Abstract
The primary challenge facing treatment of epithelial ovarian cancer (EOC) is the high frequency of chemoresistance, which severely impairs the quality of life and survival of patients with EOC. Our study aims to investigate the mechanisms by which upregulation of NR2F6 induces chemoresistance in EOC. The biological roles of NR2F6 in EOC chemoresistance were explored in vitro by Sphere, MTT and AnnexinV/PI assay, and in vivo using an ovarian cancer orthotopic transplantation model. Bioinformatics analysis, luciferase assay, CHIP and IP assays were performed to identify the mechanisms by which NR2F6 promotes chemoresistance in EOC. The expression of NR2F6 was significantly upregulated in chemoresistant EOC tissue, and NR2F6 expression was correlated with poorer overall survival. Moreover, overexpression of NR2F6 promotes the EOC cancer stem cell phenotype; conversely, knockdown of NR2F6 represses the EOC cancer stem cell phenotype and sensitizes EOC to cisplatin in vitro and in vivo. Our results further demonstrate that NR2F6 sustains activated Notch3 signaling, resulting in chemoresistance in EOC cells. Notably, NR2F6 acts as an informative biomarker to identify the population of EOC patients who are likely to experience a favorable objective response to gamma‐secretase inhibitors (GSI), which inhibit Notch signaling. Therefore, concurrent inhibition of NR2F6 and treatment with GSI and cisplatin‐based chemotherapy may be a novel therapeutic approach for NR2F6‐overexpressing EOC. In summary, we have, for the first time, identified an important role for NR2F6 in EOC cisplatin resistance. Our study suggests that GSI may serve as a potential targeted treatment for patients with NR2F6‐overexpressing EOC.
Keywords: NR2F6, epithelial ovarian cancer, cancer stem cells, chemoresistance, Notch3 signaling pathway
Short abstract
What's new?
Chemoresistance is a major challenge in women afflicted with epithelial ovarian cancer (EOC), but molecular mechanisms of EOC chemoresistance remain unclear. Here the authors connect nuclear receptor subfamily 2 group F member 6 (NR2F6) with this process. They find NR2F6 upregulated in tissues from chemoresistant EOC patients. High NR2F6 expression promoted a cancer stem cell phenotype and suppressed cisplatin‐induced apoptosis by transcriptionally upregulating Notch3 signaling, thereby promoting EOC chemoresistance.
Abbreviations
- CHIP
chromatin immunoprecipitation
- COUP‐TFs
chicken ovalbumin upstream promoter‐transcription factors
- CSCs
cancer stem cells
- DLL
delta‐like ligand
- EOC
epithelial ovarian cancer
- GSI
gamma‐secretase inhibitors
- H&E
immunohistochemistry and hematoxylin–eosin
- IP
immunoprecipitation
- MTT
3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl‐2H‐tetrazolium bromide
- NR2F6
nuclear receptor subfamily (NRs) 2 group F member 6
- OS
overall survival
- PD1/PDL1
programmed death 1/programmed cell death‐ligand 1
- SI
staining index
- TCGA
The Cancer Genome Atlas
- TIC
tumor‐initiating cell
- TR
nuclear receptor
Introduction
Ovarian cancer is one of the most prevalent and lethal gynecologic malignancies. Epithelial ovarian cancer (EOC) is the most common subtype of ovarian cancer, accounting for 85–90% of all ovarian cancer.1 Due to the lack of early diagnosis and the extremely chemoresistant phenotype common to EOC, the 5‐year survival rate for ovarian cancer patients is a dismal 29%.1, 2 The most effective treatment for EOC is cytoreductive surgery, followed by cisplatin‐based chemotherapy; however, more than 75% of treated patients experience tumor relapse and ultimately die of recurrent disease.3, 4 The development of resistance to platinum‐based chemotherapy is a serious limitation of long‐lasting, effective treatments for EOC patients. Thus, discovering novel therapeutic approaches to overcome chemoresistance is crucial to improving treatment outcomes for patients with EOC.
Nuclear receptor subfamily (NRs) 2 group F member 6 (NR2F6) belongs the chicken ovalbumin upstream promoter‐transcription factors (COUP‐TFs).5, 6 In Mammals, the COUP‐TFs regulate many key biological processes, including cell proliferation, apoptosis, gut barrier homeostasis and neuronal development.7, 8 Recent studies have also underscored the importance of NR2F6 in regulation of immunity. Victoria et al. demonstrated that NR2F6 acts as an intracellular immunity checkpoint and genetic ablation of NR2F6 acts as a “sensitizer” that enables improved therapeutic activity of clinically approved agents that block the PD‐1/PD‐L1 axis.9 Thus, targeting NR2F6 appears to be a suitable strategy to enhance the efficacy of cancer immunotherapy. However, whether NR2F6 expression is directly correlated with tumor progression, especially in EOC, remains largely unclear. Therefore, investigating the role of NR2F6 and EOC is meaningful.
Cancer stem cells (CSCs) exhibit antiapoptosis, self‐renewal and drug resistance,10, 11 and are thought to be largely responsible for tumor recurrence.11, 12 Additionally, CSCs express drug transporters and DNA repair enzymes, which protect CSCs from drug‐induced apoptosis.13, 14 Moreover, increasing evidence indicates that the Notch signaling pathway is involved in mediating CSC antiapoptosis, cell cycle control and DNA damage repair.15 The Notch pathway comprises four Notch receptors (Notch1–4) and five transmembrane Notch ligands (Jagged‐1, Jagged‐2, Delta‐like ligand [DLL] 1, DLL3 and DLL4). When Jagged binds to the Notch receptors, the Notch signaling pathway is initiated and a signaling cascade occurs. The intracellular domain of the Notch receptor is then cleaved into parts by a series of proteolytic cleavages mediated by γ‐secretase. Notch3 signaling is thought to play a crucial role in ovarian cancer, and it is related to poor clinical prognosis.16, 17 Moreover, it has been suggested that the Notch3 signaling pathway plays an important role in ovarian cancer chemoresistance.18 Furthermore, the inactivation of Notch3 signaling by γ‐secretase inhibitor (GSI) induced apoptosis and suppressed proliferation of ovarian cancer cells.17
In our study, we demonstrate that NR2F6 promotes the stem cell‐like phenotype and induces cisplatin resistance in EOC cells by activating the Notch3 pathway. These findings also suggested that NR2F6 may serve as a novel and potential therapeutic target for the treatment of EOC.
Materials and Methods
Cell lines and cell culture
The human ovarian cancer cell lines OVCAR3 and A2780 were obtained from ATCC (Manassas, VA) and from the National Institutes for Food and Drug Control (Dongcheng District, Beijing, China), respectively. These cell lines were cultured in DMEM supplemented with 10% fetal bovine serum (FBS, Life Technologies, Carlsbad, CA), 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA). All cell lines were authenticated using short tandem repeat profiling.
Chemical reagents
A gamma‐secretase inhibitor of Notch signaling, RO4929097, was purchased from Selleck Chemicals (S1575) and used at a concentration of 10 μM.
Patient information and tissue specimens
For the analysis of NR2F6 expression levels, we collected a total of 306 paraffin‐embedded EOC samples, which had been diagnosed and surgically resected at the Sun Yat‐sen University Cancer Center from 2001 to 2012. The clinical information of the samples is shown in Supporting Information Table S1. Ten freshly collected EOC tissues and three normal ovarian cancer tissues were frozen and stored in liquid nitrogen until required. To analyze the correlation between NR2F6 expression levels and chemoresistance, we obtained 29 primary tumor tissues, including 12 chemosensitive samples that had no drug resistance observed within 6 months after treatment, and 17 samples with chemoresistance.
RNA extraction, reverse transcription and quantitative real‐time PCR
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Complementary DNA was synthesized and quantified using a Bio‐Rad CFX RT‐qPCR detection system (Applied Biosystems Inc Foster City, CA), using SYBR Green Master Mix (ROX; Roche, Toronto, ON). Data were normalized using the geometric mean of the GAPDH reference gene to control for variability in expression levels. Gene expression fold changes were calculated by relative quantification: 2−[(Ct of gene) − (Ct of GAPDH)]. The primer sequences are listed in Supporting Information Table S4.
