Abstract
Despite apparent sex differences in the development and treatment of alcohol use disorder, relatively little is known about the underlying neural mechanisms. In this study, we therefore investigated neural cue‐reactivity in a sample of male (n = 28) and female (n = 27) problem drinkers (matched on age and alcohol use severity) with an average alcohol use disorder identification test score of 12 which is indicative of a likely alcohol use disorder. Neural cue‐reactivity data were extracted from four regions of interest: the ventral and dorsal striatum and the ventral and dorsal anterior cingulate cortex, with a significance level set at p < 0.05. While the cue‐reactivity paradigm induced similar levels of self‐reported craving in men and women, visual alcohol cues induced significantly stronger striatal activation in men compared to drinkers. While sex differences in ventral striatal cue‐reactivity were partly explained by sex differences in alcohol intake, cannabis use, negative affect and anxiety, this was not the case for sex differences in dorsal striatal cue‐reactivity. These results suggest that alcohol cues are differentially processed by men and women and that the neurobiological mechanisms behind cue‐reactivity differ between the sexes. Consequently, paradigms using alcohol‐related pictures may not be optimal to induce cue‐reactivity in female drinkers and may not be optimal to measure neurobiological markers of alcohol use severity and relapse. Future alcohol cue‐reactivity studies should, in addition to including both men and women, include different types of cues (e.g., stressors and imagery in addition to pictures) to assess sex differences in alcohol cue‐reactivity.
Keywords: alcohol cue‐reactivity, alcohol use disorder, craving, nucleus accumbens, sex differences, striatum
Introduction
In the past decades, differences in male and female drinking patterns have declined (Erol & Karpyak, 2015). Nonetheless, substantial sex differences in alcohol‐related physical and psychological problems are still observed, as men tend to show higher rates of alcohol‐related physical problems, whereas women tend to have a higher risk of developing comorbid psychiatric disorders (Erol & Karpyak, 2015). Moreover, women generally escalate alcohol use more rapidly once they started, compared to men (Becker, McClellan, & Reed, 2017; Lewis & Nixon, 2014), although these so‐called telescoping effects are not always evident in the general population (Keyes, Martins, Blanco, & Hasin, 2010). Importantly, compared to men, relapse rates are higher in women, often occur without apparent triggers and are more frequently related to negative affect (Becker, McClellan, & Reed, 2016; Becker et al., 2017) or events such as early life or marital stress (Buisman‐Pijlman et al., 2014; Hyman et al., 2009). Unfortunately, the underlying mechanisms that mediate these sex differences in the development, persistence and treatment of alcohol use disorder have not been studied extensively (Barker & Taylor, 2019).
Craving is a crucial characteristic of alcohol use disorder and has been related to the maintenance of abstinence, and the risk of relapse (American Psychiatric Association, 2013; Wapp, Burren, Znoj, & Moggi, 2015). A substantial amount of neural cue‐reactivity studies have reported that alcohol cues elicit robust activation of the ventral striatum (VS) and prefrontal regions, which has been suggested to underlie craving‐induction (Kühn & Gallinat, 2011; Schacht, Anton, & Myrick, 2013). These studies, however, are mainly based on male samples and studies assessing sex differences in alcohol cue‐reactivity are scarce (Barker & Taylor, 2019). The few studies that have been performed to date show that a sip of a beer induce craving in male, but not in female social drinkers (Willner, Field, Pitts, & Reeve, 1998) and show that negative mood blunts the physiological response to the smell of a preferred alcoholic drink in female but not in male drinkers (Nesic & Duka, 2006). Moreover, an EEG study demonstrated that male binge drinkers have a greater neural response to visual alcohol cues compared to female binge drinkers (Petit, Kornreich, Verbanck, & Campanella, 2013). In addition, dorsal striatum (DS) and VS activation were found to positively correlate with craving in male but not female drinkers (Seo et al., 2010), whereas VS dopamine release was found to positively correlate with the subjective effects of alcohol (Urban et al., 2010) in male, but not in female drinkers. Altogether, these few studies suggest that alcohol cues induce craving and neural cue‐reactivity in men only, which may be related to a differential role of negative effect. Moreover, while sex differences in anterior cingulate cortex (ACC) cue‐reactivity have not been reported in alcohol drinkers, cue‐reactivity studies in other substance use disorders have reported less ACC cue‐reactivity in female, compared to male, cocaine users (Volkow et al., 2011) and smokers (Dumais et al., 2017). However, also greater ACC cue‐reactivity has been found in female, compared to male, cocaine users (Kilts, Gross, & Ely, 2004) and smokers (Zanchi, Brody, Borgwardt, & Haller, 2016), whereas some studies did not find any sex differences in ACC cue‐reactivity (Wetherill et al., 2013). Importantly, strong empirical evidence for sex differences in behavioural and neural cue‐reactivity in alcohol use disorder is still lacking. Therefore, the aim of this study was to further explore sex differences in visual alcohol cue‐reactivity in a sample of male and female problem drinkers, with a minimum alcohol use disorder identification test (AUDIT) score of 12, which is indicative of a likely alcohol use disorder.
We hypothesized that male problem drinkers would show stronger striatal and frontal cue‐reactivity compared to female problem drinkers as well as stronger cue‐induced craving. This hypothesis was tested in a sample of 27 female and 28 male problem drinkers, which were matched on age and the severity of alcohol use, using an event‐related alcohol cue‐reactivity fMRI paradigm. Although sex differences have been reported in both the ventral and the dorsal parts of the striatum and ACC during alcohol, nicotine and cocaine cue‐reactivity, this study focused on these regions separately, because these regions are suggested to be related to specific processes in addiction. That is, the VS is thought to be more related to hedonic and initial alcohol use, whereas the DS is more related to habitual and compulsive alcohol use (Everitt, 2014; Vollstädt‐Klein et al., 2010). Moreover, the vACC is believed to be specifically involved in relapse and craving (Courtney, Schacht, Hutchison, Roche, & Ray, 2015; Goldstein et al., 2009; Li et al., 2013; Seo et al., 2013), whereas the dACC is suggested to be specifically related to control (Brody et al., 2007; Courtney et al., 2015; Goldstein et al., 2009). We tested the main and interaction effects of sex, drinking severity and cue‐reactivity in the regions of interest, while controlling for potential confounding demographic, personality and clinical characteristics. Because the cue‐reactivity task uses pictures of wine and beer, we furthermore tested whether preference of (alcoholic) drink type influenced alcohol cue‐reactivity.
Material and methods
Participants
Fifty‐five problem drinkers (27 women) were included in the study. Participants were recruited through Internet and poster advertisements in the local community of Amsterdam and the Psychology faculty of the University of Amsterdam, asking for individuals who wanted to reduce their alcohol intake. After providing informed consent participants received an online screening questionnaire, to assess age, drinking severity using the Alcohol Use Disorder Identification Test (AUDIT; Saunders, Aasland, Babor, De La Fuente, & Grant, 1993), alcoholic beverage of preference, drug use in the last 12 month, and their motivation to reduce or quit drinking. Participants who indicated to have used a certain drug more than 40 times in the past 12 months were asked to fill out the Drug Use Disorder Identification Test (DUDIT; Berman, Bergman, Palmstierna, & Schlyter, 2003) to assess severity of drug use. Inclusion criteria were an age between 18 and 40, a total AUDIT‐score of 12 or higher. Participants were excluded if they had a DUDIT score of 12 or higher (to exclude participants with a likely substance use disorder other than alcohol) when they preferred drinking spirits or mix‐drinks over wine or beer or when they were not motivated to reduce or to quit drinking. Participants received either a monetary compensation or a research participation credits upon completion of the study. The study was carried out in accordance with the Declaration of Helsinki and approved by the Psychology ethics committee of the University of Amsterdam. Participants were included as part of a randomized controlled study, of which the behavioural results have been published elsewhere (Kaag, Goudriaan, De Vries, Pattij, & Wiers, 2017). In this study, the baseline fMRI data are presented.
