Abstract
Significance: Pulmonary arterial hypertension (PAH) is a progressive disease arising from the narrowing of pulmonary arteries (PAs) resulting in high pulmonary arterial blood pressure and ultimately right ventricle (RV) failure. A defining characteristic of PAH is the excessive and unrelenting inward remodeling of PAs that includes increased proliferation, inflammation, and fibrosis.
Critical Issues: There is no cure for PAH nor interventions that effectively arrest or reverse PA remodeling, and intensive research over the past several decades has sought to identify novel molecular mechanisms of therapeutic value.
Recent Advances: Galectin-3 (Gal-3) is a carbohydrate-binding lectin remarkable for its chimeric structure, composed of an N-terminal oligomerization domain and a C-terminal carbohydrate-recognition domain. Gal-3 has been identified as a regulator of numerous changes in cell behavior that contributes to aberrant PA remodeling, including cell proliferation, inflammation, and fibrosis, but its role in PAH has remained poorly understood until recently. In contrast, pathological roles for Gal-3 have been proposed in cancer and inflammatory and fibroproliferative disorders, such as pulmonary vascular and cardiac fibrosis. Herein, we summarize the recent literature on the role of Gal-3 in the development of PAH. We provide experimental evidence supporting the ability of Gal-3 to influence reactive oxygen species production, NADPH oxidase enzyme expression, and redox signaling, which have been shown to contribute to both vascular remodeling and increased pulmonary arterial pressure.
Future Directions: While several preclinical studies suggest that Gal-3 promotes hypertensive pulmonary vascular remodeling, the clinical significance of Gal-3 in human PAH remains to be established. Antioxid. Redox Signal. 00, 000–000.
Keywords: pulmonary hypertension, PAH, galectin-3, vascular remodeling, reactive oxygen species, inflammation, fibrosis
Innovation.
This study utilizes novel galectin-3 (Gal-3) inhibitors to link increased Gal-3 with elevated NADPH oxidase expression (Nox1, 2, and 4) and increased reactive oxygen species (ROS) in pulmonary artery, which is important for the development of pulmonary arterial hypertension. ROS contribute to heightened vascular tone, increased inflammation, and fibrosis that collectively shape maladaptive pulmonary vascular remodeling.
Pulmonary Arterial Hypertension
Pulmonary arterial hypertension (PAH) is a progressive disease of the lung vasculature that is characterized by a sustained elevation of pulmonary arterial pressure (46). PAH is currently defined as a mean resting pulmonary artery pressure (mPAP) of ≥25 mmHg (84) and results from increased pulmonary vascular resistance that leads to compensatory right ventricular hypertrophy (45, 46). Medial hyperplasia of pulmonary artery (PA) smooth muscle cells is a hallmark feature of PAH (64), which ranges from hypertrophy to vessel occlusion (150). In most forms of PAH, muscularization of small distal PA occurs (152) and is further characterized by excessive vascular cell proliferation, fibrosis, and inflammation, leading to medial remodeling, rarefaction, and a loss of compliance of the pulmonary blood vessels (55, 131, 150, 160).
Increased resistance to blood flow and more rigid blood vessels (loss of vascular compliance) contribute to the failure of the right ventricle (RV) (104, 169). The response of the RV to the increased afterload associated with PAH is both temporal and multifaceted with increases in end-diastolic volume, hypertrophy, alterations in contractility, dilation, cardiac fibrosis, and eventual decompensation (37). Increased RV volume (diastolic and systolic) combined with increased intraluminal pressure lead to an unsustainable increase in wall stress that culminates in right heart failure and ultimately death (15, 49, 165).
Within the pulmonary vasculature, endothelial cells become dysfunctional and fail to maintain homeostasis. Vascular smooth muscle cells undergo a phenotypic switch from a quiescent contractile phenotype to a “synthetic” phenotype that is characterized by a decrease in contractile smooth muscle genes, increased secretion of matrix and proteases, and increased proliferation (70, 121). The subsequent increase in proinflammatory and profibrotic molecules drives increased fibrosis, inflammation, and the deposition of extracellular matrix (106, 127, 136, 141, 168).
The molecular triggers of altered cellular behavior in PAH remain incompletely defined. Endothelin, platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), bone morphogenetic proteins (BMPs), hypoxic signaling, altered metabolism, and reactive oxygen and nitrogen species have all been identified as contributing factors (11, 44, 102, 106), but how these seemingly disparate signals are integrated into the complex pathology underlying PAH is an increasingly important question.
A substantial body of evidence exists in support of the role of increased levels of reactive oxygen species (ROS) in both human and experimental models of pulmonary hypertension (PH) (16, 24, 34, 63, 74, 133, 184). The major ROS that are produced in the pulmonary vasculature are superoxide (O2−), hydrogen peroxide (H2O2), hydroxyl radical (OH·), and hydroperoxyl radical (HO·2) (40). Numerous mechanisms have been proposed to account for increases in ROS, including altered endothelial nitric oxide synthase activity, increased NADPH oxidase (NOX) enzyme expression and activation, and altered mitochondrial function. However, steady-state levels of ROS also reflect the balance between ROS generation and elimination or scavenging, and there is evidence to support alterations in both pathways in PH (44).
