Abstract
Background:
Severe depressive disorder is among most debilitating condition. Conventional pharmacotherapy usually takes several weeks (usually 4–12 weeks) to improve symptoms. Ketamine is an N–methyl-D aspartate receptor antagonist having rapid action on depressive symptoms.
Objectives:
The effect of subanesthetic dose of ketamine was assessed on depressive and anxiety symptoms. Illness severity and improvement were assessed after treatment with ketamine.
Materials and Methods:
Twenty-five drug-free/naïve patients of the male sex, with severe depression having no previous history of psychotic disorder, head injury, organic disorder, cardiological problem, or substance abuse were admitted for the study. Assessments were made at baseline and injection ketamine hydrochloride was given at a subanesthetic dose of 0.5 mg/kg intravenous bolus after preparation. Assessments were repeated 1 h after the first dose. Six doses were given over 2 weeks and assessments were repeated. Final assessments were made after 1 month of the last dose.
Results:
There was a significant improvement in depression, anxiety, and the severity of illness after 2 weeks and 1 month of the last dose of ketamine. Significant improvement at 1 st h of the first dose was seen in depression and anxiety and not for illness severity. There were transient adverse effects observed in some patients which subsided within 1 h.
Conclusion:
Ketamine has a robust and rapid effect on depression, which was seen immediately after the administration of ketamine and sustained at the end of 1 month.
Key words: Depression, ketamine hydrochloride, N–methyl-D aspartate
INTRODUCTION
Major depressive illness is a debilitating condition which is matter of concern worldwide affecting millions of people causing a considerable burden on health and socioeconomic status.[1] As per the World Health Organization, depression is third among global disease burden.[2] This will put tremendous pressure on societal cost due to disability. Pharmacotherapy modulating monoamine systems generally takes 4–12 weeks to start improvement. Recent studies are accepting the role of glutamate in depression, in particular, N–methyl-D aspartate (NMDA) receptors along with serotonin receptors. They are postulated to be involved in depression.[1] Glutamate is the main excitatory neurotransmitter which has a role in neurodevelopment, neurocognitive (memory learning), and neurotrophic (nerve growth differentiation, maintenance) function.[3]
Most clinical studies accept the role of drugs modulating through NMDA receptors. Ketamine is noncompetitive voltage-dependent NMDA-receptor channel blocker whose antidepressant action is evident at low doses, and with increasing doses, it mimics psychotomimetic action and eventually leads to anesthesia in higher doses.[4]
Single dose of ketamine has rapid action on depressive symptoms, and this action persists even for a week, which suggests its possible role in neuroplasticity. Many studies showed remission of depressive symptoms 1-week postinfusion.[5] A recent meta-analysis showed antidepressant efficacy from day 1 in patients of unipolar and bipolar depression.[6] The neuropsychiatric effect of subanesthetic dose of ketamine helps in the management of suicidal ideation and reduces self-harm or suicide in addition to the reduction in depressive symptoms.[7] Open-label studies have shown that one-time intravenous (i.v) ketamine led to rapid improvement of treatment-resistant depression and the quickest significant antidepressant response was noted within 2 h and slowest within 4 h.[8] Another study done in India on 27 patients of treatment-resistant depression (TRD) with single i.v dose of ketamine infusion showed short-lasting improvement in suicidal ideation and depression.[9] No significant adverse physical effects are reported in with low-dose ketamine and S-ketamine in antidepressant trials till date.[10]
Ketamine is a new and effective option in future with rapid onset of action, but studies are limited with multiple doses of ketamine which have assessed depressive and anxiety symptoms comprehensively. There are limited studies which have used i.v ketamine in bolus dose as most of the studies used it as slow i.v infusion. Hence, index study aimed to assess the efficacy of bolus i.v ketamine in patients with severe depression.