Immunohistochemistry
Three hundred and six ovarian cancer tissues fixed in 10% formalin and embedded in paraffin were cut into 4 μm slides. Immunohistochemistry staining was performed using NR2F6 antibody (60117‐1‐Ig, Proteintech, Rosemont, IL), Ki67 (#9449, CST) and Notch3 antibody (Sc‐5593). The results of the staining were evaluated and scored by two independent pathologists, who were blinded to the clinical outcome, based on both the proportion of positively stained tumor cells and the intensity of staining. Cell proportions were scored as follows: 0, no positive cells; 1, <10% positive cells; 2, 10–35% positive cells; 3, 35–75% positive cells; 4, >75% positive cells. Staining intensity was graded according to the following standard: 0, no staining; 1, weak staining (light yellow); 2, moderate staining (yellow‐brown); 3, strong staining (brown). The staining index (SI) was calculated as the product of the staining intensity score and the proportion of positive cells. Using this method of assessment, we evaluated protein expression by determining the SI, with possible scores of 0, 1, 2, 3, 4, 6, 8, 9 and 12. Samples with a SI ≥ 6 were defined as having high expression, and samples with a SI < 6 were defined as having low expression.
Luciferase activity assays
Cells (1 × 104) were seeded in 48‐well plates in triplicate, and allowed to proliferate to 60–80% confluence after 24 hr in culture. The indicated plasmids or luciferase reporter plasmids plus 1 ng of pRL‐TK renilla plasmid (Promega, Madison, WI), were transfected into cells using Lipofectamine 3000 reagent (Invitrogen), according to the manufacturer's instructions. Luciferase and renilla enzyme activity signals were measured using the Dual Luciferase Reporter Assay Kit (Promega), according to the manufacturer's protocol.
Xenograft tumor model, IHC and hematoxylin–eosin staining
The animal studies were approved by the Ethics Committee of Sun Yat‐Sen University, and all the experiments conform to the relevant regulatory standards. In the tumor model, BALB/c nude mice were randomly divided into four groups (n = 6 mice/group). The indicated luciferase expressing cells (A2780/Vector cells [1 × 106], A2780/NR2F6 cells [1 × 106], A2780/Scramble cells [1 × 106] or A2780/shRNA #1 cells [1 × 106]) were stereotactically implanted into the ovary of nude mice. After the luminescence value of xenograft tumors reached approximately 2 × 107 p/sec/cm2/sr, mice were treated with intraperitoneal injection of vehicle (control), CDDP (5 mg/kg) or a combination of CDDP (5 mg/kg) and GSI (RO4929097, 10 mg/kg for oral administration) three times per week (as per cycle), for up to 6 weeks. Tumors were detected using a Xenogen IVIS imaging system. Upon experimental endpoint, animals were euthanized, tumors were excised, weighed, paraffin‐embedded and sectioned for immunohistochemistry and hematoxylin–eosin (H&E) staining. Images were captured using the AxioVision Rel.4.6 computerized image analysis system (Carl Zeiss, Oberkochen, Germany).
Statistical analysis
All statistical analyses were carried out using SPSS version 20.0 (SPSS Inc, Chicago, IL). Statistical tests for data analysis included Fisher's exact test, Log‐rank test, Chi‐square test and Student's two‐tailed t‐test. The relationship between NR2F6 expression and clinicopathological characteristics was analyzed by the chi‐square test and Fisher's exact test. Survival curves were plotted using the Kaplan–Meier method and the log‐rank test. Univariate and multivariate Cox regression analyses were conducted using the Cox hazards model. Data are presented as mean ± SD. Values of p = 0.05 or less were considered statistically significant.
Results
NR2F6 is markedly upregulated and correlated with chemoresistance in EOC
We analyzed publicly available ovarian cancer mRNA expression profiles (GSE4122, GSE38666 and GSE1926) obtained from the NCBI (https://www.ncbi.nlm.nih.gov/geo/). We found that the mRNA expression of NR2F6 is elevated in ovarian cancer tissue compared to nontumor ovarian tissue (Figs. 1 a and 1b). NR2F6 expression is also overexpressed in chemoresistant EOC compared to chemosensitive EOC (Fig. 1 c). Consistently, real‐time PCR revealed that NR2F6 was elevated in ovarian cancer tissue, compared to normal ovarian tissue (Supplemental Fig. S1 A). To examine the potential function of NR2F6 in the relapse of chemoresistant ovarian cancer, we initially evaluated NR2F6 protein and mRNA expression in 29 primary ovarian cancer tumor tissues, including 12 samples that had no chemoresistance within 6 months, and 17 samples that exhibited chemoresistance within 6 months (Fig. 1 d, Supporting Information Figs. S1 B and S1C). Consistent with previous data, IHC staining revealed that high NR2F6 expression positively correlated with chemoresistance in patients with ovarian cancer. Correlation analysis showed that high expression of NR2F6 significantly correlated with chemoresistant relapse in EOC patients (Fig. 1 e; p < 0.001). We further analyzed NR2F6 expression in 306 paraffin‐embedded, archival ovarian cancer tissues by IHC (Supporting Information Table S1), and we found that NR2F6 expression was significantly associated with drug resistance (p < 0.001), tumor recurrence (p < 0.001), intraperitoneal metastasis (p < 0.001), intestinal metastasis (p = 0.011), ascites with tumor cells (p = 0.012) and intraperitoneal recurrence (p < 0.001; Supporting Information Table S2). Importantly, Kaplan–Meier survival analysis revealed that ovarian cancer patients (all patients and patients with chemotherapy) with high NR2F6 expression had shorter overall survival (OS) than those with low NR2F6 expression (Figs. 1 f and 1g). Furthermore, multivariate Cox regression analysis showed that NR2F6 expression was an independent prognostic factor, along with intraperitoneal metastasis, drug resistance and lymph node metastasis, for worse 5‐year OS in ovarian cancer (Supporting Information Table S3). These results indicate that overexpression of NR2F6 may contribute to ovarian cancer progression, leading to poor clinical outcomes.
Figure 1.
