Abstract
Background:
Increased consumption of fructose in recent years has increased the risk of developing metabolic syndrome. In this syndrome, induction of oxidative stress, cellular dysfunction, and decrease of antioxidant capacity can change response to pain. Therefore, this study aims to investigate the antinociceptive and antioxidant effects of eugenol on metabolic syndrome induced by a fructose-rich diet in rats.
Methods:
The rats were randomly assigned to five groups, to be under experiment for eight weeks. The first, control group, the second fructose 10% plus tween 0.5% (Fr + veh), the third fructose 10% (Fr), and the fourth fructose 10% plus a single dose of eugenol 100 mg/kg (Fr + EoS). However, the fifth obtained fructose 10% plus a continuous dose of eugenol 20 mg/kg/day (Fr + EoC) for the last 10 days of the experiment. After formalin test, blood samples were taken from the animals’ hearts followed by analysis for biochemical factors.
Results:
This study shows that fructose administration does not change any pain response and there are not any changes in pain response between Fr group and control group. However, treatment with single and continuous dose of eugenol in Fr + EoS and Fr + EoC groups significantly decreases response to pain in the first and second phase of formalin test in comparison with Fr group (P<0.05). Continuous does of eugenol improved serum malondialdehyde and total antioxidant capacity levels in Fr + Eoc group in comparison with Fr group.
Conclusions:
In the present work, new findings suggest the beneficial effects of eugenol in pain relief, improved serum glucose, insulin levels, and improved antioxidant activity in metabolic syndrome.
Keywords: Eugenol, metabolic syndrome, pain measurement
Introduction
Metabolic syndrome or syndrome X is a multifactorial disease which is related to several factors such as genetic, physiological, and environmental factors. In the last decade, obesity, diabetes, and metabolic syndrome have attracted much attention.[1] On the other hand, this syndrome has increased risk of cardiovascular risk factors, including hypertension, dyslipidemia, and obesity.[2] According to recent studies, prevalence of metabolic syndrome is 20%–25% in the world. Important factors in the development of this disorder are high carbohydrate and high-fat diet.[3]
In addition, metabolic syndrome induces oxidative stress, and in its own turn, it increases free radicals, namely, reactive oxygen species lead to cellular dysfunctioning and imbalances in antioxidant activities. Therefore, the resulted imbalance is another side effect of metabolic syndrome which may have probable effects on pain degree.[4] According to the existing studies, oxidative stress plays some important roles in the happening of different disease.[5]
Fructose is a simple monosaccharide, and today, it is used widely as a sweetener in various types of foods.[6] Consumption of this type of sugar enhances about 16% from 1986 to 2007.[7] This increase is closely related to the prevalence of obesity and metabolic syndrome.[8]
Eugenol is an aromatic molecule found in clove (Eugenia aromatic), bay leaves, and all spices.[9,10,11] It has been used for painkilling and its sedative effects among its other applications.[12] For example, in dentistry, it is widely used for its analgesic and anti-inflammatory qualities.[13,14] On the other hand, eugenol has antioxidant[13] and antiseptic properties.[13,15] Antinociception actions of eugenol is not clearly specified, but it can act as a nonspecific counterirritant which may inhibit sensory nerve activity and lead to an inhibitory action on prostacyclin production.[16,17] In the nervous system, it is a neuroprotective against excitotoxicity, ischemia, amyloid-peptide[18] and modifying neuronal and vascular dysfunctions in diabetes model.[19] Although the abilities of analgesic eugenol are evident, the mechanisms of this effects remain unclear.[20]
Regarding the role of metabolic syndrome in the induction of stress oxidative and based on the antioxidant properties of eugenol, the aim of this study is to examine the effects of eugenol on pain response to formalin test and plasma antioxidant activity in high fructose drinking water among male rats.
Methods
Animals and diets
Thirty eight male Wistar rats weighing 197.4 ± 7.2 g were the subjects of this study. These rats were used from the Animal Centre of Zahedan University of Medical Sciences, Zahedan, Iran. They were housed at a temperature of 23°C–25°C with free access to water and rat chow. The rats were acclimatized to their diet for at least 1 week before the experiment. The experimental procedure was approved by the Zahedan University Medical Sciences Ethics Committee before the experiment.
