | 13 (25) |
| • Diuretic | 4 (4) | 3 (8) | 1 (2) |
| • Urea | 9 (10) | 0 | 9 (17) |
Regarding nontolvaptan treatment options, most patients received hypertonic solution (38) alone or in combination with tolvaptan or other treatments, reaching an mOS of 12 months. Most of these patients were affected by lung tumors, but primary neoplasm was localized in a small number of other sites including the gastrointestinal tract (five patients), ovary (one patient), liver or bile duct (one patient), and prostate (one patient). A total of nine patients were treated with urea (alone or in combination) and were all affected by metastatic lung cancer reaching an mOS of 10 months. Other treatments included fluid restriction, isotonic saline, and diuretics administrated alone or in combination with other drugs used consecutively or simultaneously (Table 5).
In group A, 20 patients started tolvaptan at a dose of 15 mg/daily, 4 patients started with 30 mg/daily, 12 patients with 7.5 mg/daily, and only 1 patient started with 7.5 mg on alternate days.
No toxicity due to tolvaptan was observed in 31 patients (84%), and 6 patients (16%) experienced dry mouth. Dose changing was prescribed in 19 patients: in 4 patients the tolvaptan dose was increased to 30 mg/daily, while in the remaining 15 patients it was decreased to 7.5 mg on alternate days. In all the patients a hyponatremia improvement was observed with tolvaptan treatment, which lasted at most 30 days in 49% of patients.
Patients in group A presented more severe hyponatremia at admission: median sodium value at the time of hospitalization was 118 mmol/l in group A (range 101–134 mmol/l) and 122 mmol/l in group B (108–135 mmol/l). In addition, hyponatremia was worse in group A with serum sodium ⩽130 mmol/l in 95% patients and <120 mmol/l in 51% of patients, while in group B it was ⩽130 mmol/l in 62% of patients and <120 mmol/l in 24% of patients.
Serum sodium correction was a criterion for discharge because hyponatremia management implies the disappearance of related symptoms with an improvement of patient’s condition.
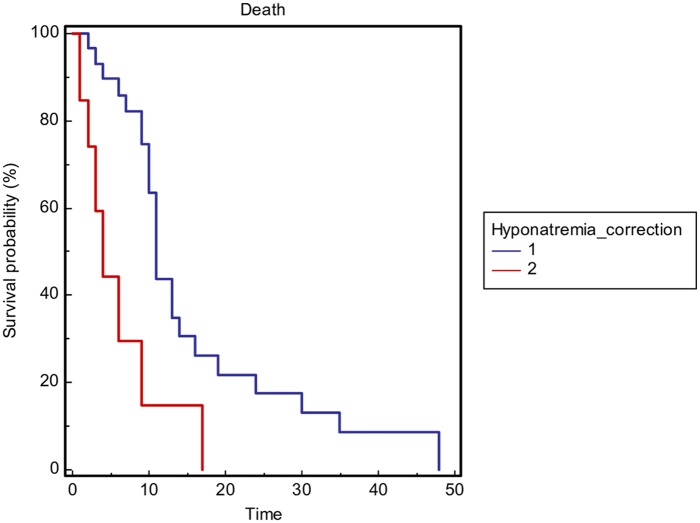
In this study the length of hospitalization was significantly longer in those patients who did not receive tolvaptan (median 10 days, range 2–41 days, in patients receiving tolvaptan versus 15 days, range 6–100 days, in patients not receiving tolvaptan; p = 0.002) and in those patients (19%) who did not reach sodium correction (considered as an improvement, >130 mmol/l, or a normalization, ⩾135 mmol/l, of sodium value) during hospitalization (p < 0.0001).
In addition, sodium normalization was obtained in 36 patients (40%) in group A and in 37 patients (41%) in group B.
Serum sodium levels were monitored over the first 24 h at regular intervals of 4–6 h, in order to control the correction speed. Rapid correction was not observed, even in the 30% of tolvaptan-treated patients that also received hypertonic saline (these treatment options were often used consecutively and not simultaneously).
The cost of hospitalization for cancer patients ranged from €220–1000/day for patients with an average of €399/day. Even though there was wide variability among institutions, the cost of hospitalization for a patient with hyponatremia calculated on the median of days of hospitalization ranged from €3990 for patients receiving tolvaptan to €5985 for patients not receiving tolvaptan.
