Abstract
Background
Dynamic contrast‐based MRI and intravoxel incoherent motion imaging (IVIM) MRI are both methods showing promise as diagnostic and prognostic tools in rectal cancer. Both methods aim at measuring perfusion‐related parameters, but the relationship between them is unclear.
Purpose
To investigate the relationship between perfusion‐ and permeability‐related parameters obtained by IVIM‐MRI, T1‐weighted dynamic contrast‐enhanced (DCE)‐MRI and T2*‐weighted dynamic susceptibility contrast (DSC)‐MRI.
Study Type
Prospective.
Subjects
In all, 94 patients with histologically confirmed rectal cancer.
Field Strength/Sequence
Subjects underwent pretreatment 1.5T clinical procedure MRI, and in addition a study‐specific diffusion‐weighted sequence (b = 0, 25, 50, 100, 500, 1000, 1300 s/mm2) and a multiecho dynamic contrast‐based echo‐planer imaging sequence.
Assessment
Median tumor values were obtained from IVIM (perfusion fraction [f], pseudodiffusion [D*], diffusion [D]), from the extended Tofts model applied to DCE data (K trans, k ep, v p, v e) and from model free deconvolution of DSC (blood flow [BF] and area under curve). A subgroup of the excised tumors underwent immunohistochemistry with quantification of microvessel density and vessel size.
Statistical Test
Spearman's rank correlation test.
Results
D* was correlated with BF (rs = 0.47, P < 0.001), and f was negatively correlated with k ep (rs = –0.31, P = 0.002). BF was correlated with K trans (rs = 0.29, P = 0.004), but this correlation varied extensively when separating tumors into groups of low (rs = 0.62, P < 0.001) and high (rs = –0.06, P = 0.68) BF. K trans was negatively correlated with vessel size (rs = –0.82, P = 0.004) in the subgroup of tumors with high BF.
Data Conclusion
We found an association between D* from IVIM and BF estimated from DSC‐MRI. The relationship between IVIM and DCE‐MRI was less clear. Comparing parameters from DSC‐MRI and DCE‐MRI highlights the importance of the underlying biology for the interpretation of these parameters.
Level of Evidence: 2
Technical Efficacy: Stage 1
J. Magn. Reson. Imaging 2019;50:1114–1124.
Keywords: perfusion, permeability, IVIM, DSC, DCE, rectal cancer
THE USE OF dynamic contrast‐enhanced magnetic resonance imaging (DCE‐MRI) has shown potential for obtaining predictive and prognostic biomarkers in rectal cancer. This method has enabled estimation of parameters with the ability to predict histopathologic treatment outcome to preoperative chemoradiotherapy1 and distinguish between tumor differentiation grades.2 These results indicate that DCE‐MRI may be a tool to enable further treatment individualization in rectal cancer at the time of diagnosis.
DCE‐MRI aims at estimating capillary permeability by measuring the rate of extravasation of an intravenously injected gadolinium‐based contrast agent (GBCA). However, for patients with impaired kidney function there are contraindications to these types of contrast agents, and in addition there are some concerns regarding the safety and long‐term retention of GBCA.3, 4 This encourages investigation of alternative noncontrast agent‐based approaches for obtaining functional MRI‐derived biomarkers.
In recent years the concept of intravoxel incoherent motion (IVIM) imaging has received growing attention. IVIM is an expansion of conventional diffusion‐weighted imaging (DWI) whereby intravascular water reflecting microcirculatory properties can be separated from extravascular water due to different diffusion properties.5 IVIM‐based DWI has shown promise as a completely noninvasive approach for obtaining perfusion‐related information6 and is thus clearly an attractive technique both in terms of reduced cost as well as patient safety compared with methods requiring contrast agent injections.
The relationship between IVIM‐derived perfusion metrics and the more conventional contrast agent (CA)‐based methods for measuring perfusion‐related parameters with MRI remains unclear. According to a recent review by Federau,7 the strongest correlation between IVIM‐ and CA‐based perfusion metrics in human studies has been reported in brain tumors using the dynamic susceptibility contrast (DSC)‐MRI method. DSC‐MRI is a CA‐based method whereby the T2*‐ (susceptibility) effect, rather than the T1‐effect of the CA, is utilized to measure perfusion‐related metrics. DSC‐MRI has mainly been used in the brain due to dominant T2*‐effects in regions with an intact blood–brain barrier,8 but has recently also shown utility in staging of rectal cancer.9 Specifically addressing brain tumors, Bisdas et al10 found good correlation between perfusion measured by DSC‐MRI and IVIM‐derived metrics but generally poorer correlation between DCE‐MRI‐derived metrics and IVIM. Also, for applications outside the central nervous system (CNS), the correlations between IVIM and DCE‐MRI‐derived metrics are generally unclear.11, 12 Although some DCE‐MRI models can be used to estimate perfusion and tissue blood volume, most DCE‐MRI studies focus on measurement of permeability‐related metrics and their correlation with IVIM‐related parameters may be less obvious. Further, DCE‐MRI is generally hampered by poor reproducibility and lack of standardization with respect to the choice of kinetic model and acquisition protocol.13
The purpose of the present work was to examine the associations between perfusion‐related parameters measured by IVIM with those obtained from DSC‐ and DCE‐MRI in patients with rectal cancer. By using a multiecho dynamic contrast‐based sequence, both DSC‐ and DCE‐derived metrics were obtained in a single acquisition, thereby potentially providing more accurate comparisons between the different methods.
