Abstract
Background:
Esophageal squamous cell carcinoma (ESCC) is the major type of esophageal cancer in Asia and demonstrates poor survival rates following a therapeutic regimen.
Methods:
Cancer stem cells (CSCs) are responsible for tumor initiation, progression, and treatment failure in cancers. Therefore, identification and characterization of CSCs may help to improve clinical outcomes for ESCC patients. Tumor sphere formation assay are performed to isolate cancer stem-like ESCC cells. QRT-PCR, tumor initiation, metastasis, CCRT treatment are used to evaluate ESCC cells’ stemness properties in vitro and in vivo.
Results:
The authors’ data demonstrates that cancer stem-like ESCC cells harbored stemness characteristics including self-renewal, differentiation, and transdifferentiation, and possess tumor initiation, metastasis, and treatment inefficiency properties. For the identification of useful biomarkers of cancer stem-like ESCC cells, the authors further identified that CLDN4 was upregulated in cancer stem-like ESCC cells when compared with bulk cancer cells. High-CLDN4 cells harbored stemness and cisplatin/concurrent chemoradiation therapy (CCRT) resistance properties and a high level of CLDN4 was correlated with poor prognosis and poor CCRT response in ESCC patients. Importantly, thiamine tetrahydrofurfuryl disulfide (TTFD) decreased CLDN4 and attenuated stemness in ESCC cells, and TTFD combined with CCRT improved CCRT response in vivo.
Conclusions:
CLDN4 was suggested as prognostic and a CCRT response indicator for ESCC patients. TTFD combined with CCRT has potential to improve ESCC patient’s clinical outcomes in the future.
Keywords: biomarker, cancer stem-like cells, concurrent chemoradiation therapy, CCRT, CLDN4, esophageal squamous cell carcinoma, thiamine tetrahydrofurfuryl disulfide (TTFD)
Introduction
Esophageal squamous cell cancer (ESCC) is among the most common malignant cancers, especially in Asia.1,2 The 5-year survival rate is <25% and has remained unchanged over the past several decades.3 The unfavorable prognosis for this type of cancer is due to the limited therapeutic options and inefficient treatments.4 Esophagectomy and concurrent chemoradiation therapy (CCRT) are the standard treatments for ESCC patients.5,6 Unfortunately, patients who receive esophagectomy alone are associated with a high rate of recurrence and a low 5-year survival rate.2 The majority of ESCC patients have advanced disease and receive CCRT alone or in combination with surgical resection.7 In total, approximately 30–35% of patients who received interventions suffered from tumor recurrence within 1 year.8 Therefore, understanding the mechanisms that lead to treatment failure, tumor recurrence, and improving therapeutic strategies are important issues for ESCC.
Cancer stem cells (CSCs) and normal stem cells share biological features including self-renewal, differentiation, and transdifferentiation potential.9 The CSC hypothesis may provide a valid explanation for therapeutic inefficiency, tumor initiation, progression, recurrence, and distal metastases in cancers.10,11 Therefore, understanding and characterizing CSCs could potentially help the development of effective therapeutic strategies for ESCC patients. However, a lack of identified CSCs biomarkers in ESCC prevents accurate estimation of prognosis and creates inefficiency in assigning therapeutic approaches for ESCC patients. A recent study reported that CSCs, marked by CD133+, contributed to glioma radioresistance through preferential activation of the DNA damage checkpoint response and an increased capacity for DNA repair.12 An increasing amount of evidence also indicates that CD133+ cancer cells harbor CSCs properties that combined with high levels of ABC transporters, contribute to chemoresistance.13–15 Because CD44+CD24– is a widely accepted biomarker of CSCs in breast cancer, its relevance is worth discussing in ESCC. In addition, p75NTR+cells were reported to harbor self-renewal capacity and chemotherapeutic resistance in corneal epithelial stem cells and ESCC, but this marker has not been thoroughly evaluated as a CSC biomarker in ESCC.16 Therefore, identification of reliable CSCs biomarkers is a critical, unmet need for improving therapeutic strategies in ESCC patients.
In this study, the authors aim to establish an in vitro culture system to isolate cancer stem-like ESCC cells and demonstrate that the isolated cells participate in tumor initiation, metastasis, chemoresistance, radioresistance, and CCRT resistance in vitro and in vivo. In addition, the authors found that CLDN4 functions as a potential biomarker of cancer stem-like ESCC cells and is positively correlated with ineffective CCRT treatment. Importantly, thiamine tetrahydrofurfuryl disulfide (TTFD) was found to decrease CLDN4, diminish CSCs characteristics and improve CCRT therapeutic effects in vitro and in vivo. Overall, the authors identified that CLDN4 can serve as a potential CSCs marker, a prognostic and CCRT response indicator for ESCC patients. Combinations of CCRT and TTFD provide a novel therapeutic strategy to improve the clinical outcome for ESCC patients in the future.
Materials and methods
Cell cultures
KYSE70 and KYSE170 cells were cultured in RPMI-1640 (Gibco, 31800-089), supplemented with 10% fetal bovine serum (FBS; Hyclone), 100 IU/ml penicillin, and 100 µg/ml streptomycin (Caisson, PSL01-100ML). CE48T cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, 12100-061), supplemented with 10% FBS, 2 mM L-glutamine and 1% P/S. All cells were maintained at 37°C in a humidified atmosphere of 5% CO2.
Tumor sphere formation assay
1 × 105 cells were cultured as spheres in 10 cm ultra-low adhesion dishes at a density containing DMEM/F12 (Gibico) with 20 ng/ml rhEGF, 10 ng/ml rhbFGF, N-2 Supplement (Gibco, 17502-048), 100 IU/ml penicillin, and 100 µg/ml streptomycin (Caisson, PSL01-100ML).14 Spheres were passaged every 7 days with 0.05% trypsin-EDTA (Invitrogen) or accutase (Gibico). The spheroid cells were maintained at 37°C in a humidified atmosphere of 5% CO2. The diameter of the tumorspheres ranged from 100 to 300 µm.
Determination of IC50 value for Cisplatin in cells
A total of 1 × 105 cells were plated in cell culture coated or ultra-low adhesion 96-well plates and exposed to different concentrations of cisplatin. After incubation for 48 h, cell viability was assessed with methyl thiazol tetrazolium (MTT) (Sigma, USA) by measuring optical density at 570 nm using an ELISA reader. Cell viability was calculated according to the following formula: Cell viability (%) = cells (sample)/cells (control) × 100 and IC50 was calculated using log formula by GraphPad Prism 6.
Evaluation of tumor initiation ability and CCRT response in the mouse model
NOD-SCID mice were obtained from the National Cheng Kung University Laboratory Animal Center. All mice were maintained using standard protocols, and the experiment was approved by the Institutional Animal Care and Use Committee, National Cheng Kung University (IACUC Approval No: 101026). To measure tumor initiation, spheroid and parental cells were subcutaneously injected into the left and right flank of nude mice (5 weeks), respectively. To evaluate metastasis, 1000 spheroid or parental cells were injected into a mouse by a tail vein. To determine the CCRT response, 1 × 105 cells were subcutaneously injected into the left and right flank of nude mice (5 weeks). After tumors reached 5 mm in diameter, the mice were exposed to 4 Gy radiation combined with 2 mg/kg cisplatin by intraperitoneal injection.17 The tumor signal from all of the mice was monitored by IVIS Spectrum in vivo imaging every week.
Clinical specimens
Primary esophageal tumors and adjacent matched normal esophageal tissues were obtained from National Cheng Kung University Hospital (Tainan, Taiwan). This study received Institutional Review Board approval (IRB numbers: A-ER-102-228; BR-100-087). Primary samples were collected with informed consent and with approval from institutional review boards. The esophageal tissue microarray was constructed using 139 specimens from patients. In addition, 22 patients donated tissue before and after CCRT treatment to evaluate the expression of CLDN4.
