Abstract
The two recent prospective randomized trials CARMENA and SURTIME have changed the therapy paradigm of metastatic renal cell carcinoma. The CARMENA trial was conducted to investigate whether cytoreductive nephrectomy (CN) is required in the targeted therapy area, whereas SURTIME studied whether deferred CN in combination with sunitinib can be used to identify patients with inherent targeted therapy resistance. In the current review, we provide a comprehensive discussion of two randomized studies and the current evidence with up-do-date algorithms for treating primary metastatic clear-cell renal cell carcinoma in the era of targeted therapy and immune-checkpoint inhibition.
Keywords: cytoreductive nephrectomy, immunotherapy, metastatic renal cell carcinoma, renal cell carcinoma, sunitinib, systemic therapy, targeted therapy, tyrosine kinase inhibitors
Introduction
CARMENA1 and SURTIME2 were two randomized controlled trials (RCTs) initiated in 2010 to investigate the necessity and sequence of cytoreductive nephrectomy (CN) in the era of vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (TKIs). Both trials presented data that superseded previous evidence that supported CN followed by interferon (IFN)-α. The European Association of Urology (EAU) renal cell carcinoma guideline panel considered data from CARMENA to be practice changing and has recently updated their recommendation for patients with primary clear-cell renal cell carcinoma (ccRCC) with the primary tumour in situ.3
Both CARMENA and SURTIME experienced their difficulties with patient recruitment. Although CARMENA recruited substantially more patients than SURTIME, both trials did not meet the planned inclusion of their calculated sample size. Meanwhile first-line treatment paradigms of treatment-naïve metastatic renal cell carcinoma (mRCC) changed with the approval of the combination of ipilimumab and nivolumab4 in the United States (US) and Europe and other combinations, including atezolizumab plus bevacizumab,5 avelumab plus axitinib6 and pembrolizumab and axitinib.7 Despite the very recent evidence from CARMENA and SURTIME, the question arises whether the management of patients with primary mRCC needs re-investigation in the era of immune-checkpoint inhibitors (ICIs). We review the current evidence and discuss potential future management of patients with primary mRCC and the tumour in place. Although kidney cancer comprises several subtypes, the highest levels of evidence exists for ccRCC only. For other subtypes CN is associated with great uncertainty and no recommendations are currently available.
The evidence prior to CARMENA and SURTIME
In the past, CN was performed in patients with single- or oligometastatic disease, either in combination with complete metastasectomy or to observe metastatic sites which very rarely disappeared after nephrectomy of the tumour-bearing kidney.8 In the cytokine era, when median overall survival (OS) of mRCC was less than 1 year,9 two trials formally assessed the efficacy of CN and randomized patients to CN plus IFN-α versus IFN-α alone. Both studies were reported in 2001 and demonstrated a 3 month and 10 month survival benefit in the larger US study and in the smaller European study, respectively.10,11 A pooled analyses of both trials revealed an advantage of approximately half a year regarding OS among those who underwent CN.12
Several hypotheses have been suggested to explain why CN might have a beneficial effect but none of these have been substantiated. Removal of the ‘immunologic sink’13 that is, diminished production of growth factors and cytokines by the tumour in situ,14,15 postponed metastatic progression16 and nephrectomy-activated azotaemia17 are among several suggested potential mechanisms.