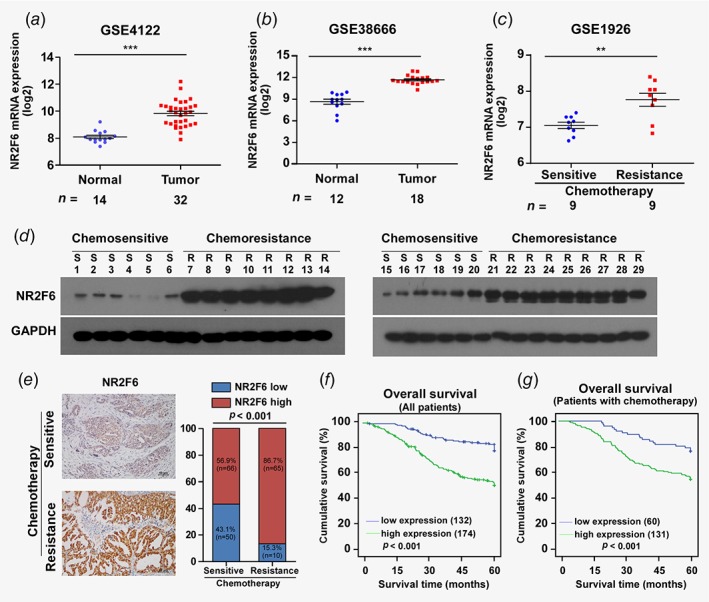
NR2F6 is overexpressed in EOC and correlates with chemoresistance. (a) Expression profiling of mRNAs in EOC tumor vs. normal tissue samples and chemosensitive tissues vs. chemoresistant tissues from Array express microarray data (GEO accession number: GSE4122, GSE38666 and GSE1926; http://www.ncbi.nlm.nih.gov/geo/). (b,c) Western blot analysis of NR2F6 protein in 29 primary ovarian cancer tissues, including 12 samples that had no chemoresistance observed within 6 months, and 17 samples with chemoresistance within 6 months. GAPDH was used as a loading control. Epithelial ovarian cancer (EOC) patients whose disease recurs in less than 6 months after initial platinum‐based chemotherapy are termed platinum resistance, and the recurrence of EOC patients after 6 months is considered platinum sensitive (Ovarian Cancer National Comprehensive Cancer Network (NCCN) 2017). (d) Representative images of EOC tumor samples with (n = 75) or without (n = 116) chemoresistance in 6 months (left panel). Correlation between chemoresistance and NR2F6 expression in patients; the Chi‐square test was used to evaluate correlation (right panel). *p < 0.05,**p < 0.01, ***p < 0.001. [Color figure can be viewed at wileyonlinelibrary.com]
NR2F6 overexpression confers cisplatin resistance to ovarian cancer in vitro
Ovarian cancer recurrence mainly arises due to chemoresistance, and abnormal regulation of apoptosis is an important mechanism of drug resistance.2, 4 To explore the antiapoptotic role of NR2F6 in ovarian cancer, we generated stable NR2F6 overexpressing cell lines and NR2F6 knockdown cell lines from the A2780 and OVCAR3 ovarian cancer cell lines (Supporting Information Fig. S2 A). Overexpression of NR2F6 drastically reduced cisplatin‐induced apoptosis and increased the colony‐formation capacity of A2780 and OVCAR3 cells (Fig. 2 a). On the contrary, IC50 value for cisplatin was increased in NR2F6‐overexpressing A2780 and OVCAR3 cells, as indicated by 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl‐2H‐tetrazolium bromide (MTT) assay, but was reduced in NR2F6‐silenced A2780 and OVCAR3 cells (Figs. 2 b and 2c). Moreover, overexpression of NR2F6 reduced cisplatin‐induced apoptosis of A2780 and OVCAR3 cells as detected by Annexin V/PI assay compared to control cells, while knockdown of NR2F6 increased cisplatin‐induced apoptosis (Fig. 2 d). Furthermore, cleaved caspase3 and PARP protein levels were significantly decreased in NR2F6 overexpressing ovarian cancer cells but were increased in NR2F6‐silenced ovarian cancer cells (Fig. 2 e). Moreover, overexpressing NR2F6 or silencing NR2F6 only induced a slight change in the apoptosis rate and colony formation capacity of ovarian cancer cells in the absence of cisplatin treatment (Supporting Information Figs. S2B–S2E). Collectively, these results demonstrate that NR2F6 expression is closely associated with cisplatin resistance in ovarian cancer cells.
Figure 2.

Overexpression of NR2F6 confers cisplatin resistance to ovarian cancer in vitro. (a) Representative images (left panel) and quantification (right panel) of the indicated cells after crystal violet staining. Each bar represents the mean ± SD of three independent experiments. *p < 0.05; **p < 0.01. (b) MTT/IC50 of cisplatin in the indicated cells. Each bar represents the mean ± SD of three independent experiments. *p < 0.05. (c) Annexin V‐FITC and PI staining of the indicated cells treated with cisplatin for 24 hr. Each bar represents the mean ± SD of three independent experiments. *p < 0.05,**p < 0.01. Abbreviations: FITC, fluorescein isothiocyanate; PI, propidium iodide. (d) Western blot analysis of cleaved caspase3 and PARP in the indicated cells; GAPDH was used as a loading control. [Color figure can be viewed at wileyonlinelibrary.com]
High expression of NR2F6 confers cisplatin resistance to ovarian cancer in vivo
We employed a nude mouse xenograft model to further explore the role of NR2F6 in ovarian cancer chemoresistance in vivo. The indicated luciferase expressing cells (A2780/Vector, A2780‐NR2F6, A2780/Scramble or A2780/shRNA#1) were orthotopically inoculated into the ovaries of nude mice. After the luminescence from the tumors reached 2 × 107 p/sec/cm2/sr (as evaluated by a Xenogen IVIS spectrum imaging system), mice were treated with 5 mg/kg cisplatin three times per week (as per cycle) for up to 6 weeks. Mice treated with the combination of NR2F6‐shRNA#1 and cisplatin exhibited a significant reduction in tumor growth. On the other hand, NR2F6 overexpression resulted in a significant increase in tumor volume, compared to the control treatment group (Figs. 3 a–3 c). Consistent with these results, IHC staining of histological tumor sections revealed that tumors developed from the A2780‐NR2F6 cells had a higher percentage of Ki‐67 positive cells than tumors from A2780/Vector cells, while tumors from the A2780/shRNA#1 group had very few Ki‐67 positive cells (Fig. 3 d). Moreover, the protein levels of NR2F6, CyclinD1 and Ki‐67 were overexpressed in the A2780‐NR2F6‐derived tumors compared to A2780/Vector tumors (Supporting Information Fig. S3 A). These results illustrate that NR2F6 overexpression contributes to ovarian cancer chemoresistance in vivo.
Figure 3.

Overexpression of NR2F6 confers cisplatin resistance on ovarian cancer in vivo. (a) Representative images of tumor‐bearing mice challenged with the indicated cells treated with 5 mg/kg cisplatin. (b) Relative changes in luminescence were evaluated by bioluminescent imaging (BLI). (c) Kaplan–Meier survival curves of mice in the indicated cell. (d–f) HE and IHC staining were used in indicated xenografts. Each bar represents the mean ± SD of three independent experiments. *p < 0.05,**p < 0.01. [Color figure can be viewed at wileyonlinelibrary.com]
NR2F6 promotes CSCs properties in ovarian cancer cells
CSCs drive chemoresistance and contribute to unfavorable clinical outcomes. Gene set enrichment analysis of ovarian cancer samples from The Cancer Genome Atlas (TCGA) revealed a significant enrichment of stem cell gene modules in NR2F6 overexpressing samples (Supporting Information Figs. S4 A and S4B). Based on our observations that NR2F6 promotes tumor sphere formation and the expression of stem cell‐related molecules, including SOX2, NANOG and OCT4 in vitro (Figs. 4 a–4 c, Supporting Information Figs. S4 C and S4D), we further explored whether NR2F6 enhances tumorigenesis of ovarian cancer cells in vivo. We injected limiting numbers (1 × 104, 1 × 105 and 1 × 106) of the indicated cells (A2780/Vector, A2780‐NR2F6, A2780/Scramble or A2780/shRNA#1) into the flanks of nude mice to assess the influence of NR2F6 expression on tumorigenicity of ovarian cancer cells. We found that expression of NR2F6 is essential for the ability of A2780 cells to seed tumors in vivo (Figs. 4 d–4 f). As anticipated, this effect was evident only when limiting numbers of tumors cells were injected in the flank of nude mice. Mice injected with 1 × 106, 1 × 105 or 1 × 104 A2780/Vector cells developed tumors in 8/8, 5/8 and 3/8 mice, respectively. In contrast, 5/8 mice injected with 1 × 104 A2780‐NR2F6 cells developed tumors; 8/8 and 8/8 mice developed tumors in the groups injected with 1 × 105 and 1 × 106 A2780‐NR2F6 cells, respectively. Mice injected with 1 × 106, 1 × 105 or 1 × 104 A2780/Scramble cells developed tumors in 8/8, 4/8 and 3/8 mice, respectively. In contrast, none of the mice injected with 1 × 104 NR2F6 silencing A2780 cells developed tumors; and fewer tumor events were observed when 1 × 106 and 1 × 105 NR2F6‐shRNA A2780 cells were injected compared to controls (8/8 and 3/8, respectively). The proportion of tumor‐initiation frequency was calculated using the ELDA software (http://bioinf.wehi.edu.au/software/elda/). These data indicate that downregulation of NR2F6 remarkably decreases the tumor‐initiating cell (TIC) frequency of A2780 cells. Conversely, overexpression of NR2F6 significantly increases the TIC frequency. Thus, our results indicated that NR2F6 plays an important role in ovarian cancer initiation in vivo.
Figure 4.