Statistical analyses
All observations were confirmed by at least three independent experiments. Data was expressed as means ± SEM. The clinical features were analyzed using the chi-squared test and Student’s t test. The association between overall survival was analyzed using log-rank Kaplan–Meier analysis. Statistical comparisons of the results were made using a Student’s t test. All tests were two-sided, and a p value <0.05 was considered to be statistically significant. SPSS version 20 (SPSS Inc.) and GraphPad Prism 6 software were used to analyze data.
Results
Isolation and characterization of cancer stem-like ESCC cells and evaluation of their oncogenic potential
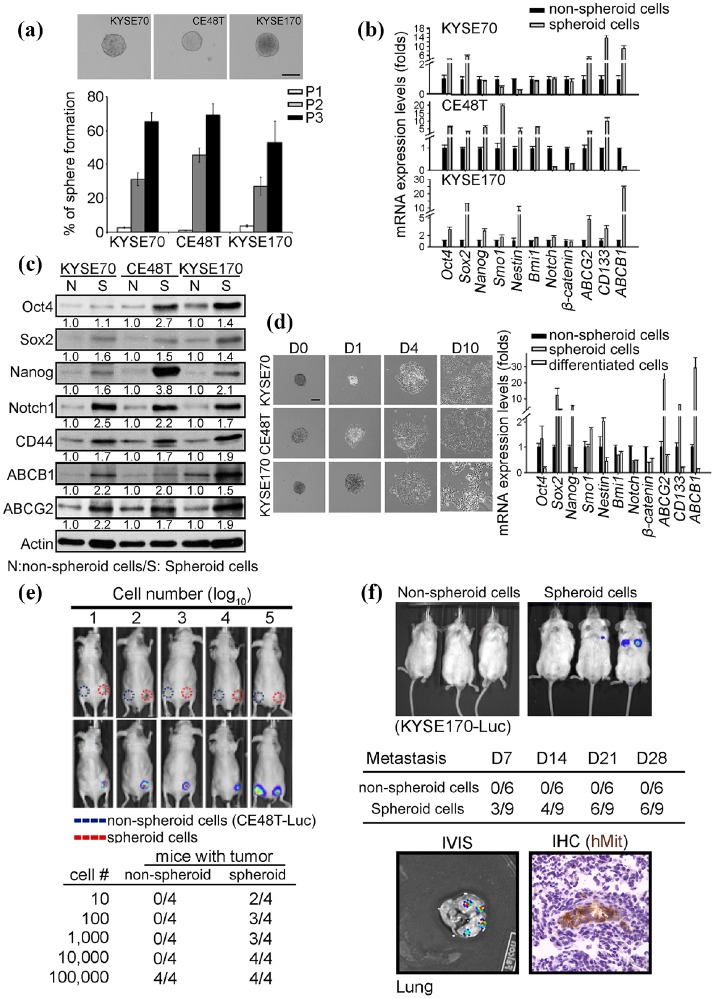
To investigate the role of CSCs in ESCC, the KYSE70, CE48T, and KYSE170 cell lines were cultured in ultra-low attachment plates to establish tumorspheres. Approximately 1–2% of cells formed tumorspheres of 100–300 mm diameter over 7 days. Notably, serial passaging enriched the population of cancer stem-like ESCC cells (Figure 1(a)), indicating that the tumorspheres harbored self-renewal capability. Because the growth of CSCs is expected to be accompanied by the upregulation of stemness and drug resistance-related genes, gene expression was evaluated in both spheroid and nonspheroid ESCC cells. The data demonstrated that the majority of the measured stemness-associated and drug resistance-related genes were upregulated in spheroid compared with nonspheroid cells, including Oct-4, SOX2, NANOG, ABCB1, ABCG2, and CD133 (Figure 1(b)). Similar to mRNA, the stemness-associated and drug resistance proteins were increased in spheroid cells (Figure 1(c)). The CSC hypothesis predicts that CSCs can differentiate into cancer cells and contribute to the heterogeneity of tumors. To examine the differentiation capacity of tumorspheres, spheroid cells were re-seeded in cell culture coated plates with DMEM medium and 10% FBS to induce cell differentiation. The spheroid cells re-attached onto the plates and the majority of the stemness-associated and drug resistance genes were significantly decreased after tumorsphere differentiation (Figure 1(d)). According to the results, ESCC tumorspheres certainly demonstrated cell differentiation ability. In addition, the spheroid cells can transdifferentiate into endothelial cells, which was confirmed by CD31 immunofluorescence staining. CSCs should also have tumor-initiating and metastasis capabilities. The tumor initiation ability of nonspheroid and spheroid cells was examined by limiting dilution cell transplantation assays. As expected, 10 spheroid cells can initiate tumors in 2 out of 4 mice. Tumor formation was also observed in mice injected with 100–10,000 spheroid cells, which suggest that cancer stem-like ESCC cells have tumor initiation ability. These results were in stark contrast to nonspheroid cells, which required injections of 1 × 105 cells to initiate tumors in 8 weeks. (Figure 1(e)). In addition, the spheroid and nonspheroid cells were injected into mice via the tail vein to investigate the metastatic ability of cancer stem-like ESCC cells. Signals from metastatic lesions were detected by IVIS in 33% of mice injected with spheroid cells 7 days after injection. The metastatic rates increased with time to 44% (14 days) and 67% (21 and 28 days) in spheroid cell-injected mice. In contrast, nonspheroid cell-injected mice did not exhibit detectable signals until 28 days after injection (Figure 1(f)). Lung tissue with positive IVIS signals was harvested to confirm that the metastatic lesions were derived from ESCC cells, by immunohistochemical staining with a human mitochondrial antibody (Figure 1(f) bottom panel). In combination this data demonstrates that the isolated cancer stem-like ESCC cells harbor self-renewal, differentiation, transdifferentiation, tumor initiation, and metastasis potential.
Figure 1.
Cancer stem-like ESCC cells promote tumor initiation and metastasis. A tumorsphere culture system was used to isolate cancer stem-like ESCC cells in KYSE70, CE48T, and KYSE170 cells. The isolated KYSE70, CE48T, and KYSE170 cells were subjected to characterize the CSCs properties in vitro and in vivo. (a) The sphere-forming assay was used to evaluate self-renewal ability in ESCC cells. The cells were seeded into 10 cm ultra-low adhesion dishes and incubated in stem cell culture medium at a density of 1 × 105 cells/dish. 7 days after seeding, tumorspheres were generated and the spheroid cells were then trypsinized and 1 × 105 spheroid cells were used for the next passage. Three passages were processed and the percentage of tumor formation was counted. Scale bar: 100 µm. (b) and (c) The indicated mRNA and protein level was examined by qPCR and western blot in nonspheroid and spheroid ESCC cells. Quantified results of western blot indicated the relative protein expression level in spheroid cells compared with nonspheroid cells. (d) Tumorspheres were incubated in a serum-induced differentiation condition medium. Representative images of the differentiate spheroid cells were taken on day 0 (d0), day 1 (d1), day 4 (d4), and day 10 (d10) after incubation. The mRNA expression levels of indicated stemness genes and drug-resistant genes were examined in nonspheroid, spheroid, and differentiated KYSE70 cells by qPCR. (e) A series of nonspheroid and spheroid KYSE170-Luc cells were subcutaneously injected into immunodeficiency mice and monitor tumor growth weekly using an in vivo Imaging Systems (IVIS) system. (f) The tail vein injection model was used to investigate the metastatic ability in vivo. A total of1000 nonspheroid and spheroid KYSE170-Luc cells were injected into immunodeficiency mice using tail vein injections, the metastases were monitored weekly using an IVIS system. Four weeks after injection, the mice were sacrificed and the lungs were harvested and the metastases were examined by immunohistochemistry using human mitochondrial antibody. (Scale bar: 100 µm).