The introduction of VEGFR-targeted therapy resulted in a considerable increase in mRCC OS, from less than 1 year18 to more than 2 years for intermediate prognostic risk patients receiving several lines of targeted therapies.19 Based on the two randomized trials in the cytokine era, CN continued to be offered by default. In a recent systematic review on CN in the targeted therapy era, 56 studies were identified with a moderate or serious risk of bias in 50.20 Of note, CN demonstrated an OS advantage in patients with mRCC in 10 retrospective comparative trials. However, these studies were biased towards a more favourable population undergoing CN when compared with those who did not undergo surgery. It was therefore uncertain whether the improvement in outcome observed with systemic therapy would benefit from an additional CN at all. To understand and investigate the contradictory indication of CN combined with VEGFR-targeted therapy, two prospective RCTs were initiated in 2010.1,2
SURTIME
The first of these trials, presented in August 2017, was SURTIME, a randomized trial investigating three cycles of sunitinib prior to the decision to perform CN in the absence of systemic progression compared with immediate CN followed by sunitinib. Patients with mRCC were randomized into either immediate CN followed by sunitinib versus three cycles sunitinib followed by CN and sunitinib. Inclusion criteria required ccRCC, a resectable primary tumour and ⩽3 risk factors associated with CN as previously reported by Culp and colleagues.21 Initially planned as a phase III randomized trial to include 458 patients with progression-free survival (PFS) as a primary endpoint, insufficient accrual led the Independent Data Monitoring Commission to recommend to report the intention-to-treat (ITT) 28-week progression-free rate as a primary endpoint instead, for which 98 patients were needed. Secondary endpoints were OS, adverse event rates and postoperative progression. The concept of reversing the sequence of CN and targeted therapy that SURTIME investigated was based on results from previous phase II studies of VEGFR-TKIs for 2–3 months of pretreatment before CN. In these studies, the Memorial Sloan Kettering Cancer Center (MSKCC) poor-risk subgroup patients had inferior outcomes regardless of surgery, whereas the response in those patients with MSKCC intermediate-risk disease could be used as litmus test to aid decisions about CN, leading to excellent survival data.22
SURTIME was closed after 5.7 years of accrual with 99 patients included and showed no difference in PFS. However, the ITT OS hazard ratio (HR) of deferred versus immediate CN was 0.57 [95% confidence interval (CI): 0.34–0.95, p = 0.032] with a median OS of 32.4 (95% CI: 14.5–65.3) and 15.0 months (95% CI: 9.3–29.5), respectively. In the deferred arm, all patients except one received sunitinib, whereas in the immediate CN arm 20% received no systemic therapy, mainly due to rapid disease progression and deterioration of performance. Despite being an underpowered trial, SURTIME supported the rationale that postponing systemic treatment by performing CN upfront may be questionable for those patients, who need early control of their progressive disease.
CARMENA
In contrast with SURTIME that investigated the sequence of CN and systemic targeted therapy, CARMENA, a phase III non-inferiority RCT, investigated the role of immediate CN followed by sunitinib versus sunitinib alone. Results of this landmark trial were reported in June 2018. The trial showed that sunitinib alone did not result in inferior survival when compared with CN followed by sunitinib. CARMENA enrolled 450 patients with metastatic ccRCC of intermediate and poor MSKCC risk, of whom 226 were randomized to immediate CN followed by sunitinib and 224 to sunitinib alone. Patients in both arms were evenly distributed and had a median of two metastatic sites and a mean tumour burden of 140 ml of measurable disease by RECIST 1.1 criteria, of which approximately 80 ml accounted for the primary tumour. The study did not reach the full accrual of 576 patients and when it became apparent that continued accrual at a slow rate would not change the interpretation of results, the Independent Data Monitoring Commission advised the trial steering committee to close the study. In the ITT analysis performed after a median follow up of 50.9 months, the median OS with CN was 13.9 months versus 18.4 months with sunitinib alone (HR 0.89; 95% CI: 0.71–1.10). For MSKCC intermediate-risk patients (n = 256) the median OS was 19.0 months with CN followed by sunitinib and 23.4 months with sunitinib alone (HR 0.92; 95% CI: 0.60–1.24) and for MSKCC poor risk (n = 193) 10.2 months and 13.3 months, respectively (HR 0.86; 95% CI: 0.62–1.17). Non-inferiority was also found in two per-protocol analyses accounting for patients in the CN arm who did either not undergo surgery (n = 16) or did not receive systemic therapy (n = 40), and patients in the sunitinib-only arm who did not receive the study drug (n = 11). Median PFS in the ITT group was 7.2 months with CN and 8.3 months with sunitinib alone (HR 0.82; 95% CI: 0.67–1.00). The clinical benefit rate, defined as disease control beyond 12 weeks was 36.6% with CN and sunitinib and 47.9% with systemic therapy alone (p = 0.022). Of note, 38 patients in the sunitinib-only arm required secondary CN. Interestingly, only seven patients required a CN due to acute symptoms. The majority had a secondary CN for complete or near-complete response. The median time from randomization to secondary CN was 11.1 months with the first secondary CN being performed 7 months after randomization.