METERIALS AND METHODS
Participants
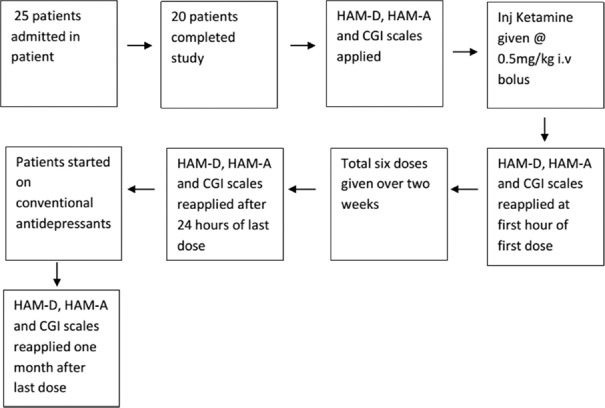
This was a hospital-based open-label prospective study, conducted from May 2016 to January 2018 at Central Institute of Psychiatry, Ranchi. Twenty-five males, aged from 18 to 60 years, were taken. Twenty patients completed the study. Patients fulfilling the International Classification of Diseases 10-Diagnostic Criteria for Research diagnostic criteria for “severe depressive episode” with pretreatment Hamilton Rating Scale for Depression (HAM-D) score >16 were included in the study. They were drug-free for at least 4 weeks (5 weeks for fluoxetine).[11] Consent was obtained and those who had any history of any major comorbid psychiatric disorder, organic disorders, or substance use disorders except (nicotine or caffeine) were excluded from the study. Patients with electrocardiogram abnormality or abnormality on fundoscopy were also excluded from the study. The study was approved by the Institutional Ethical Committee.
Sociodemographic and clinical data sheet for recording relevant data, Hamilton Depression Rating Scale (HDRS) for assessing the severity of depression,[12] Hamilton Anxiety Rating Scale (HAM-A) for anxiety,[13] and clinical global impression (CGI) scale for the severity of illness[14] were used.
Injection ketamine hydrochloride was available in vials of 5 ml each. A volume of 1 ml contained I.P equivalent to 50 mg and benzethonium chloride USP 0.01% w/v. Studies on antidepressant properties of ketamine have been conducted at lower doses, ranging from 0.1 to 1 mg/kg.[15] Here, we used i.v injection in bolus doses at 0.5 mg/kg body weight as other studies have used this medication in this bolus doses safely in postoperative pain management after tonsillectomy operation.[16] To prevent secretions, glycopyrrolate 0.4–1 mg i.v was kept on SOS basis and for the management of emergence reactions injection promethazine HCL (Phenargan) - 25 mg i.v was kept ready.[17] Other resuscitatory measures were made readily available.
Flow chart showing the outline of methodology
Procedure
Patients fit for ketamine injection were kept fasting for at least 6 h. Injection ketamine hydrochloride was calculated at a subanesthetic dose of 0.5 mg per kg of the patient's total body weight. The total amount of the drug was diluted in the ratio 1:10 with distilled water. The final volume was delivered as slow i.v bolus (within 2 min). Within 1 h of completion of the first dose of ketamine, HAM-D, HAM-A, and CGI were applied. Similarly, i.v ketamine was given on day 3, day 6, day 9, day 12, and last on day 14, respectively. Total of six sessions of i.v ketamine were given over 2 weeks. Twenty-four hours after, the last session scales were applied again. During these 2 weeks, patients were not being treated with other drugs till the last session of i.v ketamine and application of scales. At the end of the last dose of ketamine, conventional treatment like antidepressants such as selective serotonin reuptake inhibitors was started. Assessment was made after 1 month of last dose irrespective of the antidepressants they were taking.
Statistics
Data were analyzed using the IBM SPSS Statistics for Windows, version XX (IBM Corp., Armonk, NY, USA). Paired t-test was applied to see changes in various scales compared to baseline at 1 h and 2 weeks after last dose of ketamine and after 1 month of the last dose. Repeated measures ANOVA was done to assess the effect of ketamine over time.