Experimental protocol
The rats were randomly assigned to five groups for 8 weeks. The first group (n = 7) received tap water (control group), the second group (Fr + vehicle) (n = 9) received fructose 10% plus tween 0.5%.[21] The third group obtained (n = 7) fructose 10%. The fourth group obtained (n = 7) fructose 10% plus a single dose of eugenol 100 mg/kg.[22] Similarly, the fifth group (n = 8) got fructose 10% plus a continuous dose of eugenol 20 mg/kg/day for the last 10 days of the experiment.[23]
Preparation of fructose drinking water
The used fructose was D-fructose >99% (Syarikat System, Malaysia). The fresh fructose drinking water was daily prepared according to the weight/volume formula.[24] To prepare fructose 10% drinking water, 10 g of fructose was diluted in 100 ml of tap water.[24]
Formalin test in rats
Fifteen minutes after drug administration, 5-μl formalin 5% was injected subcutaneously under the plantar surface of the left hind paw. Then, the animals were placed in an acrylic observation chamber for 1 h. Next, the time spent licking, shaking, and biting the injected paw was measured with a chronometer. The duration of these activities was considered as response to nociception. The first phase of the nociceptive response is normally considered between 0 and 5 min, and the second phase 20–40 min after formalin injection.[25]
Blood biochemistry
Blood samples were taken from the heart of each animal in anesthetized rats. The rats were fasted overnight and supplemented with only tap water. The serum samples were sent to laboratory for analysis of glucose, insulin, nitrite, malondialdehyde (MDA), superoxide dismutase (SOD), and total antioxidant capacity (TAC).
The level of serum glucose was determined using quantitative diagnostic kits (Pars Azmoon, Iran). The level of insulin was measured using Mercodia Rat Insulin ELISA (Mercodia AB, Sylveniusgatan 8A, SE-754 Uppsala, Sweden). Mercodia Rat insulin ELISA is a solid two-phase immunoassay. It is based on the direct sandwich technique, in which two monoclonal antibodies are directed against separate antigenic determinants on the insulin molecule. The level of nitrite in serum (stable nitric oxide metabolite) was measured using a colorimetric assay kit (ZelBio, Germany) that involves the Griess reaction. MDA levels of serum was quantified according to the manual methodology.[26,27] Following the measurements, SOD activity and TAC in serum was measured using a colorimetric assay kit (ZelBio, Germany). In addition, for calculation of insulin resistance (IR), we measured the homeostatic model assessment (HOMA) index using the formula provided by Matthews et al.[11] Insulin (U/m) × (glucose [mmol/l]/22.5).
Drugs
Eugenol and tween 80 were purchased from Sigma St. Louis, MO, USA. Eugenol was dissolved in 0.5% Tween 80 in saline.[21]
Statistical analysis
Data are expressed as mean ± standard error of the mean. The levels of glucose, insulin, nitrite, MDA, SOD, TAC, and HOMA index were analyzed by one-way analysis of variance followed by the Dunnett test. P < 0.05 were considered statistically significant, using SPSS version 16 for the data analysis (Chicago, IL, USA).
Results
The effect of eugenol on acute and chronic pain
Evaluation of acute and chronic pain in formalin test showed that fructose administration did not change the level of pain response after 8 weeks in comparison with control group. However, treatment with single and continuous doses of eugenol could significantly decrease response to the pain in the first and second phases of formalin test in comparison with other groups, (P < 0.05); but there are not significant differences between Fr + EoS and Fr + EoC in acute pain, and chronic pain. The results suggested the noticeable role of eugenol on pain relieving [Figure 1].
Figure 1.
