Abstract
Background:
The liver effect of orlistat as a weight control treatment in patients with nonalcoholic fatty liver disease (NAFLD) with obesity remains undetermined. This study quantified liver fat improvement by orlistat in a Chinese cohort with NAFLD accompanied by obesity, diagnosed by a lower body mass index threshold than that for White patients.
Materials and methods:
We conducted a parallel-group, open-label, 24-week, randomized clinical trial registered at the Chinese Clinical Trial Registry (ChiCTR-IPR-17012258). Obese participants with NAFLD were randomized 1:1.5 to the intervention group with orlistat or conventional care. Liver fat quantification was assessed by magnetic resonance imaging-based proton density fat fraction with Dixon sequence.
Results:
Overall, 170 (n = 68, orlistat 120 mg three times/day and n = 102, conventional therapy) and 130 patients with NAFLD (n = 56, orlistat and n = 74, conventional therapy) were included for intention-to-treat (ITT) and per-protocol (PP) analysis, respectively. Orlistat reduced liver fat content to a greater degree than conventional care [−5.45% versus −1.96%, p < 0.001 (ITT analysis) and −6.66% versus −2.68%, p < 0.001 (PP analysis)]. The 6-month rate of decrease in steatosis grades was higher in the orlistat group [45.6% versus 22.5% (ITT analysis), 57.4% versus 30.3% (PP analysis), both p < 0.001]. Multivariate logistic regression analysis identified orlistat treatment [odds ratio (OR) = 2.4; 95% confidence interval (CI) 1.1–5.6, p = 0.036] as an independent predictor of steatosis improvement. Among patients with orlistat therapy, weight loss (OR = 1.2, 95% CI 1.1–1.4, p = 0.040) and severe steatosis (OR = 6.7, 95% CI: 1.1–40.3, p = 0.03) remained predictive of steatosis improvement.
Conclusions:
Orlistat can effectively promote steatosis improvement and may serve as a treatment option for controlling NAFLD.
Chinese Clinical Trial Registry identifier:
ChiCTR-IPR-17012258
Keywords: magnetic resonance imaging-derived proton density fat fraction, nonalcoholic fatty liver diseases, obesity, orlistat
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a chronic, metabolism-mediated disease characterized by excessive intrahepatic lipid accumulation, inflammation and fibrosis and can ultimately progress to cirrhosis, hepatocellular carcinoma and other extra-liver complications (such as diabetes and cardiovascular diseases).1 With the persistently increasing prevalence of obesity, NAFLD has become the most prevalent cause of chronic liver disease, affecting up to 26% of the global population.2 Given the role of obesity in the pathogenesis of NAFLD, all the guidelines recommended weight loss by lifestyle interventions as the cornerstone in management of NAFLD.1,3,4 To achieve improvement of liver histological steatosis, weight loss of at least 5% is needed, while improvement of inflammation and fibrosis requires at least 7% and 10% weight loss, respectively.3 However, only approximately 10% of patients can achieve weight loss via lifestyle interventions after 52 weeks.5 Developing adjunctive pharmaceutical interventions to promote weight loss remains a great challenge with the growing burden of NAFLD.
Orlistat, an oral gastrointestinal lipase inhibitor with weight-reducing effects, reduces the fat absorption of food and thus blocks dietary triglycerides from entering the liver.6 Several studies have explored the therapeutic effects of orlistat in NAFLD. A recent meta-analysis of 330 patients with NAFLD found that orlistat treatment significantly reduced the levels of body mass index (BMI; mean difference = −1.97; p = 0.02), but limited data were available for an analysis of hepatic steatosis.7 In a randomized, double-blind, placebo-controlled clinical trial8 from Israel, no significant decrease in body weight was observed, but ultrasound examination detected a higher reversal rate of fatty liver (24% versus 17%, p = 0.04) in patients treated with orlistat for 24 weeks compared with that in the control group. Another landmark randomized controlled trial (RCT)9 using pathological score as endpoints showed that orlistat therapy for 36 weeks was not superior to lifestyle modification in inducing weight loss or improving hepatic steatosis in White patients. Taken together, these results suggest that the efficacy of orlistat in weight management and steatosis improvement remains controversial.
Significant differences in obesity severity, distribution of adipose tissue and genetics exist between Asian and White populations.10–12 Lower BMI thresholds (BMI ⩾ 25 kg/m2) have been applied to diagnose obesity in patients from Asia with NAFLD.11 These patients also manifest more prevalent abdominal obesity in the same BMI categories than do White patients with NAFLD.10 Whether heterogeneity in obesity influences orlistat-mediated weight loss and steatosis improvement in Asian populations remains unclear.
Magnetic resonance imaging (MRI)-derived proton density fat fraction (PDFF) has been emerging as a non-invasive, quantitative and sensitive measure of the entire liver fat content, whose results show a robust correlation with magnetic resonance spectroscopy (gold standard for non-invasive hepatic fat quantification).13 This method may assist in assessing the relative reduction in liver fat content after orlistat intervention from baseline, thus estimating treatment response accurately. Therefore, the main objective of the present study was to prospectively assess the efficacy of orlistat in steatosis by MRI-PDFF in Chinese patients with NAFLD.
Materials and methods
Study design and participants
This study was a prospective, open-label, monocentric, RCT (registered at the Chinese Clinical Trial Registry, ChiCTR-IPR-17012258) conducted in the First Affiliated Hospital of Sun Yat-sen University, a tertiary NAFLD referral center in China. Patients with NAFLD who received treatment were identified between August 2017 and August 2018. The study protocol was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University and written informed consent was obtained from all patients.
Inclusion criteria included the following: (1) a diagnosis of fatty liver confirmed by MRI-PDFF; (2) age between 18 and 60 years; (3) BMI ⩾ 25 kg/m2; (4) no history of use of medication associated with obesity; and (5) complete anthropometric measurements and radiological examinations.
The exclusion criteria were as follows: (1) excessive alcohol consumption (⩾10 g/day in women and ⩾20 g/day in men); (2) use of steatosis-causing drugs such as tamoxifen and corticosterone; (3) secondary steatosis, including autoimmune liver disease (screened by nuclear antibody, mitochondrial antibody, smooth muscle antibody, liver kidney microsomal antibody) and viral hepatitis (positive hepatitis B surface antigen or antibody against hepatitis C virus); (4) serious heart, kidney, or lung comorbidities or diabetes; (5) nursing or pregnant women; and (6) radiographic evidence of cirrhosis.
Allocation and intervention
Base on a predefined computer-generated number with a 1:1.5 allocation that was concealed using serially numbered, opaque, sealed envelopes, the patients were stratified into an orlistat group and a conventional therapy group, and the selection of therapy regimen was open label. Patients in the orlistat group received orlistat (120 mg, three times daily) without additional treatment. Only patients who completed 24 weeks of orlistat treatment were included in the analysis. Orlistat intake was confirmed by prescription and records of patient interviews during clinic visits.
For the routine treatment group, patients received lifestyle modifications according to the Dietary Reference Intakes,14 the Dietary Guidelines15 and World Health Organization Global Strategy on Diet, Physical Activity and Health.16 The patients were instructed to restrict carbohydrate and fat intake and to exercise three times/week for 30 min per session. An easy-to-carry brochure with personalized exercise and dietary prescriptions based on sex, age, BMI, occupation and medical history was offered to each patient. For patients with indications for drug therapy to lipid profiles, blood pressure or uric acids, pharmacological therapy was added as recommended by guidelines.17–20 Briefly, this therapy comprised metformin or insulin for glucose control, benzbromarone for uric acid control, renin–angiotensin blockade or a calcium channel blocker for blood pressure control, and a statin for low-density lipoprotein cholesterol (LDL-C) control. The prescription of specific agents was determined by the supervising physicians.