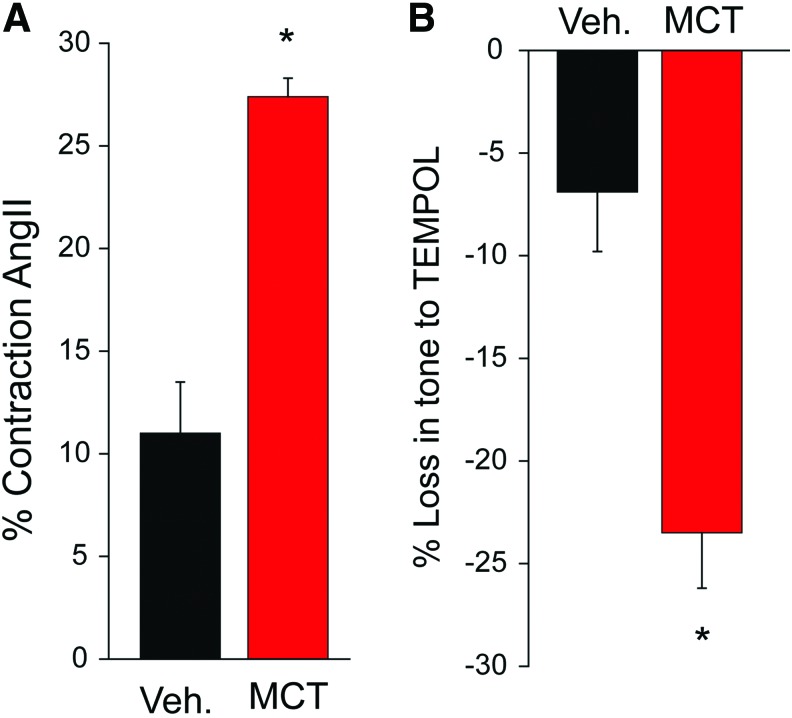
We have previously shown that ROS contribute to the development of PAH (10) and have shown in Figure 1A that vascular contraction to angiotensin II is enhanced in PAs from rats with monocrotaline (MCT)-induced PAH. Treatment of precontracted vessels with the antioxidant Tempol elicits a greater relaxation of induced tone in MCT-treated vessels compared with control (Fig. 1B), suggesting that increased oxidant tone in PAs from MCT-induced PAH augments vascular contraction. Of the ROS, O2− and H2O2 activate multiple signaling pathways promoting cell proliferation and apoptosis, elevated vascular tone, fibrosis, and inflammation, which are all hallmark signs of PAH (40). However, the cellular origin and functional significance of ROS in PAH remain poorly delineated.
FIG. 1.
Hypertensive PAs produce greater contractile force with greater dependency on ROS. (A) PA from control (vehicle) and MCT rats were mounted on a myograph (1 g passive tension) and contracted with low-dose angiotensin II (100 nM). (B) Drop in tension following the addition of ROS scavenger TEMPOL (100 mM). n = 3–4, *p < 0.05. MCT, monocrotaline; PAs, pulmonary arteries; ROS, reactive oxygen species. Color images are available online.
The human genome encodes five Nox isoforms, and four of these isoforms, NOX1, NOX2, NOX4, and NOX5, are expressed in pulmonary vascular cells. NOX4 is regarded as a constitutively active enzyme that produces levels of H2O2 that are primarily controlled by changes in gene expression (20, 117). Increased expression of NOX4 has been reported in human PAH (105), and several lines of evidence support an important role for NOX4 in the pathogenesis of PAH in rats and humans (10, 105), but this remains controversial in mice (50, 115, 166). NOX4 has been reported to be a major NADPH oxidase homolog expressed in human pulmonary arterial smooth muscle cells (PASMCs) (155), and its expression both at the mRNA level and at the protein level is significantly increased in the lungs from patients with idiopathic PAH compared with healthy lungs (105), which suggests a correlation between NOX4 and the onset of PAH.
NOX4 mediates the hypoxia-induced growth of human PASMCs (75). However, others have shown that the NOX4 expression is highest in endothelial cells and perivascular fibroblasts (1, 10, 149). Endothelial cell NOX4 is thought to be protective and supports endothelial nitric oxide synthase function (25, 143), whereas fibroblast NOX4 is highly upregulated by TGF-β and is profibrotic (10, 58). Collectively, these findings support the premise for NOX4 expression being inherently involved in pulmonary vascular remodeling by promoting arterial medial smooth muscle proliferation, endothelial proliferation, and adventitial fibroblast activation in PAH. NOX4 is also upregulated in cardiac hypertrophy and remodeling (185).
Epidemiologically, PAH is more frequent in women than in men, and untreated PAH has a survival time of 5–7 years after diagnosis (14). From a therapeutic standpoint, there are a number of vasodilator drugs that are indicated for the treatment of PAH, but none of the current therapeutics offers long-term success for survival due to limited effectiveness and unwanted side effects (142), and more importantly focus is being increasingly placed on the underlying causes of the vascular remodeling that is a hallmark of the disease (130).