New guideline recommendations for patients with primary mRCC
The EAU Renal Cancer Guideline panel cautiously interpreted both studies.3 CARMENA, despite its larger study population, did not reach the full accrual required for a statistical design using a one-sided α to show non-inferiority. Also only 0.7 patients were treated per site per year, which may have had an impact on surgical outcome.1 In addition, the eligibility criteria of CARMENA did not require patients to have a limited number of prognostic factors, such as the factors used in the validated International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) or MSKCC prognostic models or in surgical risk models.23–25 For eligibility, inclusion criteria were primary clear-cell mRCC and an Eastern Cooperative Oncology Group 0/1 performance status. Inadvertently, the trial enrolled a relatively high percentage (44% and 41 % in the immediate CN and sunitinib-alone arms) of MSKCC poor-risk patients. Although the MSKCC or IMDC risk models were never designed to inform decisions about CN, large retrospective datasets have demonstrated that poor-risk patients obtain no benefit from CN.26 This suggests physician-induced selection bias towards poorer surgical candidates prior to inclusion in CARMENA.
However, trying to select the most ideal surgical candidates for CN has an impact on trial eligibility and screen failures. SURTIME was underpowered due to poor accrual that was in part a consequence of very stringent inclusion criteria to select only the most favourable candidates for CN based on previously published albeit unvalidated surgical risk factors.9 Although the trial was successful in including predominantly MSKCC intermediate-risk patients, the sample size did not allow definite conclusions. In addition, in contrast with CARMENA, OS was a secondary endpoint, reducing the statistical robustness for this endpoint.
Yet, the outcome of the CARMENA is significant and clinically relevant. Based on the results obtained from the ITT, immediate CN should no longer be the standard of care in patients who require systemic therapy. CN is associated with morbidity and mortality and at the time of the final analysis of the prespecified variables there appeared to be no subgroup in CARMENA in which this approach was superior. CARMENA clearly demonstrates that patients with MSKCC poor risk do not obtain advantage from CN and are conceivably harmed by a surgical intervention. This subgroup analysis confirms previous retrospective data.26
SURTIME complements this conclusion. Both the subgroup analysis of the intermediate MSKCC risk group in CARMENA, and SURTIME with its predominantly MSKCC intermediate-risk patient population, support that immediate CN should not be performed in MSKCC intermediate-risk patients requiring sunitinib, or an equivalent VEGFR-TKI. The OS in the upfront CN arm in both studies was shorter than for patients receiving immediate sunitinib, although not statistically significant.