Clinical data collection
For eligible patients, we examined the basic demographic characteristics (age and sex), smoking habits, past medication use, family history of metabolic disease and anthropometric parameters (blood pressure, weight, height, waist circumference, abdominal circumference, hip circumference) from the structured interview using a questionnaire. BMI was calculated as weight (kg) divided by height (m) squared. We also documented biochemical indicators measured in the fasting state from medical electronic records, including alanine aminotransferase (ALT), aspartate amino transferase (AST), glutamyl transpeptidase, lipid profiles, blood glucose levels, plasma insulin levels and uric acids. The homeostasis model of assessment for insulin resistance (HOMA-IR) was calculated as [fasting plasma glucose level (mmol/l) × fasting plasma insulin level (μU/ml)]/22.5.21 After the initial visit, the above anthropometric indexes and biochemical indicators were measured at 1, 3 and 6 months with a maximum delay of 1 month at each arranged visit.
Liver stiffness assessment by two-dimensional shear wave elastography
Liver stiffness was measured using two-dimensional shear wave elastography (Aix-en-Provence, France) within 2 weeks after the first clinic visit. Patients were required to be fasting for over 8 h and were placed in a supine position with the right arm elevated above the head. When shear wave image frames presented a color-coded sample area, physicians selected a fixed circular region of interest (ROI) with a diameter of 2.5 cm to avoid liver capsules.
Liver fat quantification with MRI-PDFF
MRI-PDFF was utilized with a 3.0-Tesla MRI scanner (SIEMENS 3.0T MAGNETOM Verio) for the baseline and follow-up liver fat estimations. Fat–water separation images of the liver and pancreas were acquired using a T1volumetric interpolated breath-hold examination (VIBE) Dixon sequence with the following parameter settings adopted from our previous study:22 TE1 2.5 ms; TE2 3.7 ms; repetition time 5.47 ms; 5° flip angle; ±504.0 kHz per pixel receiver bandwidth; and a slice thickness of 3.0 mm. The fat content was calculated in an irregular-shaped ROI covering the entire liver in 21 consecutive slices (max-area centered) of each patient placed by two trained radiologists manually as our previous study.22 MRI-PDFF maps for all segments were also generated by placing circular ROIs with diameter of 20 mm centrally in each of the eight liver segments. For the pancreas, the fat fraction of the head, body, and tail of the pancreas were documented in square-shaped ROIs (5 × 5 mm2) from the corresponding region. The average fat content values were calculated for the entire liver and total pancreas. The steatosis grades was classified by MRI-PDFF as without (<5%) and mild (5–10%) with these cut-off values for discriminating steatosis degree were validated in the previous clinical trials for estimating the effects of different drugs on NAFLD.23–25 For distinguishing moderate and severe steatosis, as a recent meta-analysis showed that thresholds of MRI-PDFF ranged from 16.37% to 23.3% for distinguishing moderate and severe steatosis,26 we adopted 20%, 20–30% and above 30% as cut-off values for moderate, moderate-to-severe, and severe steatosis, thus there were five categories in estimating steatosis grades as without (<5%), mild (5–10%), moderate (10–20%), moderate-to-severe (20–30%) and severe (>30%). All the radiologists performing the MRI-PDFF measurements were blinded to the allocation in the two groups and the treatment of all participants. The initial content of liver fat was compared with that after treatment for 6 months, primary outcome was defined as liver fat fraction reduction with MRI-PDFF and the secondary outcome was defined as at least 1 degree of liver fat content decrease from the baseline.
Sample size calculation
According to the previous clinical trials evaluating the efficacy of various drugs (colesevelam, ezetimibe, sitagliptin and empagliflozin) with MRI-PDFF on NAFLD,27–30 a liver fat fraction reduction of 5% after follow up was set to the orlistat group, while the control group was assumed to achieve 1% reduction in liver fat after follow up. A dropout rate of 20% would be considered. With these settings calculated by the PASS software (NCSS, Kaysville, UT, USA), the estimated sample size needed to be 25 for the orlistat group and 40 for the control group to achieve a power of 90% with an alpha of 0.05, we planned to randomize at least 65 patients to ensure adequate study power, even with dropouts.
Statistical analysis
The results are presented as the mean ± standard deviation or median (interquartile range). An independent sample Student’s t-test was applied to compare baseline characteristics. Chi-square tests were used to compare the categorical variables. A paired Student’s t-test was used to assess therapeutic changes between baseline and end of treatment. The effects of orlistat on obesity-related parameters were investigated using repeated-measures analysis of variance (ANOVA). Missing laboratory data were analyzed with the last-observation-carried-forward technique. The statistical analyses in both the intention-to-treat (ITT) and per-protocol (PP) populations are given. Univariate logistic regression was performed to screen for potential covariates associated with steatosis improvement. The covariates with p < 0.1 were entered the multivariable model using the forward condition method. Multivariate linear regression analysis was conducted to reveal the estimated effect of orlistat on hepatic steatosis. All statistical analyses were performed with the R statistical package version 3.4.3 (the R Project for Statistical Computing, Vienna, Austria) with two-tailed tests. A p value <0.05 was considered statistically significant.
Results
Patient characteristics
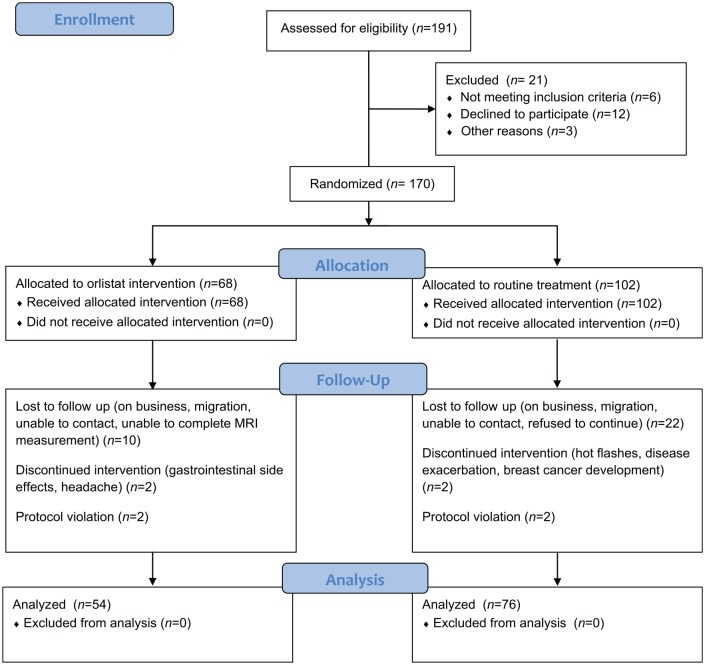
A total of 191 patients were initially enrolled in the study, and 21 patients were excluded for such reasons as refusal to participate (n = 12), use of steatosis-causing drugs (n = 3) and excess alcohol intake (n = 6). The remaining 170 patients with NAFLD with a BMI > 25 kg/m2 (68 patients receiving orlistat therapy and 102 receiving routine treatment) were randomized at baseline. Of these patients, 40 withdrew during 6 months of follow up, with 14 patients in orlistat treatment and 26 patients in routine treatment failing to return. Finally, of the remaining 130 patients, 54 (41.5%) received orlistat therapy, and 76 (58.5%) received lifestyle intervention in combination with drug therapy. The participant enrollment and allocation flow chart is presented in Figure 1. Table 1 presents the baseline characteristics for ITT and PP analysis. The mean ages of the patients at randomization in the orlistat group and routine treatment group were 46.0 years (range 28–58 years) and 45.3 years (range 25–63 years), respectively. There were no significant differences in the demographics, metabolic profiles or comorbidities of the patients between the groups in either the ITT or PP population (Table 1). No difference was found between the patients who dropped out and those who completed the treatment. In the orlistat therapy group at randomization for the ITT population, 8 patients (11.8%) were classified as having severe steatosis, and 17 (25%) were classified as having moderate-to-severe steatosis, and these percentages showed no significant differences compared with those in the routine treatment group (8.8% for severe steatosis and 24.5% for moderate-to-severe steatosis, p = 0.17). Likewise, the proportions of patients with each steatosis degree were similar in the PP analysis.