Galectin-3
Galectin-3 (Gal-3; LGALS3, Mac-2) is a member of the lectin family of proteins, which recognize and bind to specific carbohydrate motifs on glycosylated proteins as well as lipids (31). Gal-3 protein was first identified in the 3T3 mouse fibroblast cell line (134) and found to be highly expressed in the lungs (26). The gene encoding Gal-3 was cloned in 1987, and changes in mRNA expression in fibroblasts were observed in response to growth factors (77). Gal-3 protein was detected in both the cytoplasm and the nucleus of cells, and higher expression levels were observed in the nucleus of proliferating cells (107). Gal-3 expression was found to be readily induced by growth factors in young cells, but aged cells or those with replicative senescence were unresponsive (23).
In the late 1980s/early 1990s, the macrophage surface antigen, Mac-2, was determined to be identical to Gal-3 and shown to be expressed in high concentrations by subsets of proinflammatory macrophages and secreted into the extracellular space (21, 176). Mac-2 has been used extensively in tissue staining applications to identify or mark macrophages (109), and this practice continues, although to a lesser extent. Gal-3 expression, however, is not confined to macrophages, and it is found to be expressed in fibroblasts (where it was originally discovered), smooth muscle cells (5), endothelial cells (111), activated T cells (78), epithelial cells (68, 79), and selected types of tumor cells (86).
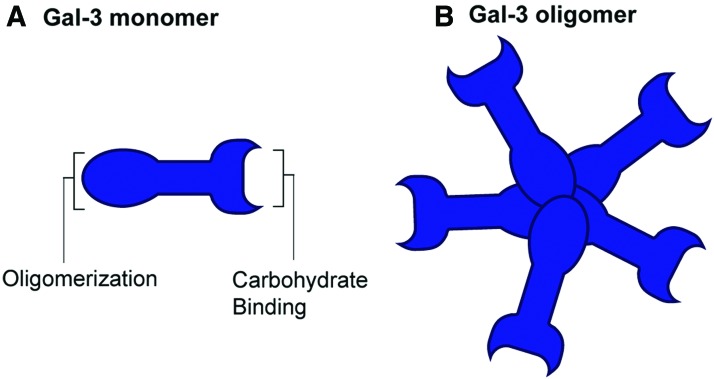
Gal-3 belongs to a family of 16 related members that all share an evolutionarily conserved carbohydrate recognition domain (CRD) that can bind β-galactosides and lactose, but they differ in their ability to bind more complex saccharides. They can be broadly classified into three types: the prototypes that contain one CRD and are monomers or homodimers (includes galectin-1, 2, 5, 7, 10, 11, 13, 14, 15, and 16), the chimeras (Gal-3 is the only member) that contain one CRD and a self-association domain, and the tandem-repeat galectins (galectin-4, 6, 8, 9, and 12) that have two CRDs connected by a linker peptide. As the only chimeric galectin, Gal-3 is composed of C-terminal CRD that is present in all members of the galectin family and a unique N-terminal domain that contains glycine- and proline-rich domains that enable Gal-3 to oligomerize with other Gal-3 molecules (Fig. 2A) or to engage in protein–protein interactions with other proteins.
FIG. 2.
Schematic illustration of Gal-3 monomer (A) and oligomer (B). Gal-3 is understood to be initially expressed as a monomer that then assembles into a larger multimers in response to carbohydrate binding and other post-translational modifications. Gal-3, galectin-3. Color images are available online.
Gal-3 is initially expressed as a monomer but can self-assemble into dimers and higher order structures in response to diverse stimuli. Cysteine 173 (previously referred to as cysteine 186) has been proposed as a critical residue that enables disulfide bonds between homodimers (175). Carbohydrate binding to the C-terminal CRD of Gal-3 triggers a structural change in the N-terminus to enable oligomerization into pentamers (Fig. 2B) (52, 87). Specific monoclonal antibodies targeting the N-terminus of Gal-3 have been shown to facilitate the multimerization of Gal-3, whereas others can induce disruption of the self-association process, thus highlighting the important role of the N-terminus in regulating the formation of Gal-3 oligomers (89).
There are also reports that the C-terminal CRD can initiate self-assembly, although more weakly via “F-faces” within the CRD (73, 87), and that this can be modified by the N-terminal domain and also impact oligomerization and substrate binding (156). Tissue transglutaminase can also directly transamidate and promote Gal-3 oligomerization, which may increase and stabilize interactions with substrates (103, 164).
Gal-3 can be found in the cell cytosol, the nucleus, and the extracellular space. How Gal-3 traffics to these different intracellular locations remains poorly understood, and it may involve post-translational modifications or through protein binding or vesicular traffic. Cytosolic Gal-3 can regulate intracellular signaling and apoptosis/cell survival (4), in the nucleus, Gal-3 has been shown to influence RNA processing (galactose/lactose-specific lectin found in association with ribonucleoprotein complexes), and in the extracellular space, Gal-3 binds to numerous ligands including receptors and integrins to influence signaling, cell:cell and cell:matrix interactions. Gal-3 does not contain a signal peptide and its secretion to the extracellular space, while polarized, has been shown to be unaffected by chemical inhibition of the classical secretory pathway (8, 69). Instead, secretion of Gal-3 is inhibited by methylamine and increased by heat shock and calcium-mobilizing agents, suggesting that exocytosis is the major export pathway (139). Recent studies support this hypothesis and show that Gal-3 can be incorporated into exosomes, which are then released into the extracellular space (8). However, several important questions remain including whether this pathway accounts for the export of both free and encapsulated Gal-3 as secreted Gal-3 is reported to be predominantly free and not packaged into extracellular vesicles (153), and how and whether Gal-3 that is present within exosomes can be released. Based on a CRISPR-Cas9 genomic screen, another proposed mechanism is that Gal-3 may bind to N-linked glycosylated proteins with signal peptides that are en route to the plasma membrane.