RESULTS
Out of 25 patients, two patients dropped after receiving two doses of ketamine as they voluntarily opted out of the study. Two patients were discharged on nonmedical reasons and one patient was dropped following switch to hypomanic episode.
Sociodemographic characteristics
Table 1 shows that the majority of the patients were Hindu (90%), with secondary education (75%). Most of them were unemployed (55%) and married (70%). They belonged to the joint family (55%) and the majority had family income below Rs. 5000 (40%). Most of them belonged to rural background (70%).
Table 1.
Frequency distribution of sociodemographic variables of patients (n=20)
| Variables | Frequency (%) |
|---|---|
| Religion | |
| Hindu | 18 (90) |
| Others | 2 (10) |
| Education (years) | |
| Illiterate | 1 (5) |
| Below secondary | 15 (75) |
| Secondary education and above | 4 (20) |
| Occupation | |
| Unemployed | 11 (55) |
| Employed | 9 (45) |
| Marital status | |
| Married | 14 (70) |
| Unmarried | 6 (30) |
| Type of family | |
| Nuclear | 9 (45) |
| Joint | 11 (55) |
| Family income (Rs.) | |
| ≤5000 | 8 (40) |
| >5000-≤10,000 | 6 (30) |
| >10,000 | 6 (30) |
| Habitat | |
| Rural | 14 (70) |
| Semiurban | 3 (15) |
| Urban | 3 (15) |
Clinical characteristics of the sample population
Table 2 shows that the mean age of the sample population was 33.55 ± 8.80 years. Mean age of onset of illness was 29.90 ± 8.73 years. Mean of the duration of illness was 6.95 ± 4.31 months. Most of the patients had diagnosis of a moderate depressive episode with the somatic syndrome (35%) or recurrent depressive disorder current episode moderate with the somatic syndrome (35%). Most of them did not have any history of medical illness or any family history of medical illness or mental illness. Majority of the patients were drug naïve (50%).
Table 2.
Descriptive statistics of the clinical characteristic of patients (n=20)
| Variables | Mean±SD |
|---|---|
| Age of patient (years) | 33.55±8.80 |
| Age of onset of illness (years) | 29.90±8.73 |
| Duration of illness (months) | 6.95±4.31 |
| Diagnosis | Frequency |
| Moderate depressive episode without somatic syndrome | 2 (10) |
| Moderate depressive episode with somatic syndrome | 7 (35) |
| Severe depressive episode without psychotic symptoms | 0 |
| Recurrent depressive disorder current episode moderate without somatic syndrome | 0 |
| Recurrent depressive disorder current episode moderate with somatic syndrome | 7 (35) |
| Recurrent depressive disorder current episode severe without psychotic symptoms | 4 (20) |
| Past history of medical illness, frequency (%) | |
| Present | 1 (5) |
| Absent | 19 (95) |
| Family history of medical illness, frequency (%) | |
| Present | 3 (15) |
| Absent | 17 (85) |
| Family history of mental illness, frequency (%) | |
| Present | 5 (25) |
| Absent | 15 (75) |
| Family history of mental illness if present, frequency (%) | |
| Mood disorders | 2 (40) |
| Psychotic disorders | 0 |
| Substance use disorders | 1 (20) |
| Others | 2 (40) |
| Past treatment history, frequency (%) | |
| Drug naïve | 10 (50) |
| Antidepressants | 3 (15) |
| Mood stabilizers | 0 |
| Antipsychotics | 2 (10) |
| Combinations | 5 (25) |
SD – Standard deviation
Effect of ketamine on psychopathology
There was significant reduction in mean scores after 1st h of the 1st dose (P < 0.01). There was significant reduction in mean scores at the end of 2 weeks (P < 0.001) and after 1 month during follow-up (P < 0.001). This implied that there was a significant improvement in depressive symptoms immediately after the first dose, and this improvement was maintained at 2 weeks (end of treatment) and continued for 1 month [Table 3a].
Table 3a.
