Abstract
Background:
Parasitic infestation is one of the serious health problems in developing countries. Parasitic infestation is usually asymptomatic and does not cause disease as it may eventually lead to the death of both organism and host.
Materials and Methods:
This was a retrospective study done over a period of 5 years from 2013 to 2018. The study included 26 cases of parasitic infestations diagnosed on fine-needle aspiration cytology (FNAC) as well as fluid cytology.
Results:
Hydatidosis, cysticercosis, and filariasis were the parasitic infestations observed in this study, of which hydatidosis was the most common infestation. The predominant age group was 20–85 years old, with a mean age of presentation being 55 years. There was male predominance with a male–female of 9:1.
Conclusion:
FNAC and fluid cytology are rapid diagnostic tools that aid in the early diagnosis of parasitic infestations. In parasitic infestations presenting as visceral cystic lesions, thorough examination with proper clinical correlation aid in early management. In cases with coexistent malignancy, cytology plays a major role in the diagnosis of silent carriers of infection.
Keywords: Fine-needle aspiration cytology, fluid cytology, parasitic infestation
INTRODUCTION
Parasitic infestation is one of the serious health problems in developing countries. Parasitic infestation is usually asymptomatic and does not cause disease as it may eventually lead to death of both organism and host. The diagnosis of parasitic infestation on cytology is usually an incidental finding. The diagnostic role of fine-needle aspiration cytology (FNAC) in cysticercosis wasfirst emphasized by Kung et al. in 1989.[1] Few cases present with superficial palpable nodules and as nodular or cystic mass in the viscera which are clinically misdiagnosed with neoplasms or reactive proliferations depending on the site. FNAC and fluid cytology are rapid diagnostic tools of evaluation in such cases. Early diagnosis of such lesions leads to a favorable outcome as they are highly responsive to specific antimicrobial therapy. The present study was done to analyze the utility of cytology in the diagnosis of clinically unsuspected cases of parasitic infestation.
MATERIALS AND METHODS
This was a retrospective study done over a period of 5 years from 2013 to 2018. The study included 26 cases of parasitic infestations diagnosed on FNAC as well as fluid cytology. Of these, 23 cases were aspirations from superficial subcutaneous nodules as well as deep visceral lesions and three cases were reported on fluid cytology. The superficial lesions were aspirated using a 22G needle attached to 20 mL syringe, while ultrasound- or computed tomography-guided FNAC was done for the visceral lesions using 23G lumbar puncture needle. The material aspirated was smeared on the glass slides. In cases of cystic lesion, smears were prepared after cytocentrifugation of the aspirated cyst fluid. The residual material within the syringes was sent for microbiological examination. The air-dried smears were stained with May-Grunwald-Giemsa, whereas hematoxylin and eosin and Papanicolaou stains were used for methanol fixed smears. Special stains such as Ziehl–Neelsen and periodic acid–Schiff were done wherever necessary. The cases diagnosed with parasitic infestation with evidence of parasite or its fragments were included in the study. Relevant clinical data were retrieved for all the cases.
RESULTS
This study included a total of 26 cases in the age group of 20–85 years old, with mean age of presentation being 55 years. There was male predominance with a male–female of 9:1. The parasitic infestations observed in the study period were hydatidosis or echinococcosis, cysticercosis, and filariasis, and their incidence is depicted in Table 1.
Table 1.