Materials and Methods
Patients
This investigation was part of a prospective biomarker study enrolling 192 patients with suspected rectal cancer between October 2013 and December 2017. Excluding cases without histologically confirmed rectal cancer (n = 19) or with study withdrawal (n = 4), nonconsistent MRI sequence due to set‐up of experiment (n = 23), poor quality of dynamic images (n = 20), difficulties in coregistration due to bowel movement or tumor volume <5 cm3 (n = 6), and other incidental difficulties encountered during the clinical MRI acquisition (eg, patients medically ineligible for or refusing contrast administration, software updates disarranging the timing of contrast administration) (n = 26), a total of 94 patients were included in this report. The MRI was acquired at baseline before any treatment had been initiated.
The study was performed in accordance with the Helsinki Declaration and written informed consent was obtained from all patients. Approval was obtained from the Institutional Review Board and the Regional Committee for Medical and Health Research Ethics.
MRI
MRI was performed with a Philips Achieva 1.5T system (Philips Healthcare, Best, The Netherlands). To reduce bowel movement, patients were given glucagon (1 mg/ml, 1 mL intramuscularly) and Buscopan (10 mg/ml, 1 mL intravenously) before scanning, and an equivalent dose of Buscopan before commencement of the dynamic acquisition.
The patients underwent MRI according to clinical procedure, including a high‐resolution T2‐weighted sequence perpendicular to the tumor axis used for tumor delineation. In addition, the patients underwent a multiecho dynamic contrast‐based MRI with GBCA injection and an extended DWI sequence.
DWI was obtained with seven b‐values, b = 0, 25, 50, 100, 500, 1000, 1300 s/mm2, echo time (TE) = 75 msec, repetition time (TR) = 3 sec, and 6 averages. The acquired matrix size was 80 × 60 over a 160 × 160 mm2 field of view and a 4 mm slice thickness, giving a spatial resolution of 2.00 × 2.67 × 4.00 mm3.
The dynamic sequence was obtained as a 3D multishot echo planar imaging sequence with three echoes, TE = 4.6, 13.9, 23.2 msec, TR = 39 msec, flip angle 39°, time resolution that varied between 1.9 and 2.5 sec, with ~60 repetitions. The acquired matrix size was 92 × 90 over a 180 × 180 mm2 field of view and a 10 mm slice thickness, giving a spatial resolution of 1.96 × 2.00 × 10 mm3. A dose of 0.2 mL/kg body weight of GBCA (Dotarem, 279.3 mg/mL, Guerbet Roissy, France) was injected as a bolus directly followed by a 20‐mL saline solution.
Image Postprocessing
The acquired multiecho data was used to extract two dynamic time series by least‐square fitting from the equation:
| (1) |
where S(t, TEn) is the acquired signal, n is the echo number, and M0(t) is the T1‐weighted signal corrected for effects from T2*‐relaxation. In addition to T1‐weighted DCE analysis, this fit also allowed estimation of the dynamic R2*(t) = 1/T2*(t) signal used for DSC analysis.
DSC‐MRI
Assuming a linear relationship between the change in R2*(t) and CA concentration to obtain semiquantitative parameters, the blood flow (BF) was calculated using the established tracer kinetic model for DSC‐MRI14:
| (2) |
where Ct(t)is the CA concentration in tissue, AIF(t) is the arterial input function (CA concentration in a feeding artery), R(t) is the residue function, and β is an unknown scaling factor. f is the fractional flow value and is related to tissue blood flow (perfusion, BF) according to:
| (3) |
where ρ is the tissue density (g/ml) and kh is the hematocrit scaling factor between large and small blood vessels,15 and was assumed to be constant. Since the values of β and kh are not known, the resulting BF values should be regarded as relative perfusion estimates.
The AIF was obtained individually from a nearby artery using an automatic cluster‐algorithm.16 The convolution integral was solved using circular singular value decomposition.17 Area under curve (AUC) for 30 and 60 seconds was calculated as an approximation of tissue blood volume as:
| (4) |
where the lower integration limit was set at bolus arrival time and the upper integration limit was set at 30 and 60 seconds, respectively.
DCE‐MRI
The M0(t) signal was then used to estimate the CA concentration using the signal equation for a spoiled gradient echo (SPGR) sequence without T2* relaxation contribution:
| (5) |
As for the DSC analysis, a linear relationship was assumed between the change in R1 = 1/T1 and CA concentration, and an average precontrast T1‐value measured from six patients was used (1350 msec in tissue).9
The extended Tofts (ET) model18 was fitted to the data with the equation:
| (6) |
where K trans is the transfer constant between blood plasma and the interstitial space, k ep the rate constant, and v p the blood plasma volume. These parameters relate to the extracellular, extravascular space, v e, as v e = K trans/k ep. The same individual AIF as for the DSC analysis was used.
The DCE and DSC‐MRI analyses were done in nordicICE (NordicNeuroLab, Bergen, Norway).
IVIM
The DWI signal was fitted to the equation:
| (7) |
where f is the perfusion fraction, D* is the pseudodiffusion coefficient, and D is the diffusion coefficient. We also calculated the product of the perfusion fraction and pseudodiffusion, f × D*. The fit was done in MatLab (R2015a, MathWorks, Natick, MA) using a Levenberg–Marquardt algorithm. To check for consistency of the results, the exponential fit was performed both with and without b = 1300 s/mm2.
Tumor delineation was done by two radiologists with 14 and 7 years of experience on T2‐weighted images, with the DWI serving as extra guidance. The resultant tumor regions were then semiautomatically coregistered to the other image sequences for optimal fit, also done in nordicICE.