The question remains whether patients with intermediate MSKCC risk mRCC who start on systemic therapy with sunitinib benefit from deferred CN in the absence of disease progression. The survival benefit in SURTIME for patients with deferred CN was 17.4 months and represents a clinically meaningful OS trend favouring this approach. Although not statistically significant, patients with intermediate MSKCC risk in CARMENA receiving sunitinib only had an OS advantage of several months compared with the CN arm. However, 38 patients in CARMENA underwent a secondary CN in the sunitinib-only arm, the majority because a near-complete response provided an opportunity to cease systemic therapy following removal of the primary tumour. Although these were only 17% of all patients in the sunitinib-alone arm, the majority of secondary CN occurred in intermediate-risk patients, suggesting that approximately 30% of patients in this risk category may require a secondary nephrectomy with this approach. Taken together, the EAU guideline panel reasoned that the course of disease in both RCTs in patients who start with sunitinib provides weak evidence that performing deferred CN in patients who do not progress after several months on VEGFR-TKI therapy confers a survival benefit.3
Treatment decisions for patients with primary mRCC in the current paradigm
After the approval of ipilimumab and nivolumab for the treatment of IMDC intermediate- and poor-risk patients with clear-cell mRCC4 as well as the recent superiority in terms of PFS and OS for pembrolizumab and axitinib for all IMDC risk groups compared with the previous standard of care sunitinib,7 ICIs now provide the new backbone of first-line therapy. This new development impacts on the treatment decisions for patients who require systemic therapy for clear-cell primary mRCC and who have the primary tumour in place.
Traditionally, several nomograms and surgical risk factors have been developed to aid in the selection of patients for CN;9,26 however, none of these nomograms or factors have been validated and have now been superseded by evidence from both CARMENA and SURTIME, which used MSKCC risk profiling as well as the recent ICI trials that introduced the concept of using IMDC risk groups to choose between the options of systemic therapies. Although the IMDC and MSKCC risk factors have never been developed to be used as a decision-making tool for CN, they reveal survival estimates for the respective risk groups and have been shown in SURTIME and CARMENA to be associated with the outcome of patients in both trials. Moreover, a recent post hoc analysis, in which patients who participated in CARMENA were reclassified in IMDC risk groups, suggests that patients with intermediate IMDC risk with one factor versus two factors have a potential benefit from upfront CN.27 Using IMDC risk group factors, 58.6% patients were intermediate and 41.4 % were poor risk. When looking at the intermediate-risk group only, 48.1% had only one risk factor (interval between diagnosis and treatment <1 year), with a median OS of 30.5 and 25.2 months in the CN and sunitinib-only arm respectively (HR 1.24, 0.81–1.90). In contrast, 51.9 % had two risk factors (mostly low haemoglobin, high corrected calcium or neutrophils in addition to the interval between diagnosis and treatment <1 year), with a median OS of 16.6 and 31.2 months in the CN and sunitinib-only arm respectively (HR 0.61, 0.41–0.91; p = 0.015). However, 40 patients had a secondary nephrectomy in the sunitinib-only arm, with median OS of 48.5 months (CI 95%: 27.9–64.4) versus 15.7 months (CI 95%: 13.3–20.5) in patients who never had surgery and therefore the long median OS of 31.2 months in the sunitinib-only arm in the IMDC intermediate-risk group with two factors must be interpreted in the light of a high percentage of deferred CN. In fact, the HR and median OS are very similar to the data observed in SURTIME, which included predominantly MSKCC intermediate-risk patients and in which patients who did not progress on systemic therapy were offered a deferred CN. These results seem to again support that initial systemic therapy with the option to perform deferred CN is the preferred treatment of choice for patients both with MSKCC and IMDC intermediate-risk groups. For patients with only one IMDC risk factor, upfront CN might be beneficial but the numbers in this particular post hoc analysis in CARMENA were small and this approach remains disputable.