Demographic and clinical assessment
In addition to drinking severity, several other measurements were taken to explore sex differences in demographic, personality and clinical characteristics. The motivation to change drinking behaviour was assessed using the Dutch translation of the Readiness to Change Questionnaire (RCQ: Heather, Gold, & Rollnick, 1991) and alcohol craving was assessed using the Desire for Alcohol Questionnaire (DAQ), which assesses alcohol craving related to the desire for alcohol, loss of control and negative reinforcement (Love, Darren, & Willner, 1998). Participants were instructed not to consume alcohol in the 12 hrs preceding the study, which was validated using a breathalyser test (which was negative for all participants). Alcohol intake in the 14 days prior to the experiment was assessed using the Time Line Follow‐Back procedure (Sobell & Sobell, 1992). Participants were also instructed to abstain from any drug (other than nicotine) in the 12 hrs preceding the study, but we did not include an objective measure to verify this. Moreover, cannabis use severity, drug use severity and smoking severity were measured using the Cannabis Use Disorder Identification Test (Adamson & Sellman, 2003), the Drug Use Disorder Identification Test (Berman et al., 2005) and the Fagerstrom Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) if participants reported to have ever used cannabis, drugs other than cannabis or were active smokers, respectively. Drug use (GHB, LSD, methamphetamine, heroin, ketamine, cannabis, cocaine, amphetamine and opiates) in the past 12 months was assessed using an ordinal scale with the categories: never, 1–2 times, 3–5 times, 6–10 times, 11–20 times, 21–40 times or more than 40 times. The Beck Depression Inventory (BDI: Beck, Steer, & Carbin, 1988) and the state and trait anxiety inventory were used to assess depressive symptoms and state and trait anxiety (STAI: Spielberger, 1985), respectively. Additionally, impulsivity was measured using the Barrat impulsivity Scale (BIS‐11: Patton, Standford, & Barrat, 1995).
Cue‐reactivity task
All MRI scans took place on Mondays between 16.00 and 21.00, to minimize the effects of diurnal variations in craving(West & Schneiders, 1987). In this study, we used a modified version of the cue‐reactivity paradigm previously developed by Cousijn et al. (2013), using full‐colour alcohol pictures (n = 30), control pictures (n = 30) and target pictures (n = 15). Alcohol (beer or wine) and control (soft‐drinks) pictures were derived from the Amsterdam Beverage Picture Set (Pronk, van Deursen, Beraha, Larsen, & Wiers, 2015) and were matched on colour, composition and type of gesture (passive or active). More specifically, the passive stimuli consisted of beer (n = 15) and wine (n = 15) pictures without social context, whereas the active stimuli consisted of males or females drinking beer (n = 15) or wine (n = 15). Importantly, the stimuli with males drinking beer or wine were only shown to the male participants, whereas the stimuli with females drinking beer or wine were only shown to the female participants. Target pictures were photographs of animals. Participants were asked to carefully pay attention to the pictures. To ensure maintained attention, they were instructed to press a key on a response box when they detected the target picture. Each image was presented for 4 s preceded by a fixation‐cross that lasted on average 4 s, jittered between 2 and 6 s. The alcohol, control and target pictures were presented in the same semirandom order (max three images of the same category in a row) for each participant. Images were projected on a screen viewed through a mirror attached to the MRI head coil. Craving was assessed inside the MRI scanner, at baseline and at the end of the experimental paradigm, using a visual analogue scale ranging from 0 (not at all) to 10 (extremely), asking “How much do you crave for alcohol right now?”.
Functional magnetic resonance imaging data acquisition and analyses
Images were acquired on a 3.0‐T Achieva full‐body scanner (Philips Medical Systems, Best, the Netherlands) using a 32 channel SENSE head coil. Echo planar images (EPIs) were taken covering the whole brain, with a total of 36 ascending axial slices (3 × 3 × 3 mm3 voxel size; slice gap 3 mm; TR/TE 2,000/28 ms; matrix 80 × 80). Also, a T1‐3D high‐resolution anatomical scan (TR/TE 8.2/3.7; matrix 240 × 187; 1 × 1 × 1 mm3 voxel; transverse slices) was taken. fMRI data were analysed using SPM8. Preprocessing included realignment, slice‐time correction, coregistration of the structural and functional scans, normalization to MNI‐space based on the segmented structural scan and smoothing with a Gaussian kernel of 8 mm full‐width at half maximum. First‐level models included separate regressors for the alcohol pictures, control pictures and target pictures. These regressors were convolved with the canonical hemodynamic response function. Six realignment parameters were included as regressors of no interest. A high‐pass filter (1/128 Hz) was included in the first‐level model to correct for low‐frequency signal drift.
For the region of interest (ROI) analyses, the first‐level contrasts for alcohol pictures and control pictures were entered in a second‐level full factorial design with stimulus type as factor. Subsequently, the Marsbar toolbox (http://marsbar.sourceforge.net) was used to extract the mean activity for the alcohol contrast and control contrast, for each ROI. The VS was defined as the nucleus accumbens from the Harvard–Oxford subcortical structure probability atlas (http://www.cma.mgh.harvard.edu/fsl_atlas. html) and the DS was defined as the caudate and putamen from the automated anatomical labelling (AAL) atlas (Tzourio‐Mazoyer et al., 2002) minus the VS. The ventral and dorsal ACC were defined as the lower (z‐coordinate < 78) and higher (z‐coordinate > 78) part of the anterior cingulate cortex defined by the AAL atlas.
Statistical analyses
Because variation in demographic, personality and clinical characteristics could potentially confound the relation between neural cue‐reactivity and sex, univariate tests were used to test for sex differences on these variables. Moreover, these same variables could confound the relation between cue‐reactivity and alcohol use severity. Therefore, correlation coefficients between total AUDIT‐scores and the other variables (BIS‐11, RCQ, DAQ, FTND, CUDIT, DUDIT, alcohol intake per week, age) were calculated. In case of a significant relation between one of these variables and AUDIT‐scores or sex, it was explored if a significant relation between cue‐reactivity, sex and AUDIT‐scores would be affected by adding this variable as a covariate in the model.
Chi‐squared tests were used to assess sex differences in substance use. Table 1 shows the number of participants that indicated to have used tobacco, cannabis and drugs other than cannabis at least once in the 12 months preceding the study.
Table 1.