Immunohistochemistry
After surgery, 17 tumors were randomly selected for immunohistochemistry (IHC). Formalin‐fixed paraffin‐embedded tissue sections (4 μm thick) were deparaffinized and hydrated followed by heat‐induced epitope retrieval (20 min at 97°C, 3‐in‐1 procedure) in Dako (Carpinteria, CA) PT‐link with target retrieval solution with high pH (code K8004). Incubation time with the primary antibody was 30 minutes and with the secondary antibody/HRP it was 20 minutes. Counterstaining was performed with Hagens hematoxylin (5 min, diluted 1:4). Staining with CD34 (mouse monoclonal antibody, clone QBEnd/10, Nordic BioSite, Sweden) was performed with the Autostainer Link 48 (Dako) using the Dako Envision Flex Code 8000 visualization kit. Appropriate controls were included and showed satisfactory results. The slides were scanned using the Aperio Scanscope AT using a 20×/0.75 objective with a 0.50 μm/pixel resolution. Microvessel density (MVD) and average vessel size were extracted from the scanned slides by an automated and adapted MatLab script.19
Statistical Analysis
Consistency in tumor delineation and reproducibility of the individual parameters for the two radiologists was evaluated with intraclass correlation tests. Correlations between parameters were investigated using Spearman rank correlation test. Results were deemed statistically significant if P < 0.01, to lower risk of false positives due to multiple testing. All statistical procedures were done in SPSS (v. 25, IBM, Armonk, NY).
Results
Patient demographics are given in Table 1. Estimated kinetic parameters obtained from the tumor volumes defined by the two radiologists were highly correlated (Table 2), and therefore only one set of parameter results is reported. The IVIM parameters showed only linear shifts towards higher D*, and lower f and D, when omitting b = 1300 s/mm2 compared with using all seven b‐values. The correlation between the set of IVIM parameters (f, D, D*) estimated from six and seven b‐values was high (>0.90) and the associations with other parameters (from DCE‐ and DSC‐MRI) showed the same trends. The results reported in the following are from all seven b‐values.
Table 1.
Patient Demographics for the Study
| Number of patients | 94 |
| Females | 33 (35%) |
| Males | 61 (65%) |
| Age (median) | 65 years |
| Disease stage | |
| T2 | 14 (15%) |
| T3 | 46 (49%) |
| T4 | 34 (36%) |
| N0 | 39 (42%) |
| N1 | 33 (35%) |
| N2 | 22 (23%) |
| M0 | 71 (76%) |
| M1 | 23 (24%) |
Table 2.
Estimated values
| Median values (25th ‐ 75th percentile) | ICC (confidence interval) | |
|---|---|---|
| IVIM | ||
| f (fraction) | 0.33 (0.30–0.37) | 0.96 (0.95–0.98) |
| D * (10‐3 mm2/s) | 12.11 (9.87–14.35) | 0.97 (0.95–0.98) |
| f x D * (10‐3 mm2/s) | 3.75 (2.93–4.57) | 0.98 (0.97–0.99) |
| D (10‐3 mm2/s) | 0.60 (0.50–0.71) | 0.98 (0.96–0.98) |
| DSC | ||
| BF (ml/min/100g) | 107.06 (78.32–135.80) | 0.97 (0.95–0.98) |
| AUC 30 (a. u.) | 0.12 (0.03–0.22) | 0.96 (0.93–0.97) |
| AUC 60 (a. u.) | 0.55 (0.31–0.80) | 0.93 (0.90–0.96) |
| DCE | ||
| K trans (min‐1) | 0.04 (0.02–0.06) | 0.96 (0.94–0.97) |
| k ep (min‐1) | 0.24 (0.11–0.38) | 0.96 (0.94–0.97) |
| v p (%) | 0.11 (0.05–0.17) | 0.96 (0.94–0.98) |
| v e (%) | 10.58 (5.68–15.49) | 0.59 (0.38–0.73) |
Median values based on tumor delineations from one radiologist and intraclass correlation coefficients (ICC) between the two radiologists. f = perfusion fraction, D * = pseudodiffusion coefficient, D = diffusion coefficient, BF = relative blood flow, AUC 30/AUC 60 = area under curve for 30 and 60 seconds, respectively, after bolus arrival (a. u. = arbitrary units), K trans = transfer constant between blood plasma and the interstitial space, k ep = rate constant, v p = blood plasma volume, v e = extracellular, extravascular space.
Median values and interquartile ranges for all calculated parameters are given in Table 2 and examples of parameter images overlaid on T2‐weighted images are shown in Fig. 1. Spearman's rank correlation coefficients (rs) and P‐values for comparisons between parameters are given in Tables 3, 4, 5, and scatterplots of the most significant findings are presented in Fig. 2. From IVIM, the pseudodiffusion coefficient D* was correlated with BF from the DSC‐analysis (rs = 0.47; P < 0.001) (Table 3), and the perfusion fraction f showed a negative correlation with k ep from the DCE‐analysis (rs = –0.31; P < 0.002) (Table 4). The comparison of parameters from DSC‐MRI and DCE‐MRI (Table 5) revealed a correlation between BF and K trans (rs = 0.29; P = 0.004), and between BF and v p (rs = 0.44; P < 0.001). Both AUC30 and AUC60 correlated with v e (rs = 0.34; P = 0.001, and rs = 0.45; P < 0.001, respectively) and, in the case of AUC60, also a negative correlation with k ep (rs = –0.45; P < 0.001). Theoretically, BF and K trans are only associated in a flow limited regime20 (low perfusion). The correlation between BF and K trans was stronger (rs = 0.62, P < 0.001) when analyzing tumors with BF < 100 ml/min/100 g, corresponding to the lower half of the population (n = 47) (Fig. 3). When analyzing the other half of the population (BF > 100 ml/min/100 g), no correlation between these parameters was found (rs = –0.06, P = 0.68).
Figure 1.