Figure 1.
Flow diagram of participant recruitment, screening and allocation.
MRI, magnetic resonance imaging.
Table 1.
Baseline characteristics.
| Group | Intention-to-treat
analysis |
Per-protocol analysis |
||||
|---|---|---|---|---|---|---|
| Routine treatment | Orlistat | p | Routine treatment |
Orlistat | p | |
| n | 102 | 68 | 76 | 54 | ||
| Age, years | 45.3 ± 12.8 | 46.0 ± 13.6 | 0.73 | 45.4 ± 13.0 | 45.4 ± 13.3 | 0.99 |
| Male | 70 (68.6%) | 37 (56.1%) | 0.08 | 56 (73.7%) | 32 (59.3%) | 0.08 |
| Smoking, yes | 14 (13.5%) | 10 (15.2%) | 0.76 | 11 (14.5%) | 9 (16.7%) | 0.73 |
| Weight, kg | 82.5 ± 11.6 | 84.8 ± 12.5 | 0.23 | 82.7 ± 11.4 | 85.5 ± 12.1 | 0.18 |
| Body mass index, kg/m2 | 29.7 ± 2.3 30.6 ± 3.0 | 30.3 ± 3.0 30.6 ± 3.0 | 0.14 | 29.9 ± 2.4 | 30.7 ± 3.0 | 0.07 |
| Waist circumference, cm | 95.6 ± 7.8 | 98.0 ± 8.3 | 0.06 | 95.9 ± 7.5 | 98.2 ± 8.6 | 0.11 |
| Bell circumference, cm | 99.4 ± 7.9 | 101.8 ± 8.5 | 0.06 | 99.7 ± 7.3 | 101.9 ± 7.8 | 0.10 |
| Waist-to-hip ratio | 0.9 ± 0.08 | 0.9 ± 0.13 | 0.10 | 0.9 ± 0.04 | 0.9 ± 0.12 | 0.35 |
| SBP, mmHg | 131.4 ± 18.1 | 133.6 ± 16.6 | 0.42 | 131.8 ± 18.3 | 132.7 ± 17.2 | 0.78 |
| DBP, mmHg | 85.6 ± 14.8 | 88.4 ± 13.6 | 0.22 | 85.1 ± 12.4 | 87.8 ± 11.6 | 0.20 |
| Liver biochemistry | ||||||
| ALT, U/l | 44.6 ± 30.0 | 48.4 ± 37.1 | 0.46 | 44.8 ± 31.9 | 48.7 ± 34.6 | 0.52 |
| AST, U/l | 36.3 ± 25.7 | 35.7 ± 17.6 | 0.87 | 35.3 ± 25.1 | 35.8 ± 17.5 | 0.89 |
| GGT, U/l | 57.8 ± 40.0 | 53.1 ± 43.5 | 0.47 |
59.9 ± 40.6 | 53.5 ± 46.4 | 0.79 |
| Alkaline phosphatase, U/L | 76.8 ± 19.4 | 85.8 ± 52.5 | 0.12 | 76.1 ± 20.9 | 83.5 ± 42.9 | 0.20 |
| Serum albumin, U/l | 46.8 ± 2.9 | 45.9 ± 3.1 | 0.08 | 46.7 ± 2.9 | 45.8 ± 2.9 | 0.09 |
| Serum globulin, U/l | 27.9 ± 4.3 | 28.4 ± 3.8 | 0.45 | 28.3 ± 4.2 | 28.9 ± 3.5 | 0.41 |
| Total bilirubin, μmol/l | 14.4 ± 6.1 | 14.6 ± 6.2 | 0.80 | 14.2 ± 6.0 | 14.0 ± 5.7 | 0.89 |
| Direct bilirubin, μmol/l | 2.7 ± 1.0 | 2.8 ± 1.1 | 0.27 | 2.7 ± 1.0 | 2.8 ± 1.1 | 0.69 |
| Metabolism | ||||||
| Uric acid, μmol/l | 426.6 ± 97.1 | 418.6 ± 119.0 | 0.63 | 420.1 ± 97.9 | 423.7 ± 114.8 | 0.85 |
| Cholesterol, mmol/l | 5.2 ± 1.0 | 5.1 ± 1.2 | 0.50 | 5.2 ± 1.1 | 5.1 ± 1.3 | 0.51 |
| Triglyceride, mmol/l | 2.1 ± 1.9 | 1.8 ± 1.5 | 0.42 | 2.3 ± 2.2 | 1.9 ± 1.6 | 0.27 |
| HDL-cholesterol, mmol/l | 1.2 ± 0.2 | 1.2 ± 0.3 | 0.20 | 1.1 ± 0.2 | 1.2 ± 0.3 | 0.24 |
| LDL-cholesterol, mmol/l | 3.6 ± 3.5 | 3.2 ± 0.8 | 0.32 | 3.8 ± 4.1 | 3.2 ± 0.8 | 0.32 |
| Apolipoprotein -A, mmol/l | 1.3 ± 0.2 | 1.3 ± 0.3 | 0.63 | 1.3 ± 0.2 | 1.3 ± 0.3 | 0.76 |
| Apolipoprotein -B, mmol/l | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.23 | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.19 |
| Free fatty acid, mmol/l | 607.0 ± 196.5 | 608.5 ± 198.2 | 0.97 | 609.8 ± 212.1 | 605.1 ± 196.2 | 0.91 |
| Fasting glucose, mmol/l | 5.2 ± 1.1 | 5.3 ± 1.2 | 0.74 | 5.3 ± 1.2 | 5.3 ± 1.2 | 0.81 |
| Fasting insulin, μU/ml | 12.9 ± 5.3 | 13.5 ± 7.0 | 0.26 | 13.9 ± 4.9 | 14.2 ± 7.2 | 0.77 |
| HOMA-IR | 3.1 ± 1.6 | 3.3 ± 2.3 | 0.25 | 3.3 ± 1.5 | 3.5 ± 2.4 | 0.57 |
| Liver fat content of a whole-liver ROI, % | ||||||
| Total | 18.4 ± 8.6 | 18.0 ± 8.9 | 0.77 | 18.8 ± 8.5 | 18.2 ± 9.0 | 0.69 |
| Pancreas fat content, % | ||||||
| Head | 2.8 ± 2.7 | 3.0 ± 2.8 | 0.67 | 2.8 ± 2.6 | 3.1 ± 3.2 | 0.60 |
| Body | 2.8 ± 2.4 | 2.6 ± 2.7 | 0.80 | 2.7 ± 2.3 | 2.8 ± 3.1 | 0.96 |
| Tail | 2.6 ± 2.0 | 2.4 ± 3.0 | 0.69 | 2.6 ± 1.8 | 2.6 ± 3.4 | 0.98 |
| Liver stiffness with 2D-SWE, kpa*
SWE, (kpa) |
5.2 (4.3–6.7) | 5.4 (4.6–6.5) | 0.43 | 5.4 (4.5–6.8) | 5.6 (4.7–6.4) | 0.27 |
Values shown are n (%) or means ± standard deviation.