Effect of ketamine treatment over time: Changes in the Hamilton Rating Scale for depression scores (n=20)
| Mean±SD | t | df | Significant (two-tailed) | |
|---|---|---|---|---|
| Pair 1 | ||||
| Hamilton rating scale for depression baseline | 23.40±5.38 | 3.38 | 19 | 0.003** |
| Hamilton rating scale for depression at the 1st h of the first dose | 21.20±6.34 | |||
| Pair 2 | ||||
| Hamilton rating scale for depression baseline | 23.40±5.38 | 14.32 | 19 | 0.000*** |
| Hamilton rating scale for depression at the end of 2 weeks | 10.25±6.40 | |||
| Pair 3 | ||||
| Hamilton rating scale for depression baseline | 23.40±5.38 | 9.79 | 19 | 0.000*** |
| Hamilton rating scale for depression at follow up after 1 month | 10.45±8.47 |
*P<0.05; **P<0.01; ***P<0.001. SD – Standard deviation
There was a significant reduction in HAM-A after 1st h of 1st dose (P < 0.01). There was a significant reduction of mean scores at the end of 2 weeks (P < 0.001) and after 1-month follow-up (P < 0.001). Thus, there was a significant improvement in anxiety symptoms immediately after the first dose, and this improvement was maintained at 2 weeks (end of treatment) and continued for 1 month [Table 3b].
Table 3b.
Effect of ketamine treatment over time: Changes in the Hamilton Anxiety Rating Scale scores (n=20)
| Mean±SD | t | df | Significant (two-tailed) | |
|---|---|---|---|---|
| Pair 4 | ||||
| Hamilton anxiety rating scale baseline | 20.00±8.27 | 2.40 | 19 | 0.027* |
| Hamilton anxiety rating scale at the 1st h of the first dose | 18.30±6.73 | |||
| Pair 5 | ||||
| Hamilton anxiety rating scale baseline | 20.00±8.27 | 9.21 | 19 | 0.000*** |
| Hamilton anxiety rating scale at the end of 2 weeks | 8.90±5.72 | |||
| Pair 6 | ||||
| Hamilton anxiety rating scale baseline | 20.00±8.27 | 7.59 | 19 | 0.000*** |
| Hamilton anxiety rating scale at follow up after 1 month | 8.70±7.09 |
*P<0.05; **P<0.01; ***P<0.001. SD – Standard deviation
There was no significant change in mean scores of CGI-severity after 1st h of 1st dose. There was a significant reduction of mean scores at the end of 2 weeks (P < 0.001) and after 1 month (P < 0.001). This showed that there was a significant reduction in illness severity at the end of 2 weeks, which even continued for 1 month [Table 3c].
Table 3c.
Effect of ketamine treatment over time: Clinical global impression - the severity of the illness scale scores (n=20)
| Mean±SD | t | df | Significant (two-tailed) | |
|---|---|---|---|---|
| Pair 7 | ||||
| Clinical global impression - severity of illness baseline | 4.20±0.41 | 1.00 | 19 | 0.330 |
| Clinical global impression - severity of illness at the 1st h of the first dose | 4.15±0.49 | |||
| Pair 8 | ||||
| Clinical global impression - severity of illness baseline | 4.20±0.41 | 13.08 | 19 | 0.000*** |
| Clinical global impression - severity of illness at the end of 2 weeks | 2.40±0.88 | |||
| Pair 9 | ||||
| Clinical global impression - severity of illness baseline | 4.20±0.41 | 7.10 | 19 | 0.000*** |
| Clinical global impression - severity of illness at follow up after 1 month | 2.55±1.28 |
*P<0.05; **P<0.01; ***P<0.001. SD – Standard deviation
There was a significant reduction in CGI-improvement scores at the end of 2 weeks (P < 0.001) and after 1 month (P < 0.001). This showed that there was a significant improvement at the end of treatment which continued for 1 month on treatment with ketamine [Table 3d].
Table 3d.