Clinical, personality and demographic characteristics
| Clinical or demographic variable | Males (n = 28) | Females (n = 27) | Sex difference p‐value | Correlation with alcohol use severity (AUDIT) | Sex*audit interaction |
|---|---|---|---|---|---|
| Age | 25.96 (6.79) | 24.85 (5.36) | F 1,52 = 0.446, p = 0.507 | r = 0.31, p = 0.021a | z = −0.28, p = 0.780, r (males) = 0.303, r (Females) = 0.373 |
| Alcohol use severity (AUDIT) | 18.93 (4.66) | 20.31 (4.63) | F 1,52 = 1.189, p = 281 | ||
| Alcohol intake (glazes per week) | 25.20 (14.56) | 18.27 (7.51) | F 1,52 = 4.720, p = 0.034a | r = 0.28, p = 0.042a | z = 0.83, p = 0.407, r (males) = 0.415, r (Females) = 0.201 |
| Days since last drink | 2.18 (1.68) | 2.59 (1.82) | F 1,53 = 0.768, p = 0.385 | r = 0.06, p = 0.671 | z = −0.95, p = 0.034 r (males) = −0.093, r (Females) = 0.175 |
| Alcohol craving (DAQ) related to | |||||
| Loss of control | 2.89 (1.46) | 2.92 (1.65) | F 1,52 = 0.005, p = 0.943 | r = 0.40, p = 0.003a | z = −1.96, p = 0.050, r (males) = 0.170, r (Females) = 0.625a |
| Desire for alcohol | 2.89 (1.11) | 3.24 (0.79) | F 1,52 = 1.780, p = 0.199 | r = 0.084, p = 0.545 | z = −0.99, p = 0.322, r (males) = −0.051, r (Females) = 0.227 |
| Negative reinforcement | 3.26 (1.20) | 3.99 (1.30) | F 1,52 = 4.617, p = 0.036a | r = 0.39, p = 0.003a | z = −0.92, p = 0.358, r (males) = 0.225, r (Females) = 0.480) |
| Readiness to change | = 0.670, p = 0.586 | = 0.058, t (53) = –0.418, p = 0.678 | = −0.173, t (53) = −1.266, p = 0.211 | ||
| Contemplation stage | N = 11 | N = 14 | |||
| Action stage | N = 16 | N = 13 | |||
| Drink of preference | = 5.505, p = 0.138 | = −0.018, t (53) = −0.130, p = 0.897 | = 0.069, t (53) = 0.500, p = 0.619 | ||
| Beer | N = 16 | N = 7 | |||
| Wine | N = 3 | N = 5 | |||
| Beer + Wine | N = 5 | N = 10 | |||
| Beer + spirits or Wine + spirits | N = 3 | N = 5 | |||
| Cannabis use severity (CUDIT) | 5.54 (6.27) | 2.42 (3.20) | F 1,52 = 5.156, p = 0.027a | r = −0.44, p = 0.752 | z = 0.46, p = 0.646, r (males) = 0.041, r (Females) = −0.09 |
| % cannabis used in the past 12 months | 71.4% | 63.0% | = 9.541, p = 0.145 | ||
| Drug Use Severity (DUDIT) | 7.04 (5.57) | 5.38 (6.45) | F 1,52 = 1.017, p = 0.318 | r = 0.218, p = 0.110 | z = −0.74, p = 0.459, r (males) = 0.14, r (Females) = 0.339 |
| % drug use in past 12 months | 75.0% | 51.9% | = 13.586, p = 0.404 | ||
| Smoking Severity (FTND) | 15.50 (9.78) | 16.46 (8.90) | F 1,52 = 0.142, p = 0.708 | r = 0.010, p = 0.941 | z = −0.20, p = 0.841, r (males) = −0.024, r (Females) = 0.032 |
| % smoking | 67.9% | 77.8% | = 0.682, p = 0.409 | ||
| Depressive mood (total BDI‐score) | 9.21 (6.23) | 14.81 (10.76) | F 1,52 = 5.566, p = 0.022a | r = 0.42, p = 0.001a | z = −182, p = 0.069, r (males) = 0.15, r (Females) = 0.586 |
| Trait Anxiety (STAI – trait) | 38.21 (8.56) | 47.61 (11.53) | F 1,52 = 11.679, p = 0.001a | r = 0.493, p < 0.001a | z = −1.19, p = 0.234, r (males) = 0.341, r (Females) = 0.602 |
| State anxiety (STAI – state) | 36.68 (9.52) | 42.26 (10.02) | F 1,52 = 4.401, p = 0.041a | r = 0.437, p < 0.001a | z = −0.82, p = 0.206, r (males) = 0.324, r (Females) = 0.515 |
| Impulsivity (BIS) | 68.68 (10.18) | 67.58 (9.11) | F 1,52 = 0.175, p = 0.678 | r = 0.31, p = 0.023a | z = −1.40, p = 161, r (males) = 0.16, r (Females) = 0.510 |
Notes. The reported values are means or medians ± the standard deviation or interquartile range.
Significant effect at p < 0.05.
To test if performance on the cue‐reactivity task (the correct identification of target pictures) was affected by sex and AUDIT‐scores, a two‐way ANOVA was performed with the number of correctly identified target trials as dependent variable, sex as independent factor and AUDIT‐scores as covariate.
To test for sex differences in “preferred drink type,” all participants were first categorized based on their preferred drink type (1: a preference for beer, 2: A preference for wine, 3: more than one drink type). Then, a chi‐squared test was performed on the distribution of these frequencies across men and women.
For all four ROIs, the mean activation of the left and right ROI was calculated. These values were entered as the dependent variable in four repeated measurements analyses, with sex as factor and AUDIT‐scores as covariate, to test for the main and interaction effects of stimulus type, sex and drinking severity. In case of a nonsignificant interaction effect, the interaction term was removed from the model. Significant interaction effects were followed by within group analyses (men vs. women and low AUDIT‐scores or high AUDIT‐scores, based on the median split) to verify the origin of the interaction effects. To explore if significant effects remained after controlling for “alcoholic drink of preference,” the analyses were repeated with “alcoholic drink of preference” (beer only, wine only or more than one drink type) included as covariate of noninterest.
To test if the relationship between cue‐reactivity and cue‐induced craving was significantly affected by sex, a repeated measurements analyses were performed with stimulus type as repeated measures, sex as independent factor and self‐reported cue‐induced craving as covariate. In case of a nonsignificant three‐way interaction, this interaction term was removed from the model.
To explore if there were any significant effects outside the predefined ROIs, a whole brain analysis was performed, with sex as independent factor and AUDIT‐scores included as regressor. These second‐level analyses were family‐wise error (FWE) rate corrected on cluster level (p < 0.05), with an initial height threshold on voxel level of p < 0.001.
Results
Clinical measures
Compared to women, men reported significantly higher weekly alcohol intake and more pronounced cannabis use severity. In contrast, women reported significantly higher craving related to negative reinforcement, depressive symptoms and state and trait anxiety. Importantly, men and women did not differ in age, severity of alcohol use, alcohol craving related to desire for alcohol and loss of control, readiness to change drinking behaviour, preferred drink type, drug use severity, smoking severity or impulsivity. Moreover, there were no differences between men and women in their drink of preference, motivation to change drinking or days since last drink (see Table 1 for precise test statistics).
Correlation analyses between alcohol use severity and the other clinical and demographic variables demonstrated that alcohol use severity was positively correlated with age, alcohol intake, craving related to loss of control, craving related to negative reinforcement, depressive symptoms, impulsivity and state and trait anxiety (see Table 1 for exact test statistics). When comparing these correlation coefficients between men and women, it became evident that there was a significant sex difference in the correlation between AUDIT‐scores and craving related to loss of control. There were no significant sex differences in the correlations between AUDIT‐scores and depressive symptoms, craving related to desire or negative reinforcement, alcohol intake per week, days since last drink, state or trait anxiety, smoking severity, cannabis use severity, drug use severity, impulsivity, age, readiness to change or drink of preference (see Table 1 for precise test statistics).