Overexpression of NR2F6 confers cancer stem cell characteristics on EOC cells in vitro and in vivo. (a) Representative images of tumor spheres formed by the indicated A2780 and OVCAR3 cells. (b) Histograms showing the mean number of spheres formed by the indicated cells from different passages. (c) Fold change in the number of cells per sphere on the indicated days. *p < 0.05, **p < 0.01, ***p < 0.001. (d) Statistical analysis of tumor incidence and tumor‐initiating cell (TIC) frequency calculated by ELDA software. (e, f) Limiting dilution assays to analyze the effect of NR2F6 on ovarian cancer tumorigenesis. (e) Indicated numbers of A2780 cells were implanted into the flank of BALB/c nude mice; tumor volumes were measured on the indicated days. (f) Five weeks later, mice were euthanized and tumors were retrieved and weighed. [Color figure can be viewed at wileyonlinelibrary.com]
NR2F6 interacts with the Notch3 promoter and enhances Notch3 transcription by increasing the enrichment of p300 and histone acetylation at the promoter
To explore the mechanism by which NR2F6 promotes CSC properties and chemoresistance, we conducted luciferase reporter assays to explore the activities of several typical stem cell‐associated pathways, including the Hedgehog, Notch, Wnt/β‐catenin and STAT3 pathways. The luciferase reporter assay revealed that NR2F6 strongly promoted the transcriptional activity of the Notch reporter, but had no effect on the Hedgehog, Wnt/β‐catenin or STAT3 reporters (Supporting Information Figs. S5 A and S5B). Furthermore, analysis of TCGA datasets showed significant correlation between NR2F6 expression and expression of Notch3, but not other Notch family members (Notch1, Notch2 and Notch4) (Supporting Information Figs. S5 C–S5 E and Fig. 5 a). Moreover, western blot and Real‐time PCR analysis indicated that overexpression of NR2F6 significantly increased Notch3, N3ICD and Hes1 expression levels, while silencing NR2F6 reduced Notch3, N3ICD and Hes1 levels. Hes1 is a well‐known direct transcriptional target of Notch3, and it plays an important role in the maintenance of cancer stem cells and tumor drug resistance.19, 20 Changes in NR2F6 expression had no impact on the expression of Jagged1 at either the protein or the mRNA level (Fig. 5 b and Supporting Information Figs. S5 F–S5 I).
Figure 5.
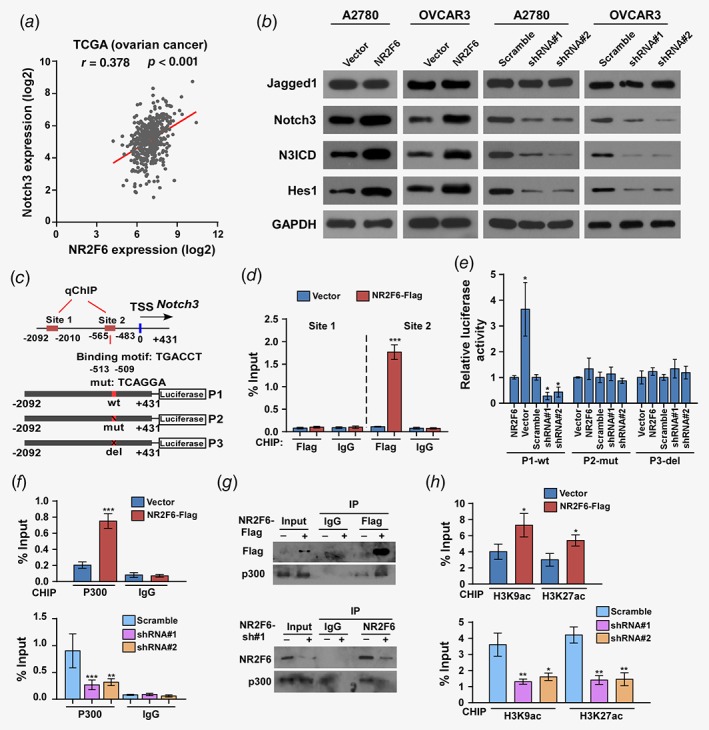
NR2F6 regulates Notch3 transcriptional activity in ovarian cancer. (a) Correlation between NR2F6 and Notch3 mRNA expression in TCGA ovarian cancer dataset. (b) Expression of Jagged1, Notch3, N3ICD, and Hes1 in the indicated A2780 and OVCAR3 cells were evaluated by western blot. GAPDH was used as loading control. (c) Schematic illustration of the PCR fragments of the human Notch3 gene promoter. (d) Chromatin immunoprecipitation (ChIP) assays were performed in A2780 cells using antibodies against Flag to identify the NR2F6 occupancy on the Notch3 gene promoter. Immunoglobulin G (IgG) was used as a negative control. (e) Luciferase reporter assays were conducted in the indicated A2780 cells to detect the effect of deletion or mutation of site 2 fragment of the Notch3 promoters on transcriptional regulation by NR2F6. (f) ChIP assays were performed in the indicated A2780 cells using antibodies against p300 acetyltransferase to identify the p300 occupancy on the Notch3 gene promoters. Immunoglobulin G (IgG) was used as a negative control. (g) Lysates from A2780 cells transfected with Flag‐NR2F6 were immunoprecipitated with anti‐Flag affinity agarose, followed by western blot analysis of p300. Immunoprecipitation assays revealed that silencing of NR2F6 substantially abrogated the interaction with p300. (h) Enrichments acetylated histone H3 (K9, K27) in site 2 fragment of the Notch3 gene promoter in the indicated A2780 cells. *p < 0.05, **p < 0.01, ***p < 0.001. [Color figure can be viewed at wileyonlinelibrary.com]
Nuclear receptor subfamily proteins serve as transcriptional regulators and promote gene transcription by recruiting acetyltransferase enzymes, thereby facilitating histone acetylation on gene promoter elements.5, 6, 21 We performed chromatin immunoprecipitation (ChIP) assays to investigate the regulatory effect of NR2F6 on Notch3 signaling. ChIP assays revealed that NR2F6‐Flag interacted with the Notch3 promoter site2 fragment, not the site 1 fragment (Figs. 5 c and 5d). However, neither overexpression nor knockdown of NR2F6 had any effect on the Notch3 promoter activity from the luciferase reporters that contained deleted or mutated site2 fragments (Fig. 5 e). Moreover, the ChIP assays showed that upregulation of NR2F6 remarkably increased, while silencing of NR2F6 significantly reduced, the occupancy of P300 acetyltransferase on the Notch3 promoter (Fig. 5 f). In addition, the immunoprecipitation (IP) assays demonstrate that NR2F6 interacts with p300; however, silencing of NR2F6 substantially abrogated the interaction with P300 (Fig. 5 g). Furthermore, the ChIP assays were performed on the Notch3 promoter site 2 fragment, and silencing of NR2F6 significantly reduced, while upregulation of NR2F6 markedly increased, the appearance of P300‐acetylated histone H3(H9,K27) located at the proximal Notch3 promoter (Fig. 5 h).
Finally, we investigated the mechanism causing NR2F6 overexpression in EOC. We explored the correlation between genomic copy number and mRNA expression in the “Ovarian Epithelial Carcinoma (TCGA, Provisional)” dataset from the cBioportal database (http://www.cbioportal.org). Notably, genetic amplification of NR2F6 was frequently found in TCGA EOC tissues, and the copy‐number of NR2F6 significantly correlated with levels of mRNA expression (Supporting Information Figs. S6 A–S6C). These results suggest that gene amplification is one mechanism responsible for NR2F6 overexpression in EOC.
Notch3 signaling is required for NR2F6‐induced chemoresistance and the clinical relevance of NR2F6 and Notch3 expression in EOC
To further verify that NR2F6 mediates ovarian cancer chemoresistance through activation of Notch3 signaling, we inhibited the Notch3 pathway in NR2F6‐overexpressing cells by treatment with shRNA or with a γ‐secretase inhibitor (GSI, RO4929097, Selleck, Houston, TX). Clonogenic survival assays, MTT cell proliferation assays and Annexin V/PI apoptosis assays revealed that Notch3 signaling is essential for NR2F6‐mediated survival and antiapoptotic effects in EOC cells after cisplatin treatment (Figs. 6 a–6 c). Tumor sphere formation by NR2F6‐overexpressing A2780 and OVCAR3 cells was robustly suppressed by the inhibition of Notch3 (Fig. 6 d).