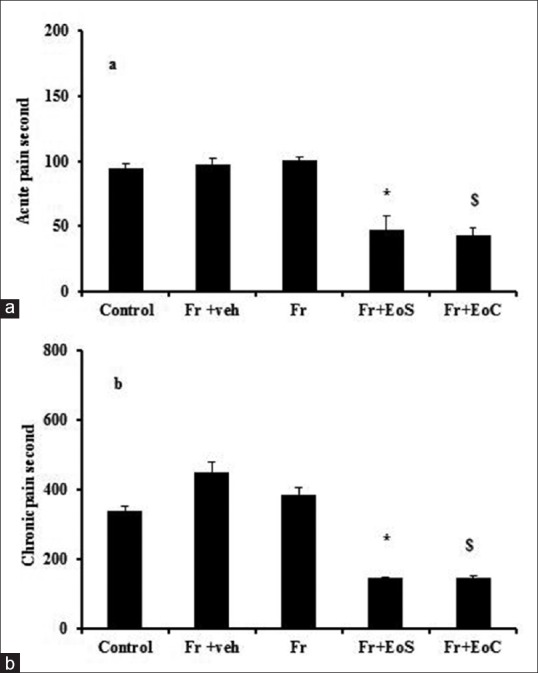
Acute pain (a) chronic pain (b). *indicates significant difference between Fr+EoS from Fr group, $ indicates significant difference between Fr+EoC from Fr group. Fr, veh, EoS and EoC stand for Fructose, vehicle, single dose of eugenol and Continuous dose of eugenol, respectively
The effect of eugenol on body weight, plasma glucose, insulin, and homeostatic model assessment index (HOMA Index)
The effects of fructose on body weight showed an increase in body weight after 8 weeks. Significant differences were observed between all fructose-received groups and the control group (P < 0.05). Meanwhile, single and continuous doses of eugenol have no effect on body weight [Table 1].
Table 1.
The effect of eugenol on body weight, plasma glucose, insulin and homa index (mean±sem)
| Group | ∆Body weight (g) | Glucose (mg/dl) | Insulin (µg/l) | HOMA index |
|---|---|---|---|---|
| Control | 106.2±4.62* | 108.3±6.00 | 0.015±0.00 | 0.14±0.00 |
| Fr +veh | 138.75±5.2 | 242±34.43 | 0.55±0.12 | 5.33±0.01 |
| Fr | 156.23±11.33 | 227.75±18.74** | 0.70±0.02** | 6.97±0.32** |
| Fr+EoS | 149.12±9.22 | 270.5±13.76 | 0.30±0.02 | 5.55±0.5 |
| Fr+EoC | 145.45±5.62 | 162.25±21.37$ | 0.06±0.04$ | 0.13±0.01$ |
*Indicates significant difference between control group with other groups (P<0.05). **Indicates significant difference from control group (P < 0.05), $indicates significant difference from Fr group (P<0.05). Fr, veh, EoS, EoC and HOMA Index stand for Fructose, vehicle, single dose of eugenol, Continuous doses of eugenol and Homeostatic model assessment index respectively
The measurement of plasma glucose levels suggested that the administration of fructose increased plasma glucose that showing a meaningful difference with the control group (P < 0.05). Here, it is worth mentioning that the treatment with a single dose of eugenol did not bring about any effect on plasma glucose. During the use of continuous doses of eugenol, blood glucose decreased and reached the same level of the control group at 0.037 level of significance [Table 1]. HOMA index was evaluated to investigate IR. The results showed that, in the presence of fructose, HOMA index increased at a significant level (P < 0.05), in comparison with the control group. On the contrary, in the presence of eugenol in the form of single dose, this index was not significantly affected. However, treatment with continuous doses of eugenol significantly decreased HOMA index (P < 0.05). Therefore, it did not show any significant differences with the control group [Table 1].
In this study, the measurement of insulin in the experimental groups suggested that the use of fructose for 8 weeks could increase insulin level in comparison with control group (P < 0.05). Similarly, the continuous dose of eugenol in comparison with Fr group could significantly decrease the insulin level at the statistically significant level of P < 0.05 [Table 1].
The effect of eugenol on serum malondialdehyde and nitrite level, superoxide dismutase activity, total antioxidant capacity
According to the data, 8 weeks after fructose administration, MDA level increased in fructose group in comparison with control group. Meanwhile, it is noteworthy that continuous dose of eugenol could decrease MDA level, but it did that show significant differences with the fructose groups. The analysis of nitrite level did not show any significant differences between the groups. The administration of fructose for 8 weeks and its subsequent treatment with eugenol did not cause any effects on serum levels of nitrite [Figure 2].
Figure 2.