Performance on the cue‐reactivity task
A two‐way ANOVA with the number of correctly identified target trials as dependent variable, sex as independent factor and AUDIT‐scores as covariate was performed to test if the performance on the cue‐reactivity task was affected by sex and alcohol use severity. Because there was no significant sex by AUDIT‐interaction effect (F 1,54 = 0.083, p = 0.775, = 0.002), this interaction term was removed from the model. This analyses did not reveal a significant main effect of sex (F 2,52 = 1.147, p = 0.289, = 0.002) or AUDIT‐scores (F 2,52 = 3.136, p = 0.082, = 0.057) on the number of correctly identified target trials.
Self‐reported cue‐induced craving
A repeated‐measures ANOVA, with time (craving before and after the cue‐reactivity task) as repeated measures, sex as independent factor and AUDIT‐scores as covariate, demonstrated that there was no significant time by sex by AUDIT‐interaction effect (F 1,52 = 2.524, p = 0.118, = 0.047); therefore, the sex by AUDIT‐interaction term was removed from the model. Doing so, we demonstrated a significant AUDIT by time interaction effect on craving (F 1,52 = 9.52, p = 0.003, = 0.16). There was no main effect of time or a significant time by sex interaction effect on craving (F 1,52 = 0.985, p = 0.326, = 0.019).
Follow‐up analyses, based on the median split of the total AUDIT‐scores, demonstrated that the cue‐reactivity task only significantly increased craving in participants with an AUDIT‐score ≥ 19 (F 1, 26 = 42.63, p < 0.001, = 0.62) but not in participants with an AUDIT–score < 19 (F 1,52 = 1.654, p = 0.210, = 0.062). In participants with an AUDIT‐score < 19, self‐reported craving increased from 4.9 to 5.3 on a 10‐point Likert‐scale, whereas in participants with an AUDIT‐score ≥ 19, self‐reported craving increased from 3.6 to 5.1 on a 10‐point Likert‐scale. The relation between alcohol use severity and cue‐induced craving remained significant after including age, alcohol intake, craving related to loss of control and negative reinforcement, depressive symptoms, impulsivity and state/trait anxiety as covariates of noninterest in the model (F 1,45 = 4.549, p = 0.039, = 0.096).
In addition, we explored if there was a significant effect of “preferred drink type” on craving, but this was not the case (F 1,51 = 0.360, p = 0.782, = 0.021).
ROI analyses on cue‐reactivity
Repeated‐measures analyses on the ROIs, with sex as factor and AUDIT as covariate, demonstrated that there was no significant sex by AUDIT by cue‐type interaction effect (DS: F 1,51 < 0.001, p = 0.996, < 0.001; VS: F 1,51 = < 0.597, p = 0.443, = 0.012; dACC: F 1,51 < 0.001, p = 1.00, < 0.001; vACC: F 1,52 = 0.311, p = 0.580, = 0.006). Hence, this interaction term was removed from the models.
Main effect of stimulus type on cue‐reactivity in the VS, DS, vACC and dACC
Both the VS (F 1,52 = 6.19, p = 0.016, = 0.12) and the DS (F 1,52 = 2.79, p = 0.033, = 0.08) responded significantly stronger to alcohol pictures compared to control pictures. There was no main effect of stimulus type in the dorsal or ventral anterior cingulate cortex (dACC: F 1,52 = 1.197, p = 0.279, = 0.023; vACC: F 1,52 = 1.270, p = 0.265, = 0.024).
Stimulus type by sex interaction effect on cue‐reactivity in VS, DS, vACC and dACC
Ventral striatum
There was a significant interaction between sex and cue‐induced activity in the VS (F 1,52 = 5.82, p = 0.019, = 0.10) (Figure 1). As indicated in Figure 1, men responded relatively stronger to alcohol cues compared to control cues, whereas women showed reduction in activity in response to alcohol cues versus control cues. Within‐group follow‐up tests demonstrated that in both men and women, there was no significant effect of cue‐type (men : F 1,27 = 2.853, p = 0.103, = 0.096; women : F 1,26 = 1.195, p = 0.284, = 0.044) and no significant effect of sex on the response of the VS to either neutral or alcohol stimuli (neutral: F 1,55 = 0.331, p = 0.569, = 0.006; alcohol: F 1,55 = 1.020, p = 0.317, = 0.019). The interaction effect, however, did not remain significant after including alcohol intake, cannabis use severity, craving related to negative reinforcement, depressive symptoms and state and trait anxiety as covariates of noninterest in the model (F 1,45 = 2.818, p = 0.100, = 0.059). None of these variables were, however, significantly correlated with cue‐reactivity in the VS (BDI: r = −0.138, p = 0.318; CUDIT: r = 0.040, p = 0.776; alcohol intake: r = 0.035, p0.804; DAQreinforcement: r = −0.113, p = 0.417; STAI‐State: r = 0.018, p = 0.896; STAI‐trait:r = −0.097, p = 0.486), suggesting that the changes in significance level are likely to be related to a loss of degrees of freedom, instead of these variables accounting for significant variance.
Figure 1.

Sex by stimulus‐type interaction effect. There was a significant sex by stimulus‐type interaction effect in the dorsal and ventral striatum, but not in the dorsal or ventral ACC. More specifically, reactivity in the ventral and dorsal striatum in response to alcohol stimuli versus soda stimuli was significantly stronger in male compared to female problem drinkers. Values indicated with an asterisk (*) represent a significant sex by stimulus‐type interaction effect at p < 0.05.
An exploratory analysis furthermore demonstrated that the significant sex by cue‐reactivity interaction effect remained after including “drink of preference” as covariate of noninterest in the model (F 1,51 = 4.963, p = 0.030, = 0.089). With other words, sex differences in neural cue‐reactivity in the VS were not explained by sex differences in alcoholic drink of preference.
Dorsal striatum
Similarly to the VS, there was a significant stimulus type by sex interaction effect (F 1,52 = 6.09, p = 0.017, = 0.11). As indicated in Figure 1, men responded relatively stronger to alcohol cues compared to control cues, whereas women showed reduced activity in response to alcohol cues versus control cues. Within group follow‐up analyses demonstrated that there was a (trend) significant effect of cue‐type in men (F 1,27 = 4.088, p = 0.053, = 0.132), but not in women (F 1,26 = 0.673, p = 0.419, = 0.025), neither was there a significant effect of sex in response to either neutral (F 1,53 = 0.916, p = 0.343, = 0.017) or alcohol stimuli (F 1,53 = 0.595, p = 0.444, = 0.011). The sex by stimulus‐type interaction effect remained significant after including alcohol intake, cannabis use severity, craving related to negative reinforcement, depressive symptoms and state and trait anxiety as covariates of noninterest in the model(F 1,45 = 4.44, p = 0.041, = 0.09). None of these variables were, however, significantly correlated with cue‐reactivity in the DS (BDI: r = −0.99, p = 0.477; CUDIT: r = 0.128, p = 0.355; alcohol intake: r = 0.017, p = 0.902; DAQreinforcement: r = 0.018, p = 0.896; STAI‐state: r = 0.065, p = 0.642; STAI‐trait:r = −0.025, p = 0.855).
An exploratory analysis furthermore demonstrated that the significant sex by cue‐reactivity interaction effect remained after including “drink of preference” as covariate of noninterest in the model (F 1,51 = 5.005, p = 0.030, = 0.089). With other words, sex differences in neural cue‐reactivity in the DS were not explained by sex differences in alcoholic drink of preference.
Ventral and dorsal anterior cingulate cortex
There was no significant stimulus type by sex interaction effect on cue‐reactivity in the ventral anterior cingulate cortex (F 1,45 = 0.604, p = 0.441, = 0.013) or dorsal anterior cingulate cortex (F 1,45 = 0.128, p = 0.722, = 0.003).