Incidence of various parasitic infestations
| Diagnosis | Site of lesion | Number of cases (n=26) | Type of procedure | Age range (years) |
|---|---|---|---|---|
| Hydatid cyst (n=16) | Liver | 9 | Guided FNAC | 15-54 |
| Lung | 5 | Guided FNAC | 22-65 | |
| Spleen | 1 | Guided FNAC | 60 | |
| Subcutaneous nodule in the chest wall | 1 | FNAC | 50 | |
| Cysticercosis (n=5) | Subcutaneous nodules in various sites | 5 | FNAC | 20-60 |
| Filariasis (n=5) | Pleural fluid | 3 | Pleural fluid cytology | 56-80 |
| Subcutaneous nodule in the arm and neck | 2 | FNAC |
FNAC: Fine-needle aspiration cytology
Of 26 cases, 16 cases were diagnosed with echinococcosis, and the predominant site was the liver followed by the lung with isolated cases in the spleen and chest wall. Imaging revealed multiloculated cystic lesions ranging from 3 to 7 cm. Clinically, vague abdominal or chest pain, dry cough, mild fever, and generalized weakness were the presenting complaints. Provisional clinical diagnosis of benign cystic lesion was made in seven cases, whereas the majority were clinically misdiagnosed as inflammatory lesions and tumors with cystic degeneration. Laboratory findings revealed leukocytosis with peripheral blood eosinophilia in eight cases. Liver function tests revealed elevated liver enzymes in cases of hepatic hydatidosis. In cases involving the lung, chest X-ray revealed large homogenous opacities almost replacing the lung. Aspirated material was clear with few granular deposits in most cases and few cases yielded hemorrhagic fluid. Allergic reaction or any other complication was not observed in any of the cases following FNAC. On cytology, the smears were paucicellular and showed multiple fragments of the laminated membrane with delicate undulating parallel striations. Background showed amorphous necrotic material with many protoscolices. Degenerated protoscolices and detached hooklets were seen as refractile structures [Figure 1]. The diagnosis of hydatidosis was made which was further confirmed on subsequent excision biopsies. Subsequent review of clinical history revealed frequent contact with dogs and farm animals in seven cases.
Figure 1.
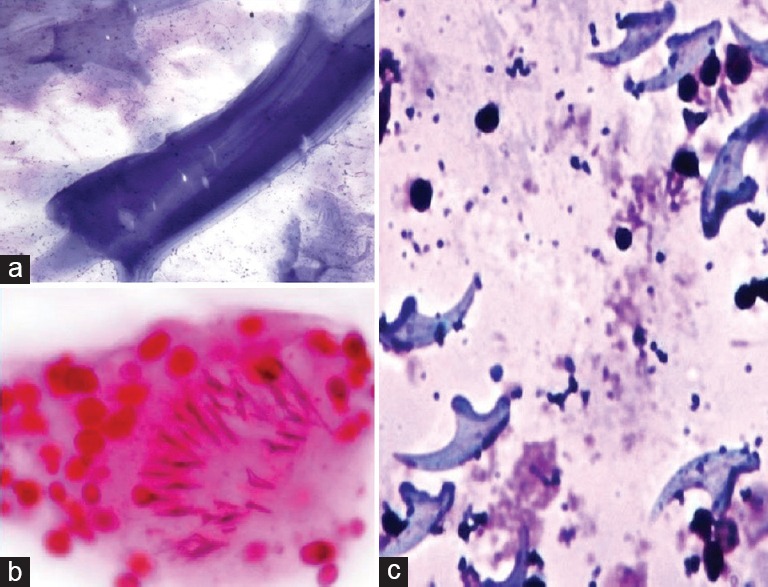
Hydatidosis – (a) Fragment of the acellular lamellated membrane; (b) Scolex with hooklets (H and E, ×400); (c) Scattered refractile hooklets (May-Grunwald-Giemsa, ×400)
Five out of 26 cases were diagnosed with cysticercosis and was the most common infestation presenting as painless, subcutaneous nodules in various sites such as peripheral limbs, chest, and abdominal wall. Ultrasound examination revealed well-defined, thin-walled, cystic lesions within the subcutaneous plane. The adjacent soft tissues were thickened with significant exudative fluid collection. A provisional diagnosis of benign cystic lesion was made and the FNAC was done. Cytology smears revealed fragments of bladder wall of cysticercus and very occasional hooklets and scolex. Background showed varying proportion of mixed inflammatory response comprised histiocytes, multinucleated giant cells, ill-defined granulomas, and amorphous necrotic material [Figure 2]. A diagnosis of cysticercosis was made which was further confirmed on subsequent excision biopsies in three cases. The other two cases responded well with medical management. Review of clinical history revealed that most of these patients were urban slum dwellers, and there was the occasional intake of pork in one case.