Patients who experienced another episode of hyponatremia following tolvaptan dose modification/discontinuation (35%) requiring hospitalization presented a significantly lower serum sodium value at the time of hospitalization (p = 0.002) and a significantly lower minimum sodium value during hospitalization (p = 0.006) compared to patients who have not experienced another episode. In addition, the minimum sodium value during hospitalization was significantly lower in patients that required other treatments for hyponatremia, apart from tolvaptan (78%), (p < 0.0001) compared with patients that required only tolvaptan.
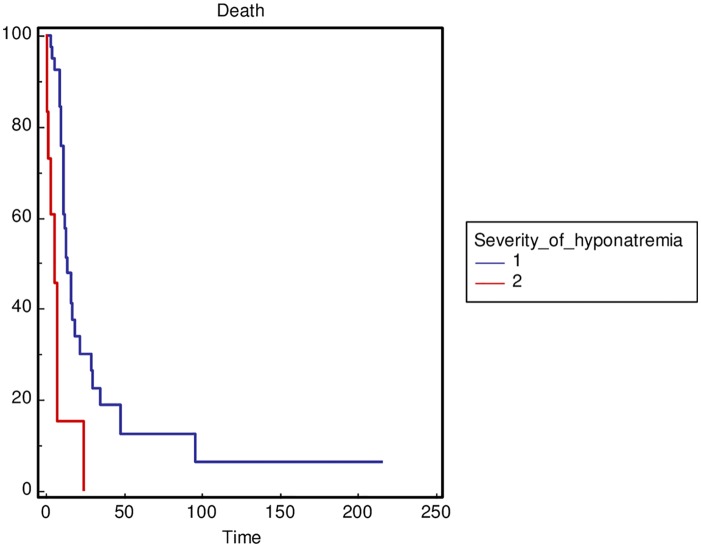
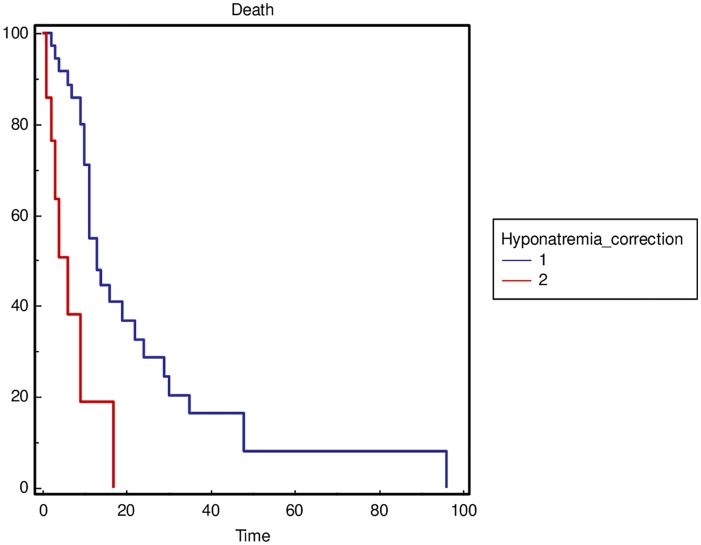
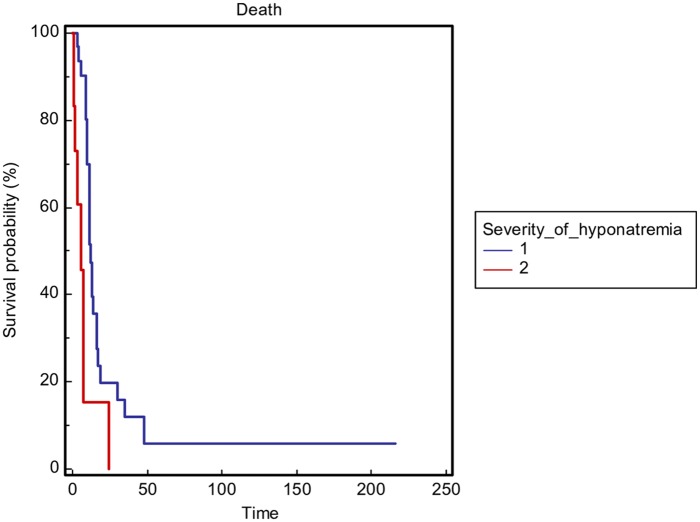
The severity of hyponatremia, defined as the minimum sodium value during hospitalization with a cut off value of 110 mmol/l (p = 0.0001) (Figure 2), and not obtaining sodium correction during hospitalization (p = 0.0003) (Figure 3) was significantly correlated with OS in the general population. In addition, to reduce the heterogeneity of the performed analysis, the authors carried out survival comparisons in the lung cancer patients subgroup confirming the findings (Figures 4 and 5).