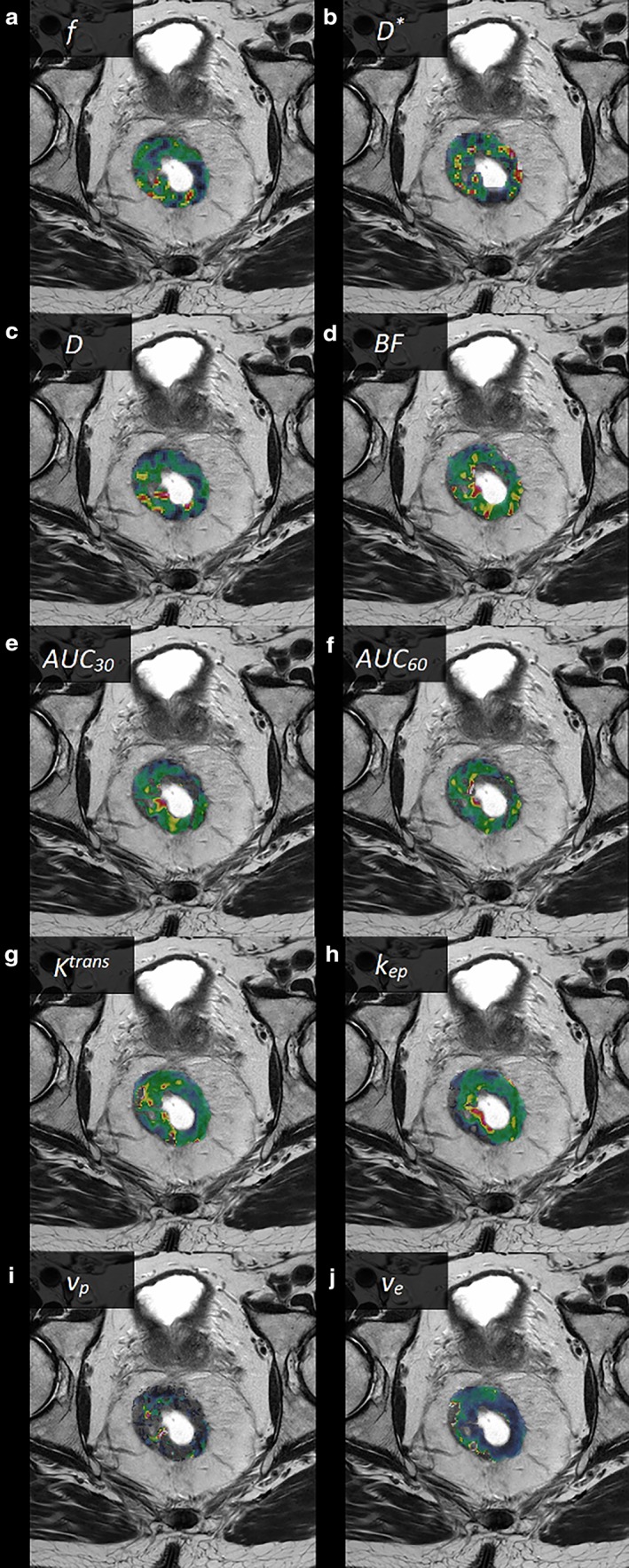
Examples of parametric images from a male patient with a T3 rectal tumor (parametric images of the tumor area shown as color overlays on T2‐weighted images), (a) f, (b) D*, (c) D, (d) BF, (e) AUC30, (f) AUC60, (g) K trans, (h) k ep, (i) v p, and (j) v e.
Table 3.
Correlations between IVIM‐ and DSC‐MRI
| BF | AUC 30 | AUC 60 | |
|---|---|---|---|
| f | –0.03 (0.80) | –0.03 (0.79) | 0.12 (0.26) |
| D * | 0.47 (<0.001) | 0.15 (0.15) | 0.22 (0.03) |
| f x D * | 0.38 (<0.001) | 0.09 (0.41) | 0.23 (0.03) |
| D | –0.23 (0.03) | 0.05 (0.61) | ‐0.07 (0.52) |
Spearman's Rank Correlation Coefficients rs (P‐values in parenthesis)
Table 4.
Correlations between IVIM‐ and DCE‐MRI
| K trans | k ep | v p | v e | |
|---|---|---|---|---|
| f | –0.22 (0.03) | –0.31 (0.002) | –0.17 (0.10) | 0.23 (0.03) |
| D * | –0.18 (0.09) | 0.06 (0.54) | 0.12 (0.27) | –0.16 (0.13) |
| f x D * | –0.28 (0.007) | –0.10 (0.32) | 0.01 (0.97) | –0.01 (0.98) |
| D | 0.03 (0.81) | –0.11 (0.28) | –0.12 (0.25) | 0.13 (0.19) |
Spearman's Rank Correlation Coefficients rs (P‐values in parenthesis)
Table 5.
Correlations between DSC‐ and DCE‐MRI
| K trans | k ep | v p | v e | |
|---|---|---|---|---|
| BF | 0.29 (0.004) | 0.05 (0.67) | 0.44 (<0.001) | 0.10 (0.35) |
| AUC 30 | 0.14 (0.17) | –0.23 (0.03) | 0.08 (0.44) | 0.34 (0.001) |
| AUC 60 | 0.15 (0.14) | –0.45 (<0.001) | 0.08 (0.47) | 0.45 (<0.001) |
Spearman's Rank Correlation Coefficients rs (P‐values in parenthesis)
Figure 2.