CSCs, cancer stem cells; ESCC, esophageal squamous cell carcinoma
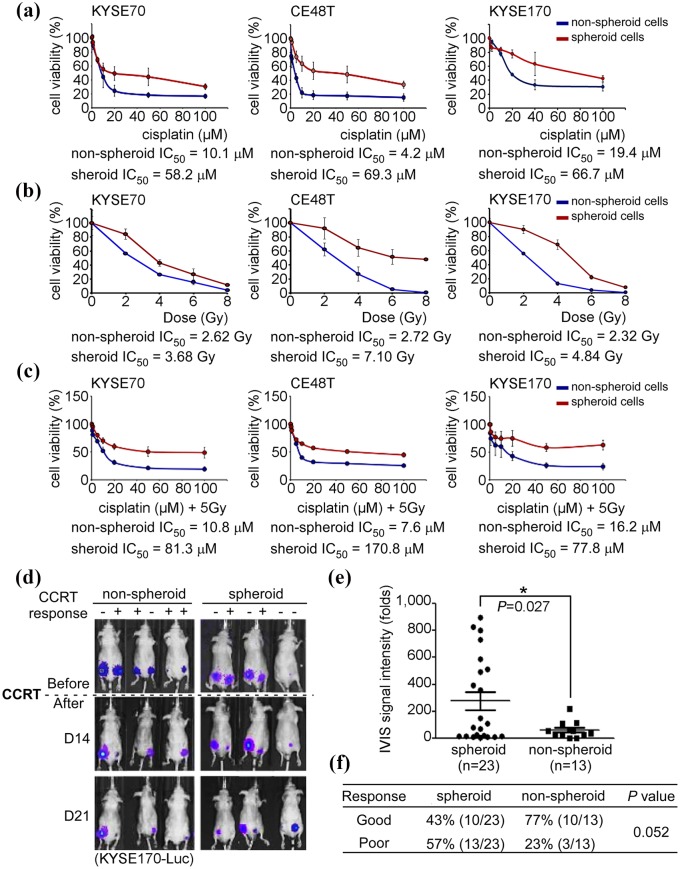
Cancer stem-like ESCC cells are resistant to cisplatin, radiation and concurrent chemoradiotherapy in vitro and in vivo
CCRT is the standard treatment for ESCC, but the therapeutic benefit is insufficient.2,5,6 CSCs have been reported to be highly associated with therapeutic failure and poor clinical outcomes.9 Spheroid cells were isolated and single cells were subsequently used for investigating the response to chemotherapy, radiotherapy, and CCRT compared with nonspheroid cells in vitro and in vivo. Three ESCC cell lines were treated with different concentrations of cisplatin, and the spheroid cells demonstrated chemoresistance with IC50 values of 58.2, 69.3, and 66.7 µM, compared with nonspheroid cell IC50 values of 10.1, 4.2, and 19.4 µM in KYSE70, CE48T, and KYSE170 cells, respectively (Figure 2(a)). In addition, spheroid cells also demonstrated radioresistance. The IC50 values were 3.68, 7.1 and 4.84 Gy in spheroid cells and 2.62, 2.72 and 2.32 Gy in nonspheroid cells in KYSE70, CE48T and KYSE170 cells, respectively (Figure 2(b)). CCRT is widely used for ESCC treatment, and the authors’ results demonstrated, in addition, that spheroid cells were resistant to CCRT treatment. The IC50 values were 4.8-fold to 22-fold higher in spheroid cells compared with nonspheroid cells in vitro (Figure 2(c)). Experiments measuring CCRT response in vivo were conducted to confirm the results from cell models. Spheroid and nonspheroid cells were xenotransplanted into the left and right flank of nude mice. The mice were monitored weekly using an IVIS system and treated by CCRT after the tumor size reached 5 mm in diameter. A therapeutic responder was defined as an animal, in which the tumor size was reduced by > 30%. This group is indicated as ‘response (+)’ (Figure 2(d)). Plotting quantitative data from IVIS analysis, in addition, demonstrated that the average tumor sizes of tumor sphere-generated tumors were significantly larger than the nonspheroid cell-generated tumors (paired Student’s t test; p = 0.027; Figure 2(e)). Following 3 weeks of observation, 77% (10/13) of nonspheroid cell-generated tumors were inhibited by CCRT, and 23% (3/13) of nonspheroid cell-generated tumors kept growing. In contrast, only 43% (10/23) of tumor sphere-generated tumors were inhibited by CCRT, while 57% (13/23) of tumorsphere generated tumors did not respond to CCRT (chi-squared analysis, p = 0.052) (Figure 2(f)). The results supported the theory that CSCs are resistant to CCRT treatment and promote metastasis. Thus, CSCs may contribute to poor clinical outcomes in ESCC.
Figure 2.
Cancer stem-like ESCC cells are resistant to concurrent chemoradiation therapy (CCRT) in vitro and in vivo. Nonspheroid and spheroid cells of KYSE70, CE48T, and KYSE170 were used to evaluate the sensitivity of cisplatin (a), radiation (b), and cisplatin combined with radiation (c). After treatment for 48 h, cell viability of cisplatin and CCRT were verified using MTT and cell viability to radiation was evaluated by colony-forming assay, and IC50 of cisplatin, radiation or CCRT was used to evaluate the sensitivity. Cells were treated with different dosages of cisplatin or radiation. In CCRT, cells were treated with different dosages of cisplatin combined with 5 Gy of irradiation. (d) A total of 1 × 105 KYSE170-Luc cells were subcutaneously injected into immunodeficiency mice. Tumor sizes were measured weekly using an in vivo Imaging Systems (IVIS) system. The mice were treated with 4 Gy of irradiation and 2 mg/kg cisplatin when the diameters of the tumors were > 5 mm. Tumor sizes reduced more than 30% were defined as a CCRT good response (labeled as ‘+’); tumor sizes reduced <30% were defined as a CCRT poor response (labeled as ‘–’). (e) The quantitative data from IVIS was plotted following CCRT treatment. (f) The correlation between nonspheroid/spheroid cells and CCRT response was analyzed using a chi-squared test. *p < 0.05.