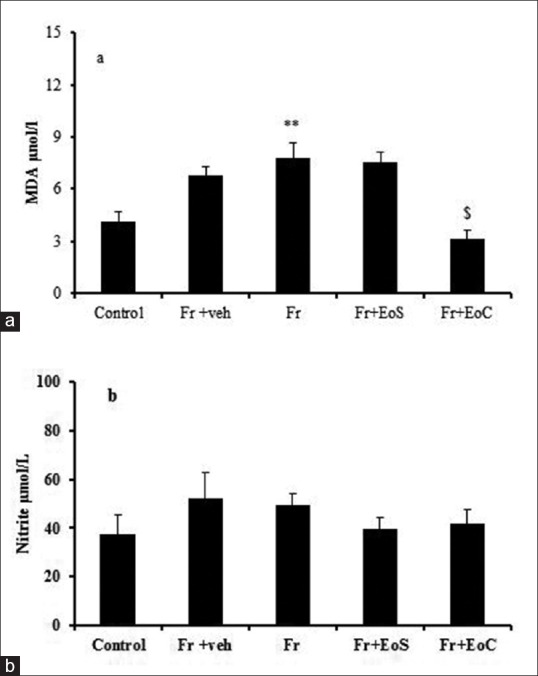
Serum level of MDA (a) and Nitrite (b). **indicates significant difference from control group, $indicates significant difference from Fr group. Fr, veh, EoS and EoC stand for Fructose, vehicle, single dose of eugenol and Continuous dose of eugenol respectively. MDA stands for Malondialdehyde
Considering the activity of serum SOD showed that its activity after fructose administration did not change. Similarly, there is no significant difference between groups [Figure 3]. Furthermore, TAC study showed that fructose had no effect on TAC level, but the treatment of rats with continuous does of eugenol could increase its level showing a significant difference with Fr group [Figure 3].
Figure 3.
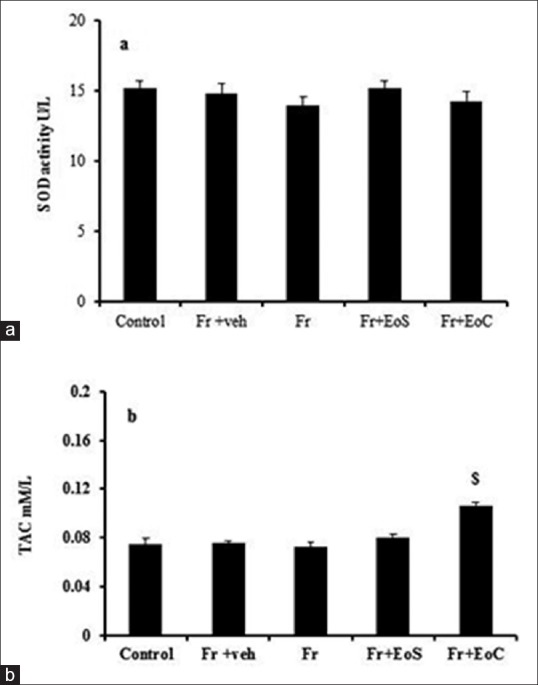
Serum SOD activity (a) and TAC level (b). $indicates significant difference from Fr group Fr. veh, EoS and EoC stand for Fructose, vehicle, single dose of eugenol and Continuous dose of eugenol respectively. SOD and TAC stand for Superoxide dismutase and Total Antioxidant Capacity, respectively
Discussion
Two most important results of this study are as follows: First, single and continuous eugenol applications were found to be significantly effective in relieving pain. Second, treatment with continuous doses of eugenol decreased plasma glucose leading to improved IR and antioxidant levels.