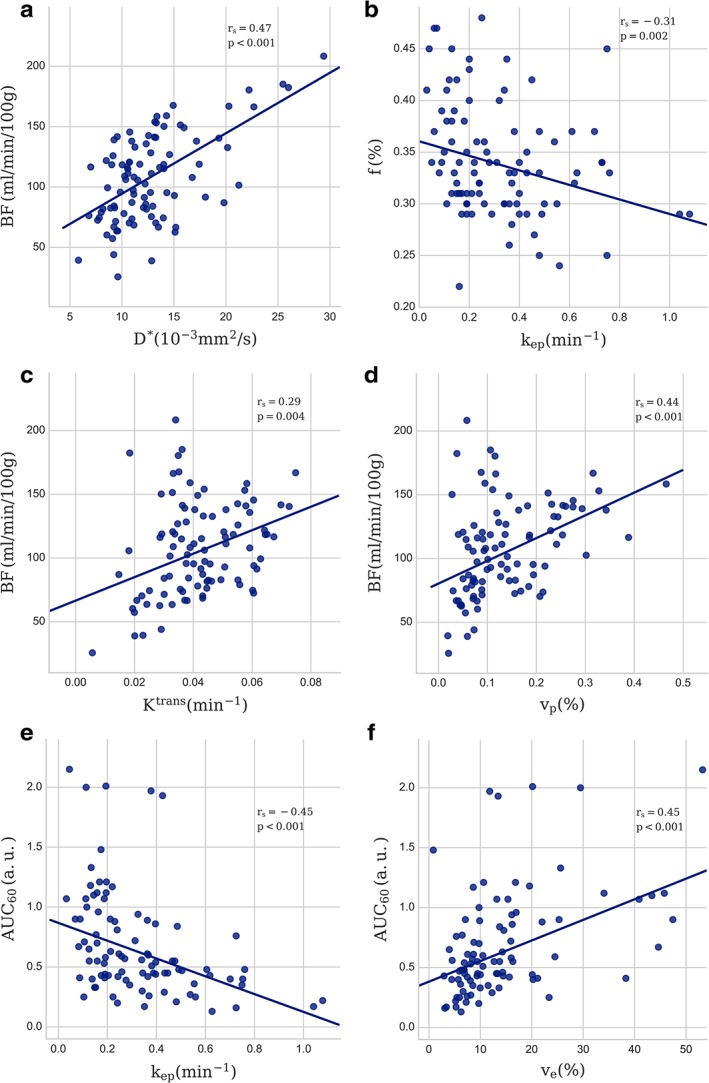
Scatterplot with the least square regression line of the most prominent results, (a) BF vs. D*, (b) f vs. k ep, (c) BF vs. K trans, (d) BF vs. v p, (e) AUC60 vs. k ep, (f) AUC60 vs. v e. Spearman's rank correlation coefficients (rs) and P‐values are indicated.
Figure 3.
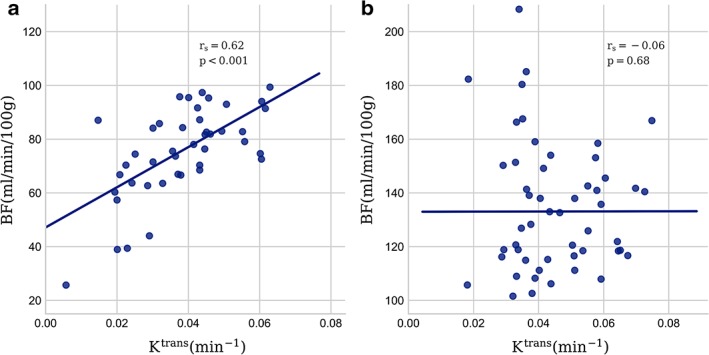
Scatterplot showing the correlation between BF and K trans separated in populations with (a) low (<100) and (b) high (>100 ml/min/100 g) BF. Spearman's rank correlation coefficients (rs) and P‐values are indicated.
Of the 17 tumor specimens analyzed by IHC, 12 had a full set of MRI parameters for correlation analysis. There was no correlation between MVD or vessel size and any MRI‐derived parameters. However, when looking at tumors with BF > 100 ml/min/100 g (n = 10), we observed a negative correlation between vessel size and K trans (rs = –0.82, P = 0.004) (Fig. 4).
Figure 4.
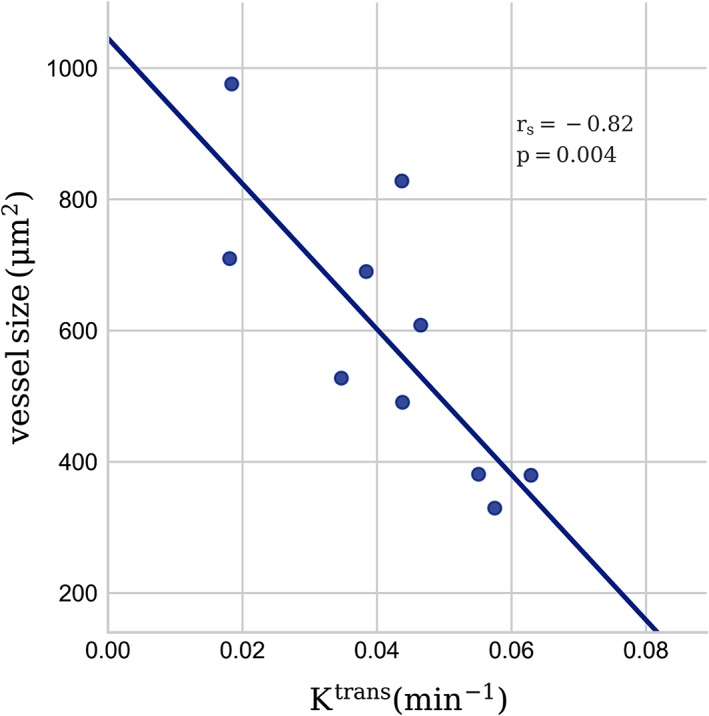
Scatterplot showing the correlation between vessel size quantified from CD34‐based immunohistochemistry of excised tumors and K trans from DCE‐MRI. Spearman's rank correlation coefficient (rs) and P‐values are indicated.