Figure 6.
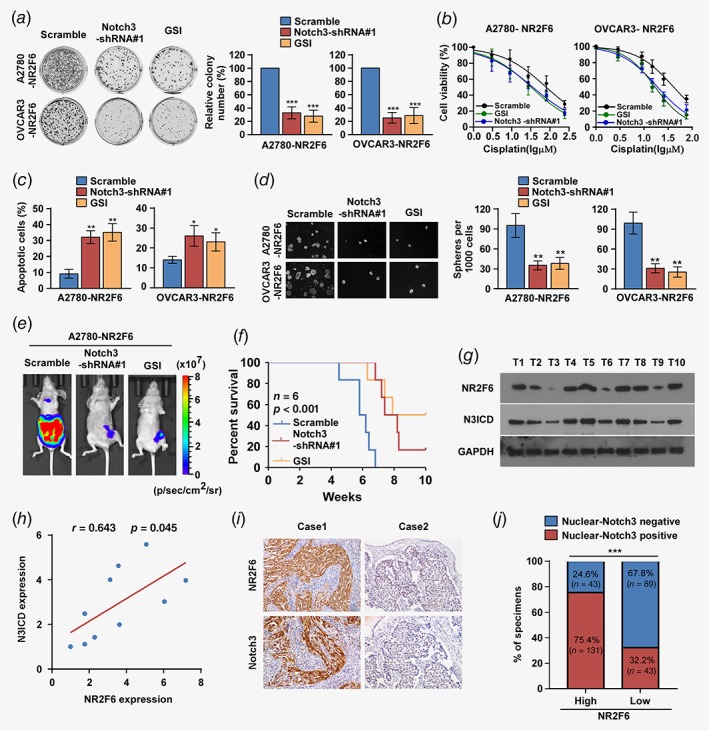
Notch3 is required for NR2F6‐induced cisplatin resistance and the clinical relevance of NR2F6 and Notch3. (a) Representative images (left panel) and quantification (right panel) after crystal violet staining of the indicated cells treated with Notch3 shRNAs and Notch3 inhibitor (GSI). (b) MTT/IC50 of cisplatin in the indicated cells treated with Notch3 shRNAs and Notch3 inhibitor (GSI). Each bar represents the mean ± SD of three independent experiments. *p < 0.05. (c). Annexin V‐FITC and PI staining of the indicated cells treated with Notch3 shRNAs and Notch3 inhibitor (GSI). Each bar represents the mean ± SD of three independent experiments. (d) Representative micrographs and quantification of the tumor spheres formed by A2780‐NR2F6 and OVCAR3‐NR2F6 cells subjected to the indicated treatments. (e, f) Representative images (e) and Kaplan–Meier survival curves (f) of tumor‐bearing mice subjected to Notch3 shRNAs and Notch3 inhibitor (GSI) treatments. (g) Expression analysis and (h) correlation of NR2F6 and Notch3 expression in 10 freshly collected human ovarian cancer tissue samples; GAPDH was used as a loading control. Each bar represents the mean ± SD of three independent experiments. (i, j) Immunohistochemical staining showing that NR2F6 expression correlates positively with Notch3 expression in 306 clinical ovarian cancer specimens. Percentage of human ovarian cancer specimens showing low or high NR2F6 expression relative to the levels of Notch3. Scale bars = 50 μm; ***p < 0.001. [Color figure can be viewed at wileyonlinelibrary.com]
Furthermore, we evaluated the effect of Notch3 inhibition on NR2F6‐induced chemoresistance in vivo. Inhibition of Notch3 resulted in a significant reduction in tumor volume and mice in the GSI treated group exhibited significantly longer survival compared to the control group (Figs. 6 e and 6f).
Finally, we examined whether the NR2F6/Notch3 axis was clinically relevant in ovarian cancer. We found that NR2F6 levels were positively correlated with Notch3 expression (Figs. 6 g and 6h; r = 0.643, p = 0.045). Furthermore, we evaluated nuclear‐Notch3 and NR2F6 expression by IHC staining in 306 paraffin‐embedded, archived EOC samples. Clinical samples with high levels of NR2F6 expression showed a higher percentage of nuclear‐Notch3, whereas samples with low NR2F6 expression exhibited lower percentage of nuclear‐Notch3 expression (Figs. 6 i and 6j). In addition, we analyze NR2F6 expression in five pairs of sensitive and resistive tumors collected from the same patient, which had been diagnosed and surgically resected at Sun Yat‐sen University Cancer Center. These five EOC patients were platinum‐sensitive during the first postoperative chemotherapy, and these patients eventually relapse and develop platinum resistance. For these platinum resistance patients, secondary tumor cytoreductive surgery and second‐line drug are preferred. Then we detect the expression of NR2F6 and Notch 3 ICD in the pairs of sensitive and resistant tumors collected from the same patient. We found that the expression of both NR2F6 and Notch 3 ICD are upregulated in cisplatin‐resistance ovarian cancer patient tissues than cisplatin‐sensitive ovarian cancer patient tissues (Supporting Information Fig. S7).
Taken together, these observations suggest that NR2F6 interacts with the promoter of Notch3, and further increases the enrichment of p300 and histone acetylation at the Notch3 promoter, inducing Notch3 signaling activation and increasing CSCs properties, thus promoting cisplatin resistance in ovarian cancer cells (Supporting Information Fig. S8).
Discussion
In our study, we report a novel molecular mechanism to potentially explain the emergence of chemoresistance in ovarian cancer patients. NR2F6 is elevated in chemoresistant ovarian cancer tissues, and expression of NR2F6 significantly correlates with poor OS and disease‐free survival (DFS). Moreover, we observed that overexpression of NR2F6 in ovarian cancer cells correlated with cisplatin resistance‐induced relapse in patients with EOC. Mechanically, NR2F6 activates Notch3 signaling by promoting the Notch3 transcription, thus enhancing the pluripotency of cancer cells and promoting the development of chemoresistance. More importantly, our study illustrates that inhibition of Notch3 signaling significantly resensitizes EOC cells to cisplatin chemotherapy, resulting in suppressed tumor growth. These findings suggest that NR2F6 may act as a potential therapeutic target to enhance the cisplatin response for patients with chemoresistant EOC.
NR2F6 is overexpressed in cervical cancer,22 colorectal cancer23 and leukemia.24 Previous studies have suggested that NR2F6 may function to positively regulate tumor cell survivability and to induce cancer progression. On the contrary, some reports have demonstrated that NR2F6 can act as a strong negative regulator for the function of the thyroid hormone (T3) nuclear receptor (TR), which regulates cell growth, differentiation and development25 Notably, Victoria et al.9 reported that NR2F6 serves as safeguard to protect gut barrier homeostasis. Our knowledge of the maintenance of intestinal barrier homeostasis is dependent on the differentiation and maturation of stem cells from the intestinal recess. Thus, Victoria et al. suggested that NR2F6 plays a fundamental and nonredundant role in the maintenance of the stem cell phenotype. These prior researches implied that NR2F6 might be closely involved in the pathophysiology of malignant diseases and may play a dual role in cancer progression. Our present results are in line with previous studies,9, 23, 24, 25 which indicate that NR2F6 expression promotes the CSC phenotype and induces cisplatin resistance of ovarian cancer cells by positively regulating transcriptional activation of the Notch3 signaling pathway.
We further analyzed NR2F6 expression in other tumor types in TCGA datasets using the GEPIA software (http://gepia.cancer‐pku.cn/). We found that NR2F6 is also overexpressed in cervical squamous cell carcinoma, stomach adenocarcinoma, uterine corpus endometrial carcinoma, bladder urothelial carcinoma and breast invasive carcinoma tissue. Moreover, previous studies also indicated that Notch3 signaling is activated in these tumor types.26, 27, 28, 29, 30, 31 These findings suggest that the NR2F6/Notch3 axis might also be relevant in tumor types in addition to EOC, and plays an important role in tumor progression.