Continuous variables are expressed as median with 25–75% interquartile range for non-Gaussian distribution.
2D-SWE, two-dimensional shear wave elastography; ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; DBP, diastolic blood pressure; GGT, gamma-glutamyl transferase; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein; ROI, region of interest; SBP, systolic blood pressure.
Effects of orlistat on steatosis, weight loss and metabolic indicators
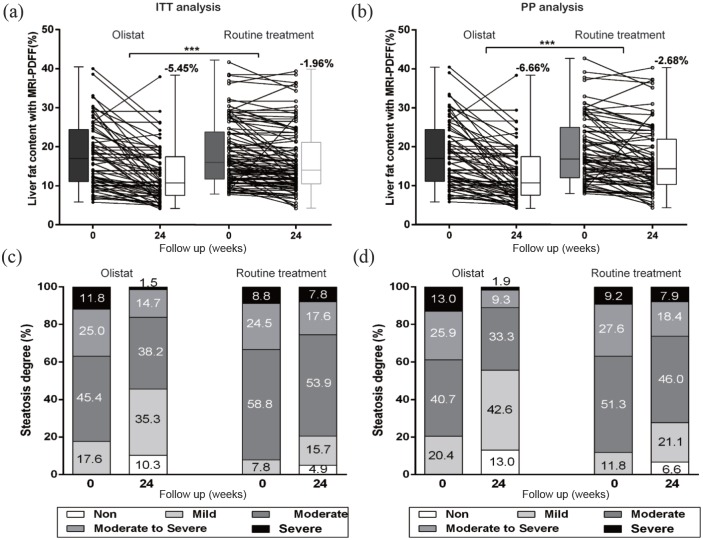
After intervention for 6 months, a significantly greater decrease in total liver fat content (Figure 2) according to MRI-PDFF using the whole liver as the ROI was found in the orlistat treatment group compared with the routine treatment group [−5.45% versus −1.96%, p < 0.001 for ITT analysis; Figure 3(a), and −6.66% versus −2.68%, p < 0.001 for PP analysis; Figure 3(b)]. Orlistat improved the steatosis grade by four grades in 1.5% of patients, by two grades in 11.8% of patients and by one grade in 44.1% of patients in the ITT population, while steatosis improved by four grades in 1.9%, two grades in 14.8% and one grade in 53.7% of patients in the PP subgroup. Steatosis improved in a higher percentage of patients receiving orlistat treatment than in the routine treatment group [57.3% versus 23.5%, p < 0.001 for ITT analysis, and 70.4% versus 32.9%, p < 0.001 for PP analysis, Figure 3(c, d)]. By reassessing our MRI-PDFF measurements with centrally placed ROIs in all liver segments, decreases in the mean liver fat fraction of −6.41% and −2.46% were observed in the orlistat therapy and routine treatment groups, respectively, in the PP population, which were similar to the decreases observed using the whole-liver ROI (p = 0.84 and p = 0.69, respectively). Furthermore, there was a strong correlation between the liver fat fraction changes measured by these two different methods (orlistat group: Pearson’s r = 0.96, p < 0.0001; routine treatment group: Pearson’s r = 0.92, p < 0.0001). No significant difference in the reduction of the fat fraction across liver segments was detected by ANOVA (p = 0.98, Table 2).
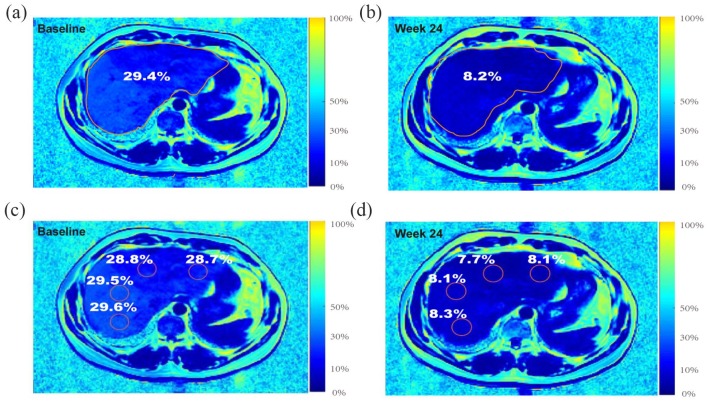
Figure 2.
Example of measuring the percentage change in liver fat fraction with MRI-PDFF. A 45-year-old male with moderate-to-severe steatosis was treated with orlistat. (a) Pretreatment MRI-PDFF demonstrated a total liver fat fraction of 29.4%. (b) The 6-month follow-up MRI-PDFF imaging exhibited a 21.2% decrease in the liver fat content of the total segments of liver (total liver fat fraction of 8.2%). (c) A pretreatment MRI-PDFF map. (d) A 6-month follow-up MRI-PDFF imaging map, placing ROIs centrally in all liver segments for the same patient.
MRI-PDFF, magnetic resonance imaging-derived proton density fat fraction; ROI, region of interest.
Figure 3.
Changes in liver fat fraction with MRI-PDFF in the ITT analysis (a) and PP analysis (b). Proportion of fatty liver grade reduction from baseline to 6 months of treatment in the ITT analysis (c) and PP analysis (d). Paired Student’s t-tests were used to determine whether changes in liver fat fraction were significantly different between cohorts. ***p < 0.001.
ITT, intention to treat; MRI-PDFF, magnetic resonance imaging-derived proton density fat fraction; PP, per protocol.
Table 2.
Segmental changes of liver fat fractions by orlistat therapy and routine treatment.
| Routine treatment
(n = 76) |
Orlistat
(n = 54) |
||||||
|---|---|---|---|---|---|---|---|
| Liver segment | Before | After | Changes | Before | After | Changes | p value for changes |
| I | 17.9 ± 8.4 | 15.6 ± 7.6 | –2.3 ± 5.6 | 18.5 ± 8.7 | 12.2 ± 7.3 | –6.3 ± 6.8 | 0.004 |
| II | 18.0 ± 8.4 | 16.7 ± 7.5 | –2.3 ± 5.6 | 17.8 ± 8.6 | 11.4 ± 7.2 | –6.4 ± 6.8 | 0.003 |
| III | 18.0 ± 8.3 | 15.7 ± 7.5 | –2.3 ± 5.6 | 17.7 ± 8.7 | 11.4 ± 7.3 | –6.3 ± 6.8 | 0.004 |
| IV | 18.1 ± 8.4 | 16.9 ± 7.5 | –2.2 ± 5.5 | 17.8 ± 8.6 | 11.5 ± 7.2 | –6.3 ± 6.8 | 0.002 |
| V | 18.8 ± 8.6 | 16.0 ± 7.7 | –2.8 ± 6.2 | 18.6 ± 9.4 | 11.9 ± 7.5 | –6.7 ± 7.2 | 0.001 |
| VI | 18.4 ± 8.7 | 15.6 ± 7.5 | –2.8 ± 6.3 | 18.9 ± 9.2 | 12.6 ± 7.5 | –6.3 ± 6.9 | 0.003 |
| VII | 19.1 ± 8.8 | 16.6 ± 7.8 | –2.5 ± 6.3 | 19.1 ± 9.2 | 12.5 ± 7.4 | –6.6 ± 7.0 | <0.001 |
| VIII | 19.1 ± 8.6 | 15.6 ± 7.8 | –2.5 ± 6.1 | 18.9 ± 9.3 | 12.8 ± 7.5 | –6.1 ± 7.1 | 0.002 |
| Liver fat content, average in all segments, % | 18.5 ± 8.5 | 16.0 ± 7.6 | –2.46 ± 5.8 | 18.4 ± 8.9 | 12.0 ± 7.3 | –6.41 ± 6.9 | <0.001 |
Values were devied from the per-protocol analysis cohort.