Effect of ketamine treatment over time: Clinical global impression - improvement scale scores (n=20)
| Mean±SD | t | df | Significant (two-tailed) | |
|---|---|---|---|---|
| Pair 10 | ||||
| Clinical global impression - improvement at the 1st h of the first dose | 3.35±0.75 | 11.96 | 19 | 0.000*** |
| Clinical global impression - improvement at the end of 2 weeks | 1.75±0.79 | |||
| Pair 11 | ||||
| Clinical global impression - improvement at the 1st h of the first dose | 3.35±0.75 | 6.097 | 19 | 0.000*** |
| Clinical global impression - improvement at follow-up after 1 month | 1.85±1.14 |
*P<0.05; **P<0.01; ***P<0.001. SD – Standard deviation
The above findings were corroborated when repeated measures ANOVA was carried out to see the effect of Ketamine over time. Effect-Size estimation revealed moderate to good effect size on all rating scales, for example, for HDRS, it was (0.825), HARS was (0.760), CGI-severity of illness was (0.794), and CGI-improvement was (0.731).
DISCUSSION
There are studies using ketamine at 0.5 mg/kg but by slow infusion over 40 min using the infusion pump.[18] One study used ketamine in the form of slow i.v bolus injection but single dose of 0.2 mg/kg.[19] Most of the studies showed rapid antidepressant action within 24 h and few studies assessed its effects immediately, closest one is an assessment done at 2 h,[20] but here we assessed in the 1st h after the first dose.
Our findings suggest that there was a significant improvement in terms of depression, anxiety, and disease severity after 1 h as compared with baseline. Rapid decrease in HAM-D scores suggests overall improvement in depressive symptoms supporting rapid antidepressant effect of ketamine as similar results were found in a randomized placebo-controlled trial by Zarate et al. in 19 patients of TRD although, in our study, there was better effect size.[21] In another study, rapid antidepressant effect was also seen within 24-h postdose (18.9 ± 6.6, P < 0.001) on 67 patients of major depressive disorder (MDD).[22] Although, both the studies used ketamine delivery by infusion pump over 40 min. In another randomized controlled trial by on 27 patients of MDD following a single i.v dose there was significant decrease in symptom severity compared to placebo with peak effect within 24 h with poorer effect size (Cohen´s d) of 0.62 than index study.[23]
To the best of our knowledge, studies on repeated dosing are limited. This study also included long term repeated doses of ketamine as evident that there was significant reduction in depression, anxiety and illness severity scores after 2 weeks with six repeated doses of ketamine. Similar findings were also found in an open-label study on patients of MDD, showed that patients who responded to single dose of i.v ketamine which was maintained after total six doses over 12 days though the sample size was limited.[24] In another recent randomized control trial on 67 treatment-resistant depression patients using twice/thrice weekly infusions of ketamine over 15 days showed significant improvement in depressive symptoms over 15 days.[25] Both the above studies used injection ketamine as an i.v infusion over 40 min, in contrast, to slow i.v bolus ketamine in our study.
Limited studies have investigated the role of ketamine in anxiety symptoms. Our study found significant decrease anxiety with the first dose at 1 h which maintained after repeated dosing at the end of 2 weeks. An open-label study on 14 patients with symptoms of depression with anxiety were given daily oral tablet ketamine hydrochloride (0.5 mg/kg) for 28 days which showed that eight patients who completed trial showed significant response on anxiety symptoms which was seen on day 3.[26] In a similar study on 12 patients of generalized anxiety disorder and social phobia, those were not depressed improvement in anxiety occurred within 1 h, which persisted till 1 week.[27] This study instead found improvement on repeated slow i.v bolus ketamine on anxiety symptoms present in depressive disorders.
To the best of our knowledge, we could not find any studies which have assessed CGI-severity of illness and any study which has comprehensively assessed depressive symptoms, anxiety symptoms, and illness severity simultaneously.