Consistent with the results of this study, Park et al. reported that different doses of eugenol decreased acetic-acid-induced writhing, but the best response is related to the dosage of 10 mg/kg eugenol. In addition, eugenol decreases response to pain in the first and second phases of formalin test. In this study, the remarkable point is that eugenol 10 mg/kg has no effect on the first phase of formalin test, but in the second phase, it causes 75% decrease in response to the pain. Findings of this study are perfectly consistent with above-mentioned previous findings. Furthermore, they have shown that blocking opioid and α-2 adrenergic receptors can inhibit the effect of eugenol. Thus, it can be concluded that these receptors can mediate the effect of eugenol on the pain response.[28]
Another study, confirming our results, reported that 30–300 mg/kg eugenol caused decreased acetic-acid-induced abdominal constriction in mice. Furthermore, applying 0.3–100 mg/kg eugenol decreased response to glutamate pain. On the other hand, injecting intraspinal eugenol, after 30 min, could cause long inhibition in pain response to glutamate, α-amino-3-hydroxy-5-methyl-4-isoxazolepro pionic acid, kinate, and substance P but had no effect on pain caused by N-methyl-D-aspartate and Trans-ACPD.[22] Other studies in this field have shown that eugenol can cause long blocking biting response, induced due to TNF-α intraspinal usage.[22] It is noteworthy that the administration of naloxone nonselective antagonist opioids, 20 min before eugenol treatment, can neutralize the effect of eugenol on pain response.[22,28] This is in agreement with our results. In this regard, Guénette et al. suggested that eugenol can relieve neuropathic pain.[29]
Pain behavior related to the first phase of formalin test is normally caused by the direct effect of peripheral pain receptors and its efferent fibers, while the second phase is related to tonic inflammation. Several other studies have similarly reported on medications affecting the environment such as aspirin and glucocorticoids, having palliative effect on the first phase of formalin test. In this regard, another study reported aminopyrine and mefenamic acid, having peripheral and central effects and decrease pain response in both phases of formalin test. Therefore, eugenol is at least a compound material with its central useable effects which is more effective on the second phase of formalin test.[25,30,31,32] In addition, the previous studies reported that only opioid α adrenergic, but not serotonergic receptors, were involved in pain relief from eugenol.[28]
There are also reports that the administration of fructose for 8 weeks can induce metabolic syndrome approved by the increase of plasma glucose, obesity, the increase of body mass index,[33] the increase of insulin secretion, and the increase of fasting insulin/fasting glucose and triglyceride.[34] Hence, these studies are also consistent with the results of our study. In diabetic-induced rats with streptozotocin, eugenol decreased plasma glucose and increased insulin secretion, in all of doses.[35]
The studies in the field of antioxidant effects of eugenol have shown that lipid peroxidation level, the activation of Glutathione peroxidase, glutathione reeducates, SOD, Glutathione, and catalase arrived at their normal range, 15 days after eugenol administration.[36] Similarly, in our study, 8 weeks after administrating eugenol, the same results are obtained. Another study reported that 90 days after eugenol administration, glutathione-transfer activity increased, suggesting that eugenol is a nontoxic component with its uses in scavenging toxic components.[36] In addition, Gülçin has shown that eugenol inhibited 97% lipid peroxidation, and in accordance with this study, eugenol is a strong antioxidant having radical-scavenging activity which is consistent with our study.[37]
In brief, it can be concluded that eugenol improves pain response and antioxidant activity by improving serum levels of insulin and glucose. At the same time, considering the role of metabolic syndrome in inducing oxidative stress, the role of inflammation is questionable. In this regard, further studies are needed to identify the involved inflammation mechanisms in the induction of pain.
Conclusions
In the present work, the findings indicated the beneficial effects of eugenol in pain relief, improved serum glucose, insulin levels, and improved antioxidant activity. In fact, eugenol improves antioxidant activity and relieves pain by improving serum levels of insulin leading to improving glucose levels which in turn improves antioxidant activity. Further investigation is required on the beneficial effects of treatment with eugenol on oxidative stress and inflammation.