While inhibition of N-linked glycosylation reduced surface expression of Gal-3, it did not reduce its presence in the extracellular media, indicating that N-linked glycosylation is not required for secretion but essential for extracellular membrane binding (153). An alternative mechanism for secretion is the reported ability of Gal-3 to penetrate lipid bilayers, which may enable it to exit (as well as enter) cells and traffic to the nucleus and other intracellular organelles (93).
Gal-3 is subjected to several post-translation modifications. It is cleaved by matrix metalloproteinases 2 and 9 between Ala62 and Tyr63 to yield an intact CRD and N-terminal peptides, which results in increased carbohydrate binding but reduced oligomerization (120). Gal-3 is also a substrate for other proteases including MMP-7, MMP-13, MT1-MMP, PSA, and proteases encoded by parasites (48). Gal-3 is primarily phosphorylated on Ser6 and to a lesser extent Ser12 (68) and Tyr107 (6, 7), although this may be signal dependent. Phosphorylation can impact the subcellular localization of Gal-3 by promoting the translocation from the nucleus to the cytoplasm (158), thus influencing its ability to regulate apoptosis in the cytoplasm (181). Ser6 phosphorylation can impact the ability of Gal-3 to recognize carbohydrate motifs, and the phosphorylation of Tyr107 may impair protease-dependent cleavage (48).
Gal-3 Ligands
Ligand-binding specificity is encoded by the CRD of Gal-3, and while there are overlapping substrates, it has been shown to bind to distinct subsets of glycoproteins than other galectin family members (154). Gal-3 binds to numerous substrates including (but not limited to) signaling molecules (Ras, TGF-β), transcriptional regulators (β-catenin), ribonucleoproteins (RNA splicing), cell surface receptors (integrins [β1], TGF-β, deleted in malignant brain tumors 1, vascular endothelial growth factor (VEGF), epidermal growth factor receptor [EGFR]), lysosomal proteins, and matrix proteins (fibronectin, collagen, laminin) (19, 35, 62, 100, 114, 119, 135). In addition to glycosylated proteins, Gal-3 can also bind to glycosphingolipids present on mammalian cells, which may enable interaction with ABO blood group antigens and the HNK-1 antigen in the neurons and leukocytes (22).
Gal-3 influences a variety of processes including RNA splicing proliferation, altered signaling, migration, apoptosis, fibrosis, and inflammation (4, 18, 28, 59, 60, 72). As a result, a pathogenic role for Gal-3 has been proposed in numerous diseases such as cancer (76, 183) and inflammatory (113, 122) and fibroproliferative disorders such as pulmonary, cardiac, and hepatic fibrosis (54, 60, 116, 148, 161, 162).
How Gal-3 alters signaling depends on its intracellular location and also the ability of its CRD to recognize specific glycosylation motifs on substrates and the ability to form oligomers. Gal-3 binds to the EGFR in mesenchymal cells, which results in a mitogenic response and increased collagen (85). Gal-3 also binds with high avidity to advanced glycosylation end-product binding proteins in a variety of cell types, including macrophages and endothelial cells (129, 167). A notable marker for cell adhesion, CD98, has also been reported to be a receptor for Gal-3 (29), and there is support for a mechanistic link between CD98, interleukin-4, and Gal-3 to elicit macrophage activation and drive both inflammatory and fibrotic diseases (33). Furthermore, studies show that Gal-3 can bind to CD45, which induces cellular apoptosis (177).
With regard to ligands, extracellular matrix glycoproteins (laminins and integrins) have been identified as matrices that interact with Gal-3 (99, 180). Specifically, increased Gal-3 expression and subsequent binding increase β1 integrin-mediated cell adhesion to both laminin and fibronectin (99), and other studies show that Gal-3 may be involved in an integrin-induced epithelial–mesenchymal transition process that upregulates fibronectin and cellular migration (99). LGALS3BP (Gal-3 binding protein) is another Gal-3 ligand that mediates the induction of VEGF to promote angiogenesis (128), alters signal transduction in cancer cells to promote cell survival, migration, proliferation (128), and cell adhesion (71), and enhances antiviral response/innate immunity (91). LGALS3BP forms ring-shaped oligomers of varying sizes (mostly decamers) that interact with Gal-3 and also fibronectin, collagens, and integrins (108).
In the nucleus, Gal-3 has been detected as part of the spliceosome complex where it is important for pre-mRNA splicing. This is mediated via a specific interaction with the U1 small nuclear ribonucleoprotein (snRNP), which facilitates pre-mRNA splicing (56). Lysosome membrane permeabilization (LMP) occurs in response to excessive lipids, osmotic changes, and increased ROS (145). Following lysosomal damage, β-galactosides containing proteins, normally protected within the lumen of the lysosomes, are exposed to the cytosol where they are recognized by galectins including Gal-3 (3). Autophagy can prevent excessive cell death following LMP by sequestering membrane remnants (145).