Stimulus type by alcohol use severity interaction on cue‐reactivity in the VS, DS, vACC and dACC
Ventral striatum
There was a significant interaction between stimulus type and AUDIT‐scores on cue‐reactivity in the VS (F 1,52 = 7.01, p = 0.011, = 0.12), indicating that cue‐reactivity in the VS was significantly affected by alcohol use severity (Figure 2). Follow‐up analyses based on the median split of the AUDIT‐scores demonstrated that the VS in participants with an AUDIT‐score < 19 responded stronger, but nonsignificantly, to control pictures (F 1,25 = 1.268, p = 0.271, = 0.048), whereas the VS in participants with an AUDIT‐score ≥ 19 responded stronger, but nonsignificantly, to alcohol pictures compared to control pictures (F 1,26 = 2.695, p = 0.113, = 0.094). The interaction between AUDIT‐scores and cue‐induced activity in the VS remained significant after including age, alcohol intake, craving related to loss of control, craving related to negative reinforcement, depressive symptoms, impulsivity and state/trait anxiety as covariates of noninterest in the model (F 1,43 = 9.803, p = 0.003, = 0.186).
Figure 2.
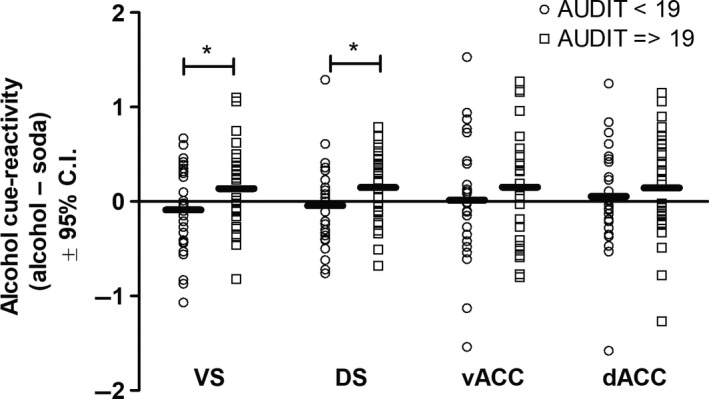
Alcohol use severity by stimulus‐type interaction effect. There was a significant AUDIT (alcohol use severity) by stimulus‐type interaction effect in the ventral and dorsal striatum. More specifically, in individuals with the highest scores on alcohol use severity, the ventral and dorsal striatum responded stronger to alcohol stimuli compared to soda stimuli, whereas in individuals with the lowest scores on alcohol use severity, the ventral and dorsal striatum responded stronger to soda stimuli compared to alcohol stimuli. For visualization purposes, these data are plotted based on the median split of the AUDIT‐scores. Values indicated with an asterisk (*) represent a significant AUDIT by stimulus‐type interaction effect at p < 0.05
Dorsal striatum
Similarly, there was a significant stimulus type by AUDIT‐interaction effect in the DS (F 1,52 = 6.15, p = 0.016, = 0.11), indicating that cue‐reactivity in the DS was significantly affected by alcohol use severity (Figure 2). Follow‐up analyses based on the median split of the AUDIT‐scores demonstrated that participants with an AUDIT‐score < 19 responded stronger, but nonsignificantly, to control pictures (F 1,25 = 0.327, p = 0.572, = 0.013), whereas participants with an AUDIT‐score ≥ 19 responded significantly stronger to alcohol pictures (F 1,26 = 4.42, p = 0.045, = 0.15). The interaction between AUDIT‐scores and cue‐induced activity in the DS remained significant after including age, alcohol intake, craving related to loss of control and negative reinforcement, depressive symptoms, impulsivity and state/trait anxiety as covariates of noninterest in the model (F 1,46 = 9.407, p = 0.004, = 0.180).
Ventral and dorsal anterior cingulate cortex
There was no significant stimulus type by AUDIT‐interaction effect on cue‐reactivity in the ventral anterior cingulate cortex (F 1,52 = 1.943, p = 0.169, = 0.036) or dorsal anterior cingulate cortex (F 1,52 = 2.069, p = 0.157, = 0.038) (Figure 2).
Stimulus type by sex by craving interaction effect on cue‐reactivity in the VS, DS, vACC and dACC
A repeated‐measures analyses with stimulus type as repeated measure, sex as independent factor and cue‐induced craving as covariate did not demonstrated a significant stimulus type by sex by craving interaction effect on neural cue‐reactivity (VS: F 1,51 = 0.067, p = 0.796, = 0.001; DS: F 1,51 = 0.181, p = 0.672, = 0.004; vACC: F 1,51 = 0.137, p = 0.713, = 0.003; dACC:F 1,51 = 0.343, p = 0.561, = 0.007) or a significant stimulus type by craving interaction effect on neural cue‐reactivity (VS: F 1,52 = 1.312, p = 0.257, = 0.025; DS: F 1,52 = 1.257, p = 0.267, = 0.024; vACC: F 1,52 = 2.244, p = 0.140, = 0.041; dACC: F 1,52 = 3.322, p = 0.074, = 0.060). In other words, we did not demonstrate a significant association between cue‐induced craving and cue‐reactivity in the ROIs, nor was this differentially affected by sex.
Whole brain analysis on cue‐reactivity
The whole brain analysis did not show a significant sex by stimulus type or AUDIT by stimulus‐type interaction effect outside the predefined regions of interest.
Discussion
The aim of this study was to investigate sex differences in visual alcohol cue‐induced craving and cue‐reactivity in the VS, DS, vACC and dACC in a sample of male and female problem drinkers. In support of our hypothesis, we demonstrated that alcohol cues induced significantly less striatal activity in female compared to male problem drinkers. These findings are important, as (neural) cue‐reactivity has been suggested to strongly predict craving, severity of dependence and/or behavioural problems associated with substance use disorders, including alcohol use disorder (Courtney et al., 2015; Engelmann et al., 2012; Jasinska, Stein, Kaiser, Naumer, & Yalachkov, 2014; Kühn & Gallinat, 2011; Schacht et al., 2013; Yalachkov, Kaiser, & Naumer, 2012). Nonetheless, while both male and female problem drinkers have been included in some of the previous studies, sex differences in cue‐reactivity have never been accounted for in these reviews or meta‐analyses. The current study, however, suggests that female problem drinkers, on a neural level, are insensitive to alcohol‐related cues, despite similar levels in alcohol use severity, which is in line with the findings of one earlier EEG study in alcohol use disorder (Petit et al., 2013). Moreover, the current findings are also in line with several neural cue‐reactivity studies in nicotine dependence (Cosgrove et al., 2014; Dumais et al., 2017). Animal studies have also reported that acute and chronic alcohol exposure only alters striatal mRNA expression in male, but not in female rats (Baxter‐Potter et al., 2017) and that there is a differential involvement of serotonergic and noradrenergic signalling in cue‐induced reinstatement (Kohtz & Aston‐Jones, 2017). Altogether, the current study supports the hypothesis that different (neural) mechanisms may underlie the development and persistence of substance use disorders in males and females.