Characterization and validation the surface markers of cancer stem-like ESCC cells
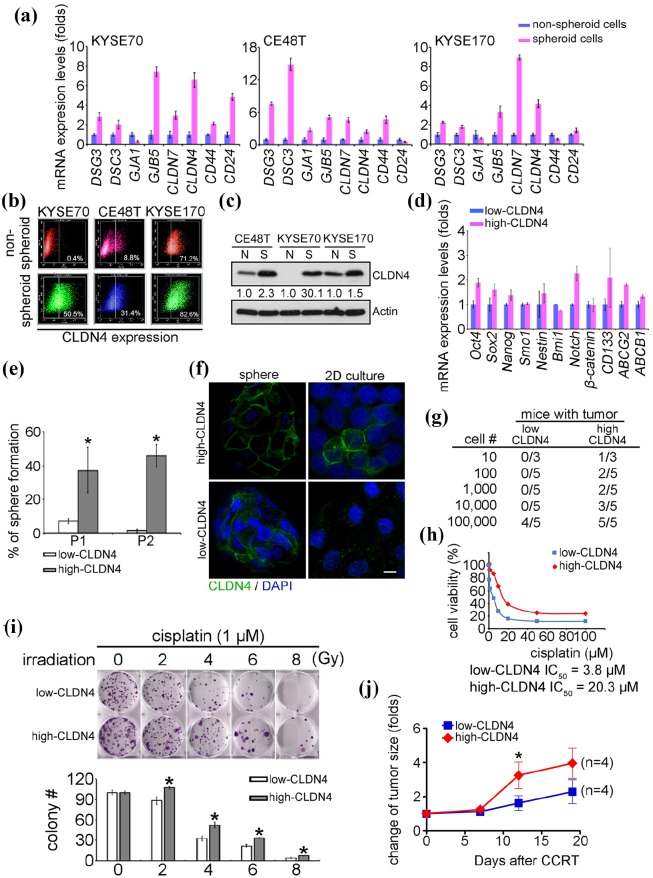
Despite the importance of identifying CSCs in tumors, current CSC biomarkers are not suitable for detection in ESCC. The results demonstrated that CD24–CD44+ cells neither increased the stemness gene expression nor enhanced CCRT resistance (Supplemental Data 1). Therefore, the authors tried to identify surface biomarkers of cancer stem-like ESCC cells. By comparing spheroid cells and nonspheroid cells using microarray, the differential expression of epithelial-to-mesenchymal transition (EMT), differentiation, stemness, and drug resistance genes were measured in both KYSE70 and CE48T cells, following which they were validated by qPCR (Supplemental Data 2 and Figure 3(a)). Among these genes, CLDN4 exhibited high level in KYSE70, CE48T, and KYSE170-derived spheroid cells, and was correlated with poor survival in esophagus and breast cancers (Figure 3(a) and Supplemental Data 3(a)). In addition, the microarray data (GSE86099) showed that CLDN4 had increased in paclitaxel-resistant cells, recalling the drug-resistant character of CSCs (Supplemental Data 3(b)).4 In combination, this data suggested that CLDN4 is a potential marker for cancer stem-like ESCC cells. Because cancer stem-like cells possess sphere formation capacity, the authors measured the population of high-CLDN4 cells in spheroid and nonspheroid cultures. The data demonstrated that the high-CLDN4 population and the CLDN4 protein level increased at least twofold in spheroid cells compared with nonspheroid cells, as shown by both flow cytometry (KYSE70 increased from 0.4% to 50.5%, CE48T increased from 8.8% to 31.4%, and KYSE170 increased from 71.2% to 82.6%, respectively) and immunoblotting analysis (Figure 3(b) and (c)). The high-CLDN4 and low-CLDN4 cells were then sorted by flow cytometry and separately subjected to the spheroid formation assay (Supplemental Data 4). Figure 3(d) shows that high-CLDN4 cells had high expression levels of stemness and drug resistance-related genes compared with low-CLDN4 cells, supporting the hypothesis that high-CLDN4 cells harbor CSCs properties. The tumorsphere assay was also performed to examine stemness in both high-CLDN4 and low-CLDN4 cells. High-CLDN4, but not low-CLDN4 cells showed 40–50% tumorsphere formation ability and maintained stemness in the first and second passage in 3D culture (Figure 3(e)). The expression level of CLDN4 was evaluated using both 3D and 2D culture systems and found that CLDN4 was essential for sphere formation (Figure 3(f)). Limiting dilution cell transplantation assays were used to determine the tumor initiation ability of high-CLDN4 and low-CLDN4 cells. The results demonstrated that 10 high-CLDN4 cells can initiate tumor formation in 1 out of 3 mice. Tumor formation was also observed in mice injected with 100–10,000 high-CLDN4 cells, suggesting that high-CLDN4 cells, exhibit tumor initiation ability. Low-CLDN4 cells required injections of 1 × 105 cells to initiate tumors in 8 weeks (Figure 3(g)). However, the authors found that cancer stem-like ESCC cells were resistant to cisplatin and CCRT, and further tested the cisplatin and CCRT sensitivity of high-CLDN4 cells to therapeutics. The cisplatin and CCRT responses were examined in both high-CLDN4 and low-CLDN4 cells in vitro and in vivo. By treating with different concentrations of cisplatin, High-CLDN4 cells were found to be resistant to cisplatin, with an IC50 value of 20.3 µM, compared with low-CLDN4 cells, with an IC50 value of 3.8 µM (Figure 3(h). A similar result was found when cisplatin was combined with radiation treatment. High-CLDN4 cells were resistant to CCRT treatment compared with low-CLDN4 cells (Figure 3(i). In addition, high-CLDN4 cells also showed CCRT resistance compared with low-CLDN4 cells in xenograft mouse models (Figure 3(j)). In combination, the data indicates that CLDN4 can serve as a biomarker for cancer stem-like ESCC cells, and high-CLDN4 cells carry tumor initiation and CCRT resistance properties.
Figure 3.
CLDN4 enhances cancer stem cell properties in ESCC. (a) The candidate cell surface genes selected from microarray were examined in both nonspheroid and spheroid cells. (b) The population of high-CLDN4 cells was examined in both nonspheroid and spheroid cells by flow cytometry. (c) The CLDN4 expression level of nonspheroid and spheroid cells was measured by immunoblotting. Quantified results of immunoblotting indicated the relative CLDN4 expression level in spheroid cells compared with nonspheroid cells. (d) and (e) The stemness and drug resistance genes expression levels and sphere formation ability was evaluated in isolated high-CLDN4 or low-CLDN4 cells by flow cytometry. (f) Immunofluorescence was performed to evaluate the CLDN4 levels in ultra-low or 2D cultured-high-CLDN4 or low-CLDN4 cells. (g) The indicated numbers of high-CLDN4 or low-CLDN4 cells were xenotransplanted into immunodeficiency mice to evaluate tumor initiation ability in vivo. Flow cytometry was used to isolate high-CLDN4 or low-CLDN4 cells to evaluate the sensitivity of cisplatin (h) and CCRT in vitro (i) and cisplatin (7 mg/Kg) combined with radiation (2 Gy) in vivo (j). The change of tumor size was normalized with the tumor size before treatment.
CCRT, cisplatin/concurrent chemoradiation therapy; ESCC, esophageal squamous cell carcinoma
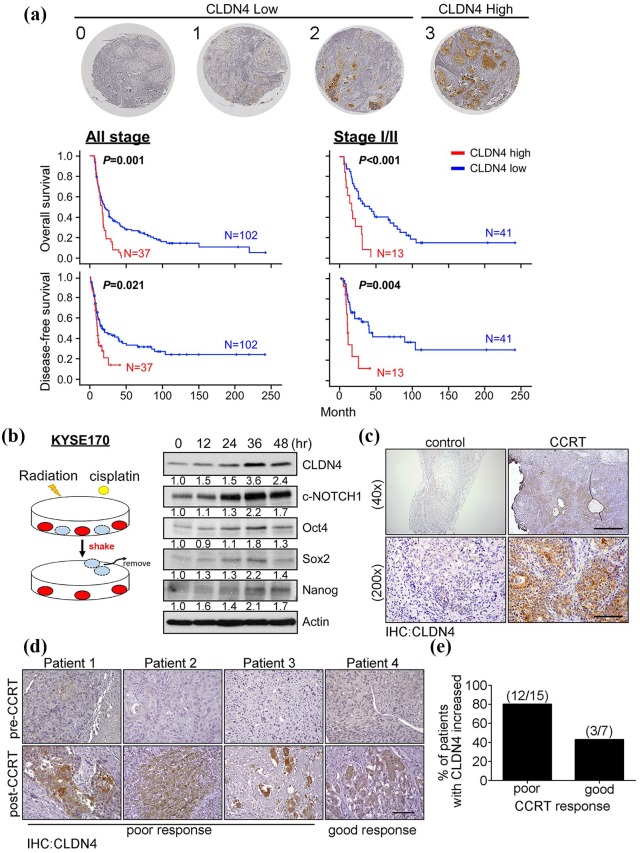
CCRT treatment enhances CLDN4 expression, and high CLDN4 is associated with poor CCRT response in ESCC patients
To examine the prognostic role of CLDN4, 139 clinical specimens were collected for further analysis (Table 1). IHC staining of CLDN4 was scored on a scale from 0 to 3 and subdivided into low (0 to 2) and high (3) expressing groups. Categorized by IHC scoring, patient survival was analyzed by Kaplan–Meier analysis (Figure 4(a)). The results demonstrated that patients with high levels of CLDN4 generally had poor survival and early recurrence, even if they had the early-stage disease, suggesting that CLDN4 can serve as a prognostic biomarker for ESCC patients. The authors’ data support the hypothesis that CSCs confer resistance to therapy. CCRT treatment can eliminate bulk cancer cells but not CSCs, resulting in a dominant CSC population in the tumor. This would explain why and how ESCC patients with high CLDN4 expression showed high recurrence rates after CCRT treatment. Therefore, the KYSE170 cells were treated with CCRT and the surviving cells were collected and subjected to immunoblotting analysis at the indicated time points (Figure 4(b)). The results showed that stemness-associated proteins (Oct-4, SOX2, NANOG, and c-Notch) and CLDN4 were time-dependently upregulated in CCRT-treated cells, supporting the theory that CSCs were enriched under CCRT treatment. Tissue from the xenograft mouse models with or without CCRT treatment was collected to evaluate the percentage of cancer stem-like cells. As expected, CLDN4 was extremely upregulated in the tumor tissue after CCRT treatment, but not in the control group, supporting the hypothesis that the cancer stem-like cells population is increased after CCRT treatment (Figure 4(c)). Finally, specimens collected from patients before and after CCRT treatment were used to validate this hypothesis. After receiving CCRT therapy, ESCC patients were subdivided into good or poor CCRT response. The level of CLDN4 was examined by immunohistochemistry in both poor and good responders (Figure 4(d) and Supplemental Data 5(a) and (b)). The results demonstrated that CLDN4 was increased in 12 out of 15 (80%) CCRT poor responders and 3 out of 7 (43%) CCRT good responders (Figure 4(e)). In combination, this data demonstrates that CLDN4 serves as not only a prognostic biomarker but also as a predictive indicator of CCRT response for ESCC patients.