The tripartite motif (TRIM) family of proteins are regulators of autophagy that assemble activated ULK1 and Beclin 1 on target complexes. Gal-3 binds to TRIM16 enabling it to recognize endomembrane damage and connect LMP to the autophagic response (19). Elevated ROS, which can induce LMP, have been shown to increase the lysosomal localization of Gal-3 (125).
Gal-3 Inhibitors
Some of the earliest used, and least specific, approaches to inhibiting the actions of Gal-3 have included the use of high concentrations (mM) of sugars including lactose and N-acetyllactosamine (83), which overwhelm the ability of the CRD on Gal-3 to recognize substrates. Major limitations with the use of high concentrations of sugars as Gal-3 inhibitors are the high potential for off-target effects through inhibition of other galectin family members as well as other molecules and mechanisms that may be affected.
Complex plant-derived polysaccharides including modified citrus pectins and galactomannans can also inhibit Gal-3. They are complex carbohydrate polymers that can offer greater structural complexity and diversity that enable the specific binding/absorption of different galectin isoforms with differing affinities. Inhibitors from Galectin Therapeutics including GR-MD-02 (galactoarabino-rhamnogalaturonan) and GM-CT-01 (galactomannan) are part of this class of molecules that are more specific for Gal-3 (9, 54, 162). Glycomimetics are another class of inhibitor that contain chemical substitutions (i.e., aromatic amido or 4 amido-1,2,3-triazolyl groups) that can mimic the structure of carbohydrates. They also offer advantages with regard to specificity and potency as well as the potential for intracellular actions (30).
Highly specific small-molecule inhibitors of Gal-3 have recently been developed including TD139 (95), which is a potent inhibitor that binds specifically to the galactoside-binding pocket of Gal-3. TD139 has very attractive pharmacokinetics including intracellular actions and can be inhaled, which provides advantages with lung targeting and minimization of systemic actions. Neutralizing Gal-3 antibodies have also been developed that are highly isoform specific and can bind extracellular Gal-3 and prevent many of its actions (179). The ability of neutralizing antibodies to interfere with the intracellular actions of Gal-3 may be a limitation. Finally, genetic approaches to targeting Gal-3 include standard silencing approaches using siRNA and the germline knockout of Gal-3 in mice and rats (9, 66).
The Role of Gal-3 in Inflammation
Inflammation underlies the pathology of many if not most chronic diseases. Vascularized tissue and individual cells respond to injury, infection, and irritation by initiating an inflammatory response. Acute inflammation is the early response, which usually resolves within a short period of time to enable the transition to the process of healing. In contrast, chronic inflammation is the failure of acute inflammation to resolve itself, resulting in harmful or deleterious conditions usually through persistence of an inflammatory stimulus (112).
Gal-3 is an important regulator of the immune system, and it modulates many immune reactions and directly impacts immune cell function via both autocrine and paracrine actions. Gal-3 is highly expressed in myeloid cells including monocytes, macrophages, dendritic cells, and neutrophils and has been shown to have important roles in regulating innate immunity as well as contributing to both acute and chronic inflammations. Gal-3 binds directly to CD11b on macrophages (33) and CD66 on neutrophils and can regulate inflammatory cell extravasation (140). Gal-3 regulates immune cell differentiation as well as the binding to numerous pathogens including lipopolysaccharide (LPS), the endotoxin from gram-negative bacteria (38), Helicobacter pylori (123), pathogenic fungi, and Trypanosoma cruzi (27) to name but a few.
Gal-3 can also function as a pattern-recognition receptor and a danger-associated molecular pattern (32) that can promote the assembly of inflammasomes, which produce interleukin (IL)-1β and IL-18 and activate the unfolded protein response that can amplify inflammatory responses by potentiating nuclear factor kappa light chain enhancer of activated B cells (NFκB) as well as other pathways. Gal-3 is generally regarded as a proinflammatory molecule and has been reported to activate T and B lymphocytes (65), mast cells, (43) monocytes and macrophages (138), and neutrophils (178). Gal-3 is expressed on the surface of human monocytes, and differentiation to macrophages is accompanied by increased expression levels. Moreover, Gal-3 is important in regulating macrophage polarization toward the M2 phenotype, and macrophages lacking Gal-3 show an impaired ability to express M2 gene sets in response to IL-4 (96).
Gal-3 is important for phagocytosis, and macrophages lacking Gal-3 exhibit reduced ability to remodel actin fibers post, suggesting that intracellular Gal-3 contributes to macrophage phagocytosis (137). Gal-3 can also function as chemoattractant, and high levels can promote the inward migration of monocytes and macrophages (138). The effects of Gal-3 on the behavior of immune cells can be mediated by extracellular Gal-3 binding to membrane receptors on the cell surface or by intracellular Gal-3 modulating the activity of intracellular proteins. To recognize intracellular bacterial pathogens that reside and replicate with specialized vacuoles, the innate immune system has to be able to distinguish pathogen-containing vacuoles from endogenous vesicles.