Discussion
A main result from this study of 94 rectal tumors is the correlation between D* from IVIM imaging and BF from DSC‐MRI. This is in line with the findings of Bisdas et al,10 where a strong correlation between these parameters was found in glioblastomas. To our knowledge, this relationship has so far not been examined outside the CNS. The validity of measuring perfusion with the IVIM‐method has been questioned,21 and we show here that there at least exists a linear relationship between the pseudodiffusion coefficient and perfusion estimated by contrast‐based MRI.
Previous studies have examined the link between IVIM imaging and DCE‐MRI, with varying results,10, 11, 22 possibly reflecting the numerous qualitative and quantitative methods used to analyze DCE data. In addition, the interpretation of the parameters from DCE‐MRI can vary depending on the underlying biology. In particular, K trans will reflect tissue blood flow if tissue perfusion is low relative to the permeability surface area product (flow‐limited regime), and conversely will reflect permeability if tissue perfusion is high relative to the permeability surface area product (permeability‐limited regime).20 In the limiting condition of a pure permeability‐limited regime, perfusion and permeability are therefore expected to be uncorrelated. The results from our data suggest that blood flow in this population of rectal tumors spans both these regimes, where low BF in tumors was strongly correlated with K trans, and high tumor BF was not correlated, suggesting that the heterogeneity of rectal cancers makes it challenging to interpret results in studies focusing only on DCE‐MRI. There was no significant correlation between MVD and K trans, in line with a report by Kim et al,23 but we observed a strong negative correlation between K trans and vessel size in tumors with high perfusion. The cases for this analysis were in the high BF group where K trans is only expected to be dependent on permeability and surface area of the vessels.18 The higher leakage from smaller vessels may be explained by their larger ratio of circumference to cross‐section measures compared with larger vessels, and hence larger surface area per unit blood volume. No other MRI‐derived parameters were correlated with MVD or vessel size, which may reflect that MRI parameters in general are measured in in vivo functional tissue, and therefore will not be adequately represented in ex vivo resected tissue.
We further observed a correlation between BF and v p. This is not surprising, since an increase in plasma volume would lead to a similar increase in tissue perfusion for constant blood supply. It should be noted that the ET model used to estimate v p and permeability is only strictly valid in the limiting case of very high tissue blood flow (negligible mean capillary transit time, MTT).20 Since blood flow estimates are independent of MTT, any correlations between BF and DCE‐parameters derived using the ET model may be biased by local variations in tissue MTT. In particular, the measured parameter v p may therefore reflect a mixture of blood volume and plasma flow. Using a more complex kinetic model, like the two‐compartment exchange model, might account for MTT variations and also provide direct estimates of tissue perfusion from the DCE data,20 but we question whether our data supports the use of an even more complex model.
In some reports about the relationship between IVIM‐ and DCE‐MRI parameters,24, 25 the focus has been on the correlation between the perfusion fraction f from IVIM and blood volume from DCE, as this is the expected theoretical relationship according to one of the original works of Le Bihan and Turner.26 The hypothesis that v p derived from the ET model likely reflects a combination of both blood flow and blood volume could be a possible reason for the absence of this correlation between f and v p in our data. In the model‐free deconvolution approach in DSC‐MRI the blood volume is normally estimated by the integration over the contrast curve, normalized by the area of the AIF. This approach assumes that the contrast agent is confined to the intravascular space for the duration of the measurement, and thus the DSC approach has to date been mostly used in CNS applications where the intact blood–brain barrier prevents CA leakage. We used a similar approach to estimate tissue AUC, integrated over both 30 and 60 seconds, normalized to the area of the AIF. However, due to CA extravasation, this AUC does not reflect tissue blood volume alone but rather the combined volume of the intravascular and extravascular, extracellular volume fractions. This is reflected in our results by the correlation between both AUC30 and AUC60 and the extracellular, extravascular volume fraction v e and the negative correlation with the CA reflux constant k ep. We did not correct for CA leakage in the DSC‐based perfusion analysis, since BF obtained from AIF deconvolution, to a first approximation, can be assumed to be independent of CA leakage.27 Further, current kinetic model‐based leakage correction methods applied to blood volume estimates from normalized tissue response time integrals rely on identification of a reference area with no CA leakage effects.28 These methods are thus not readily applicable to non‐CNS applications since nonleaky reference regions cannot be obtained.
From this, it is concluded that estimation of pure intravascular blood volume is challenging using both DCE‐ and DSC‐MRI outside the CNS, limiting the ability to show the expected correlation with IVIM‐derived perfusion fraction f.26 We did, however, find a weak negative correlation between f and the rate constant k ep, and between f × D* and K trans, without being able to find a biological reasoning behind this result. Recently, Sun et al22 investigated the relationship between IVIM and DCE‐MRI in a comparable cohort of rectal cancer patients and found a positive correlation between f × D* and K trans. Interestingly, they report an f‐value about half the value of our estimations (17.02 ± 8.37% vs. 33 ± 3.4%). Given that their investigations are with a superior diffusion sequence (3.0T and 16 b‐values), one would assume their estimate to be more accurate. However, Xu et al,29 who also did IVIM‐analysis on 3.0T with 16 b‐values, reports an f‐value similar to ours (>30%), and it is therefore not likely that the diffusion sequence we used is the source of disagreement. Parameters from the IVIM analysis should, at least in theory, be more comparable between centers than for instance parameters from DCE‐ and DSC‐MRI, and the repeatability and conformance of these parameters are of great interest and importance when comparing studies. It is therefore interesting, but not easily addressed, whether these differences could be attributed to the imaging performance or the algorithms used for analyzing the data.