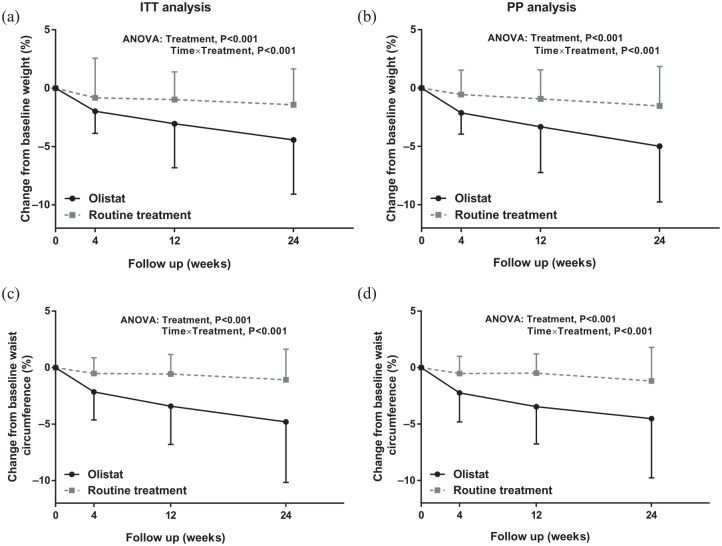
Regarding obesity-related parameters, the mean percentage changes in BMI and waist circumference decreased significantly over time in the two groups, although more remarkably in the orlistat group (repeated-measures ANOVA, p < 0.001, in both the ITT and PP cohorts; Figure 4) than in the routine treatment group. The mean changes in ALT, AST, total cholesterol, triglyceride, LDL-C, fasting blood glucose levels, fasting plasma insulin levels, uric acids, free fatty acids, and the fat fraction of the pancreas over the 6-month intervention in the orlistat and routine treatment groups were similar after ITT and PP analysis (Table 3).
Figure 4.
Body mass index (a) and waist circumference (c) at baseline and after 1, 3 and 6 months of treatment with orlistat or routine treatment in the ITT analysis. Body mass index (b) and waist circumference (d) at baseline and after 1, 3 and 6 months of treatment with orlistat or routine treatment in the PP analysis. Bars show the mean, and error bars represent standard deviations of relative reduction to baseline. Data points in each group with repeated measures at each time point are shown with an interconnecting line.
ANOVA, analysis of variance; ITT, intention-to treat; PP, per protocol.
Table 3.
Changes of obesity, liver biochemistry and other metabolic outcomes with orlistat or routine treatment at week 24.
| Group | Intention-to-treat
analysis |
Per-protocol analysis |
||||
|---|---|---|---|---|---|---|
| Routine treatment |
Orlistat | p | Routine treatment |
Orlistat | p | |
| n | 102 | 68 | 76 | 54 | ||
| Body mass index, kg/m2 | –0.4 ± 0.9 | –1.3 ± 1.4 | <0.001 | –0.5 ± 1.0 | –1.5 ± 1.4 | <0.001 |
| Waist circumference, cm | –1.1 ± 2.8 | –4.6 ± 5.3 | <0.001 | –1.2 ± 2.9 | –4.5 ± 5.2 | <0.001 |
| Bell circumference, cm | –1.1 ± 2.7 | –4.8 ± 5.4 | <0.001 | –1.1 ± 2.9 | –4.7 ± 5.1 | <0.001 |
| SBP, mmHg | –1.2 ± 9.0 | –0.6 ± 15.5 | 0.75 | –1.1 ± 9.9 | 0.3 ± 16.4 | 0.57 |
| DBP, mmHg | –0.1 ± 7.6 | –2.5 ± 13.1 | 0.14 | 0.0 ± 7.8 | –1.8 ± 12.9 | 0.32 |
| Liver biochemistry | ||||||
| ALT, U/l | –6.4 ± 20.5 | –15.1 ± 38.5 | 0.06 | –5.6 ± 20.2 | –16.5 ± 39.8 | 0.06 |
| AST, U/l | –3.7 ± 8.4 | –7.0 ± 16.9 | 0.09 | –3.2 ± 7.8 | –8.3 ± 17.1 | 0.08 |
| GGT, U/l | –9.5 ± 14.2 | –12.8 ± 23.8 | 0.31 | –8.6 ± 14.7 | –15.7 ± 26.0 | 0.07 |
| ALP, U/l | –1.6 ± 8.6 | –10.8 ± 48.9 | 0.06 | –1.2 ± 9.7 | –5.4 ± 42.0 | 0.43 |
| Metabolism | ||||||
| Uric acid, μmol/l | –23.3 ± 63.8 | –25.2 ± 132.3 | 0.91 | –22.9 ± 58.4 | –12.3 ± 32.9 | 0.56 |
| Cholesterol, mmol/l | –0.2 ± 0.9 | –0.5 ± 1.4 | 0.09 | –0.2 ± 0.7 | –0.4 ± 1.1 | 0.21 |
| Triglyceride, mmol/l | –0.4 ± 1.3 | –0.1 ± 1.3 | 0.24 | –0.4 ± 1.5 | –0.1 ± 1.4 | 0.21 |
| LDL-cholesterol, mmol/l | –0.5 ± 3.3 | –0.4 ± 0.9 | 0.84 | –0.6 ± 4.0 | –0.3 ± 0.8 | 0.63 |
| Apolipoprotein -A, mmol/l | 0.0 ± 0.3 | –0.1 ± 0.3 | 0.20 | 0.0 ± 0.4 | 0.0 ± 0.2 | 0.51 |
| Apolipoprotein -B, mmol/l | 0.0 ± 0.1 | –0.1 ± 0.3 | 0.06 | 0.0 ± 0.1 | 0.1 ± 0.2 | 0.11 |
| Free fatty acid, mmol/l | –36.6 ± 150.4 | –95.1 ± 257.7 | 0.063 | –41.6 ± 159.5 | –88.3 ± 257.7 | 0.27 |
| Fasting glucose, mmol/l | –0.2 ± 0.6 | –0.5 ± 1.5 | 0.07 | –0.2 ± 0.6 | –0.3 ± 1.2 | 0.42 |
| HOMA-IR | –0.2 ± 1.2 | –0.6 ± 1.3 | 0.07 | –0.2 ± 1.3 | –0.7 ± 1.6 | 0.06 |
| Pancreas fat content, % | ||||||
| Head | –0.6 ± 1.9 | –0.2 ± 3.7 | 0.41 | –0.8 ± 2.1 | –0.2 ± 3.9 | 0.26 |
| Body | –1.1 ± 1.5 | –0.3 ± 3.4 | 0.07 | –1.2 ± 1.5 | –0.3 ± 3.5 | 0.21 |
| Tail | –0.8 ± 1.6 | 0.0 ± 3.2 | 0.06 | –1.0 ± 1.7 | 0.0 ± 3.3 | 0.21 |
ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; DBP, diastolic blood pressure; GGT, gamma-glutamyl transferase; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein; SBP, systolic blood pressure.