Table 1.
Clinical features of tumors and association with CLDN4 expression level (n = 139).
| Feature | CLDN4 | p value | |
|---|---|---|---|
| Low | High | ||
| Sex (M/F) | 97/5 | 35/2 | 0.905 |
| Survival (A/D) | 18/84 | 0/37 | 0.006 * |
| Recurrence (Y/N) | 63/39 | 26/11 | 0.356 |
| Stage (I–IIb/III–IVB) | 41/58 | 13/22 | 0.658 |
| Age (mean ± SD) | 56.31 ± 10.80 | 57.89 ± 12.43 | 0.466 |
| Survival month (mean ± SD) | 40.46 ± 46.69 | 18.13 ± 9.88 | 0.005 # |
| Recurrence month (mean ± SD) | 33.67 ± 47.20 | 11.12 ± 7.91 | 0.005 # |
Chi-squared; #Student’s t test.
F, female; M, male;
Figure 4.
The high CLDN4 level is correlated with poor prognosis and poor CCRT response in ESCC. (a) Representative immunohistochemistry images of ESCC specimens showing CLDN4 expression scores from 0 to 3. Score 0, 1, and 2 were classified to CLDN4 low expression and score 3 is defined to CLDN4 high expression. Overall survival (left-upper) and disease-free survival (left-lower) of 139 ESCC patients were stratified by CLDN4 expression level by Kaplan–Meier analysis. Overall survival (right-upper) and disease-free survival (right-lower) of the 54 patients with stage I/II ESCC was stratified with CLDN4 expression level by Kaplan–Meier analysis. (b) The diagram shows the procedure to select CCRT resistance cells. The KYSE170 cells were received cisplatin combined with irradiation for indicating times and the lethal cells were removed before harvest protein lysate. The CCRT resistance cells were examined the CLDN4 levels and stemness genes by immunoblotting. Quantified results of immunoblotting indicated the relative protein expression level at different time points. (c) Immunohistochemistry showed the CLDN4 levels in xenotransplanted mice with or without CCRT treatment. (d) The tissues from CCRT responder or nonresponder ESCC patients were used to examine the CLDN4 levels by immunohistochemistry. (e) The percentage of increased CLDN4 was used to evaluate in both CCRT poor and good responders.
CCRT, cisplatin/concurrent chemoradiation therapy; ESCC, esophageal squamous cell carcinoma
TTFD diminishes stemness and improves CCRT therapeutic efficacy
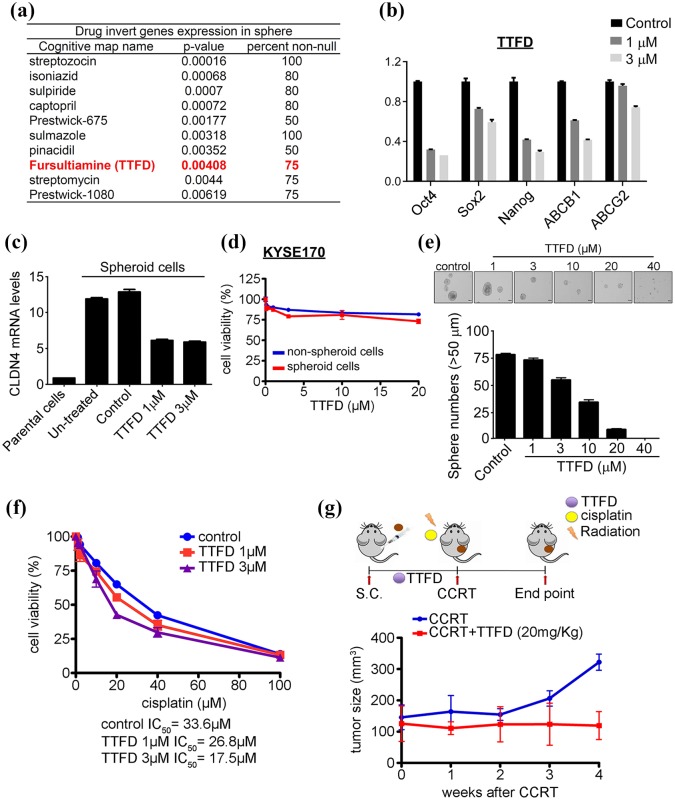
Because CSCs were highly correlated with CCRT resistance and unfavorable clinical outcomes, diminishing CSCs numbers may help to improve ESCC patient response to therapeutics. The differential expression of stemness-associated and drug resistance genes was measured in spheroid cells compared with nonspheroid cells by microarray. The gene profiles were compared in spheroid and nonspheroid KYSE70 and CE48T cells. A total of 248 upregulated and 117 downregulated genes were found in both KYSE70 and CE48T cells. This suggests these genes can serve as a gene signature for cancer stem-like ESCC cells. Connectivity Map (Cmap) was utilized to identify small molecules, which can invert the gene expression patterns in CSCs.18 As shown in Figure 5(a), potential small molecules were examined for their effects on altering stemness and drug-associated gene expression. The results indicated that only TTFD treatment can dose-dependently decrease stemness and drug-associated genes (Figure 5(b) and Supplemental Data 6(a)). CLDN4 mRNA and protein levels were also downregulated by TTFD treatment, but not other small molecules (Figure 5(c) and Supplemental Data 6(b) and (c). TTFD treatment can dose-dependently attenuate sphere formation ability from 70% to almost complete abolishment, without affecting cell motility (Figure 5(d) and (e)). Because TTFD diminished stemness, the authors then examined the therapeutic effect of TTFD and CCRT combinational treatment in CSCs. The IC50 values of TTFD and CCRT combination treatment were 26.8 µM (TTFD 1 µM + CCRT) and 17.5 µM (TTFD 3 µM + CCRT) compared with control of 33.6 µΜ (CCRT alone) (Figure 5(f)). Importantly, TTFD improved CCRT response not only in cell models but also in vivo in a xenograft mouse model. The spheroid cells were xenotransplanted into mice, which were orally treated with TTFD and CCRT after tumor growth reached approximately 100 mm3. The tumor burden was significantly decreased in the TTFD treatment group (100 mm3, TTFD + CCRT) compared with the control group (300 mm3, CCRT only) after treatment for 4 weeks (Figure 5(g)). Overall the authors demonstrated that TTFD can diminish stemness and enhance CCRT efficacy in ESCC. These results may help to improve the CCRT response and benefit the clinical outcome of ESCC patients in the future.
Figure 5.
Thiamin tetrahydrofurfuryl disulfide (TTFD) diminishes cancer stem cell formation and conquers CCRT resistance. (a) The connectivity map (Cmap)was used to predict drugs able to invert the gene’s signature in sphere cells. (b) The stemness and drug resistance genes were examined after TTFD 1 mM or 3 mM treatment for 24 h. (c) The CLDN4 mRNA expression level was evaluated in spheroid cells with indicated treatment and nonspheroid cells. (d) The cell viability was determined in nonspheroid and spheroid KYSE170 cells, which were treated with the indicated TTFD concentrations for 5 days by MTT assay. (e). The sphere formation ability was measured by the treatment of indicated TTFD concentrations. (f) The spheroid KYSE170 cells treated with indicated cisplatin dosage and combined with TTFD 1 µM or 3 µM to measure cellular viability by MTT assay. (g) The tumorsphere cells were subcutaneously injected into mice and fed with TTFD (20 mg/kg) or an equal volume of water in the control group. After tumor growth to the proper size, tumors received CCRT treatment (4 Gy, 2 mg/kg) and tumor sizes were measured weekly.