Figure 2.
Overall survival stratified by the severity of hyponatremia as minimum sodium value during hospitalization (1 ⩾ 110 mmol/l versus 2 < 110 mmol/l) in the general population.
Figure 3.
Overall survival stratified by sodium correction (considered as an improvement, >130 mmol/l, or a normalization, ⩾135 mmol/l, of sodium value) during hospitalization (1 = yes versus 2 = no) in the general population.
Figure 4.
Overall survival stratified by the severity of hyponatremia as minimum sodium value during hospitalization (1 ⩾ 110 mmol/l versus 2 < 110 mmol/l) in the lung cancer patients subgroup.
Figure 5.
Overall survival stratified by sodium correction (considered as an improvement, >130 mmol/l, or a normalization, ⩾135 mmol/l, of sodium value) during hospitalization (1 = yes versus 2 = no) in the lung cancer patients subgroup.
Discussion
Hyponatremia represents the most common electrolyte disorder encountered in cancer patients3 and most cases of hyponatremia are caused by SIADH, which occurs with a wide range of malignancies that affects 1–2% of the entire cancer population.2,20
This study represents, to the best of the authors’ knowledge, the largest multicenter study investigating SIADH in cancer patients with the aim of increasing knowledge about treatments, outcomes, the medical and economic implications of SIADH in malignancies and reflecting the national perspective.
Although several studies have focused on hyponatremia in cancer patients, only a limited number of case reports of SIADH are available in this setting and only a limited amount of data on SIADH in cancer patients are available in the literature. In addition, relatively little is known about the relationship between abnormal serum sodium and medical costs.
In this study, the length of hospitalization was significantly longer in the patients (19%) who did not reach sodium normalization during hospitalization. Furthermore, the severity of hyponatremia and not obtaining sodium correction during hospitalization significantly correlated with OS in the general population as well in the lung cancer patients subgroup.
Many studies suggest that hyponatremia could be an indicator of poor prognosis not only in small cell lung cancer (SCLC),21 but also in other types of neoplasms including non-small cell lung cancer (NSCLC),22,23 pleural mesothelioma,24 renal cell carcinoma,25 and gastrointestinal cancer.26
In addition, it might have a negative effect on quality of life, increasing morbidity and the length of hospital stay with a considerable effect on costs.4,16,17
Hyponatremia due to SIADH could also have a negative effect on the response to anticancer treatments.25,27,28 Furthermore, there is an increasing body of evidence indicating that the correction of sodium and amelioration of the clinical symptoms is important in improving quality of life and prognosis in patients with extensive disease as well as preventing and reversing the neurologic sequelae.29–31
Current treatment options for hyponatremia are often limited because of poor compliance or undesirable side effects. A recent randomized controlled trial demonstrated that the vasopressin-2-receptor antagonist tolvaptan is effective in correcting SIADH-associated hyponatremia among patients with cancer.32 Tolvaptan appears to offer a more tolerable approach to the management of hyponatremia due to SIADH.
In the authors’ analysis, no considerable toxicity due to tolvaptan was observed, except for dry mouth in a few cases. The authors also observed a significantly increased length of hospital stay in those patients who did not receive tolvaptan.
Patients who experienced another episode of hyponatremia following tolvaptan dose modification/discontinuation (35%) presented a significantly lower serum sodium value at the time of hospitalization and a significantly lower minimum sodium value. In addition, the minimum sodium value during hospitalization was significantly lower in patients that required other treatment for hyponatremia, apart from tolvaptan (78%), compared with patients who required only tolvaptan.