Gal-3 can recognize the carbohydrate modifications on the luminal side of vacuolar membranes and enable the delivery of antimicrobial GTPases to pathogen-containing vacuoles (36). While these actions of Gal-3 are important in defending from pathogens and maintaining the health of an organism, not all are helpful. Gal-3 expression is increased by influenza, and while helpful for an antiviral response, it has been shown to facilitate the binding of Streptococcus pneumoniae to the pulmonary epithelium resulting in increased susceptibility of influenza patients to pneumonia (118). Similarly, Gal-3 has numerous roles in inflammatory diseases such as atherosclerosis, sepsis, arthritis, asthma, and systemic sclerosis, which are reviewed in more details elsewhere (144). Another mechanism by which Gal-3 regulates the function of the immune system is through the regulation of ROS production, which is discussed below.
The Role of Gal-3 in Fibrotic Disease
Fibrosis refers to the deposition of excessive amounts of connective tissue as part of a reparative process, often secondary to inflammation, that results in the scarring or hardening of a tissue or organ, which impairs the ability to function efficiently. Gal-3 has long been identified as a driver of fibrosis (81). By activating fibroblasts, Gal-3 induces secretion of collagen leading to fibrosis (59, 60). In PAH, fibrosis occurs in both the lung vasculature and the right ventricle (13). Vascular fibrosis results from a diverse range of stimuli including oxidative stress, inflammatory cells and the release of inflammatory cytokines, compromised endothelial function, and the production of endothelium-derived vasoactive substances including the renin–angiotensin aldosterone system (17).
Elevated aldosterone can increase Gal-3 expression in vascular smooth muscle cells and drive vascular fibrosis (18). Collagen I expression is increased by Gal-3 in rat vascular smooth muscle cells, and in hypertensive aldosterone-treated rats, Gal-3 expression is increased along with the onset of vascular hypertrophy, inflammation, and fibrosis, which is reversed in the presence of Gal-3 inhibitors and absent in Gal-3 knockout (KO) mice (18). Wang et al. investigated the effect of Gal-3 on pulmonary vascular fibrosis in the MCT-treated rat model of PAH and found evidence of increased vascular fibrosis and in vitro that Gal-3 mediated TGF-β1-induced vascular fibrosis via the STAT3 and MMP9 signaling pathways (171). Myocardiac fibrosis is best understood in the left ventricle (LV) and occurs in response to injury; ischemia, chronic stress, or excessive deposition of matrix reduces tissue compliance, limits contractility, and accelerates the progression to heart failure.
In the setting of PAH, the RV, under conditions of prolonged increases in volume overload and excessive afterload, undergoes a plethora of compensatory and eventually decompensatory pathophysiological and morphological remodeling changes that eventually lead to failure. Among these alterations in cardiac morphology is the development of fibrosis (124). While fibrosis of the right ventricle can be commonly detected in human and animal models of PAH, the relationship between fibrosis and cor pulmonale is not as clearly defined as for the LV.
Recent studies have observed increased circulating levels of Gal-3 in cardiac fibrosis, which may provide utility as a clinical biomarker providing diagnostic information for the potential onset of heart failure (61, 92). Increased levels of Gal-3 are seen in fibrotic hearts, and multiple lines of evidence suggest that it contributes to myocardial fibrosis. In mice, knockout or pharmacological inhibition of Gal-3 reduces LV fibrosis and improves function (182). In rats, infusion of recombinant Gal-3 for 4 weeks promotes cardiac fibroblast proliferation, collagen production, and cyclin D1 expression leading to LV dysfunction (146). Mechanistically, hyaluronic acid has been reported to be a major component of myocardial fibrosis, and Gal-3 upregulates CD44, which increases the levels of hyaluronic acid (67, 170).
PAH Is Associated with Increased Levels of Gal-3
Increasing evidence supports a role for Gal-3 in the development of PAH. In humans with PAH, circulating Gal-3 is elevated and correlates with RV ejection fraction, end diastolic and systolic volumes (37), and this is supported by other studies showing that Gal-3 levels correlate with the severity of PAH, are a biomarker of disease progression (17), and are a strong predictor of mortality (101) in PAH.
Circulating levels of Gal-3 correlate with RV dysfunction (2). These data are in agreement with an already recognized role of Gal-3 as a promising indicator of left-sided cardiac failure (12, 41). Gal-3 expression has also been shown to be upregulated in different established experimental rat models of PAH. Luo et al. (94) have reported that Gal-3 is upregulated in lung tissue from the hypoxia-induced rat model of PAH, and we have reported increased Gal-3 expression in the MCT rat model and the Sugen 5416/hypoxia rat model of PAH (9). Elevated Gal-3 expression has also been reported in the hypoxia-induced mouse model of PAH (53).
Gal-3 Has a Functional Role in the Development of PAH
Hao et al. reported that chronic hypoxia increased both RV hypertrophy and right ventricle systolic pressure (RVSP) in wild-type (WT) mice (53); however, in Gal-3 KO mice, both these indices were not elevated by hypoxia, suggesting an amelioration of PAH. Similarly, in the hypoxia-induced rat model of PAH, where both mPAP and RVSP as well as the Fulton Index (RV/LV+S, an index of RV hypertrophy) were increased by hypoxia but inhibited by N-Lac, a nonselective galectin inhibitor (94). Furthermore, Luo et al. found that Gal-3 inhibition by N-Lac attenuated the medial hypertrophy as well as collagen deposition in the PA, suggesting that Gal-3 expression is involved in both PA proliferation and fibrosis, possibly via a TGF-β signaling pathway (94). While intriguing, a caveat of these studies is the reversibility of hypoxia models of pulmonary hypertension upon return to normoxia, which is distinct from the progressive remodeling seen in human PAH.