We used the same AIF for analysis of the DSC and the DCE data, without adjusting for the different AIF amplitude of the R2*‐response compared with the R1‐response, as this will only have a linear and constant scaling effect on all parameters. Since the study was focused on parameter correlations between the different methods and not on absolute quantification, this scaling effect should not influence the results.
In a recent article,30 Le Bihan mentions a common concern with the IVIM approach; the attenuation of the signal as it approaches higher b‐values results in the signal reaching the "noise floor," where the signal is completely masked by the noise. We therefore checked our results by doing the curve fitting to the biexponential curve both with and without the highest b = 1300 s/mm2 value, which only resulted in linear shifts in the absolute value for all parameters and suggested that the curve fitting was adequately robust.
Our results would have been strengthened by the inclusion of a gold standard for perfusion measurements. There is, however, at present no clear gold standard for this purpose outside the CNS, and for this reason both DCE‐, DSC‐, and IVIM‐MRI are proposed as methods for obtaining hemodynamic parameters that reflect perfusion. All these methods have limitations. Perfusion and volume estimates by DSC‐MRI may be particularly challenging outside the CNS due to both kinetic model limitations (CA extravasation), uncertainty in AIF detection, and technical challenges relating to susceptibility artifacts and motion. Dynamic MRI acquisitions are particularly challenging in the pelvic cavity region due to susceptibility artifacts from gas‐pockets and bowel motion; in addition, large susceptibility effects can occur when the contrast agent accumulates in the bladder. Bowel motion and susceptibility artifacts from gas‐pockets are also a concern with DWI for the IVIM approach. Motion and susceptibility artifacts made it difficult to coregister smaller tumor volumes than 5 cm3, and they were excluded. We also experienced timing issues with the contrast administration after a software update, since this multiecho sequence was taken as part of a split dynamics sequence, interleaved with high spatial resolution images.31 However, by focusing on comparing these models against each other and finding a moderate correlation regardless of the limitations and uncertainties in these estimations, the hypothesis that both these methods can be used for perfusion imaging in rectal tumors is strengthened. We have further shown that by acquiring multiecho contrast‐based MRI data, two separate dynamic series can be generated reflecting different contrast mechanisms and possibly different underlying hemodynamic tissue properties.
As this was a single‐center study, no comparison of parameters between imaging modalities from different vendors or with different field strength could be done. The patient population was heterogeneous, comprised of patients from many stages of rectal cancer, and so the underlying biology of the tumors may differ, and the biological interpretation of the measured parameters may vary within the patient population. However, this heterogeneity also ensures that the patient population is representative of the average rectal cancer patient in the clinic.
In conclusion, in rectal tumors, we observed a linear correlation between D* from IVIM imaging and BF obtained from the R2*‐curve. In addition, we found several other correlations between parameters derived from DSC‐MRI, DCE‐MRI, and IVIM. However, the interpretation of these parameters may depend on the underlying biology, the choice of kinetic models, and the MRI acquisition protocols used. The observed negative correlation between the contrast agent transfer constant, K trans, from DCE‐MRI and mean vessel size from IHC supports a dependence of K trans on mean tumor vessel surface area.
Acknowledgments
Contract grant sponsor: South‐Eastern Norway Regional Health Authority; Contract grant numbers: 2014012, 2015048, 2016050; Contract grant sponsor: Akershus University Hospital; Contract grant numbers: 267940, 268938; Contract grant sponsor: Olav Raagholt and Gerd Meidel Raagholt Research Foundation.
References
- 1. Tong T, Sun Y, Gollub MJ, et al. Dynamic contrast‐enhanced MRI: Use in predicting pathological complete response to neoadjuvant chemoradiation in locally advanced rectal cancer. J Magn Reson Imaging 2015;42:673–680. [DOI] [PubMed] [Google Scholar]
- 2. Shen FU, Lu J, Chen L, Wang Z, Chen Y. Diagnostic value of dynamic contrast‐enhanced magnetic resonance imaging in rectal cancer and its correlation with tumor differentiation. Mol Clin Oncol 2016;4:500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1‐weighted MR images: Relationship with increasing cumulative dose of a gadolinium‐based contrast material. Radiology. 2014;270:834–841. [DOI] [PubMed] [Google Scholar]
- 4. Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC. Progressive Increase of T1 Signal Intensity of the Dentate Nucleus on Unenhanced Magnetic Resonance Images Is Associated With Cumulative Doses of Intravenously Administered Gadodiamide in Patients With Normal Renal Function, Suggesting Dechelation. Invest Radiol. 2014;49:685–690. [DOI] [PubMed] [Google Scholar]
- 5. Bihan DL, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval‐Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988;168:497–505. [DOI] [PubMed] [Google Scholar]
- 6. Sun H, Xu Y, Song A, Shi K, Wang W. Intravoxel incoherent motion MRI of rectal cancer: Correlation of diffusion and perfusion characteristics with prognostic tumor markers. AJR Am J Roentgenol 2018;210:W139–w147. [DOI] [PubMed] [Google Scholar]