An alternative explanation could be that substance‐related cues may not be optimal to induce cue‐reactivity in women and therefore could result in a paradigm that may not provide neurobiological markers for substance use severity or relapse in women. The stimuli used in the current study have been validated previously in 193 female and 86 male social drinkers, with a mean AUDIT‐score of 9.2 and 12.0, respectively (Pronk et al., 2015). In this study, it was demonstrated that the alcohol pictures induced a stronger urge to drink in men compared to women, whereas women reported a stronger positive valence towards the nonalcoholic pictures, which could partly explain the reported sex differences in neural cue‐reactivity. Moreover, the current findings also support the hypothesis that substance use and relapse in men is predominantly driven by external substance‐related cues (positive reinforcement), whereas substance use and relapse in women is driven predominantly by internal negative emotional states (negative reinforcement) (Cosgrove et al., 2014; Hardee et al., 2017). In line with this, animal research demonstrated that corticosterone treatment, which is consistent with a mild physiological stressor, only induced alcohol‐reinstatement in female but not in male rats (Bertholomey, Nagarajan, & Torregrossa, 2015). Moreover, studies in smokers have demonstrated that smoking cues induce more negative effect in female compared to male smokers (Doran, 2014) and that nicotine craving in women is more strongly induced by stress cues opposed to smoking cues in men (Wray et al., 2014). This not only emphasizes the importance of including both men and women in cue‐reactivity studies (Goel, Workman, Lee, Innala, & Viau, 2014), but also underlines that different cues should be used to induce cue‐reactivity (e.g., stressors and imagery in addition to substance‐related pictures), as including these will result in a clearer picture on what constitutes cue‐reactivity and how this multifaceted construct is related, for instance, to the course of addictive disorders.
In contrast to our hypothesis, we did not find significant sex differences in cue‐reactivity in the ACC. This is surprising as other studies have consistently reported ACC activation by alcohol cues (Schacht et al., 2013). A possible explanation could be that we included a nonclinical population of problem drinkers, although in this population significant cue‐reactivity has been reported in the ACC (Schacht et al., 2013). Another explanation of the lack of neural cue‐reactivity in the ACC could lie in the type of stimuli used. For this study, we used images from the Amsterdam Beverage Picture Set (Pronk et al., 2015). This set contains pictures of alcoholic drinks and control pictures (passive and active) in the absence of any additional context. While these pictures have been validated to induce craving in problem drinkers, they have been optimized to measure cognitive bias and not cue‐reactivity in alcohol use disorder (Pronk et al., 2015). Because these images contain no context, they may be less emotionally arousing and hence could primarily trigger dopamine‐related activity in the striatum, and not neural activity related to other cognitive processes. Indeed, the ventral and dorsal ACC are crucially involved in the inhibition and expression of negative emotions, respectively (Etkin, Egner, & Kalisch, 2011) and neural activation of these regions induced by alcohol‐related cues may actually reflect the processing of negative emotional aspects of these cues, instead of alcohol of craving‐related aspects. If cue‐reactivity in women is, indeed, more related to negative emotional states, it could be expected that sex differences in ACC activation are more pronounced when using alcohol pictures with a negative emotional valence.
Our choice of visual alcohol cues may also explain why we did not find a significant sex difference in self‐reported cue‐induced craving (Willner et al., 1998) and why we did not find a differential association between cue‐reactivity and cue‐induced craving in men and women (Seo et al., 2010; Urban et al., 2010). That is, while we used visual alcohol cues, the studies that demonstrated a differential association between men and women in cue‐induced craving, either used an alcohol‐challenge as alcohol‐related cue (Urban et al., 2010; Willner et al., 1998) or individually tailored imagery scripts (Seo et al., 2010). Moreover, the cue‐reactivity paradigm in the current study only induced a significant increase in craving in participants with an AUDIT‐score above 19. Therefore, the alcohol stimuli used in the current study may have been sufficient to induce neural cue‐reactivity but may have induced too little craving, to demonstrate a significant relation between neural cue‐reactivity and cue‐induced self‐reported craving. Alternatively, the small increase in self‐reported craving that we did demonstrate could have been the result from a report bias (for example: the participants could report an increase in craving because they assume this is expected from them). This could explain why we did not find any significant relation between self‐reported craving and neural cue‐reactivity, in addition to the finding that we did not demonstrate any neural cue‐reactivity in women, despite the fact that they did report a significant increase in self‐reported craving. Future studies would therefore benefit from using alcohol stimuli that are more craving inducing, for instance by including emotional context or using oral alcohol challenges.
To our knowledge, this is one of the first studies that have extensively studied sex differences in neural cue‐reactivity in problem drinkers. Although we included a nonclinical population of problem drinkers, the average AUDIT‐score in this population (19, ranging from 12 to 29) is strongly indicative of an alcohol use disorder (Johnson, Lee, Vinson, & Seale, 2013). Nonetheless, the current results warrant replication in a clinical population. Another limitation is that, while men and women in the current study did not significantly differ in their preference of alcohol‐containing beverages, we did not match men and women on their preference. Despite the fact that drink of preference did not significantly affect the sex by cue‐type interaction effect in the VS and DS, future cue‐reactivity studies should match men and women on drink of preference. Another limitation of the current study is that we did not control for menstrual cycle of the included women, while there are important indications that the phase of the menstrual cycle affects the sensitivity to reward‐ and substance‐related stimuli (Banis & Lorist, 2017; Dreher et al., 2007; Franklin et al., 2014; Ossewaarde et al., 2010; Sakaki & Mather, 2013). Hence, future studies could either include women that are in the same phase of the menstrual cycle or should at least assess in which phase of the menstrual cycle the women are, to properly control for differences in menstrual cycle. Lastly, future studies addressing sex differences in alcohol cue‐reactivity should employ alcohol cues that are relevant for both men and women, for instance using cues that contain an emotional context.
In summary, in line with previous studies, we demonstrated that AUDIT‐scores were positively correlated with striatal cue‐reactivity, suggesting that alcohol cue‐reactivity in this region is related to problematic alcohol use severity. More importantly, we showed that alcohol cues induce significantly stronger activation of the striatum in male drinkers, compared to female problem drinkers, an effect that was not explained by sex differences in clinical or demographic characteristics. Nonetheless, male and female problem drinkers reported similar levels of cue‐induced subjective craving, which suggests that different neural pathways underlie craving‐induction in male and female problem drinkers. Altogether, these results point to sex differences in alcohol cue‐reactivity that stress the fact that both men and women should be included when assessing the clinical relevance of cue‐reactivity in alcohol use and other substance use disorders.
Conflict of interest
The authors report no conflict of interest.
Author contribution
AMK collected and analysed the data, and wrote the first draft of the paper. AMK, RWW, TJDV, TP and AEG were involved in the design of the studies and actively participated in writing and revising the manuscript for publication. All authors critically reviewed the manuscript for content and approve the final version for publication.
Abbreviations
- AUDIT
Alcohol Use Disorder Identification Test
- dACC
dorsal Anterior Cingulate Cortex
- DAQ
Desire for Alcohol Questionnaire
- DS
Dorsal Striatum
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- DUDIT
Drug Use Disorder Identification Test
- EEG
Electro Encephalo Graphic
- fMRI
functional Magnetic Resonance Imaging
- FTND
Fagerstrom Test for Nicotine Dependence
- RCQ
Readiness to Change Questionnaire
- ROI
Region of Interest
- vACC
ventral anterior Cingulate Cortex
- VS
Ventral Striatum
Supporting information
Acknowledgements
This work was supported by a research grant from the Amsterdam Brain and Mind Project, a joint initiative of the University of Amsterdam and VU University Amsterdam.
The authors would like to thank Thomas Rouw, Sabine Wigbers, and Gwen Schroyen for their assistance in data collection.
Edited by Rita Goldstein. Reviewed by Anna B. Konova, New York University, USA; and Reagan Wetherill, University of Pennsylvania, USA.
All peer review communications can be found with the online version of the article.
Data accessibility
Data will be made available upon request.