We also found that with these dosing pattern, only 9 out of 19 patients' experienced mild transient adverse reactions which resolved within 1 h of injection of ketamine which suggests that ketamine is well tolerated at 0.5 mg/kg given i.v bolus in patients of depression as reported in one such study.[28]
Neurobiologically, our findings support for NMDA receptor antagonism. It has also paved way towards understanding of other possible mechanisms of rapid and sustained effects of ketamine in depression. In a meta-analysis in MDDs using 1H-magnetic resonance spectroscopy, it was found that there is down regulation of glutamate and glutamine in the anterior cingulate cortex (standardized mean difference for glutamate).[29]
Searching for functional correlates of the mechanism proposed Abdallah et al. (2017) on 18 patients with MDD who were given a single dose of ketamine infusion, did functional magnetic resonance imaging and showed improvement in “Global functional connectivity” after 24 h of infusion.[30] This change in brain functional connectivity may explain the sustained effects of ketamine and could be a marker for synaptic connectivity seen in animal studies and can be well replicated in a similar model used in our study supplementing with functional connectivity markers.
CONCLUSION
It can be postulated that administration of slow i.v ketamine in patients with severe depression results in significant improvement in depression and anxiety symptoms immediately 1 h after single dose and this effect is sustained with repeated six doses over 2 weeks and there is an overall decrease in illness severity after 2 weeks. This provides substantiative evidence for ketamine's rapid and sustained effective role in depressive disorders and its tolerability at a subanesthetic dose of 0.5 mg/kg body weight by i.v bolus injection. This study has several limitations. Open-label design limits the interpretation of efficacy. Specifically, it is not known to what extent the observed decrease in depression and anxiety severity would have occurred even under placebo conditions. Other includes limited sample size and absence of female sex, and hence, the finding cannot be generalized. Notwithstanding the important limitations, we believe that the current report contributes significantly to the small but growing literature on the clinical impact of ketamine in patients with severe depression.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This research was carried out with the sincere help of consultant anesthetist of Central Institute of Psychiatry, Ranchi. No other financial help was obtained.
REFERENCES
- 1.Naughton M, Clarke G, O'Leary OF, Cryan JF, Dinan TG. A review of ketamine in affective disorders: Current evidence of clinical efficacy, limitations of use and pre-clinical evidence on proposed mechanisms of action. J Affect Disord. 2014;156:24–35. doi: 10.1016/j.jad.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Baskaran A, Milev R, McIntyre RS. The neurobiology of the EEG biomarker as a predictor of treatment response in depression. Neuropharmacology. 2012;63:507–13. doi: 10.1016/j.neuropharm.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Serafini G, Howland RH, Rovedi F, Girardi P, Amore M. The role of ketamine in treatment-resistant depression: A systematic review. Curr Neuropharmacol. 2014;12:444–61. doi: 10.2174/1570159X12666140619204251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller OH, Moran JT, Hall BJ. Two cellular hypotheses explaining the initiation of ketamine's antidepressant actions: Direct inhibition and disinhibition. Neuropharmacology. 2016;100:17–26. doi: 10.1016/j.neuropharm.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Vasavada MM, Leaver AM, Espinoza RT, Joshi SH, Njau SN, Woods RP, et al. Structural connectivity and response to ketamine therapy in major depression: A preliminary study. J Affect Disord. 2016;190:836–41. doi: 10.1016/j.jad.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romeo B, Choucha W, Fossati P, Rotge JY. Meta-analysis of short – And mid-term efficacy of ketamine in unipolar and bipolar depression. Psychiatry Res. 2015;230:682–8. doi: 10.1016/j.psychres.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 7.Rajkumar R, Fam J, Yeo EY, Dawe GS. Ketamine and suicidal ideation in depression: Jumping the gun? Pharmacol Res. 2015;99:23–35. doi: 10.1016/j.phrs.