Predictors of steatosis improvement
We analyzed the factors in the binary logistic regression model of steatosis improvement in the PP cohort. For all patients, orlistat treatment, hypercholesterolemia, moderate-to-severe and severe steatosis according to MRI-PDFF and change in body weight were identified as predictors of steatosis improvement in the univariate analysis (Table 4). After multivariate analysis, orlistat treatment [odds ratio (OR) = 3.1, 95% confidence interval (CI) 1.3–7.4, p = 0.009], moderate-to-severe and severe steatosis according to MRI-PDFF (OR = 3.6, 95% CI 1.5–8.6, p = 0.003) and weight loss (OR = 1.3, 95% CI 1.1–1.5, p < 0.001) were identified as predictors of steatosis improvement (Table 4). A univariate analysis of the change in the fat fraction according to MRI-PDFF as a continuous variable also revealed significant correlations of the orlistat treatment, weight change and moderate-to-severe and severe steatosis with the whole-liver fat content. The multivariate linear regression analysis revealed that the estimated effect of orlistat on hepatic steatosis was −2.1% (p = 0.04) after controlling for baseline steatosis (β = −4.5, p < 0.001) and weight loss (β = −0.6, p < 0.001).
Table 4.
Predictors of steatosis grades decrease by orlistat therapy and routine treatment.
| Per-protocol cohort |
Routine treatment |
Orlistat |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
Univariate |
Multivariate |
|||||||
| Variables | OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| Orlistat treatment | 4.8 (2.3–10.3) | <0.001111 | 3.1 (1.3–7.4) | 0.009 | – | – | – | – | ||||
| Female | 1.0 (0.5–2.0) | 0.89 | 0.4 (0.1–1.3) | 0.21 | 2.7 (0.7–9.9) | 0.13 | ||||||
| Smoking | 1.4 (0.5–3.6) | 0.53 | 1.9 (0.5–6.9) | 0.34 | 0.8 (0.2–3.7) | 0.79 | ||||||
| BMI > 30 kg/m2 | 1.4 (0.7–2.9) | 0.30 | 1.1 (0.4–3.0) | 0.82 | 1.1 (0.3–3.5) | 0.91 | ||||||
| WHR > 0.9 | 0.8 (0.4–1.7) | 0.65 | 1.0 (0.4–2.6) | 0.95 | 0.5 (0.1–1.9) | 0.31 | ||||||
| Hypertension | 1.1 (0.5–2.3) | 0.83 | 1.3 (0.4–3.7) | 0.67 | 1.0 (0.4–2.2) | 0.54 | ||||||
| ALT > 2×ULNa | 1.7 (0.5–5.5) | 0.38 | 0.4 (0.0–4.2) | 0.48 | 3.4 (0.4–7.1) | 0.27 | ||||||
| HOMA-IR > 3 | 1.2 (0.5–3.6) | 0.62 | 0.7 (0.2–2.0) | 0.47 | 0.8 (0.2–3.7) | 0.79 | ||||||
| CHOL > 5.7 mmol/l | 0.4 (0.1–0.9) | 0.032 | 0.4 (0.1–1.3) | 0.14 | 0.3 (0.1–0.9) | 0.041 | 0.3 (0.3–1.2) | 0.10 | 1.0 (0.2–6.5) | 1.0 | ||
| TG > 1.7 mmol/l | 0.7 (0.3–1.6) | 0.46 | 0.9 (0.3–2.6) | 0.91 | 1.0 (0.2–4.4) | 0.97 | ||||||
| Hyperuricemia | 0.7 (0.3–1.6) | 0.38 | 0.6 (0.2–1.7) | 0.35 | 0.6 (0.3–1.4) | 0.53 | ||||||
| Steatosis degree | ||||||||||||
| Mild to moderate | Reference | – | Reference | – | Reference | – | – | |||||
| Moderate to severe and severe | 2.6 (1.3–5.5) | 0.009 | 3.6 (1.5–8.6) | 0.003 | 2.6 (1.0–7.0) | 0.058 | 2.7 (0.9–0.8) | 0.076 | 3.9 (1.0–15.9) | 0.048 | 8.1 (1.4–46.2) | 0.018 |
| Weight loss (%) | 1.3 (1.2–1.5) | <0.001 | 1.3 (1.1–1.5) | <0.001 | 1.4 (1.1–1.7) | 0.006 | 1.3 (1.1–1.6) | 0.044 | 1.2 (1.1–1.4) | 0.045 | 1.3 (1.0–1.6) | 0.016 |
Normal ALT levels represent 40 U/l for women and men.
ALT, alanine aminotransferase; BMI, body mass index; CHOL, cholesterol; CI, confidence interval; HOMA-IR, homeostasis model assessment of insulin resistance; OR, odds ratio; TG, triglyceride; ULN, upper limit of normal; WHR, waist-to-hip ratio.
Among patients receiving orlistat therapy, we found that weight loss and moderate-to-severe and severe steatosis according to MRI-PDFF were favorable predictors of steatosis improvement in the univariate analysis (Table 4). Multivariate analysis after adjustment for confounders confirmed that weight loss (OR = 1.3, 95% CI 1.0–1.6, p = 0.016) and moderate-to-severe and severe steatosis according to MRI-PDFF (OR = 8.1, 95% CI 1.4–46.2, p = 0.018) remained independent predictors of steatosis improvement (Table 4).
Discussion
In this single-center study, 130 patients with NAFLD receiving orlistat therapy or routine treatment were prospectively analyzed. The results showed that orlistat treatment resulted in a higher degree of amelioration of steatosis grade than routine therapy. We further identified that increased weight loss within 6 months and severe steatosis were predictors of steatosis response. To the best of the authors’ knowledge, this is the first study to examine the true extent of the effect of orlistat therapy on steatosis in a cohort of Asian patients using MRI-PDFF as the endpoint.