Chemoresistance to platinum therapy is a major obstacle in treating EOC patients and conveys a poor prognosis. The high rates and patterns of therapeutic failure seen in EOC patients are consistent with a steady accumulation of drug‐resistant CSCs.24, 32 Previous reports demonstrated that the Notch3 signaling pathway is critical for the regulation of CSCs and facilitating tumor resistance to platinum.29, 30, 31 Therefore, targeting Notch3 signaling appears to be a rational and innovative approach for the treatment of EOC. Inhibitors of Notch signaling are presently undergoing clinical trials. GSI with drugs including RO4929097, a small and potent inhibitor of γ‐secretase, lead to the blockade of Notch signaling in tumor cells, and the significant antitumor effects of GSIs have been verified in preclinical studies.33, 34 Nevertheless, in a current phase II study performed in patients with pancreatic adenocarcinoma, colorectal cancer and EOC, RO4929097 showed minimal clinical activity and the median progression‐free survival time was not superior relative to historical treatment data.35, 36 Based on our data, we propose two reasons that may partly explain the underwhelming clinical response to RO4929097, and we suggest strategies to improve the objective responses to this GSI. On the one hand, these negative clinical trials were conducted in unselected populations of patients. However, Notch3 inhibition may only be effective in patients whose tumors are primarily driven by altered Notch3 signaling. Thus, prescreening for the presence of Notch3 activation should be considered indispensable when considering treatment with a GSI. Our study also shows that NR2F6 may serve as a valuable biomarker to indicate the activation of Notch3 signaling pathway. Moreover, GSI was used as a monotherapy in these patients and showed limited efficacy; we suggest that combination therapy may improve GSI efficacy. Additionally, previous studies have reported that autoinduction of RO4929097 at a high dose level that limited the ability to escalate dose impaired the ability to fully evaluate clinical observations. However, in spite of this autoinduction, at all dose levels, the C max appeared to be above the level required for Notch inhibition in the plasma. Thus, the initial dose appeared to be sufficient to have on‐target effects.37, 38 Previous studies have also indicated that unbound RO4929097 is pharmacologically active, and monitoring the unbound plasma drug concentrations are recommended to avoid misleading conclusions based on total drug levels.39 Wang et al. showed that the combination of GSIs and Tocilizumab, an IL6R antagonist, significantly decreases the breast cancer stem cell population and inhibits tumor growth, and thus this combination therapy might serve as a novel therapeutic strategy for patients with Notch3‐expressing breast cancer.40 However, there is no research about the correlation between RO4929097 efficacy and IL6 targeting in ovarian cancer patients. We believe this is an interesting and meaningful point, and we will investigate the relationship between Notch3 activation and IL6 targeting in future studies. Taken together, we suggest that prescreening for patients with high NR2F6 expression may indicate a cancer subpopulation susceptible to GSI treatment in combination with cytotoxic chemotherapy, and may yield higher response rates.
Recently, cancer immunotherapy has joined the ranks of surgery, chemotherapy and radiotherapy as a potent strategy for cancer treatment, and is poised to revolutionize cancer therapy in the future.41 Accumulating evidence indicates that EOC is an immunogenic tumor, and therefore an excellent candidate for immunotherapy.42 In the past two decades, there has been a dramatic increase in the number of immunotherapy clinical trials performed in EOC, including blockade of the inhibitory immune checkpoints CTLA4‐CD80/86 and programmed death 1/programmed cell death‐ligand 1 (PD1/PDL1), Treg‐depleting strategies, and cancer vaccine.43, 44 Although both passive and active immunotherapeutic modalities have indicated potential clinical benefit in EOC patients, the clinical benefit in addition to chemotherapy seems limited. Therefore, there is an enormous and urgent need to develop combinatorial approaches of traditional chemotherapy and immunotherapy to enhance treatment efficacy and prolong survival in EOC patients. Of note, several studies reported that NR2F6 serves as an intracellular “immune checkpoint”, and targeting NR2F6 enhances the activity of PD1/PD‐L1 checkpoint blockade and effector T‐cell subsets to augment antitumor immune responses.10 Therefore, this unique feature of NR2F6 in influencing the sensitivity of chemotherapy and immunotherapy may improve cancer therapy in the future.
Based on previous studies and our present research, we believe that NR2F6 may also serve as a valuable druggable target. Recent studies indicate that NR2F6 serves as an immune checkpoint regulator that suppresses adaptive anticancer immune responses; this sets the stage for clinical validation of targeting NR2F6 for next‐generation immune‐oncological treatment regimens.10, 45 Notably, Hermann‐Kleiter et al.45 demonstrated that NR2F6 appears to be an intracellular immune checkpoint in effector T cells, directly repressing the NFAT/AP‐1 complex on both the interleukin 2 and interferon and cytokine promoters, attenuating their transcription. Moreover, adoptive transfer of Nr2f6‐deficient T cells into tumor‐bearing immune competent mice was shown to be sufficient to delay tumor outgrowth.45 Importantly, a previous study indicated that the ligand binding domain (LBD) on NR2F6 is highly evolutionarily conserved and appears to be essential for NR2F6 transcriptional activity.46 Therefore, we believe that the potential druggability of the NR2F6 LBD for a “small molecule checkpoint blockade drug” may provide a rational mechanistic basis to target the manipulation of NR2F6 in T cells as a promising intracellular checkpoint targeting strategy. Such a strategy would improve the efficacy and broaden the applicability of cancer immunotherapy regimens, and facilitate prolonged patient survival.
In addition, NR2F6 belongs to the family of nuclear receptors (NRs) which are transcription factors that transmit physiological signals from a wide variety of ligands, such as classical steroid hormones, retinoic acid, thyroid hormone and vitamin D.47, 48 Moreover, it there is evidence that transcription factors direct binding accessibility on DNA loci, and are overactive in most human cancer cells, which makes them promising targets for the development of anticancer drugs.49, 50 In our study, we demonstrate that NR2F6 serves as a transcription factor, directs binding of transcriptional elements to the TGACCT motif on the Notch3 promoter, and induces chemoresistance of ovarian cancer cells. We suggest that a synthesized DNA fragment with TGACCT‐repeat motifs might act as a “sponge” to restrict the function of NR2F6; however, this hypothesis remains to be further investigated. However, research on a small‐molecule inhibitor targeting NR2F6 remains elusive. Therefore, we believe that our present research provides a rational mechanistic basis to target the manipulation of NR2F6 in EOC, and could represent a compelling therapeutic strategy suitable to improve the efficacy of cancer therapy regimes. We will evaluate the future application of targeting NR2F6 in future studies.
In brief, our study illustrates that NR2F6 plays an important role in facilitating the pluripotency of EOC cells to gain chemoresistance by promoting Notch3 transcription. Moreover, we demonstrate that NR2F6 may be a valuable biomarker to identify a population of EOC patients who are likely to have good objective responses to GSI treatment. Notably, NR2F6 acts as an “immunity checkpoint”, and its genetic ablation or direct targeting could be a “sensitizer” to support combined therapy regimens to improve immunotherapy activity and improve cancer therapy. We propose that the important roles of NR2F6 in chemoresistance and immunotherapy, justify the evaluation of NR2F6 as a potent druggable target for the treatment of cancer.
Author contributions
HL and YZ developed the experimental ideas and drafted the article. WZ, CN and XW conducted the experiments and contributed to the analysis of data. CL, YD and YJ contributed to data analysis. YL, LY, YO and LS contributed to data analysis and revised the article. JC and JQ edited the article. All authors contributed to revising the article and approved the final version for publication.
Ethical approval
Prior patient consent and ethics approval was obtained from the Institutional Research Ethics Committee of Sun Yat‐sen University Cancer Center for the use of the clinical specimens for research purposes. The ethics approval number for the patient study is GZR2018‐010. The ethics approval statements for animal work were provided by The Institutional Animal Care and Use Committee of Sun Yat‐sen University Cancer Center. The ethics approval number for animal work is L102012018000P.