References
- Adamson, S. J. , & Sellman, J. D. (2003). A prototype screening instrument for cannabis use disorder: The Cannabis Use Disorders Identification Test (CUDIT) in an alcohol‐dependent clinical sample. Drug and Alcohol Review, 22, 309–315. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association and DSM‐5 Task Force . (2013). Diagnostic and statistical manual of mental disorders : DSM‐5. Philadelphia, PA: American Psychiatric Association. [Google Scholar]
- Banis, S. , & Lorist, M. M. (2017). The combined effects of menstrual cycle phase and acute stress on reward‐related processing. Biological Psychology, 125, 130–145. [DOI] [PubMed] [Google Scholar]
- Barker, J. M. , & Taylor, J. R. (2019). Sex differences in incentive motivation and the relationship to the development and maintenance of alcohol use disorders. Physiology and Behavior, 203, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter‐Potter, L. N. , Henricks, A. M. , Berger, A. L. , Bieniasz, K. V. , Lugo, J. M. , & McLaughlin, R. J. (2017). Alcohol vapor exposure differentially impacts mesocorticolimbic cytokine expression in a sex‐, region‐, and duration‐specific manner. Neuroscience, 346, 238–246. [DOI] [PubMed] [Google Scholar]
- Beck, A. T. , Steer, R. A. , & Carbin, M. G. (1988). Psychometric properties of the Beck Depression Inventory: Twenty‐five years of evaluation. Clinical Psychology Review, 8, 77–100. [Google Scholar]
- Becker, J. B. , McClellan, M. , & Reed, B. G. (2016). Sociocultural context for sex differences in addiction. Addiction Biology, 21, 1052–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, J. B. , McClellan, M. L. , & Reed, B. G. (2017). Sex differences, gender and addiction. Journal of Neuroscience Research, 95, 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman, A. H. , Bergman, H. , Palmstierna, T. , & Schlyter, F. (2003). The drug use disorders identification test: Manual 1–16. [DOI] [PubMed]
- Berman, A. H. , Bergman, H. , Palmstierna, T. , & Schlyter, F. (2005). Evaluation of the Drug Use Disorders Identification Test (DUDIT) in criminal justice and detoxification settings and in a Swedish population sample. European Addiction Research, 11, 22–31. [DOI] [PubMed] [Google Scholar]
- Bertholomey, M. L. , Nagarajan, V. , & Torregrossa, M. M. (2015). Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacology (Berl), 344, 1173–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody, A. , Mandelkern, M. A. , Olmstead, R. , Jou, J. , Tiongson, E. , Allen, V. , … Cohen, M. S. (2007). Neural substrates of resisting craving during cigarette cue exposure. Nature Reviews Cancer, 62, 642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisman‐Pijlman, F. T. A. , Sumracki, N. M. , Gordon, J. J. , Hull, P. R. , Carter, C. S. , & Tops, M. (2014). Individual differences underlying susceptibility to addiction: Role for the endogenous oxytocin system. Pharmacology, Biochemistry and Behavior, 119, 22–38. [DOI] [PubMed] [Google Scholar]
- Cosgrove, K. P. , Wang, S. , Kim, S.‐J. , McGovern, E. , Nabulsi, N. , Gao, H. , … Morris, E. D. (2014). Sex differences in the brain's dopamine signature of cigarette smoking. Journal of Neuroscience, 34, 16851–16855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney, K. E. , Schacht, J. P. , Hutchison, K. , Roche, D. J. O. , & Ray, L. A. (2015). Neural substrates of cue reactivity: Association with treatment outcomes and relapse. Addiction Biology, 21, 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn, J. , Goudriaan, A. E. , Ridderinkhof, K. R. , van den Brink, W. , Veltman, D. J. , & Wiers, R. W. (2013). Neural responses associated with cue‐reactivity in frequent cannabis users. Addiction Biology, 18, 570–580. [DOI] [PubMed] [Google Scholar]
- Doran, N. (2014). Sex differences in smoking cue reactivity: Craving, negative affect, and preference for immediate smoking. The American Journal on Addict, 23, 211–217. [DOI] [PubMed] [Google Scholar]
- Dreher, J.‐C. , Schmidt, P. J. , Kohn, P. , Furman, D. , Rubinow, D. , & Berman, K. F. (2007). Menstrual cycle phase modulates reward‐related neural function in women. Proceedings of the National Academy of Sciences of the United States of America, 104, 2465–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais, K. M. , Franklin, T. R. , Jagannathan, K. , Hager, N. , Gawrysiak, M. , Betts, J. , … Wetherill, R. R. (2017). Multi‐site exploration of sex differences in brain reactivity to smoking cues: Consensus across sites and methodologies. Drug and Alcohol Dependence, 178, 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann, J. M. , Versace, F. , Robinson, J. D. , Minnix, J. A. , Lam, C. Y. , Cui, Y. , … Cinciripini, P. M. (2012). NeuroImage Neural substrates of smoking cue reactivity : A meta‐analysis of fMRI studies. NeuroImage, 60, 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol, A. , & Karpyak, V. M. (2015). Sex and gender‐related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug and Alcohol Dependence, 156, 1–13. [DOI] [PubMed] [Google Scholar]
- Etkin, A. , Egner, T. , & Kalisch, R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt, B. J. (2014). Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories – indications for novel treatments of addiction. European Journal of Neuroscience, 40, 2163–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin, T. R. , Jagannathan, K. , Wetherill, R. R. , Johnson, B. , Kelly, S. , Langguth, J. , … Childress, A. R. (2014). Influence of menstrual cycle phase on neural and craving responses to appetitive smoking cues in naturally cycling females. Nicotine and Tobacco Research, 17, 390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel, N. , Workman, J. L. , Lee, T. T. , Innala, L. , & Viau, V. (2014). Sex differences in the HPA axis. Comprehensive Physiology, 4, 1121–1155. [DOI] [PubMed] [Google Scholar]
- Goldstein, R. Z. , Alia‐Klein, N. , Tomasi, D. , Carrillo, J. H. , Maloney, T. , Woicik, P. A. , … Volkow, N. D. (2009). Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proceedings of the National Academy of Sciences of the United States of America, 106, 9453–9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardee, J. E. , Cope, L. M. , Munier, E. C. , Welsh, R. C. , Zucker, R. A. , & Heitzeg, M. M. (2017). Sex differences in the development of emotion circuitry in adolescents at risk for substance abuse: A longitudinal fMRI study. Social Cognitive and Affective Neuroscience, 12, 965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heather, N. , Gold, R. , & Rollnick, S. (1991). Readiness to change questionnaire: User's manual. KensingtonAustralia Natl. Drug Alcohol Res. Centre, Univ. New South Wales.