Liver fat content has been established as an important risk factor for diabetes and ischemic heart disease.22,31 Improvement of steatosis was associated with remission of hepatic apoptosis and necroinflammation, suggesting that reducing hepatic fat may be critical for improving hepatic inflammation and even the long-term prognosis.25 According to our research, significant reductions in liver fat content were observed with orlistat treatment for 24 weeks compared with routine treatment. Similarly to our results, ultrasound grading improvements in fatty liver were reported at higher rates in 30 patients (66.67%) in an orlistat group than in 14 patients (43.75%) in a diet control group in a previous study.32 Furthermore, another RCT from Israel8 demonstrated that patients treated with orlistat for 24 weeks had a higher reversal rate of fatty liver (24% versus 17%, p = 0.04) than those in a hypocaloric diet group. However, another landmark RCT9 assessed with histopathology showed that orlistat therapy for 36 weeks was not superior to lifestyle modification in inducing improvements in hepatic steatosis in White patients. The reason for the conflicting results may be due in part to the method used to measure steatosis improvements. Although abdominal ultrasound is the preferred first-line screening method for NAFLD, it is subjective and qualitative in assessing NAFLD, especially for mild steatosis.33 Therefore, it may detect treatment efficacy with low accuracy. Liver biopsy with histology scoring is the gold standard for estimating steatosis improvements; however, liver biopsy cannot sample exactly the same anatomic location at baseline and after treatment and therefore allows only a semi-quantification of steatosis.34 The reductions in steatosis grade may vary by different liver fat distributions and pathologists. As MRI-PDFF provides a measure of the fat infiltration severity across the whole liver with a mean value of the fat fraction, we were able to more sensitively investigate the change in liver fat content, strengthening the argument that orlistat treatment may be beneficial in lowering the fat fraction in NAFLD. Our study is the first to report an estimated mean difference of 4% in the change in fat content (orlistat: −6.66% versus −2.68% for the routine treatment) in orlistat treatment compared with the routine treatment after 6 months of therapy in an Asian cohort. It was reported that histologic responders in a clinical trial in NAFLD presented a marked reduction according to MRI-PDFF of −4.1% ± 4.9 versus −0.6 ± 4.1 (difference of 3.5%, p < 0.04).35 Therefore, a reduction in fat content of 4% may reflect histologic remission; however, this hypothesis should be interpreted with caution because paired biopsies were not performed in our research. Interestingly, the estimated mean difference of 4% in fat content was also similar to the percentages in two recent reports advocating novel effective treatments for lowering the liver fat fraction.30,36 Therefore, orlistat was beneficial for improving intrahepatic lipid accumulation in NAFLD.
Currently, weight loss is recognized as a key determinant of steatosis improvement after lifestyle changes. However, whether the effect of orlistat on steatosis depends on weight loss is unclear. Several other studies have reported that weight, BMI and waist-to-hip ratio were not significantly decreased between orlistat groups (intervention for 16 or 24 weeks) and control groups,8,32 although a statistically significant reversal of sonographic fatty liver in the orlistat group was observed. In contrast, the orlistat-treated patients in our study obtained a significant reduction of 2.1 in BMI after 6 months, and weight reduction was identified as an independent determinant of steatosis improvement. Our results are well in line with a recent meta-analysis7 of approximately 330 patients with NAFLD with a follow up ranging from 16 to 36 weeks that demonstrated that orlistat intervention enabled a significant reduction in BMI of 1.97 (95% CI = −3.60 to −0.33). In another study,9 only weight loss of 9% following orlistat treatment was associated with a histological response (decrease in NAFLD activity score after treatment). These discrepancies may be related to differences in dietary habits, the orlistat intervention period and genetic background across studies, as most other studies performed on White individuals administered orlistat for less than 24 weeks and the diets and genetic backgrounds of the participants were different from those of the Chinese population. In this study, we found that weight loss induced by orlistat was associated with steatosis improvements in Chinese obese patients with NAFLD. Moreover, after controlling for baseline steatosis and weight loss, the multivariate linear regression analysis demonstrated the independent effect of orlistat on hepatic steatosis. This finding provides the important perspective that while hepatic fat loss was driven primarily by weight loss, there is an effect of orlistat on the NAFLD liver in addition to its primary effect on body weight in NAFLD.
Aggregations of visceral adiposity are now known to be highly correlated with NAFLD. In a recent clinical trial aimed at evaluating the efficacy of orlistat in Japanese participants with excessive visceral fat accumulation but no metabolic diseases, visceral fat area measured by computed tomography, waist circumference, and body weight were more effectively reduced in the orlistat group than in the placebo group.37 These findings were consistent with those of our research using simpler nonspecific fat indexes, including body weight, abdominal circumference and BMI. However, MRI techniques in our center could not quantify the visceral adiposity area, and the relationship between fat reduction in the right lobe of the liver and changes in the visceral adiposity area requires further study. Moreover, determining whether splanchnic-derived fatty acids are specifically inhibited by orlistat will require further physiological and mechanistic experiments.
In the logistic regression model analysis, patients with severe steatosis were more likely to obtain steatosis improvement from orlistat therapy, with an OR of 2.3 in the current study. Although it might be reasonable to attribute the primary efficacy of orlistat in patients with NAFLD to weight reduction,38 severe steatosis at baseline remained a statistically significant determinant even after adjusting for weight loss. Another potential explanation may be the reduction in the serum levels of lipopolysaccharide, periostin and tumor necrosis factor-α by orlistat, as well as the increase in protective endocrine cytokines, such as adiponectin,32 which were not measured in our study. These potentially relevant cytokines have been reported to correlate with steatosis grade, and changes in their levels promote amelioration of steatosis by orlistat treatment. In patients with mild or moderate steatosis, baseline cytokine levels were only mildly abnormal, and improving the fatty liver degree by reducing these levels is difficult with such a short duration of orlistat treatment.
Liver fat overaccumulation is caused by excessive lipid uptake and de novo lipogenesis combined with decreased lipid export or oxidation in the liver.39 The lipids that overflow to the liver originate from the release of free fatty acids (FFAs) by peripheral adipose tissue, which is mainly driven by both increased total body adiposity and an increased rate of FFA release.39 Orlistat treatment significantly reduced the BMI (mean difference = −1.97; p = 0.02) and visceral fat area in patients with obesity, therefore effectively lowering the mass of adipose tissues. Moreover, overfeeding with saturated FFAs markedly increased the severity of hepatic steatosis in humans,40 while a fat-lowering diet with an isocaloric strategy appears to reduce liver fat content.41 This evidence supports the idea that orlistat, as an inhibitor of fat uptake from the gut, would also be beneficial in preventing steatosis.
Although the two groups (receiving orlistat or routine treatment) exhibited similar baseline characteristics, several limitations existed in our study. The nonalcoholic steatohepatitis (NASH) proportion at baseline and histological remission could not be evaluated, as liver biopsy was not routinely performed in most of the included patients. With the open-label nature of the study design, the lack of blinding may have introduced a performance bias. The radiologists evaluating the MRI scans were blinded to the patients’ allocation to the two groups, and the outcome measurements by MRI-PDFF were therefore objective, which could partly reduce the ascertainment bias of the findings of this study. We also conducted ITT analyses, which naturally include some deviation from the protocol to lessen the impact of the open-label design. Although the sample size was similar to that of previous studies, it remained small, and the small number of patients in the subgroup analysis may weaken the power of the conclusions in our study.
In conclusion, our study suggested that orlistat can be an important treatment option for promoting steatosis improvement and achieving weight loss goals in Chinese patients with NAFLD in a general medical setting. A weight reduction greater than 5% after 6 months and severe steatosis at baseline might predict high steatosis improvement rates with orlistat treatment. More multicenter, prospective studies are needed to verify the long-term efficacy of orlistat treatment in NAFLD.
Acknowledgments
Junzhao Ye and Yanqin Wu contributed equally to this article.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by Special Project on the Integration of Industry, Education and Research of Guangzhou, China (grant number 201604020155), Science and Technology Program of Guangdong province, China (grant numbers 2013B021800290, 2014A020212118, and 2017A020215015) and National Natural Science Foundation of China (grant numbers 81870404, 81670518, and 81170392).
Conflict of interest statement: Junzhao Ye, Yanqin Wu, Fuxi Li, Tingfeng Wu, Congxiang Shao, Yansong Lin, Wei Wang, Shiting Feng, and Bihui Zhong declare that they have no conflict of interest.