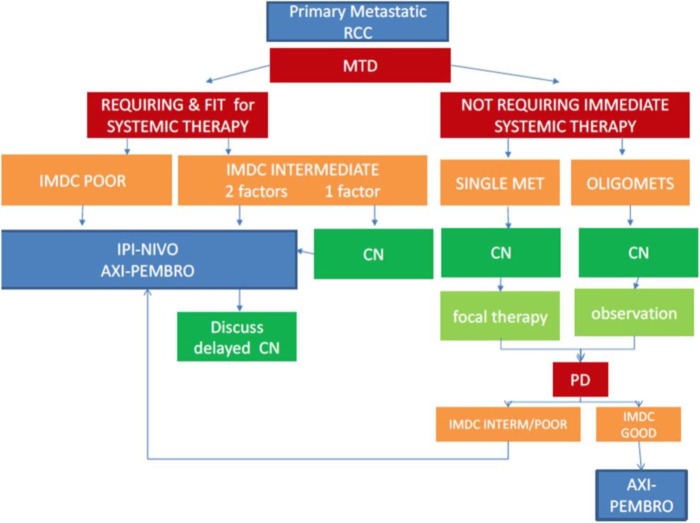
With VEGFR-TKI reduced to a secondary role in patients presenting with intermediate and poor-risk primary mRCC, the question occurs how to best treat patients with the primary tumour in place in the era of immune-checkpoint inhibition. Although performed with a certain class of drugs only, both CARMENA and SURTIME support the concept that patients who require systemic therapy should start with drug treatment whereupon they could undergo a deferred CN at a later stage depending on a beneficial course of the disease (Figure 1). This concept makes sense from a clinical point of view in the new era of ICI therapy, although this would require formal testing in an RCT (Table 1). In reality, however, in the pivotal CheckMate 214 trial, 194 mRCC patients were included with their primary tumour in place demonstrating the safety of this approach and a survival benefit with immune-checkpoint inhibition.
Figure 1.
Decision algorithm for patients with primary mRCC of clear-cell subtype and good performance status.
AXI, axitinib; CN, cytoreductive nephrectomy; IMDC, International Metastatic Database Consortium risk model; IPI, ipilimumab; MET, metastases; mRCC, metastatic renal cell carcinoma; MTD, multidisciplinary team decision; NIVO, nivolumab; PD, progressive disease; PEMBRO, pembrolizumab; RCC, renal cell carcinoma.
Table 1.
Rationale for CN in the era of immune-checkpoint inhibition.
| Scenario | Rationale of CN | Probability |
|---|---|---|
| CR of primary and metastases | CN not required | Unlikely, but has been reported in presurgical trials |
| CR at metastatic sites only | Deferred CN advised in all instances: • to stop treatment • potentially curative |
May occur in up to 11% |
| SD or PR but median OS substantially longer than in VEGFR-TT era with 10–20% ‘cured’ | Deferred CN may be of benefit: • in case of symptoms • potentially curative |
Very likely in a high percentage |
CN, cytoreductive nephrectomy; CR, complete response; OS, overall survival; PR, partial response; SD, stable disease; TT, targeted therapy; VEGFR, vascular endothelial growth factor receptor.
Patients with single- or oligometastatic disease were not eligible for both CARMENA and SURTIME because the trials required that they must have had a clinical need to start systemic therapy with sunitinib. Therefore, CARMENA and SURTIME do not answer the question of CN in patients with low volume but unresectable metastatic disease, a good performance, favourable and intermediate risk, who do not require immediate treatment with VEGFR-TKIs or ICIs but may be observed instead.28 In these cases, immediate CN may be justified, as prospective single-arm studies have shown that the time of observation of the metastases until progression requires systemic treatment can be substantial28 (Figure 1). For patients with a primary tumour in place, good performance status and resectable single- or oligometastasis it is generally accepted that CN and focal therapy, which can be metastasectomy, ablation or stereotactic body radiotherapy, should be employed to render them disease-free.