However, the current study has some limitations. First, the retrospective nature of this study could result in unwanted methodological biases. In addition, the therapy choices in hyponatremic patients were not randomized, therefore, conclusions about the relative efficacy of the treatments, including tolvaptan, are limited. Finally, specific costs for every recovery were not available and there was variability between centers. The inevitable weighting errors in evaluation of costs could also affect the economic analysis. These findings are in accordance with previous studies suggesting that the correction of hyponatremia could improve patient outcomes. It is controversial whether the development and severity of hyponatremia correlate with tumor burden and the extent of metastatic disease.
Conclusion
To the best of the authors’ knowledge, this is the largest multicenter study analyzing SIADH in cancer patients to demonstrate that hyponatremia due to SIADH could result in an increase in length of hospitalization. It could also result in a decreased OS if not adequately corrected and that tolvaptan has the potential to be an effective treatment with a potential effect in improving both. Based on the present findings, a national study is underway to confirm the result prospectively (ASSERT trial).
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declare that there is no conflict of interest.
Contributor Information
Rossana Berardi, Clinica Oncologica, Università Politecnica delle Marche, Azienda Ospedaliero-Universitaria Ospedali Riuniti Umberto I – GM Lancisi – G Salesi di Ancona, Via Conca 71, Ancona, 60126, Italy.
Candida Mastroianni, Azienda Ospedaliera di Cosenza, Italy.
Giuseppe Lo Russo, Thoracic Oncology Fondazione IRCCS Istituto dei Tumori, Milano, Italy.
Roberta Buosi, Oncology Unit- S. Spirito Hospital - Casale M.to – Alessandria, Italy.
Daniele Santini, Medical Oncology Unit, Campus Bio-Medico University of Rome, Rome, Italy.
Agnese Montanino, Thoracic Medical Oncology, Istituto Nazionale Tumori ‘Fondazione G Pascale’, IRCCS, Napoli, Italy.
Carlo Carnaghi, Istituto Clinico Humanitas, Rozzano, Italy.
Marcello Tiseo, U.O. Oncologia Medica Azienda Ospedaliero-Universitaria di Parma, Italy.
Rita Chiari, Medical Oncology, Santa Maria della Misericordia Hospital, Azienda Ospedaliera di Perugia, Perugia, Italy.
Andrea Camerini, Medical Oncology, Azienda USL Toscana nord-ovest, Ospedale Versilia, Italy.
Sandro Barni, Oncology Unit, ASST Bergamo Ovest, Treviglio, Italy.
Valeria De Marino, U.O. Pneumologia ad indirizzo Oncologico. Azienda dei colli-Monaldi, Napoli, Italy.
Daris Ferrari, U.O. Oncologia Medica ASST Santi Paolo e Carlo, Presidio Ospedaliero San Paolo, Milano, Italy.
Antonella Cristofano, Department of Oncology, Ospedale Regionale della Valle d’Aosta, Italy.
Laura Doni, Ospedale Careggi, Firenze, Italy.
Federica Freddari, Oncologia Medica, Ospedale Civile di Senigallia, Senigallia, Italy.
Daniele Fumagalli, U.O. Oncologia Multimedica Sesto San Giovanni (MI), Italy.
Luigi Portalone, Pneumologia Oncologica 2, Az. Osp. S. Camillo Forlanini, Roma, Italy.
Roberta Sarmiento, Ospedale San Filippo Neri, Roma, Italy.
Giovanni Schinzari, Polo Scienze Oncologiche ed Ematologiche, UOC di Oncologia Medica, Università Cattolica del Sacro Cuore, Fondazione Policlinico Universitario Agostino Gemelli, Rome, Italy.
Francesca Sperandi, U.O. Oncologia Medica, Azienda Ospedaliero-Universitaria S.Orsola-Malpighi, Bologna, Italy.
Marcello Tucci, Division of Medical Oncology, Department of Oncology, San Luigi Gonzaga Hospital, University of Turin, Turin, Italy.
Alessandro Inno, Medical Oncology, Ospedale Sacro Cuore Don Calabria, Negrar, Verona, Italy.
Libero Ciuffreda, Azienda Ospedaliero Universitaria Città della Salute e della Scienza, Torino, Italy.
Marita Mariotti, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori, Meldola, Italy.