ORCID iD: Bihui Zhong  https://orcid.org/0000-0002-4638-7699
https://orcid.org/0000-0002-4638-7699
Contributor Information
Junzhao Ye, Department of Gastroenterology, the First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China.
Yanqin Wu, Department of Interventional Oncology, the First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China.
Fuxi Li, Department of Gastroenterology, the First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China.
Tingfeng Wu, Department of Gastroenterology, the First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China.
Congxiang Shao, Department of Gastroenterology, the First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China.
Yansong Lin, Department of Gastroenterology, the First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China.
Wei Wang, Department of Medical Ultrasonics, the First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China.
Shiting Feng, Department of Radiology, the First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China.
Bihui Zhong, Department of Gastroenterology, the First Affiliated Hospital, Sun Yat-sen University, No. 58 Zhongshan II Road, Yuexiu District, Guangzhou, 510080, China.
References
- 1. Wong VW, Chan WK, Chitturi S, et al. Asia-Pacific working party on non-alcoholic fatty liver disease guidelines 2017-part 1: definition, risk factors and assessment. J Gastroenterol Hepatol 2018; 33: 70–85. [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Koenig AB, Abdelatif D. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64: 73–84. [DOI] [PubMed] [Google Scholar]
- 3. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 2016; 59: 1121–1140. [DOI] [PubMed] [Google Scholar]
- 4. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018; 67: 328–357. [DOI] [PubMed] [Google Scholar]
- 5. Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol 2017; 67: 829–846. [DOI] [PubMed] [Google Scholar]
- 6. Khera R, Murad MH, Chandar AK, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA 2016; 315: 2424–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang H, Wang L, Cheng Y, et al. Efficacy of orlistat in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Rep 2018; 9: 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zelber-Sagi S, Kessler A, Brazowsky E, et al. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2006; 4: 639–644. [DOI] [PubMed] [Google Scholar]
- 9. Harrison SA, Fecht W, Brunt EM, et al. Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology 2009; 49: 80–86. [DOI] [PubMed] [Google Scholar]
- 10. Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev 2002; 3: 141–146. [DOI] [PubMed] [Google Scholar]
- 11. Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol 2017; 67: 862–873. [DOI] [PubMed] [Google Scholar]
- 12. Xu Z, Duan Q, Cui J, et al. Analysis of genetic and nongenetic factors influencing triglycerides-lowering drug effects based on paired observations. BMC Proc 2018; 12: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caussy C, Reeder SB, Sirlin CB, et al. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology 2018; 68: 763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chinese Nutrition Society. Chinese Dietary Reference Intakes (2013). Beijing: Science Press, 2013, pp. 660. [Google Scholar]
- 15. Chinese Nutrition Society. Chinese Dietary Guidelines (2007). Beijing: The Tibet People’s Publishing House, 2008, pp. 205. [Google Scholar]
- 16. Waxman A. and World Health Assembly. WHO global strategy on diet, physical activity and health. Food Nutr Bull 2004; 25: 292–302. [DOI] [PubMed] [Google Scholar]
- 17. Multidisciplinary Expert Task Force on Hyperuricemia and Related Diseases. Chinese multidisciplinary expert consensus on the diagnosis and treatment of hyperuricemia and related diseases. Chin Med J (Engl) 2017; 130: 2473–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park S, Buranakitiaroen P, Chen CH, et al. Expert panel consensus recommendations for home blood pressure monitoring in Asia: the hope Asia network. J Hum Hypertens 2018; 32: 249–258. [DOI] [PubMed] [Google Scholar]
- 19. Weng J, Ji L, Jia W, et al. Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev 2016; 32: 442–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu KH, Chen DY, Chen JH, et al. Management of gout and hyperuricemia: multidisciplinary consensus in Taiwan. Int J Rheum Dis 2018; 21: 772–787. [DOI] [PubMed] [Google Scholar]
- 21. Li M, Zhang S, Wu Y, et al. Prevalence of insulin resistance in subjects with nonalcoholic fatty liver disease and its predictors in a Chinese population. Dig Dis Sci 2015; 60: 2170–2176. [DOI] [PubMed] [Google Scholar]
- 22. Dong Z, Luo Y, Zhang Z, et al. MR quantification of total liver fat in patients with impaired glucose tolerance and healthy subjects. PLoS ONE 2014; 9: e111283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caussy C, Alquiraish MH, Nguyen P, et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology 2018; 67:1348–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin SC, Heba E, Wolfson T, et al. Noninvasive diagnosis of nonalcoholic fatty liver disease and quantification of liver fat using a new quantitative ultrasound technique. Clin Gastroenterol Hepatol 2015; 13:1337–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jayakumar S, Middleton MS, Lawitz EJ, et al. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: analysis of data from a phase II trial of selonsertib. J Hepatol 2019; 70: 133–141. [DOI] [PubMed] [Google Scholar]
- 26. Gu J, Liu S, Du S, et al. Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis. Eur Radiol 2019; 29: 3564–3573. [DOI] [PubMed] [Google Scholar]
- 27. Le TA, Chen J, Changchien C, et al. San Diego Integrated NAFLD Research Consortium (SINC). Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology 2012; 56: 922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loomba R, Sirlin CB, Ang B, et al. ; San Diego Integrated NAFLD Research Consortium (SINC). Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 2015; 61: 1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cui J, Philo L, Nguyen P, et al. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol 2016; 65: 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuchay MS, Krishan S, Mishra SK, et al. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT Trial). Diabetes Care 2018; 41: 1801–1808. [DOI] [PubMed] [Google Scholar]
- 31. Lauridsen BK, Stender S, Kristensen TS, et al. Liver fat content, non-alcoholic fatty liver disease, and Ischaemic heart disease: Mendelian randomization and meta-analysis of 279 013 individuals. Eur Heart J 2018; 39: 385–393. [DOI] [PubMed] [Google Scholar]
- 32. Ali Khan R, Kapur P, Jain A, et al. Effect of orlistat on periostin, adiponectin, inflammatory markers and ultrasound grades of fatty liver in obese NAFLD patients. Ther Clin Risk Manag 2017; 13: 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bedossa P, Patel K. Biopsy and noninvasive methods to assess progression of nonalcoholic fatty liver disease. Gastroenterology 2016; 150: 1811–1822. [DOI] [PubMed] [Google Scholar]
- 34. Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 2011; 54: 1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patel J, Bettencourt R, Cui J, et al. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Therap Adv Gastroenterol 2016; 9: 692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yan J, Yao B, Kuang H, et al. Liraglutide, sitagliptin, and insulin glargine added to metformin: the effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Hepatology. 2019; 69: 2414–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shirai K, Fujita T, Tanaka M, et al. Efficacy and safety of lipase inhibitor orlistat in Japanese with excessive visceral fat accumulation: 24-week, double-blind, randomized, placebo-controlled study. Adv Ther 2019; 36: 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bedossa P. Current histological classification of NAFLD: strength and limitations. Hepatol Int 2013; 7: 765–770. [DOI] [PubMed] [Google Scholar]
- 39. Mittendorfer B, Magkos F, Fabbrini E, et al. Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity 2009; 17: 1872–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosqvist F, Iggman D, Kullberg J, et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes 2014; 63: 2356–2368. [DOI] [PubMed] [Google Scholar]
- 41. Utzschneider KM1, Bayer-Carter JL, Arbuckle MD, et al. Beneficial effect of a weight-stable, low-fat/low-saturated fat/low-glycaemic index diet to reduce liver fat in older subjects. Br J Nutr 2013; 109: 1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]