Future CN trials
Future trials investigating CN will be challenging to perform in view of the rapid evolvement of new treatment paradigms. Like the previous SWOG and EORTC trials in the cytokine era, both CARMENA and SURTIME took 8 years to accrue enough patients for academically valid information on how these patients should be best managed. Both trials, however, did not reach full accrual which was especially apparent in the more complex designed SURTIME trial. With new treatment options replacing sunitinib as first-line treatment of mRCC, the continued conduct of both CARMENA and SURTIME would have introduced ethical dilemmas and both trials had to stop, let alone for reasons of poor accrual. The current changes in treatment paradigms are likely to appear at a faster pace than previously anticipated. Anyone designing and embarking on a phase III RCT with primary metastatic patients, needs to consider the changed epidemiology with fewer patients eligible than 20 years ago as well as a high likelihood that the first-line therapy chosen at the outset of the trial will change during its course. Based on past experience, it is therefore doubtful whether we should be conducting these large trials in this very selective, but heterogeneous, patient population anymore. In addition, both trials revealed the detrimental effect of immediate CN in patients who require systemic therapy. Based on the ratio of priming the immune system through pretreatment of tumour tissue, it would be more appropriate to try to answer whether deferred CN is superior to no CN. Alternatively, studies could be downsized according to certain biomarker profiles which would enable calculation of smaller sample sizes based on higher HRs that could lead to faster read-outs of study results.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: Axel Bex took part in advisory boards of Pfizer, Novartis, Ipsen, Eisai and Roche. He is the Principal Investigator of the EORTC SURTIME trial sponsored in part by a grant from Pfizer to the EORTC. Thomas Powles received honoraria from Bristol-Myers Squibb, MERCK, and Roche/Genentech; has consulting or advisory roles at AstraZeneca, Bristol-Myers Squibb, Genentech/Roche, Merck, and Novartis; received research funding from AstraZeneca/MedImmune and Roche/Genentech; and has other relationships with Bristol-Myers Squibb and Ipsen.
Contributor Information
Teele Kuusk, Royal Free Hospital, Department of Urology, Renal Cancer Unit, London, UK.
Bernadett Szabados, Barts Experimental Cancer Medicine Centre, Barts Cancer Institute, Queen Mary University of London, London, UK.
Wing Kin Liu, Royal Free Hospital, Department of Medical Oncology, London, UK.
Thomas Powles, Barts Experimental Cancer Medicine Centre, Barts Cancer Institute, Queen Mary University of London, London, UK.
Axel Bex, Royal Free Hospital, Department of Urology, Renal Cancer Unit, University College London, Division of Surgical and Interventional Sciences, London, UK; The Netherlands Cancer Institute Division of Surgical Oncology, Department of Urology, Plesmanlaan 121, 1066 CX Amsterdam, the Netherlands.
References
- 1. Méjean A, Ravaud A, Thezenas S, et al. Sunitinib alone or after nephrectomy in metastatic renal cell carcinoma. N Engl J Med 2018; 379: 417–427. [DOI] [PubMed] [Google Scholar]
- 2. Bex A, Mulders P, Jewett M, et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: the SURTIME randomized clinical trial. JAMA Oncol 2019; 5(2): 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bex A, Albiges L, Ljungberg B, et al. Updated European association of urology guidelines for cytoreductive nephrectomy in patients with synchronous metastatic clear-cell renal cell carcinoma. Eur Urol 2018; 74: 805–809. [DOI] [PubMed] [Google Scholar]
- 4. Hammers HJ, Plimack ER, Infante JR, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 study. J Clin Oncol 2017; 35: 3851–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019; 393(10189): 2404–2415. [DOI] [PubMed] [Google Scholar]