CCRT, cisplatin/concurrent chemoradiation therapy; MTT, methyl thiazol tetrazolium
Discussion
CSCs are known to promote tumor initiation, metastasis, and drug resistance in a number of cancers.9,10,19 Identifying the cellular roles of CSCs in treatment resistance and metastasis may help to develop effective therapies for ESCC patients. In this study, the authors demonstrated the cancer stem-like ESCC cells can promote tumor initiation, metastasis, and contribute to the ineffectiveness of CCRT treatment in ESCC. This data also revealed that CLDN4, a surface protein, was significantly increased in spheroid cells, which suggests that CLDN4 can serve as a marker of stem-like ESCC cells. Xenotransplanted mice and clinical specimen analyses demonstrated that CLDN4 was increased after CCRT treatment and supported that cancer stem-like ESCC cells were enriched after treatment. In addition, the CLDN4 expression level was correlated with unfavorable clinical outcomes and CCRT treatment failure in ESCC patients. Overall the authors’ data reveals that high-CLDN4 cancer stem-like ESCC cells are responsible for tumor initiation, metastasis, and drug resistance properties in vitro and in vivo. Of note, TTFD was identified by Cmap as a small molecule that is able to reverse the CSCs gene expression signature. TTFD treatment can diminish cancer stem-like ESCC cells formation and enhance CCRT response, but not affect cell viability. In combination, the authors demonstrated that high-CLDN4 cancer stem-like ESCC cells harbored tumor initiation, metastasis, and CCRT resistance properties and that TTFD can diminish stemness as well as improve CCRT response in ESCC.
Based on the CSC hypothesis, CSCs represent a limited population of cells that are associated with tumor initiation, metastasis, and drug resistance properties. Therefore, identifying CSCs is a critical issue in cancers.9,10 Over the last decade, several cell surface markers were found to identify CSCs in cancers. CD44+CD24–/low was first shown to act as a CSCs marker in breast cancer. These cells showed a high capacity to initiate tumor burden in xenotransplanted mice.20 In addition ALDH1+CD44+CD24– cells were reported to initiate tumors and promote metastasis, and ALDH1 was correlated with poor prognosis in patients with breast cancer.21,22 However, the authors’ data indicate that CD44+CD24– cells do not exhibit CSC properties in ESCC (Supplemental Data 2). The role of CLDN4 in ESCC has been reported previously;23,24 however, the opposite findings might be caused by the clinical characteristics of different enrolled groups. It is widely accepted that the 5-year survival rate of ESCC patients is still <25% which is similar in the enrolled ESCC patients in this study(Figure 4). However, CLDN4 was directly identified by comparing the surface markers of cancer stem-like ESCC cells and bulk tumor cells (Figure 3). In addition, the authors found that high-CLDN4 cells were shown to harbor tumor initiation, metastasis, and CCRT resistance capabilities (Figures 3 and 4). Moreover, high-CLDN4 cells were enriched under the selective pressure of CCRT treatment, and CLDN4 was correlated with poor prognosis and early recurrence, even in early disease ESCC. In combination the results indicate that the CLDN4 not only serves as a marker of cancer stem-like ESCC cells but also acts as a prognostic and CCRT treatment predictive biomarker for ESCC patients.
The origin of CSCs is still a controversial issue. CSCs may be derived from the transformation of normal stem cells or the dedifferentiation of cancer cells.25 An increasing number of studies indicate that CSCs can be induced by extrinsic regulation including inflammation, hypoxia, and cancer-associated fibroblasts (CAFs).9,26–29 In addition, EMT has been broadly indicated to mediate transformation of mature cancer cells into CSCs.30–32 In this study, isolated high-CLDN4 cells showed CSCs properties, however, few cells can harbor stemness properties and show CLDN4 expression from isolated low-CLDN4 cells, suggesting the CLDN4 is essential for cancer stem-like ESCC cells. Of note, CCRT treatment was shown to enrich the cancer stem-like ESCC cell population in ESCC patients leading to CCRT treatment failure. In agreement with this finding, Roesch and colleagues reported that JARID1B+ cells showed more CSC features than JARID1B– cells in melanoma, and further that the expression of JARID1B was dynamic. JARID1B– cells can switch to JARID1B+ cells and, with the switch, begin to exhibit CSC properties.33 Together, the CSCs can be enriched under-stimulation such as CCRT. Therefore, investigating the regulatory mechanisms of CLDN4 may help to improve therapeutic strategies for ESCC patients.
Previous studies indicated that CSCs express high levels of multidrug resistance (MDR) genes such as ABCB1 and ABCG2 to facilitate drug efflux.34,35 This theory is consistent with this study’s results that show high-CLDN4 cancer stem-like ESCC cells cause treatment failure. Therefore, targeting CSCs may be a more efficient way to overcome cancer resistance and prevent the spread of ESCC. In fact, several drugs were developed to target CSCs through Wnt, Notch or Hedgehog signaling, underlining the importance of targeting CSCs.36 The authors’ revealed that TTFD, a disulfide derivative of thiamine, can reverse the stemness gene profile, downregulate CLDN4 levels, and diminish stemness potential (Figure 5). Reducing CSCs may improve therapeutic responses and this study’s data that pre-treatment of TTFD improves the CCRT response in vitro and in vivo supported this concept (Figure 5). Importantly, unlike unknown molecules that may be used as CSC-targeting drugs, TTFD is known to be relatively safe for humans.
In combination the authors’ findings demonstrate that CLDN4 is not only a cancer stem-like ESCC cell marker but also acts as a prognostic and CCRT response indicator for ESCC patients. CCRT treatment enriched high-CLDN4 cells, potentially conferring CCRT resistance in ESCC patients. Importantly, attenuating stemness properties of cancer cells by TTFD treatment may improve CCRT response. These results may provide an opportunity to improve therapeutic outcomes for ESCC patients.