Supporting information
Appendix S1: Supporting Information
Figure S1 (A) Real‐time PCR analysis of NR2F6 expression in ovarian cancer tissues and normal ovarian tissues. Expression levels were normalized to GAPDH. (B, C) The mRNA level of NR2F6 was significantly higher in ovarian cancer tissues of patients with chemoresistance relapse compared to those without chemoresistance relapse. Each bar represents the mean ± SD of three independent experiments. ***p < 0.001.
Figure S2 (A) Western blot analysis of NR2F6 levels in the indicated cells; GAPDH was used as a loading control. (B) Representative images (left panel) and quantification (right panel) of the indicated cells after crystal violet staining. Each bar represents the mean ± SD of three independent experiments. (C, D) AnnexinV‐FITC and PI staining of the indicated cells without cisplatin treatment. (C) Histograms showing the percentage of apoptotic cells in the indicated groups. Each bar represents the mean ± SD of three independent experiments.
Figure S3 (A) Western blot analysis showing that the protein level of NR2F6, CyclinD1, and KI‐67 are upregulated in A2780‐NR2F6‐derived tumors compared to the control vector tumors
Figure S4 (A, B) Gene set enrichment analysis (GSEA) of gene expression from the TCGA dataset showed significant enrichment of stemness gene modules (BYSTRYKH_STEM_CELL and BEIER_STEM_CELL_UP), in samples with high expression of NR2F6. NES, normalized enrichment score. (C, D) The mRNA expression of several stem cancer cell markers, including SOX2, NANOG, and OCT4 in indicated cells detected by RT‐PCR. Each bar represents the mean ± SD of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure S5 (A, B) Luciferase reporter assays of Hedgehog/GLI, Notch/CSL, TOP FLASH, and STAT3 reporters. (C–E) Correlation between mRNA expression of NR2F6 and three Notch paralogues (Notch1, Notch2, and Notch4) in TCGA ovarian cancer datasets. (F–I) Real‐time PCR analysis of Jagged1, Notch3, and Hes1 in the indicated cells. Transcript levels were normalized to GAPDH expression. Each bar represents the mean ± SD of three independent experiments. **p < 0.01, ***p < 0.001.
Figure S6 (A,B) cBioPortal (http://www.cbioportal.org/) analysis showed that NR2F6 copy‐number alteration is associated with NR2F6 mRNA expression. (C) cBioPortal analysis showed that the NR2F6 gene locus was amplified in EOC tissues in the TCGA dataset.
Figure S7 (A)Western blot analysis of the expression of NR2F6 and Notch 3 ICD in the five pairs of sensitive and resistant tumors collected from the same patient.
Figure S8 Proposed model. Overexpressed NR2F6 interacts with the Notch3 promoter, and further increases the enrichment of p300 and histone acetylation at the promoter, inducing Notch3 signaling activation and increasing CSCs properties, promoting cisplatin resistance in ovarian cancer cells.
Table S1. Clinicopathological characteristics and expression of NR2F6 in epithelial ovarian cancer.
Table S2. Correlation between NR2F6 expression and the clinicopathological features of epithelial ovarian cancer.
Table S3. Cox regression univariate and multivariate analyses of prognostic factors in epithelial ovarian cancer.
Table S4 Supplementary Table 4
Conflict of interest: The authors declare that they have no competing interests.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Mezzanzanica D. Ovarian cancer: a molecularly insidious disease. Chin J Cancer 2015;34:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nick AM, Coleman RL, Ramirez PT, et al. A framework for a personalized surgical approach to ovarian cancer. Nat Rev Clin Oncol 2015;12:239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amate P, Huchon C, Dessapt AL, et al. Ovarian cancer: sites of recurrence. Int J Gynecol Cancer 2013;23:1590–6. [DOI] [PubMed] [Google Scholar]
- 5. Tsai SY, Tsai MJ. Chick ovalbumin upstream promoter‐transcription factors (COUP‐TFs): coming of age. Endocr Rev 1997;18:229–40. [DOI] [PubMed] [Google Scholar]
- 6. Germain P, Staels B, Dacquet C, et al. Overview of nomenclature of nuclear receptors. Pharmacol Rev 2006;58:685–704. [DOI] [PubMed] [Google Scholar]
- 7. Takamoto N, You LR, Moses K, et al. COUP‐TFII is essential for radial and anteroposterior patterning of the stomach. Development 2005;132:2179–89. [DOI] [PubMed] [Google Scholar]
- 8. Zhou C, Tsai SY, Tsai MJ. COUP‐TFI: an intrinsic factor for early regionalization of the neocortex. Genes Dev 2001;15:2054–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klepsch V, Hermann‐Kleiter N, Do‐Dinh P, et al. Nuclear receptor NR2F6 inhibition potentiates responses to PD‐L1/PD‐1 cancer immune checkpoint blockade. Nat Commun 2018;9:1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marotta LL, Polyak K. Cancer stem cells: a model in the making. Curr Opin Genet Dev 2009;19:44–50. [DOI] [PubMed] [Google Scholar]
- 11. Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105–11. [DOI] [PubMed] [Google Scholar]
- 12. Polyak K, Hahn WC. Roots and stems: stem cells in cancer. Nat Med 2006;12:296–300. [DOI] [PubMed] [Google Scholar]
- 13. Kobayashi Y, Seino K, Hosonuma S, et al. Side population is increased in paclitaxel‐resistant ovarian cancer cell lines regardless of resistance to cisplatin. Gynecol Oncol 2011;121:390–4. [DOI] [PubMed] [Google Scholar]
- 14. Hu L, McArthur C, Jaffe RB. Ovarian cancer stem‐like side‐population cells are tumourigenic and chemoresistant. Br J Cancer 2010;102:1276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Espinoza I, Pochampally R, Xing F, et al. Notch signaling: targeting cancer stem cells and epithelial‐to‐mesenchymal transition. Onco Targets Ther 2013;6:1249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park JT, Chen X, Trope CG, et al. Notch3 overexpression is related to the recurrence of ovarian cancer and confers resistance to carboplatin. Am J Pathol 2010;177:1087–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park JT, Li M, Nakayama K, et al. Notch3 gene amplification in ovarian cancer. Cancer Res 2006;66:6312–8. [DOI] [PubMed] [Google Scholar]
- 18. McAuliffe SM, Morgan SL, Wyant GA, et al. Targeting notch, a key pathway for ovarian cancer stem cells, sensitizes tumors to platinum therapy. Proc Natl Acad Sci USA 2012;109:E2939–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sang L, Coller HA, Roberts JM. Control of the reversibility of cellular quiescence by the transcriptional repressor HES1. Science 2008;321:1095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee SH, Hong HS, Liu ZX, et al. TNFalpha enhances cancer stem cell‐like phenotype via notch‐Hes1 activation in oral squamous cell carcinoma cells. Biochem Biophys Res Commun 2012;424:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang L, Zhou Y, Li Y, et al. Mutations of p53 and KRAS activate NF‐kappaB to promote chemoresistance and tumorigenesis via dysregulation of cell cycle and suppression of apoptosis in lung cancer cells. Cancer Lett 2015;357:520–6. [DOI] [PubMed] [Google Scholar]
- 22. Niu C, Sun X, Zhang W, et al. NR2F6 expression correlates with pelvic lymph node metastasis and poor prognosis in early‐stage cervical cancer. Int J Mol Sci 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li XB, Jiao S, Sun H, et al. The orphan nuclear receptor EAR2 is overexpressed in colorectal cancer and it regulates survivability of colon cancer cells. Cancer Lett 2011;309:137–44. [DOI] [PubMed] [Google Scholar]
- 24. Ichim CV, Atkins HL, Iscove NN, et al. Identification of a role for the nuclear receptor EAR‐2 in the maintenance of clonogenic status within the leukemia cell hierarchy. Leukemia 2011;25:1687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu XG, Park KS, Kaneshige M, et al. The orphan nuclear receptor ear‐2 is a negative coregulator for thyroid hormone nuclear receptor function. Mol Cell Biol 2000;20:2604–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liang Y, Zhang T, Shi M, et al. Low expression of NCOA5 predicts poor prognosis in human cervical cancer and promotes proliferation, migration, and invasion of cervical cancer cell lines by regulating notch3 signaling pathway. J Cell Biochem 2018;120:6237–49. [DOI] [PubMed] [Google Scholar]