Cinzia Mariani, Oncologia Medica, Ospedale di Macerata, Macerata, Italy.
Miriam Caramanti, Clinica Oncologica, Università Politecnica delle Marche, Azienda Ospedaliero-Universitaria Ospedali Riuniti Umberto I, GM Lancisi, G Salesi, Ancona, Italy Current address: UOC Oncologia, Ospedale E.Profili di Fabriano Asur, Marche Area Vasta 2, Italy.
Mariangela Torniai, Clinica Oncologica, Università Politecnica delle Marche, Azienda Ospedaliero-Universitaria Ospedali Riuniti Umberto I, GM Lancisi, G Salesi, Ancona, Italy.
Rosaria Gallucci, Thoracic Oncology Fondazione IRCCS Istituto dei Tumori, Milano, Italy.
Chiara Bennati, Dipartimento di Oncologia-Ematologia, AUSL della Romagna, Ravenna, Italy.
Paola Bordi, U.O. Oncologia Medica Azienda Ospedaliero-Universitaria di Parma, Italy.
Lucio Buffoni, Azienda Ospedaliero Universitaria Città della Salute e della Scienza, Torino, Italy.
Achille Galeassi, Humanitas Materdomini, Castellanza, Italy.
Michele Ghidini, Medical Oncology and Hematology Unit, Humanitas Cancer Center, Humanitas Clinical and Research Center-IRCCS, Rozzano (Milan), Italy.
Emidio Grossi, Division of Oncological Endocrinology, Department of Medical Sciences, University of Turin, Italy.
Alessandro Morabito, Thoracic Medical Oncology, Istituto Nazionale Tumori ‘Fondazione G Pascale’, IRCCS, Napoli, Italy.
Bruno Vincenzi, Medical Oncology Unit, Campus Bio-Medico University of Rome, Rome, Italy.
Emanuela Arvat, Division of Oncological Endocrinology, Department of Medical Sciences, University of Turin, Italy.
References
- 1. Berardi R, Rinaldi S, Caramanti M, et al. Hyponatremia in cancer patients: time for a new approach. Crib Rev Oncol Hematol 2016; 102: 15–25. [DOI] [PubMed] [Google Scholar]
- 2. Berghmans T, Paesmans M, Body J. A prospective study on hyponatremia in medical cancer patients: epidemiology, aetiology and differential diagnosis. Support Care Cancer 2000; 8: 192–197. [DOI] [PubMed] [Google Scholar]
- 3. Platania M, Verzoni E, Vitali M. Hyponatremia in cancer patients. Tumori 2015; 101: 246–248. [DOI] [PubMed] [Google Scholar]
- 4. Doshi SM, Shah P, Lei X, et al. Hyponatremia in hospitalized cancer patients and its impact on clinical outcomes. Am J Kidney Dis 2012; 59: 222–228. [DOI] [PubMed] [Google Scholar]
- 5. Oronsky B, Caroen S, Oronsky A, et al. Electrolyte disorders with platinum-based chemotherapy: mechanisms, manifestations and management. Cancer Chemother Pharmacol 2017; 80: 895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berardi R, Santoni M, Rinaldi S, et al. Risk of hyponatraemia in cancer patients treated with targeted therapies: a systematic review and meta-analysis of clinical trials. PLoS One 2016; 11: e0152079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wanchoo R, Karam S, Uppal NN, et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol 2017; 45: 160–169. [DOI] [PubMed] [Google Scholar]
- 8. Sørensen JB, Andersen MK, Hansen HH. Syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in malignant disease. J Intern Med 1995; 238: 97–110. [DOI] [PubMed] [Google Scholar]
- 9. Bartter FC, Schwartz WB. The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med 1967; 42: 790–806. [DOI] [PubMed] [Google Scholar]
- 10. Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med 2013; 126: S1–42. [DOI] [PubMed] [Google Scholar]
- 11. Castillo JJ, Vincent M, Justice E. Diagnosis and management of hyponatremia in cancer patients. Oncologist 2012; 17: 756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weismann D, Schneider A, Höybye C. Clinical aspect of symptomatic hyponatremia. Endocr Connect 2016; 5: R35–R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thajudeen B, Salahudeen AK. Role of tolvaptan in the management of hyponatremia in patients with lung and other cancers: current data and future perspectives. Cancer Manag Res 2016; 8: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Corona G, Giuliani C, Parenti G, et al. Moderate hyponatremia is associated with increased risk of mortality: evidence from a meta-analysis. PLoS One 2013; 8: e80451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holland-Bill L, Christiansen CF, Heide-Jørgensen U, et al. Hyponatremia and mortality risk: a Danish cohort study of 279 508 acutely hospitalized patients. Eur J Endocrinol 2015; 173: 71–81. [DOI] [PubMed] [Google Scholar]