The rat MCT and Sugen/hypoxia models are irreversible leading to cor pulmonale and are more inflammatory and fibrotic (82, 152, 159, 174). Barman et al. observed an increase in Gal-3 expression in isolated PAs from both the MCT rat models of PAH, Sugen/hypoxia model and human PAH, which was found primarily within the medial smooth muscle layer (9). Inhibitors of Gal-3, which have been used in animal models of fibrosis and human nonalcoholic steatohepatitis (NASH) (54, 161, 162), were used to assess a functional role of Gal-3 in these models. Inhibition of Gal-3 in prevention studies reduced PA vascular remodeling and ameliorated in vivo indices of PAH. Inhibition of Gal-3 in reversal studies also showed significant efficacy at slowing disease progression (9).
To provide a complementary genetic approach that is more selective, Gal-3 was knocked out in the Sprague-Dawley rat (SDR) using CRISPR Cas9 technology. In rats, noninvasive indices of PAH were assessed in vivo using high-resolution digital ultrasound in both WT and Gal-3 KO rats treated with or without MCT. MCT-treated WT rats exhibited a time-dependent increase in PAH that was absent in Gal-3 KO rats (9). In addition, while RVSP was significantly increased in WT rats exposed to Sugen/hypoxia, there was no difference between control WT rats and Sugen/hypoxia-exposed Gal-3 KO rats (9). Collectively, these results advance the hypothesis that Gal-3 expression is increased in PAH from rodent models and human PAH, and it contributes to the vascular remodeling of PAs and the development of PAH in multiple models.
Gal-3 Promotes the Development of PAH Through Multiple Mechanisms
The ability of Gal-3 to regulate cell proliferation has been well documented (35, 72, 126). Gal-3 levels are higher in some proliferating cancer cells (76, 132, 144, 172). In PAs from rodents and humans with PAH, Gal-3 expression was detected within the hyperproliferative smooth muscle layer. This correlated with increases in numerous cellular markers of proliferation in isolated PAs, and proliferation was significantly decreased in PAs isolated from MCT-treated rats in which Gal-3 function was suppressed through pharmacological inhibition using GR or from Gal-3 KO rats. In addition, isolated PASMCs from knockout rats have reduced the capacity to proliferate, and this deficit is rescued by recombinant Gal-3, and increased expression of Gal-3 via adenoviral-mediated gene transfer stimulates human PASMC proliferation (9).
The ability of PDGF to stimulate PASMC proliferation has been shown to be dependent on increased expression of Gal-3 and is reduced by silencing of Gal-3 (51). Inhibition of Gal-3 in human pulmonary arterial vascular smooth muscle cells (HPASMCs) reduces proliferation by decreasing cyclin D1 expression and increasing p27 expression and promoting a contractile phenotype (53). Gal-3 has also been shown to mediate the ability of TGF-β to increase the proliferation of pulmonary fibroblasts (94).
As discussed above, ROS contribute to the development of PAH. Gal-3 has been shown to promote ROS generation in a range of cells. Recombinant Gal-3 stimulates dose-dependent ROS production in neutrophils (80, 178) and monocytes (90). In mast cells, extracellular Gal-3 but not Gal-1 induces apoptosis via the release of superoxide (157). While the ability of Gal-3 to induce ROS production appears to be mediated by its extracellular actions, the mechanisms by which Gal-3 promotes ROS production are not completely understood.