Supplemental Material
Supplemental material, 20181217_Lin_Supplemental_Data_1 for High-CLDN4 ESCC cells harbor stem-like properties and indicate for poor concurrent chemoradiation therapy response in esophageal squamous cell carcinoma by Cheng-Han Lin, Hao-Yi Li, Yu-Peng Liu, Pei-Fung Kuo, Wen-Ching Wang, Forn-Chia Lin, Wei-Lun Chang, Bor-Shyang Sheu, Yi-Ching Wang, Wan-Chun Hung, Hui-Chuan Cheng, Yun-Chin Yao, Marcus J. Calkins, Michael Hsiao and Pei-Jung Lu in Therapeutic Advances in Medical Oncology
Supplemental Material
Supplemental material, 20181217_Lin_Supplemental_Data_2.jpg for High-CLDN4 ESCC cells harbor stem-like properties and indicate for poor concurrent chemoradiation therapy response in esophageal squamous cell carcinoma by Cheng-Han Lin, Hao-Yi Li, Yu-Peng Liu, Pei-Fung Kuo, Wen-Ching Wang, Forn-Chia Lin, Wei-Lun Chang, Bor-Shyang Sheu, Yi-Ching Wang, Wan-Chun Hung, Hui-Chuan Cheng, Yun-Chin Yao, Marcus J. Calkins, Michael Hsiao and Pei-Jung Lu in Therapeutic Advances in Medical Oncology
Supplemental Material
Supplemental material, 20181217_Lin_Supplemental_Data_3 for High-CLDN4 ESCC cells harbor stem-like properties and indicate for poor concurrent chemoradiation therapy response in esophageal squamous cell carcinoma by Cheng-Han Lin, Hao-Yi Li, Yu-Peng Liu, Pei-Fung Kuo, Wen-Ching Wang, Forn-Chia Lin, Wei-Lun Chang, Bor-Shyang Sheu, Yi-Ching Wang, Wan-Chun Hung, Hui-Chuan Cheng, Yun-Chin Yao, Marcus J. Calkins, Michael Hsiao and Pei-Jung Lu in Therapeutic Advances in Medical Oncology
Supplemental Material
Supplemental material, 20181217_Lin_Supplemental_Data_4 for High-CLDN4 ESCC cells harbor stem-like properties and indicate for poor concurrent chemoradiation therapy response in esophageal squamous cell carcinoma by Cheng-Han Lin, Hao-Yi Li, Yu-Peng Liu, Pei-Fung Kuo, Wen-Ching Wang, Forn-Chia Lin, Wei-Lun Chang, Bor-Shyang Sheu, Yi-Ching Wang, Wan-Chun Hung, Hui-Chuan Cheng, Yun-Chin Yao, Marcus J. Calkins, Michael Hsiao and Pei-Jung Lu in Therapeutic Advances in Medical Oncology
Supplemental Material
Supplemental material, 20181217_Lin_Supplemental_Data_5 for High-CLDN4 ESCC cells harbor stem-like properties and indicate for poor concurrent chemoradiation therapy response in esophageal squamous cell carcinoma by Cheng-Han Lin, Hao-Yi Li, Yu-Peng Liu, Pei-Fung Kuo, Wen-Ching Wang, Forn-Chia Lin, Wei-Lun Chang, Bor-Shyang Sheu, Yi-Ching Wang, Wan-Chun Hung, Hui-Chuan Cheng, Yun-Chin Yao, Marcus J. Calkins, Michael Hsiao and Pei-Jung Lu in Therapeutic Advances in Medical Oncology
Supplemental Material
Supplemental material, 20181217_Lin_Supplemental_Data_6_20190507 for High-CLDN4 ESCC cells harbor stem-like properties and indicate for poor concurrent chemoradiation therapy response in esophageal squamous cell carcinoma by Cheng-Han Lin, Hao-Yi Li, Yu-Peng Liu, Pei-Fung Kuo, Wen-Ching Wang, Forn-Chia Lin, Wei-Lun Chang, Bor-Shyang Sheu, Yi-Ching Wang, Wan-Chun Hung, Hui-Chuan Cheng, Yun-Chin Yao, Marcus J. Calkins, Michael Hsiao and Pei-Jung Lu in Therapeutic Advances in Medical Oncology
Supplemental Material
Supplemental material, Supplemental_Data_7_20190530-R1 for High-CLDN4 ESCC cells harbor stem-like properties and indicate for poor concurrent chemoradiation therapy response in esophageal squamous cell carcinoma by Cheng-Han Lin, Hao-Yi Li, Yu-Peng Liu, Pei-Fung Kuo, Wen-Ching Wang, Forn-Chia Lin, Wei-Lun Chang, Bor-Shyang Sheu, Yi-Ching Wang, Wan-Chun Hung, Hui-Chuan Cheng, Yun-Chin Yao, Marcus J. Calkins, Michael Hsiao and Pei-Jung Lu in Therapeutic Advances in Medical Oncology
Acknowledgments
Cheng-Han Lin and Hao-Yi Li contributed equally to this work. We thank the proteomics core facility of the Clinical Medicine Research Center at National Cheng Kung University Hospital for assisting with protein expression experiment processing. We are also grateful to Dr. Kuen-Jer Tsai and Ya-Chun Hsiao for the services of the image acquiring and analyzing in the Center of Clinical Medicine, National Cheng Kung University Hospital.
Footnotes
Author contributions: Study concept and design- Lu PJ, Lin CH, Li HY
Acquisition of data- Lin CH, Li HY, Liu YP, Kuo PF, Hung WC, Cheng HC
Analysis and interpretation of data- Lin CH, Li HY, Liu YP
Drafting of the manuscript- Lin CH, Li HY, Calkins MJ
Technical and material support- Lin FC, Chang WL, Wang WC, Wang YC, Sheu BS, Yao YC
Study supervision- Lu PJ, Hsiao M
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by the Ministry of Science and Technology, R.O.C. (MOST 105-2320-B-006-054, MOST 106-2320-B-006-066-MY3) to Pei-Jung Lu, and supported by Academia Sinica and Ministry of Science and Technology [MOST 106-0210-01-15-02, MOST 107-0210-01-19-01] to Michael Hsiao.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
ORCID iDs: Yi-Ching Wang  https://orcid.org/0000-0002-7694-2067
https://orcid.org/0000-0002-7694-2067
Michael Hsiao  https://orcid.org/0000-0001-8529-9213
https://orcid.org/0000-0001-8529-9213
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Cheng-Han Lin, Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan.
Hao-Yi Li, Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan.
Yu-Peng Liu, Institute of Clinical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung.
Pei-Fung Kuo, Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan.
Wen-Ching Wang, Department of Surgery, Chi Mei Medical Center, Tainan.
Forn-Chia Lin, Department of Radiation Oncology, National Cheng Kung University Hospital, Tainan.
Wei-Lun Chang, Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan; Department of Internal Medicine, National Cheng Kung University Hospital, Tainan.
Bor-Shyang Sheu, Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan; Department of Internal Medicine, National Cheng Kung University Hospital, Tainan.
Yi-Ching Wang, Department of Pharmacology, College of Medicine, National Cheng Kung University, Tainan.
Wan-Chun Hung, Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan.
Hui-Chuan Cheng, Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan.
Yun-Chin Yao, Clinical Medicine Research Center, National Cheng Kung University, Tainan.
Marcus J. Calkins, Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan
Michael Hsiao, Genomics Research Center, Academia Sinica, Taipei; Department of Biochemistry, College of Medicine, Kaohsiung Medical University, Kaohsiung.
Pei-Jung Lu, Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, No. 35, Siaodong Road, 704, Tainan; Department of Clinical Medicine Research, National Cheng Kung University Hospital, Tainan.