Financial support and sponsorship
The research was supported by Zahedan University of Medical Sciences (Grant no. 7653).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Howie GJ, Sloboda DM, Kamal T, Vickers MH. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol. 2009;587:905–15. doi: 10.1113/jphysiol.2008.163477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Björntorp P. Metabolic implications of body fat distribution. Diabetes Care. 1991;14:1132–43. doi: 10.2337/diacare.14.12.1132. [DOI] [PubMed] [Google Scholar]
- 3.Federation ID. Lifestyle changes for theprevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155–61. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 4.Perrone S, Bellieni CV, Negro S, Longini M, Santacroce A, Tataranno ML, et al. Oxidative stress as a physiological pain response in full-term newborns. Oxid Med Cell Longev. 2017;2017:3759287. doi: 10.1155/2017/3759287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–23. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 6.Lê KA, Tappy L. Metabolic effects of fructose. Curr Opin Clin Nutr Metab Care. 2006;9:469–75. doi: 10.1097/01.mco.0000232910.61612.4d. [DOI] [PubMed] [Google Scholar]
- 7.Tappy L, Lê KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 8.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–43. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 9.Ohkubo T, Shibata M. The selective capsaicin antagonist capsazepine abolishes the antinociceptive action of eugenol and guaiacol. J Dent Res. 1997;76:848–51. doi: 10.1177/00220345970760040501. [DOI] [PubMed] [Google Scholar]
- 10.Kim SS, Oh OJ, Min HY, Park EJ, Kim Y, Park HJ, et al. Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells. Life Sci. 2003;73:337–48. doi: 10.1016/s0024-3205(03)00288-1. [DOI] [PubMed] [Google Scholar]
- 11.Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 12.Sneddon IB, Glew RC. Contact dermatitis due to propanidid in an anaesthetist. Practitioner. 1973;211:321–3. [PubMed] [Google Scholar]
- 13.Li W, Tsubouchi R, Qiao S, Haneda M, Murakami K, Yoshino M, et al. Inhibitory action of eugenol compounds on the production of nitric oxide in RAW264.7 macrophages. Biomed Res. 2006;27:69–74. doi: 10.2220/biomedres.27.69. [DOI] [PubMed] [Google Scholar]
- 14.Frisch J, Bhaskar SN. Tissue response to eugenol-containing periodontal dressings. J Periodontol. 1967;38:402–8. doi: 10.1902/jop.1967.38.5.402. [DOI] [PubMed] [Google Scholar]
- 15.Moleyar V, Narasimham P. Antibacterial activity of essential oil components. Int J Food Microbiol. 1992;16:337–42. doi: 10.1016/0168-1605(92)90035-2. [DOI] [PubMed] [Google Scholar]
- 16.Ozeki M. The effects of eugenol on the nerve and muscle in crayfish. Comp Biochem Physiol C. 1975;50:183–91. [PubMed] [Google Scholar]
- 17.Trowbridge H, Edwall L, Panopoulos P. Effect of zinc oxide-eugenol and calcium hydroxide on intradental nerve activity. J Endod. 1982;8:403–6. doi: 10.1016/S0099-2399(82)80094-0. [DOI] [PubMed] [Google Scholar]
- 18.Wie MB, Won MH, Lee KH, Shin JH, Lee JC, Suh HW, et al. Eugenol protects neuronal cells from excitotoxic and oxidative injury in primary cortical cultures. Neurosci Lett. 1997;225:93–6. doi: 10.1016/s0304-3940(97)00195-x. [DOI] [PubMed] [Google Scholar]
- 19.Nangle MR, Gibson TM, Cotter MA, Cameron NE. Effects of eugenol on nerve and vascular dysfunction in streptozotocin-diabetic rats. Planta Med. 2006;72:494–500. doi: 10.1055/s-2005-916262. [DOI] [PubMed] [Google Scholar]
- 20.Ohkubo T, Kitamura K. Eugenol activates Ca(2+)-permeable currents in rat dorsal root ganglion cells. J Dent Res. 1997;76:1737–44. doi: 10.1177/00220345970760110401. [DOI] [PubMed] [Google Scholar]
- 21.Kurian R, Arulmozhi D, Veeranjaneyulu A, Bodhankar S. Effect of eugenol on animal models of nociception. Indian J Pharmacol. 2006;38:341. [Google Scholar]