Figure 2.
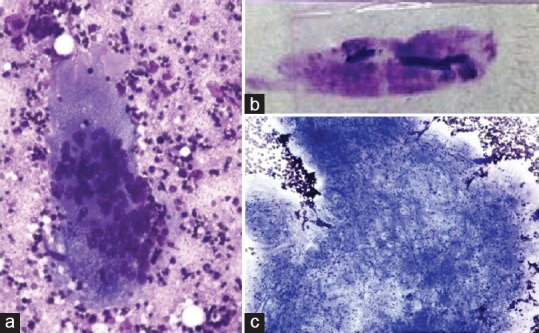
Cysticercosis – (a) inflammatory cells with multinucleate giant cells and necrotic debris (H and E, ×400); (b) fragments of parasite on naked eye examination of smear; (c) Bladder wall with tiny parasitic nuclei (May-Grunwald-Giemsa, ×400)
Filariasis was diagnosed in five cases, of which three cases were present in the lung diagnosis of pleural fluid cytology. Mild fever, dry cough with wheeze, and chest pain were the predominant presenting features. Chest X-ray revealed massive pleural effusion. Provisional diagnosis of malignant effusion was made and pleural tap was done. Fluid yielded was mostly thick, turbid, and in one case was hemorrhagic. The other two cases presented as painless, subcutaneous nodules in the neck, and arm with a provisional diagnosis of benign soft-tissue tumors on ultrasonography. Peripheral blood smear examination of nocturnal venous blood revealed no microfilaria in all cases, and mild peripheral eosinophilia was seen in three cases. On cytology, smears showed microfilaria in a background of mixed inflammatory infiltrate. There was coexistent adenocarcinoma in two cases of microfilaria diagnosed on pleural fluid cytology [Figure 3]. The other three cases responded well to diethylcarbamazine with marked relief from symptoms.
Figure 3.
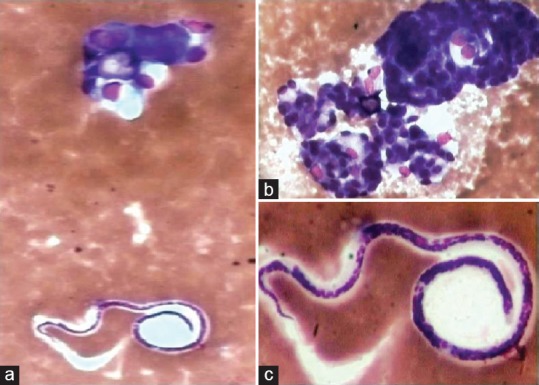
Filariasis – Microfilaria adjacent to cluster of malignant glandular cells on fluid cytology (a: H and E, ×100); (b and c: H and E, ×400)
DISCUSSION
Cytological examination is a rapid, efficient, and widely acceptable diagnostic tool for evaluation of parasitic infestation, especially in the unsuspected cases. Most of the superficial parasitic nodules are often clinically misinterpreted as benign or malignant mesenchymal tumors or as inflammatory lesions. In the present study, most of the patients were older adults with male predominance similar to Yadav et al., but studies by Suchitha et al. showed no gender predilection and most of the cases were children.[2,3,4] Echinococcosis, cysticercosis, and filariasis were the three parasitic infestations diagnosed in the present study. Infestations such as leishmaniasis and enterobiasis were not reported in this study, and very few cases diagnosed on cytology are reported in the literature.[5,6]
Echinococcosis also known as hydatid disease or hydatidosis was the most common parasitic infestation in this study. Liver is the most commonly affected site followed by the lung and rarely involves lymph node, soft tissue, bone, brain, breast, thyroid, salivary gland, and kidney.[7,8,9] It is caused by Echinococcus species which inhabit the small intestine of carnivorous definitive hosts, such as dogs, coyotes, or wolves. Herbivores such as sheep, cattle, and goats are intermediate hosts. Humans are accidental intermediate hosts and acquire infection following contact with infected dogs or after ingestion of contaminated food. The other previous studies showed a decrease incidence of hydatidosis as FNAC is usually not recommended in a suspected case of hydatid disease. The possible reason is to prevent acute anaphylactic reaction due to spillage of hydatid fluid during the procedure. However, no complications were encountered during FNAC in this study which is similar to other studies.[8] In most of these cases, FNAC can be avoided if there was a proper clinical correlation, thereby preventing their misdiagnosis. Most cases are usually asymptomatic as the parasites usually die and calcify, and the host reaction is very minimal. Clinical presentations vary depending on the anatomic location, pressure effect, and size of the growing cysts.[9] Subcutaneous hydatidosis is very rare, and we had one case which was misdiagnosed as benign tumor. The characteristic clear fluid on aspiration with or without granular deposits should raise the suspicion of parasitic etiology. Suchitha et al. in their study emphasized the importance of thorough processing of every clear fluid aspirated during FNAC.[3] Cytology smears reveal the presence of a laminated membrane with parallel striations, dispersed retractile hooklets, amorphous granular necrotic debris, and inflammatory cells such as neutrophils, eosinophils, and multinucleated giant cells.[8]