- 7. Federau C. Intravoxel incoherent motion MRI as a means to measure in vivo perfusion: A review of the evidence. NMR Biomed 2017;30:e3780. [DOI] [PubMed] [Google Scholar]
- 8. Østergaard L. Principles of cerebral perfusion imaging by bolus tracking. J Magn Reson Imaging 2005;22:710–717. [DOI] [PubMed] [Google Scholar]
- 9. Grøvik E, Redalen KR, Storås TH, et al. Dynamic multi‐echo DCE‐ and DSC‐MRI in rectal cancer: Low primary tumor Ktrans and peak are significantly associated with lymph node metastasis. J Magn Reson Imaging 2017;46:194–206. [DOI] [PubMed] [Google Scholar]
- 10. Bisdas S, Braun C, Skardelly M, et al. Correlative assessment of tumor microcirculation using contrast‐enhanced perfusion MRI and intravoxel incoherent motion diffusion‐weighted MRI: Is there a link between them? NMR Biomed 2014;27:1184–1191. [DOI] [PubMed] [Google Scholar]
- 11. Marzi S, Stefanetti L, Sperati F, Anelli V. Relationship between diffusion parameters derived from intravoxel incoherent motion MRI and perfusion measured by dynamic contrast‐enhanced MRI of soft tissue tumors. NMR Biomed 2016;29:6–14. [DOI] [PubMed] [Google Scholar]
- 12. Patel J, Sigmund EE, Rusinek H, Oei M, Babb JS, Taouli B. Diagnosis of cirrhosis with intravoxel incoherent motion diffusion MRI and dynamic contrast‐enhanced MRI alone and in combination: Preliminary experience. J Magn Reson Imaging 2010;31:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lambregts DMJ, Maas M, Stokkel MPM, Beets‐Tan RGH. Magnetic resonance imaging and other imaging modalities in diagnostic and tumor response evaluation. Semin Radiat Oncol 2016;26:193–198. [DOI] [PubMed] [Google Scholar]
- 14. Østergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med 1996;36:715–725. [DOI] [PubMed] [Google Scholar]
- 15. Rempp KA, Brix G, Wenz F, Becker CR, Gückel F, Lorenz WJ. Quantification of regional cerebral blood flow and volume with dynamic susceptibility contrast‐enhanced MR imaging. Radiology 1994;193:637–641. [DOI] [PubMed] [Google Scholar]
- 16. Bjørnerud A, Emblem KE. A fully automated method for quantitative cerebral hemodynamic analysis using DSC‐MRI. J Cereb Blood Flow Metab 2010;30:1066–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu O, Østergaard L, Weisskoff RM, Benner T, Rosen BR, Sorensen AG. Tracer arrival timing‐insensitive technique for estimating flow in MR perfusion‐weighted imaging using singular value decomposition with a block‐circulant deconvolution matrix. Magn Reson Med 2003;50:164–174. [DOI] [PubMed] [Google Scholar]
- 18. Tofts PS. Modeling tracer kinetics in dynamic Gd‐DTPA MR imaging. J Magn Reson Imaging 1997;7:91–101. [DOI] [PubMed] [Google Scholar]
- 19. Mikalsen LT, Dhakal HP, Bruland OS, Nesland JM, Olsen DR. Quantification of angiogenesis in breast cancer by automated vessel identification in CD34 immunohistochemical sections. Anticancer Res 2011;31:4053–4060. [PubMed] [Google Scholar]
- 20. Sourbron SP, Buckley DL. On the scope and interpretation of the Tofts models for DCE‐MRI. Magn Reson Med 2011;66:735–745. [DOI] [PubMed] [Google Scholar]
- 21. Henkelman RM. Does IVIM measure classical perfusion? Magn Reson Med 1990;16:470–475. [DOI] [PubMed] [Google Scholar]
- 22. Sun H, Xu Y, Xu Q, et al. Correlation between intravoxel incoherent motion and dynamic contrast‐enhanced magnetic resonance imaging parameters in rectal cancer. Acad Radiol 2018; doi: 10.1016/j.acra.2018.08.012 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 23. Kim YE, Lim JS, Choi J, et al. Perfusion parameters of dynamic contrast‐enhanced magnetic resonance imaging in patients with rectal cancer: Correlation with microvascular density and vascular endothelial growth factor expression. Korean J Radiol 2013;14:878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu C, Wang K, Chan Q, et al. Intravoxel incoherent motion MR imaging for breast lesions: Comparison and correlation with pharmacokinetic evaluation from dynamic contrast‐enhanced MR imaging. Eur Radiol 2016;26:3888–3898. [DOI] [PubMed] [Google Scholar]
- 25. Fujima N, Yoshida D, Sakashita T, et al. Intravoxel incoherent motion diffusion‐weighted imaging in head and neck squamous cell carcinoma: Assessment of perfusion‐related parameters compared to dynamic contrast‐enhanced MRI. Magn Reson Imaging 2014;32:1206–1213. [DOI] [PubMed] [Google Scholar]
- 26. Bihan DL, Turner R. The capillary network: A link between ivim and classical perfusion. Magn Reson Med 1992;27:171–178. [DOI] [PubMed] [Google Scholar]
- 27. Bjørnerud A, Sorensen AG, Mouridsen K, Emblem KE. T1‐ and T2*‐dominant extravasation correction in DSC‐MRI: Part I—Theoretical considerations and implications for assessment of tumor hemodynamic properties. J Cereb Blood Flow Metab 2011;31:2041–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leu K, Boxerman JL, Cloughesy TF, et al. Improved leakage correction for single‐echo dynamic susceptibility contrast perfusion MRI estimates of relative cerebral blood volume in high‐grade gliomas by accounting for bidirectional contrast agent exchange. Am J Neuroradiol 2016;37:1440–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu Q, Xu Y, Sun H, et al. Quantitative intravoxel incoherent motion parameters derived from whole‐tumor volume for assessing pathological complete response to neoadjuvant chemotherapy in locally advanced rectal cancer. J Magn Reson Imaging 2018;48:248–258. [DOI] [PubMed] [Google Scholar]
- 30. Le Bihan D. What can we see with IVIM MRI? NeuroImage 2017;187:56–67. [DOI] [PubMed] [Google Scholar]
- 31. Grøvik E, Bjørnerud A, Storås TH, Gjesdal K‐I. Split dynamic MRI: Single bolus high spatial‐temporal resolution and multi contrast evaluation of breast lesions. J Magn Reson Imaging 2014;39:673–682. [DOI] [PubMed] [Google Scholar]