- 22.Dal Bó W, Luiz AP, Martins DF, Mazzardo-Martins L, Santos AR. Eugenol reduces acute pain in mice by modulating the glutamatergic and tumor necrosis factor alpha (TNF-α) pathways. Fundam Clin Pharmacol. 2013;27:517–25. doi: 10.1111/j.1472-8206.2012.01052.x. [DOI] [PubMed] [Google Scholar]
- 23.Joushi S, Salmani ME. Effect of eugenol on lithium-pilocarpine model of epilepsy: Behavioral, histological, and molecular changes. Iran J Basic Med Sci. 2017;20:745–52. doi: 10.22038/IJBMS.2017.9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodríguez L, Otero P, Panadero MI, Rodrigo S, Álvarez-Millán JJ, Bocos C, et al. Maternal fructose intake induces insulin resistance and oxidative stress in male, but not female, offspring. J Nutr Metab 2015. 2015:158091. doi: 10.1155/2015/158091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunskaar S, Fasmer OB, Hole K. Formalin test in mice, a useful technique for evaluating mild analgesics. J Neurosci Methods. 1985;14:69–76. doi: 10.1016/0165-0270(85)90116-5. [DOI] [PubMed] [Google Scholar]
- 26.Nematbakhsh M, Ebrahimian S, Tooyserkani M, Eshraghi-Jazi F, Talebi A, Ashrafi F, et al. Gender difference in cisplatin-induced nephrotoxicity in a rat model: Greater intensity of damage in male than female. Nephrourol Mon. 2013;5:818–21. doi: 10.5812/numonthly.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadeghi F, Nematbakhsh M, Noori-Diziche A, Eshraghi-Jazi F, Talebi A, Nasri H, et al. Protective effect of pomegranate flower extract against gentamicin-induced renal toxicity in male rats. J Renal Inj Prev. 2015;4:45–50. doi: 10.12861/jrip.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SH, Sim YB, Lee JK, Kim SM, Kang YJ, Jung JS, et al. The analgesic effects and mechanisms of orally administered eugenol. Arch Pharm Res. 2011;34:501–7. doi: 10.1007/s12272-011-0320-z. [DOI] [PubMed] [Google Scholar]
- 29.Guénette SA, Ross A, Marier JF, Beaudry F, Vachon P. Pharmacokinetics of eugenol and its effects on thermal hypersensitivity in rats. Eur J Pharmacol. 2007;562:60–7. doi: 10.1016/j.ejphar.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 30.Hunskaar S, Hole K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–14. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 31.Choi SS, Lee JK, Suh HW. Antinociceptive profiles of aspirin and acetaminophen in formalin, substance P and glutamate pain models. Brain Res. 2001;921:233–9. doi: 10.1016/s0006-8993(01)03126-2. [DOI] [PubMed] [Google Scholar]
- 32.Chung KM, Lee KC, Choi SS, Suh HW. Differential roles of spinal cholera toxin- and pertussis toxin-sensitive G proteins in nociceptive responses caused by formalin, capsaicin, and substance Pin mice. Brain Res Bull. 2001;54:537–42. doi: 10.1016/s0361-9230(01)00441-5. [DOI] [PubMed] [Google Scholar]
- 33.Mamikutty N, Thent ZC, Sapri SR, Sahruddin NN, Mohd Yusof MR, Haji Suhaimi F, et al. The establishment of metabolic syndrome model by induction of fructose drinking water in male wistar rats. Biomed Res Int. 2014;2014:263897. doi: 10.1155/2014/263897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shahraki MR, Harati M, Shahraki AR. Prevention of high fructose-induced metabolic syndrome in male wistar rats by aqueous extract of Tamarindus indica seed. Acta Med Iran. 2011;49:277–83. [PubMed] [Google Scholar]
- 35.Srinivasan S, Sathish G, Jayanthi M, Muthukumaran J, Muruganathan U, Ramachandran V, et al. Ameliorating effect of eugenol on hyperglycemia by attenuating the key enzymes of glucose metabolism in streptozotocin-induced diabetic rats. Mol Cell Biochem. 2014;385:159–68. doi: 10.1007/s11010-013-1824-2. [DOI] [PubMed] [Google Scholar]
- 36.Vidhya N, Devaraj SN. Antioxidant effect of eugenol in rat intestine. Indian J Exp Biol. 1999;37:1192–5. [PubMed] [Google Scholar]
- 37.Gülçin İ. Antioxidant activity of eugenol: A structure-activity relationship study. J Med Food. 2011;14:975–85. doi: 10.1089/jmf.2010.0197. [DOI] [PubMed] [Google Scholar]