- Heatherton, T. F. , Kozlowski, L. T. , Frecker, R. C. , & Fagerstrom, K. (1991). The fagerstrom test for nicotine dependence: A revision of the fagerstrom tolerance questionnaire. British Journal of Addiction, 86, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hyman, S. M. , Paliwal, P. , Chaplin, T. M. , Mazure, C. M. , Bruce, J. , & Sinha, R. (2009). Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug and Alcohol Dependence, 92, 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska, A. J. , Stein, E. a. , Kaiser, J. , Naumer, M. J. , & Yalachkov, Y. (2014). Factors modulating neural reactivity to drug cues in addiction: A survey of human neuroimaging studies. Neuroscience and Biobehavioral Reviews, 38, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, J. A. , Lee, A. , Vinson, D. , & Seale, J. P. (2013). Use of AUDIT‐based measures to identify unhealthy alcohol use and alcohol dependence in primary care: A validation study. Alcoholism, Clinical and Experimental Research, 37, 253–259. [DOI] [PubMed] [Google Scholar]
- Kaag, A. M. , Goudriaan, A. E. , De Vries, T. J. , Pattij, T. , & Wiers, R. W. (2017). A high working memory load prior to memory retrieval reduces craving in non‐treatment seeking problem drinkers. Psychopharmacology (Berl), 235, 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes, K. M. , Martins, S. S. , Blanco, C. , & Hasin, D. S. (2010). Telescoping and gender differences in alcohol dependence: New evidence from two national surveys. American Journal of Psychiatry, 167, 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts, C. D. , Gross, R. E. , & Ely, T. D. (2004). The neural correlates of cue‐induced craving in cocaine‐dependent women. American Journal, 161, 233–241. [DOI] [PubMed] [Google Scholar]
- Kohtz, A. S. , & Aston‐Jones, G. (2017). Cocaine seeking during initial abstinence is driven by noradrenergic and serotonergic signaling in hippocampus in a sex‐dependent manner. Neuropsychopharmacology, 42, 408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn, S. , & Gallinat, J. (2011). Common biology of craving across legal and illegal drugs – a quantitative meta‐analysis of cue‐reactivity brain response. European Journal of Neuroscience, 33, 1318–1326. [DOI] [PubMed] [Google Scholar]
- Lewis, B. , & Nixon, S. J. (2014). Characterizing gender differences in treatment seekers. Alcoholism, Clinical and Experimental Research, 38, 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Hartwell, K. J. , Borckardt, J. , Prisciandaro, J. J. , Saladin, M. E. , Morgan, P. S. , … George, M. S. (2013). Volitional reduction of anterior cingulate cortex activity produces decreased cue craving in smoking cessation: A preliminary real‐time fMRI study. Addiction Biology, 18, 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, A. , Darren, J. , & Willner, P. (1998). A comparison of two alcohol craving questionnaires. Addiction, 93, 1091–1102. [DOI] [PubMed] [Google Scholar]
- Nesic, J. , & Duka, T. (2006). Gender specific effects of a mild stressor on alcohol cue reactivity in heavy social drinkers. Pharmacology, Biochemistry and Behavior, 83, 239–248. [DOI] [PubMed] [Google Scholar]
- Ossewaarde, L. , Hermans, E. J. , van Wingen, G. A. , Kooijman, S. C. , Johansson, I. M. , Bäckström, T. , & Fernández, G. (2010). Neural mechanisms underlying changes in stress‐sensitivity across the menstrual cycle. Psychoneuroendocrinology, 35, 47–55. [DOI] [PubMed] [Google Scholar]
- Patton, J. , Standford, M. , & Barrat, E. (1995). Factor structure of the Barratt impulsive‐ ness scale. Journal of Clinical Psychology, 51, 768–774. [DOI] [PubMed] [Google Scholar]
- Petit, G. , Kornreich, C. , Verbanck, P. , & Campanella, S. (2013). Gender differences in reactivity to alcohol cues in binge drinkers: A preliminary assessment of event‐related potentials. Psychiatry Research, 209, 494–503. [DOI] [PubMed] [Google Scholar]
- Pronk, T. , van Deursen, D. S. , Beraha, E. M. , Larsen, H. , & Wiers, R. W. (2015). Validation of the Amsterdam beverage picture set: A controlled picture set for cognitive bias measurement and modification paradigms. Alcoholism, Clinical and Experimental Research, 39, 2047–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki, M. , & Mather, M. (2013). How reward and emotional stimuli induce different reactions across the menstrual cycle. Social and Personality Psychology Compass, 6, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders, J. B. , Aasland, O. G. , Babor, T. F. , De La Fuente, J. R. , & Grant, M. (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction, 88, 791–804. [DOI] [PubMed] [Google Scholar]
- Schacht, J. P. , Anton, R. F. , & Myrick, H. (2013). Functional neuroimaging studies of alcohol cue reactivity: A quantitative meta‐analysis and systematic review. Addiction Biology, 18, 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, D. , Lacadie, C. M. , Tuit, K. , Hong, K.‐I. , Constable, R. T. , & Sinha, R. (2013). Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry, 70, 727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, D. , Zshiru, J. , Lacadie, C. M. , Tsou, K. A. , Bergquist, K. L. , & Sinha, R. (2010). Sex differences in neural responses to stress and alcohol context cues. Human Brain Mapping, 119, 5124–5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell, L. , & Sobell, M. (1992). Timeline follow‐back: A technique for assessing self‐reported alcohol consumption In Measuring alcohol consumption. pp. 41–72.
- Spielberger, C. D. (1985). Assessment of state and trait anxiety: Conceptual and methodological issues. South Psychology, 2, 6–16. [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15, 273–289. [DOI] [PubMed] [Google Scholar]
- Urban, N. B. L. , Kegeles, L. S. , Slifstein, M. , Xu, X. , Martinez, D. , Sakr, E. , … Abi‐Dargham, A. (2010). Sex differences in striatal dopamine release in young adults after oral alcohol challenge: A positron emission tomography imaging study with [11C]raclopride. Biological Psychiatry, 68, 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow, N. D. , Tomasi, D. , Wang, G. , Fowler, J. S. , Telang, F. , Rita, Z. , … Wong, C. (2011). Reduced metabolism in brain “control networks” following cocaine‐cues exposure in female cocaine abusers. Control, 6, e16573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstädt‐Klein, S. , Wichert, S. , Rabinstein, J. , Bühler, M. , Klein, O. , Ende, G. , … Mann, K. (2010). Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction, 105, 1741–1749. [DOI] [PubMed] [Google Scholar]
- Wapp, M. , Burren, Y. , Znoj, H. , & Moggi, F. (2015). Association of alcohol craving and proximal outcomes of a residential treatment program for patients with alcohol use disorders. Journal of Substance Use, 20, 11–15. [Google Scholar]
- West, P. , & Schneiders, N. (1987). Craving for cigarettes. British Journal of Addiction, 82, 407–415. [DOI] [PubMed] [Google Scholar]
- Wetherill, R. R. , Young, K. A. , Jagannathan, K. , Shin, J. , O'Brien, C. P. , Childress, A. R. , & Franklin, T. R. (2013). The impact of sex on brain responses to smoking cues: A perfusion fMRI study. Biology of Sex Differences, 4, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner, P. , Field, M. , Pitts, K. , & Reeve, G. (1998). Mood, cue and gender influences on motivation, craving and liking for alcohol in recreational drinkers. Behavioural Pharmacology, 9, 631–642. [DOI] [PubMed] [Google Scholar]
- Wray, J. M. , Gray, K. M. , Mcclure, E. A. , Carpenter, M. J. , Tiffany, S. T. , & Saladin, M. E. (2014). Gender differences in responses to cues presented in the natural environment of cigarette smokers. Nicotine and Tobacco Research, 17, 438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalachkov, Y. , Kaiser, J. , & Naumer, M. J. (2012). Functional neuroimaging studies in addiction: Multisensory drug stimuli and neural cue reactivity. Neuroscience and Biobehavioral Reviews, 36, 825–835. [DOI] [PubMed] [Google Scholar]
- Zanchi, D. , Brody, A. , Borgwardt, S. , & Haller, S. (2016). Sex effects on smoking cue perception in non‐smokers, smokers, and ex‐smokers: A pilot study. Frontiers in Psychiatry, 7, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon request.