- 27. Yeasmin S, Nakayama K, Rahman MT, et al. Expression of nuclear Notch3 in cervical squamous cell carcinomas and its association with adverse clinical outcomes. Gynecol Oncol 2010;117:409–16. [DOI] [PubMed] [Google Scholar]
- 28. Kang H, An HJ, Song JY, et al. Notch3 and Jagged2 contribute to gastric cancer development and to glandular differentiation associated with MUC2 and MUC5AC expression. Histopathology 2012;61:576–86. [DOI] [PubMed] [Google Scholar]
- 29. Mitsuhashi Y, Horiuchi A, Miyamoto T, et al. Prognostic significance of Notch signalling molecules and their involvement in the invasiveness of endometrial carcinoma cells. Histopathology 2012;60:826–37. [DOI] [PubMed] [Google Scholar]
- 30. Zhang H, Liu L, Liu C, et al. Notch3 overexpression enhances progression and chemoresistance of urothelial carcinoma. Oncotarget 2017;8:34362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamaguchi N, Oyama T, Ito E, et al. NOTCH3 signaling pathway plays crucial roles in the proliferation of ErbB2‐negative human breast cancer cells. Cancer Res 2008;68:1881–8. [DOI] [PubMed] [Google Scholar]
- 32. Steg AD, Bevis KS, Katre AA, et al. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin Cancer Res 2012;18:869–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luistro L, He W, Smith M, et al. Preclinical profile of a potent gamma‐secretase inhibitor targeting notch signaling with in vivo efficacy and pharmacodynamic properties. Cancer Res 2009;69:7672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krop I, Demuth T, Guthrie T, et al. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (notch) inhibitor MK‐0752 in adult patients with advanced solid tumors. J Clin Oncol 2012;30:2307–13. [DOI] [PubMed] [Google Scholar]
- 35. De Jesus‐Acosta A, Laheru D, Maitra A, et al. A phase II study of the gamma secretase inhibitor RO4929097 in patients with previously treated metastatic pancreatic adenocarcinoma. Invest New Drugs 2014;32:739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strosberg JR, Yeatman T, Weber J, et al. A phase II study of RO4929097 in metastatic colorectal cancer. Eur J Cancer 2012;48:997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tolcher AW, Messersmith WA, Mikulski SM, et al. Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J Clin Oncol 2012;30:2348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. LoConte NK, Razak AR, Ivy P, et al. A multicenter phase 1 study of gamma‐secretase inhibitor RO4929097 in combination with capecitabine in refractory solid tumors. Invest New Drugs 2015;33:169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu J, Lorusso PM, Matherly LH, et al. Implications of plasma protein binding for pharmacokinetics and pharmacodynamics of the gamma‐secretase inhibitor RO4929097. Clin Cancer Res 2012;18:2066–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang D, Xu J, Liu B, et al. IL6 blockade potentiates the anti‐tumor effects of gamma‐secretase inhibitors in Notch3‐expressing breast cancer. Cell Death Differ 2018;25:330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang L, Conejo‐Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003;348:203–13. [DOI] [PubMed] [Google Scholar]
- 43. Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte‐associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA 2003;100:4712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti‐PD‐L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hermann‐Kleiter N, Klepsch V, Wallner S, et al. The nuclear orphan receptor NR2F6 is a central checkpoint for cancer immune surveillance. Cell Rep 2015;12:2072–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hermann‐Kleiter N, Meisel M, Fresser F, et al. Nuclear orphan receptor NR2F6 directly antagonizes NFAT and RORgammat binding to the Il17a promoter. J Autoimmun 2012;39:428–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kumar R, Thompson EB. The structure of the nuclear hormone receptors. Steroids 1999;64:310–9. [DOI] [PubMed] [Google Scholar]
- 48. Warnmark A, Treuter E, Wright AP, et al. Activation functions 1 and 2 of nuclear receptors: molecular strategies for transcriptional activation. Mol Endocrinol 2003;17:1901–9. [DOI] [PubMed] [Google Scholar]
- 49. Darnell JE Jr. Transcription factors as targets for cancer therapy. Nat Rev Cancer 2002;2:740–9. [DOI] [PubMed] [Google Scholar]
- 50. Vilaboa N, Bore A, Martin‐Saavedra F, et al. New inhibitor targeting human transcription factor HSF1: effects on the heat shock response and tumor cell survival. Nucleic Acids Res 2017;45:5797–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Figure S1 (A) Real‐time PCR analysis of NR2F6 expression in ovarian cancer tissues and normal ovarian tissues. Expression levels were normalized to GAPDH. (B, C) The mRNA level of NR2F6 was significantly higher in ovarian cancer tissues of patients with chemoresistance relapse compared to those without chemoresistance relapse. Each bar represents the mean ± SD of three independent experiments. ***p < 0.001.
Figure S2 (A) Western blot analysis of NR2F6 levels in the indicated cells; GAPDH was used as a loading control. (B) Representative images (left panel) and quantification (right panel) of the indicated cells after crystal violet staining. Each bar represents the mean ± SD of three independent experiments. (C, D) AnnexinV‐FITC and PI staining of the indicated cells without cisplatin treatment. (C) Histograms showing the percentage of apoptotic cells in the indicated groups. Each bar represents the mean ± SD of three independent experiments.
Figure S3 (A) Western blot analysis showing that the protein level of NR2F6, CyclinD1, and KI‐67 are upregulated in A2780‐NR2F6‐derived tumors compared to the control vector tumors
Figure S4 (A, B) Gene set enrichment analysis (GSEA) of gene expression from the TCGA dataset showed significant enrichment of stemness gene modules (BYSTRYKH_STEM_CELL and BEIER_STEM_CELL_UP), in samples with high expression of NR2F6. NES, normalized enrichment score. (C, D) The mRNA expression of several stem cancer cell markers, including SOX2, NANOG, and OCT4 in indicated cells detected by RT‐PCR. Each bar represents the mean ± SD of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure S5 (A, B) Luciferase reporter assays of Hedgehog/GLI, Notch/CSL, TOP FLASH, and STAT3 reporters. (C–E) Correlation between mRNA expression of NR2F6 and three Notch paralogues (Notch1, Notch2, and Notch4) in TCGA ovarian cancer datasets. (F–I) Real‐time PCR analysis of Jagged1, Notch3, and Hes1 in the indicated cells. Transcript levels were normalized to GAPDH expression. Each bar represents the mean ± SD of three independent experiments. **p < 0.01, ***p < 0.001.
Figure S6 (A,B) cBioPortal (http://www.cbioportal.org/) analysis showed that NR2F6 copy‐number alteration is associated with NR2F6 mRNA expression. (C) cBioPortal analysis showed that the NR2F6 gene locus was amplified in EOC tissues in the TCGA dataset.
Figure S7 (A)Western blot analysis of the expression of NR2F6 and Notch 3 ICD in the five pairs of sensitive and resistant tumors collected from the same patient.
Figure S8 Proposed model. Overexpressed NR2F6 interacts with the Notch3 promoter, and further increases the enrichment of p300 and histone acetylation at the promoter, inducing Notch3 signaling activation and increasing CSCs properties, promoting cisplatin resistance in ovarian cancer cells.
Table S1. Clinicopathological characteristics and expression of NR2F6 in epithelial ovarian cancer.
Table S2. Correlation between NR2F6 expression and the clinicopathological features of epithelial ovarian cancer.
Table S3. Cox regression univariate and multivariate analyses of prognostic factors in epithelial ovarian cancer.
Table S4 Supplementary Table 4