Cysticercosis was the most common parasitic infestation presenting as subcutaneous nodules in this study which was similar to previous studies by Yadav et al.[2] It is an ancient disease and is particularly common in Asia, Sub-Saharan Africa, and Latin America.[10] Cysticercosis is a tissue infection caused by the larval stage of the cestode Taenia solium or pork tapeworm. Humans are the only definitive host and can also act as intermediate hosts by ingestion of cysts in poorly cooked pork and contaminated water. Personal hygiene and sanitation, cooking pork well, and improved access to clean water are the measures to prevent infection. Most commonly, it manifests as subcutaneous or intramuscular nodules which are often misdiagnosed clinically as inflammatory lesions or tumors. Other sites such as the skeletal muscle, eye, central nervous system, oral cavity, tongue, submandibular gland, and heart can be rarely involved.[11,12,13] Neurocysticercosis involves the brain and spinal cord with seizures, back pain, and radiculopathy as the presenting clinical features.[14] Cytological smears show the parasitic fragments which include bladder wall and tegument thrown into rounded wavy folds with tiny, parasitic nuclei calcospherules and hooklets.[15,16,17,18] A mixed inflammatory infiltrate of eosinophils, neutrophils, histiocytes, giant cells, and necrotic material is usually seen in the background. The presence of these findings is variable and depends on the host immune response, but a subcutaneous nodule with dirty necrotic background on cytology should raise the suspicion of a parasitic infestation. Three of our cases showed hooklets and all cases showed the bladder wall on cytology.
Filariasis is a major public health problem in subtropical and tropical countries like India. Filariasis is caused by nematodes Wuchereria bancrofti and Brugia malayi and transmitted by the bite of Culex mosquito. Most patients are initially asymptomatic and following repeated episodes of inflammation and lymphedema develop chronic swelling and elephantiasis of the legs, arms, scrotum, vulva, and breasts. All five cases of filariasis in this study were caused by W. bancrofti. Microfilaremia and eosinophilia are common in the acute phase, but only three cases showed peripheral eosinophilia with scattered eosinophils in the aspirate smears. Hence, eosinophils are not necessarily seen in the lesions of filariasis. Microfilaria can be detected in various sites such as the thyroid, breast, salivary glands, lymph nodes, urine, skin, soft tissue, epididymis, and effusion fluids.[19,20,21,22,23] In 2 out of 3 cases reported on pleural fluid cytology, there was coexistent malignant effusion by pulmonary adenocarcinoma which is a very rare finding. Microfilaria in a malignant pleural effusion has been reported by Singh et al.[24] Appearance in tissue fluids could be secondary to lymphatic obstruction by scars or tumors or secondary to damage due by inflammation, trauma, or stasis. Few case reports of coexistence of microfilariae with neoplastic lesions such as squamous cell carcinoma of maxillary antrum, carcinoma of the pharynx, follicular carcinoma of the thyroid, carcinoma of the breast, carcinoma of the pancreas, carcinoma of the gall bladder, transitional cell carcinoma of the bladder, metastatic melanoma to the bladder, seminoma of undescended testis, leukemia, meningioma, and craniopharyngioma are reported in the literature.[24,25,26] The presence of microfilaria in metastatic lymph nodes could be due to transmigration of microfilaria along with metastatic tumor emboli. Larvae may be present in the vasculature and seen on cytology following rupture of vessels during aspiration, especially in highly vascular tumors. Most of these cases harbor infection and remain silent and cytology can be an effective tool for the detection of such silent carriers helping in disease eradication.