- 6. Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019; 380: 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal cell carcinoma. N Engl J Med 2019; 380: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 8. Kurauchi H, Mori Y, Suzuki M, et al. Nephrectomy in renal carcinoma with distant metastasis. Br J Urol 1989; 63: 600–604. [DOI] [PubMed] [Google Scholar]
- 9. Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999; 17: 2530–2540. [DOI] [PubMed] [Google Scholar]
- 10. Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med 2001; 345: 1655–1659. [DOI] [PubMed] [Google Scholar]
- 11. Mickisch GH, Garin A, van Poppel H, et al. European Organisation for Research and Treatment of Cancer (EORTC) Genitourinary Group. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet 2001; 358: 966–970. [DOI] [PubMed] [Google Scholar]
- 12. Flanigan RC, Mickisch G, Sylvester R, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol 2004; 171: 1071–1076. [DOI] [PubMed] [Google Scholar]
- 13. Lahn M, Fisch P, Köhler G, et al. Pro-inflammatory and T cell inhibitory cytokines are secreted at high levels in tumor cell cultures of human renal cell carcinoma. Eur Urol 1999; 35: 70–80. [DOI] [PubMed] [Google Scholar]
- 14. Uzzo RG, Rayman P, Kolenko V, et al. Mechanisms of apoptosis in T cells from patients with renal cell carcinoma. Clin Cancer Res 1999; 5: 1219–1229. [PubMed] [Google Scholar]
- 15. Xia Y, Zhang Q, Zhen Q, et al. Negative regulation of tumor-infiltrating NK cell in clear cell renal cell carcinoma patients through the exosomal pathway. Oncotarget 2017; 8: 37783–37795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rackley R, Novick A, Klein E, et al. The impact of adjuvant nephrectomy on multimodality treatment of metastatic renal cell carcinoma. J Urol 1994;152: 1399–1403. [DOI] [PubMed] [Google Scholar]
- 17. Gatenby RA, Gawlinski ET, Tangen CM, et al. The possible role of postoperative azotemia in enhanced survival of patients with metastatic renal cancer after cytoreductive nephrectomy. Cancer Res 2002; 62: 5218–5222. [PubMed] [Google Scholar]
- 18. Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007; 356: 115–124. [DOI] [PubMed] [Google Scholar]
- 19. Motzer RJ, Barrios CH, Kim TM, et al. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol 2014; 32: 2765–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhindi B, Abel EJ, Albiges L, et al. Systematic review of the role of cytoreductive nephrectomy in the targeted therapy era and beyond: an individualized approach to metastatic renal cell carcinoma. Eur Urol 2019; 75: 111–128. [DOI] [PubMed] [Google Scholar]
- 21. Culp SH, Karam JA, Wood CG. Population-based analysis of factors associated with survival in patients undergoing cytoreductive nephrectomy in the targeted therapy era. Urol Oncol 2014; 32: 561–568. [DOI] [PubMed] [Google Scholar]
- 22. Powles T, Blank C, Chowdhury S, et al. The outcome of patients treated with sunitinib prior to planned nephrectomy in metastatic clear cell renal cancer. Eur Urol 2011; 60: 448–454. [DOI] [PubMed] [Google Scholar]
- 23. Pérez-Valderrama B, Arranz Arija JA, Rodríguez Sánchez A, et al. Validation of the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) prognostic model for first-line pazopanib in metastatic renal carcinoma: the Spanish Oncologic Genitourinary Group (SOGUG) SPAZO study. Ann Oncol 2016; 27: 706–711. [DOI] [PubMed] [Google Scholar]
- 24. Ko JJ, Xie W, Kroeger N, et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol 2015; 16: 293–300. [DOI] [PubMed] [Google Scholar]
- 25. Lee HJ, Lee A, Huang HH, et al. External validation of the updated Leibovich prognostic models for clear cell and papillary renal cell carcinoma in an Asian population. Urol Oncol 2019; 37: 356.e9–356.e18. [DOI] [PubMed] [Google Scholar]
- 26. Heng DY, Wells JC, Rini BI, et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol 2014; 66: 704–710. [DOI] [PubMed] [Google Scholar]
- 27. Mejean A, Thezenas S, Chevreau C, et al. Cytoreductive nephrectomy (CN) in metastatic renal cancer (mRCC): update on CARMENA trial with focus on intermediate IMDC risk population. J Clin Oncol 2019; 37: 4508. [Google Scholar]
- 28. Rini BI, Dorff TB, Elson P, et al. Active surveillance in metastatic renal cell carcinoma: a prospective, phase 2 trial. Lancet Oncol 2016; 17:1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]