References
- 1. Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013; 381: 400–412. [DOI] [PubMed] [Google Scholar]
- 2. Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 2014; 371: 2499–2509. [DOI] [PubMed] [Google Scholar]
- 3. Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol 2007; 8: 545–553. [DOI] [PubMed] [Google Scholar]
- 4. Wang R, Sumarpo A, Saiki Y, et al. ABCB1 is upregulated in the acquisition of taxane resistance: lessons from esophageal squamous cell carcinoma cell lines. Tohoku J Exp Med 2016; 240: 295–301. [DOI] [PubMed] [Google Scholar]
- 5. Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011; 12: 681–692. [DOI] [PubMed] [Google Scholar]
- 6. van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–2084. [DOI] [PubMed] [Google Scholar]
- 7. Strong VE, D’Amico TA, Kleinberg L, et al. Impact of the 7th edition AJCC staging classification on the NCCN clinical practice guidelines in oncology for gastric and esophageal cancers. J Natl Compr Canc Netw 2013; 11: 60–66. [DOI] [PubMed] [Google Scholar]
- 8. Lou F, Sima CS, Adusumilli PS, et al. Esophageal cancer recurrence patterns and implications for surveillance. J Thorac Oncol 2013; 8: 1558–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nassar D, Blanpain C. Cancer stem cells: basic concepts and therapeutic implications. Annu Rev Pathol 2016; 11: 47–76. [DOI] [PubMed] [Google Scholar]
- 10. Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med 2011; 17: 313–319. [DOI] [PubMed] [Google Scholar]
- 11. Krause M, Dubrovska A, Linge A, et al. Cancer stem cells: radioresistance, prediction of radiotherapy outcome and specific targets for combined treatments. Adv Drug Deliv Rev 2017; 109: 63–73. [DOI] [PubMed] [Google Scholar]
- 12. Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006; 444: 756–760. [DOI] [PubMed] [Google Scholar]
- 13. Bertolini G, Roz L, Perego P, et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci U S A 2009; 106: 16281–16286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu YP, Yang CJ, Huang MS, et al. Cisplatin selects for multidrug-resistant CD133+ cells in lung adenocarcinoma by activating Notch signaling. Cancer Res 2013; 73: 406–416. [DOI] [PubMed] [Google Scholar]
- 15. Sarvi S, Mackinnon AC, Avlonitis N, et al. CD133+ cancer stem-like cells in small cell lung cancer are highly tumorigenic and chemoresistant but sensitive to a novel neuropeptide antagonist. Cancer Res 2014; 74: 1554–1565. [DOI] [PubMed] [Google Scholar]
- 16. Huang SD, Yuan Y, Liu XH, et al. Self-renewal and chemotherapy resistance of p75NTR positive cells in esophageal squamous cell carcinomas. BMC Cancer 2009; 9: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin CH, Tsai CH, Yeh CT, et al. MiR-193a-5p/ERBB2 act as concurrent chemoradiation therapy response indicator of esophageal squamous cell carcinoma. Oncotarget 2016; 7: 39680–39693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lamb J, Crawford ED, Peck D, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science 2006; 313: 1929–1935. [DOI] [PubMed] [Google Scholar]
- 19. Chen D, Wu M, Li Y, et al. Targeting BMI1+ cancer stem cells overcomes chemoresistance and inhibits metastases in squamous cell carcinoma. Cell stem cell 2017; 20: 621–634.e626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003; 100: 3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Charafe-Jauffret E, Ginestier C, Iovino F, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res 2010; 16: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell stem cell 2007; 1: 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shi M, Wang Z, Song L, et al. Low expression of claudin-4: an indicator of recurrence in esophageal squamous cell carcinoma after Ivor Lewis esophagectomy? Med Oncol 2014; 31: 951. [DOI] [PubMed] [Google Scholar]
- 24. Sung CO, Han SY, Kim SH. Low expression of claudin-4 is associated with poor prognosis in esophageal squamous cell carcinoma. Ann Surg Oncol 2011; 18: 273–281. [DOI] [PubMed] [Google Scholar]
- 25. Friedmann-Morvinski D, Verma IM. Dedifferentiation and reprogramming: origins of cancer stem cells. EMBO Rep 2014; 15: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell 2007; 129: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Liu Y, Malek SN, et al. Targeting HIF1alpha eliminates cancer stem cells in hematological malignancies. Cell Stem Cell 2011; 8: 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015; 16: 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shimoda M, Principe S, Jackson HW, et al. Loss of the Timp gene family is sufficient for the acquisition of the CAF-like cell state. Nat Cell Biol 2014; 16: 889–901. [DOI] [PubMed] [Google Scholar]
- 30. Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009; 9: 265–273. [DOI] [PubMed] [Google Scholar]
- 31. Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol 2012; 22: 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139: 871–890. [DOI] [PubMed] [Google Scholar]
- 33. Roesch A, Fukunaga-Kalabis M, Schmidt EC, et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell 2010; 141: 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou JJ, Deng XG, He XY, et al. Knockdown of NANOG enhances chemosensitivity of liver cancer cells to doxorubicin by reducing MDR1 expression. Int J Oncol 2014; 44: 2034–2040. [DOI] [PubMed] [Google Scholar]
- 35. Bleau AM, Hambardzumyan D, Ozawa T, et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell 2009; 4: 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takebe N, Miele L, Harris PJ, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol 2015; 12: 445–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, 20181217_Lin_Supplemental_Data_1 for High-CLDN4 ESCC cells harbor stem-like properties and indicate for poor concurrent chemoradiation therapy response in esophageal squamous cell carcinoma by Cheng-Han Lin, Hao-Yi Li, Yu-Peng Liu, Pei-Fung Kuo, Wen-Ching Wang, Forn-Chia Lin, Wei-Lun Chang, Bor-Shyang Sheu, Yi-Ching Wang, Wan-Chun Hung, Hui-Chuan Cheng, Yun-Chin Yao, Marcus J. Calkins, Michael Hsiao and Pei-Jung Lu in Therapeutic Advances in Medical Oncology
Supplemental material, 20181217_Lin_Supplemental_Data_2.jpg for High-CLDN4 ESCC cells harbor stem-like properties and indicate for poor concurrent chemoradiation therapy response in esophageal squamous cell carcinoma by Cheng-Han Lin, Hao-Yi Li, Yu-Peng Liu, Pei-Fung Kuo, Wen-Ching Wang, Forn-Chia Lin, Wei-Lun Chang, Bor-Shyang Sheu, Yi-Ching Wang, Wan-Chun Hung, Hui-Chuan Cheng, Yun-Chin Yao, Marcus J. Calkins, Michael Hsiao and Pei-Jung Lu in Therapeutic Advances in Medical Oncology
Supplemental material, 20181217_Lin_Supplemental_Data_3 for High-CLDN4 ESCC cells harbor stem-like properties and indicate for poor concurrent chemoradiation therapy response in esophageal squamous cell carcinoma by Cheng-Han Lin, Hao-Yi Li, Yu-Peng Liu, Pei-Fung Kuo, Wen-Ching Wang, Forn-Chia Lin, Wei-Lun Chang, Bor-Shyang Sheu, Yi-Ching Wang, Wan-Chun Hung, Hui-Chuan Cheng, Yun-Chin Yao, Marcus J. Calkins, Michael Hsiao and Pei-Jung Lu in Therapeutic Advances in Medical Oncology
Supplemental material, 20181217_Lin_Supplemental_Data_4 for High-CLDN4 ESCC cells harbor stem-like properties and indicate for poor concurrent chemoradiation therapy response in esophageal squamous cell carcinoma by Cheng-Han Lin, Hao-Yi Li, Yu-Peng Liu, Pei-Fung Kuo, Wen-Ching Wang, Forn-Chia Lin, Wei-Lun Chang, Bor-Shyang Sheu, Yi-Ching Wang, Wan-Chun Hung, Hui-Chuan Cheng, Yun-Chin Yao, Marcus J. Calkins, Michael Hsiao and Pei-Jung Lu in Therapeutic Advances in Medical Oncology
Supplemental material, 20181217_Lin_Supplemental_Data_5 for High-CLDN4 ESCC cells harbor stem-like properties and indicate for poor concurrent chemoradiation therapy response in esophageal squamous cell carcinoma by Cheng-Han Lin, Hao-Yi Li, Yu-Peng Liu, Pei-Fung Kuo, Wen-Ching Wang, Forn-Chia Lin, Wei-Lun Chang, Bor-Shyang Sheu, Yi-Ching Wang, Wan-Chun Hung, Hui-Chuan Cheng, Yun-Chin Yao, Marcus J. Calkins, Michael Hsiao and Pei-Jung Lu in Therapeutic Advances in Medical Oncology
Supplemental material, 20181217_Lin_Supplemental_Data_6_20190507 for High-CLDN4 ESCC cells harbor stem-like properties and indicate for poor concurrent chemoradiation therapy response in esophageal squamous cell carcinoma by Cheng-Han Lin, Hao-Yi Li, Yu-Peng Liu, Pei-Fung Kuo, Wen-Ching Wang, Forn-Chia Lin, Wei-Lun Chang, Bor-Shyang Sheu, Yi-Ching Wang, Wan-Chun Hung, Hui-Chuan Cheng, Yun-Chin Yao, Marcus J. Calkins, Michael Hsiao and Pei-Jung Lu in Therapeutic Advances in Medical Oncology
Supplemental material, Supplemental_Data_7_20190530-R1 for High-CLDN4 ESCC cells harbor stem-like properties and indicate for poor concurrent chemoradiation therapy response in esophageal squamous cell carcinoma by Cheng-Han Lin, Hao-Yi Li, Yu-Peng Liu, Pei-Fung Kuo, Wen-Ching Wang, Forn-Chia Lin, Wei-Lun Chang, Bor-Shyang Sheu, Yi-Ching Wang, Wan-Chun Hung, Hui-Chuan Cheng, Yun-Chin Yao, Marcus J. Calkins, Michael Hsiao and Pei-Jung Lu in Therapeutic Advances in Medical Oncology