CONCLUSION
FNAC and fluid cytology are rapid diagnostic tools that aid in the early diagnosis of parasitic infestations. Aspiration of clear fluid from a subcutaneous nodule, the presence of acellular tissue fragments, and amorphous necrotic background on cytology should raise the suspicion of parasitic etiology. Thorough examination and proper clinical correlation in case of visceral cystic lesions reduce the role of invasive surgeries as these are often cured with medical management and respond very well to antimicrobial therapy. In cases with coexistent malignancy, cytology plays a major role in the diagnosis of silent carriers of infection.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kung IT, Lee D, Yu HC. Soft tissue cysticercosis. Diagnosis by fine-needle aspiration. Am J Clin Pathol. 1989;92:834–5. doi: 10.1093/ajcp/92.6.834. [DOI] [PubMed] [Google Scholar]
- 2.Yadav YK, Gupta O, Aggarwal R. Cytological diagnosis of parasites presenting as superficial nodular swelling: Report of 35 cases. J Parasit Dis. 2012;36:106–11. doi: 10.1007/s12639-011-0095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suchitha S, Vani K, Sunila R, Manjunath GV. Fine needle aspiration cytology of cysticercosis-a case report. Case Rep Infect Dis. 2012;2012:854704. doi: 10.1155/2012/854704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verma K, Kapila K. Fine needle aspiration diagnosis of cysticercosis in soft tissue swellings. Acta Cytol. 1989;33:663–6. [PubMed] [Google Scholar]
- 5.Pal S, Biswas B. Fine-needle aspiration cytology of leishmanial lymphadenitis in an HIV-reactive patient: Report of a rare case. Trop Parasitol. 2018;8:50–2. doi: 10.4103/tp.TP_61_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta B, Jain S. Perianal nodule due to Enterobius vermicularis: Cytomorphological spectrum on fine needle aspiration cytology with a review of literature. Trop Parasitol. 2018;8:53–5. doi: 10.4103/tp.TP_33_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sultana N, Hashim TK, Jan SY, Khan Z, Malik T, Shah W. Primary cervical hydatid cyst: A rare occurrence. Diagn Pathol. 2012;7:157. doi: 10.1186/1746-1596-7-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tekin M, Osma U, Yaldiz M, Topcu I. Preauricular hydatid cyst: An unusual location for echinoccosis. Eur Arch Otorhinolaryngol. 2004;261:87–9. doi: 10.1007/s00405-003-0650-7. [DOI] [PubMed] [Google Scholar]
- 9.Cancelo MJ, Martín M, Mendoza N. Preoperative diagnosis of a breast hydatid cyst using fine-needle aspiration cytology: A case report and review of the literature. J Med Case Rep. 2012;6:293. doi: 10.1186/1752-1947-6-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García HH, Gonzalez AE, Evans CA, Gilman RH. Cysticercosis Working Group in Peru. Taenia solium cysticercosis. Lancet. 2003;362:547–56. doi: 10.1016/S0140-6736(03)14117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nigam S, Singh T, Mishra A, Chaturvedi KU. Oral cysticercosis – Report of six cases. Head Neck. 2001;23:497–9. doi: 10.1002/hed.1066. [DOI] [PubMed] [Google Scholar]