- 16. Berardi R, Caramanti M, Castagnani M, et al. Hyponatremia is a predictor of hospital length and cost of stay and outcome in cancer patients. Support Care Cancer 2015; 23: 3095–3101. [DOI] [PubMed] [Google Scholar]
- 17. Wald R, Jaber BL, Price LL, et al. Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med 2010; 170: 294–302. [DOI] [PubMed] [Google Scholar]
- 18. Boscoe A, Paramore C, Verbalis JG. Cost of illness of hyponatremia in the United States. Cost Eff Resour Alloc 2006; 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mantovani L. Health Technology Assessment Principi, concetti, strumenti operativi. Milano: Il Sole 24 ore Spa 2010; 210. [Google Scholar]
- 20. Glover DJ, Glick JH. Metabolic oncologic emergencies. CA Cancer J Clin 1987; 37: 302–320. [DOI] [PubMed] [Google Scholar]
- 21. Tiseo M, Buti S, Boni L, et al. Prognostic role of hyponatremia in 564 small cell lung cancer patients treated with topotecan. Lung Cancer 2014; 86: 91–95. [DOI] [PubMed] [Google Scholar]
- 22. Berardi R, Santoni M, Newsom-Davis T, et al. Hyponatremia normalization as an independent prognostic factor in patients with advanced non-small cell lung cancer treated with first-line therapy. Oncotarget 2017; 8: 23871–23879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kobayashi N, Usui S, Yamaoka M, et al. The influence of serum sodium concentration on prognosis in resected non-small cell lung cancer. Thorac Cardiovasc Surg 2014; 62: 338–343. [DOI] [PubMed] [Google Scholar]
- 24. Berardi R, Caramanti M, Fiordoliva I, et al. Hyponatraemia is a predictor of clinical outcome for malignant pleural mesothelioma. Support Care Cancer 2015; 23: 621–626. [DOI] [PubMed] [Google Scholar]
- 25. Jeppesen AN, Jensen HK, Donskov F, et al. Hyponatremia as a prognostic and predictive factor in metastatic renal cell carcinoma. Br J Cancer 2010; 102: 867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farid SG, Prasad KR. Prognostic impact of hyponatraemia in patients with colorectal cancer. Colorectal Dis 2015; 17: 451. [DOI] [PubMed] [Google Scholar]
- 27. Schutz FA, Xie W, Donskov F, et al. The impact of low serum sodium on treatment outcome of targeted therapy in metastatic renal cell carcinoma: results from the International Metastatic Renal Cell Cancer Database Consortium. Eur Urol 2014; 65: 723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Svaton M, Fiala O, Pesek M, et al. Predictive and prognostic significance of sodium levels in patients with NSCLC treated by erlotinib. Anticancer Res 2014; 34: 7461–7465. [PubMed] [Google Scholar]
- 29. Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate and severe hyponatremia. Am J Med 2009; 122: 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hansen O, Sørensen P, Hansen KH. The occurrence of hyponatremia in SCLC and the influence on prognosis: a retrospective study of 453 patients treated in a single institution in a 10-year period. Lung Cancer 2010; 68: 111–114. [DOI] [PubMed] [Google Scholar]
- 31. Petereit C, Zaba O, Teber I, et al. Is hyponatremia a prognostic marker of survival for lung cancer? Pneumologie 2011; 65: 565–571. [DOI] [PubMed] [Google Scholar]
- 32. Salahudeen AK, Ali N, George M, et al. Tolvaptan in hospitalized cancer patients with hyponatremia: a double-blind, randomized, placebo-controlled clinical trial on efficacy and safety. Cancer 2014; 120: 744–751. [DOI] [PubMed] [Google Scholar]