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Hasselmann HW. Ketamine as antidepressant? Current state and future perspectives. Curr Neuropharmacol. 2014;12:57–70. doi: 10.2174/1570159X113119990043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thakurta RG, Das R, Bhattacharya AK, Saha D, Sen S, Singh OP, et al. Rapid response with ketamine on suicidal cognition in resistant depression. Indian J Psychol Med. 2012;34:170–5. doi: 10.4103/0253-7176.101793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katalinic N, Lai R, Somogyi A, Mitchell PB, Glue P, Loo CK. Ketamine as a new treatment for depression: A review of its efficacy and adverse effects. Aust N Z J Psychiatry. 2013;47:710–27. doi: 10.1177/0004867413486842. [DOI] [PubMed] [Google Scholar]
- 11.Niciu MJ, Luckenbaugh DA, Ionescu DF, Guevara S, Machado-Vieira R, Richards EM, et al. Clinical predictors of ketamine response in treatment-resistant major depression. J Clin Psychiatry. 2014;75:e417–23. doi: 10.4088/JCP.13m08698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 14.Guy W. ECDEU assessment manual for psychopharmacology. Washington, DC: US Department of Health, Education, and Welfare; 1976. pp. 534–7. [Google Scholar]
- 15.Wan LB, Levitch CF, Perez AM, Brallier JW, Iosifescu DV, Chang LC, et al. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry. 2015;76:247–52. doi: 10.4088/JCP.13m08852. [DOI] [PubMed] [Google Scholar]
- 16.Javid MJ, Hajijafari M, Hajipour A, Makarem J, Khazaeipour Z. Evaluation of a low dose ketamine in post tonsillectomy pain relief: A randomized trial comparing intravenous and subcutaneous ketamine in pediatrics. Anesth Pain Med. 2012;2:85–9. doi: 10.5812/aapm.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kothari D, Mehrotra A, Jain A, Dixit S. Role of promethazine in post ketamine emergence phenomenon-a clinical evaluation. Indian J Anaesth. 2003;47:456. [Google Scholar]
- 18.Murrough JW, Abdallah CG, Mathew SJ. Targeting glutamate signalling in depression: Progress and prospects. Nat Rev Drug Discov. 2017;16:472–86. doi: 10.1038/nrd.2017.16. [DOI] [PubMed] [Google Scholar]
- 19.Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14:1127–31. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- 20.Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, Aan Het Rot M, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74:250–6. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. Arandomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 22.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–42. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sos P, Klirova M, Novak T, Kohutova B, Horacek J, Palenicek T. Relationship of ketamine's antidepressant and psychotomimetic effects in unipolar depression. Neuro Endocrinol Lett. 2013;34:287–93. [PubMed] [Google Scholar]
- 24.Aan Het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–45. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 25.Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, et al. Adouble-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173:816–26. doi: 10.1176/appi.ajp.2016.16010037. [DOI] [PubMed] [Google Scholar]
- 26.Irwin SA, Iglewicz A, Nelesen RA, Lo JY, Carr CH, Romero SD, et al. Daily oral ketamine for the treatment of depression and anxiety in patients receiving hospice care: A 28-day open-label proof-of-concept trial. J Palliat Med. 2013;16:958–65. doi: 10.1089/jpm.2012.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glue P, Medlicott NJ, Harland S, Neehoff S, Anderson-Fahey B, Le Nedelec M, et al. Ketamine's dose-related effects on anxiety symptoms in patients with treatment refractory anxiety disorders. J Psychopharmacol. 2017;31:1302–5. doi: 10.1177/0269881117705089. [DOI] [PubMed] [Google Scholar]
- 28.Chilukuri H, Reddy NP, Pathapati RM, Manu AN, Jollu S, Shaik AB. Acute antidepressant effects of intramuscular versus intravenous ketamine. Indian J Psychol Med. 2014;36:71–6. doi: 10.4103/0253-7176.127258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luykx JJ, Laban KG, van den Heuvel MP, Boks MP, Mandl RC, Kahn RS, et al. Region and state specific glutamate downregulation in major depressive disorder: A meta-analysis of (1)H-MRS findings. Neurosci Biobehav Rev. 2012;36:198–205. doi: 10.1016/j.neubiorev.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology. 2017;42:1210–9. doi: 10.1038/npp.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]