- 12.Sawhney M, Agarwal S. Cysticercosis: Hooked by a hooklet on fine needle aspiration cytology – A case report. Case Rep Infect Dis. 2013;2013:315834. doi: 10.1155/2013/315834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandhu VK, Sharma U, Singh N, Goyal G. Fine-needle aspiration cytology of cysticercosis in submandibular gland. J Oral Maxillofac Pathol. 2017;21:264–6. doi: 10.4103/jomfp.JOMFP_140_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prasad KN, Prasad A, Verma A, Singh AK. Human cysticercosis and Indian scenario: A review. J Biosci. 2008;33:571–82. doi: 10.1007/s12038-008-0075-y. [DOI] [PubMed] [Google Scholar]
- 15.Handa U, Garg S, Mohan H. Fine needle aspiration in the diagnosis of subcutaneous cysticercosis. Diagn Cytopathol. 2008;36:183–7. doi: 10.1002/dc.20792. [DOI] [PubMed] [Google Scholar]
- 16.Kraft R. Cysticercosis: An emerging parasitic disease. Am Fam Physician. 2007;76:91–8. [PubMed] [Google Scholar]
- 17.Kamal MM, Grover SV. Cytomorphology of subcutaneous cysticercosis. A report of 10 cases. Acta Cytol. 1995;39:809–12. [PubMed] [Google Scholar]
- 18.Singh N, Arora VK, Bhatia A. Are all subcutaneous parasitic cysts cysticercosis? Acta Cytol. 2006;50:114–5. [PubMed] [Google Scholar]
- 19.Yenkeshwar PN, Kumbhalkar DT, Bobhate SK. Microfilariae in fine needle aspirates: A report of 22 cases. Indian J Pathol Microbiol. 2006;49:365–9. [PubMed] [Google Scholar]
- 20.Kadam PN, Rathod KG, Chavan YH, Panchamhalkar AC, Bagre DH. Isolated left supraclavicular lymphadenopathy – A rare presentation of lymphatic filariasis in children. J Evol Med Dent Sci. 2013;29:5426–30. [Google Scholar]
- 21.Katti TV, Athanikar VS, Ananthrao AS, Rathod CV. Cytodiagnosis of microfilarial lymphadenitis coexistent with metastatic squamous cell carcinoma in a left cervical lymph node: An unusual presentation. Ann Niger Med. 2012;6:47–9. [Google Scholar]
- 22.Sahoo N, Saha A, Mishra P. Coexistence of microfilaria with metastatic adenocarcinomatous deposit from breast in axillary lymph node cytology: A rare association. J Cytol. 2017;34:43–5. doi: 10.4103/0970-9371.197617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navatha V, Mallesh T. Filarial pleural effusion with cervical lymphadenopathy. J Cont Med A Dent. 2014;2:77–9. [Google Scholar]
- 24.Singh SK, Pujani M, Pujani M. Microfilaria in malignant pleural effusion: An unusual association. Indian J Med Microbiol. 2010;28:392–4. doi: 10.4103/0255-0857.71833. [DOI] [PubMed] [Google Scholar]
- 25.Sinha R, Sengupta S, Pal S, Adhikari A. Incidental diagnosis of filariasis in association with carcinoma of gall bladder: Report of a case evidenced on ultrasound-guided fine-needle aspiration cytology with review of the literature. J Cytol. 2014;31:174–5. doi: 10.4103/0970-9371.145662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bika S, Kumar V, Dubey S, Kapoor A, Kumar HS. Microfilariae coexistent with the cytology of adenocarcinoma gall bladder: An unnoticed comorbidity. Clin Cancer Invest J. 2016;5:193–5. [Google Scholar]