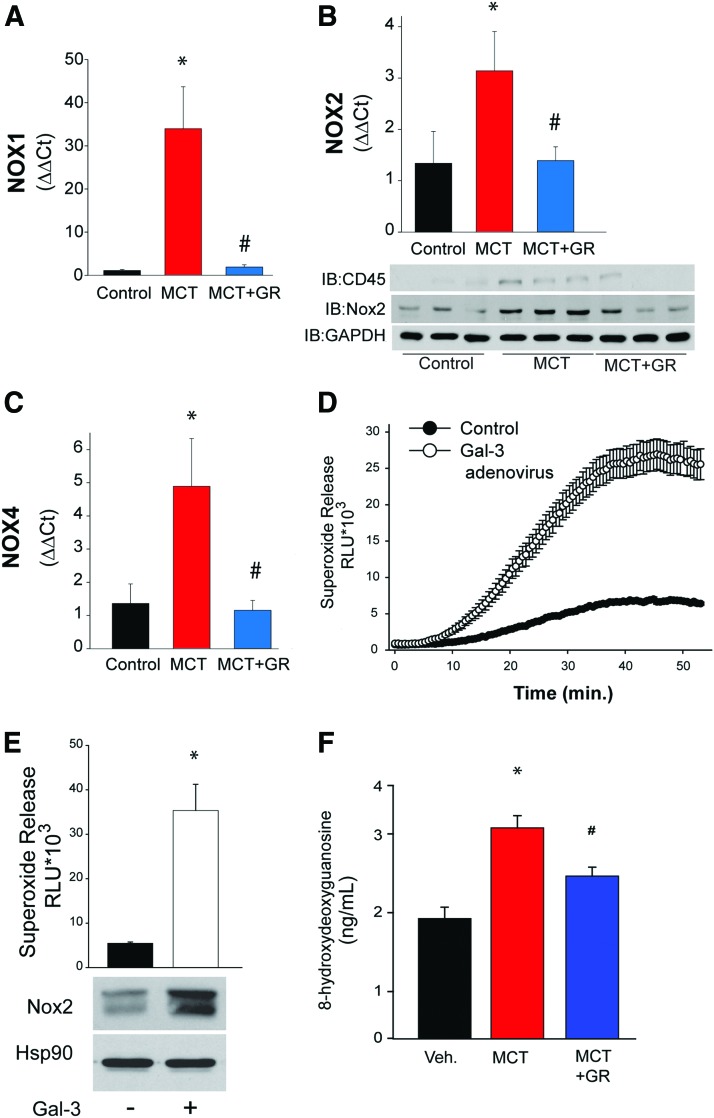
In the MCT model of PAH, we found increased expression of NOX1, NOX2, and NOX4 mRNA in isolated PAs (Fig. 3A–C). Pretreatment with an inhibitor of Gal-3 that ameliorates PAH (9) leads to significant reductions in NOX1, NOX2, and NOX4 expression (Fig. 3A–C). Increased intracellular and extracellular Gal-3 can contribute to superoxide production. Transduction of mouse peritoneal macrophages with a Gal-3 adenovirus resulted in increased phorbol myristate acetate-stimulated superoxide production. Alternatively, extracellular recombinant Gal-3 increased superoxide production in mouse peritoneal macrophages, which was accompanied by increased expression of NOX2, the major oxidoreductase in immune cells (Fig. 3E).
FIG. 3.
Gal-3 increases the expression of NOX enzymes and reactive oxygen-specific production in PAs from a rat model of pulmonary hypertension. The expression of NOX enzymes was determined in PAs isolated from rats treated with MCT for 4 weeks. Relative expression of (A) NOX1 mRNA, (B) NOX2 mRNA and protein, and (C) NOX4 mRNA was determined in PAs isolated from control, MCT-treated, and MCT-treated with the Gal-3 inhibitor GR by real-time PCR (n = 3–4). In (D), mouse peritoneal macrophages were transduced with control (GFP) or Gal-3 adenovirus, and the ability to generate ROS was determined using enhanced L-012 chemiluminescence (n = 8). In (E), mouse peritoneal macrophages were incubated with recombinant Gal-3 (10 μg/mL), and 24 h later, basal superoxide production was determined using L-012 versus NOX2 expression (n = 8). In (F), the levels of 8-hydroxydeoxyguanosine, a molecular footprint of ROS production in vivo, were measured by ELISA in lung tissue isolated from control, MCT-treated rats, and MCT-treated rats plus the Gal-3 inhibitor GR (n = 5). *p < 0.05 versus vehicle/control, #p < 0.05 versus MCT. NOX, NADPH oxidase; PCR, polymerase chain reaction. Color images are available online.
To assess whether Gal-3 contributes to vascular ROS production in PAH, we measured the expression levels of 8-hydroxy deoxyguanosine, a molecular footprint of DNA damage due to ROS, in the lungs from control rats and rats treated with MCT and MCT plus a Gal-3 inhibitor. We found that MCT increased the levels of ROS as estimated by 8-hydroxy deoxyguanosine and that pretreatment with a Gal-3 inhibitor reduced these levels to control (Fig. 3F). Collectively, these results suggest that in vivo Gal-3 contributes to the elevation of ROS via the upregulation of multiple NOX isoforms that contribute to aberrant vascular remodeling.
Others have shown a relationship between Gal-3 and oxidative stress in blood vessels (39). For example, monocytes treated with phorbol myristate acetate, which induces NADPH-oxidase-dependent ROS, increased both Gal-3 mRNA and protein expression, which was inhibited by the putative NADPH inhibitor, apocynin (98). Evidence also shows a possible relationship between Gal-3 and Nox4 to promote RV remodeling. He et al. found that a positive correlation exists between serum Nox4 and Gal-3 levels in PAH patients (57). Furthermore, in the MCT-induced rat model of PAH, both Gal-3 and Nox4 expressions were upregulated in the right ventricular myocardium with specific staining of both moieties in the intracellular myocardial matrix (57).
In specific cell types, it has been proposed that Gal-3 stimulates cardiac fibroblasts to promote RV fibrosis via interacting with Nox4, and it has been observed that knockdown of Gal-3 can inhibit Nox4 protein expression and Nox4-derived production of ROS, which is greatly increased in cardiac fibrosis (57). While we found that the inhibition of Gal-3 robustly decreased the expression level of NOX4 (Fig. 3C), we did not observe an ability of Gal-3 to upregulate NOX4 expression in fibroblasts (Fig. 5D), suggesting that other cell types or mechanisms are involved.
FIG. 5.
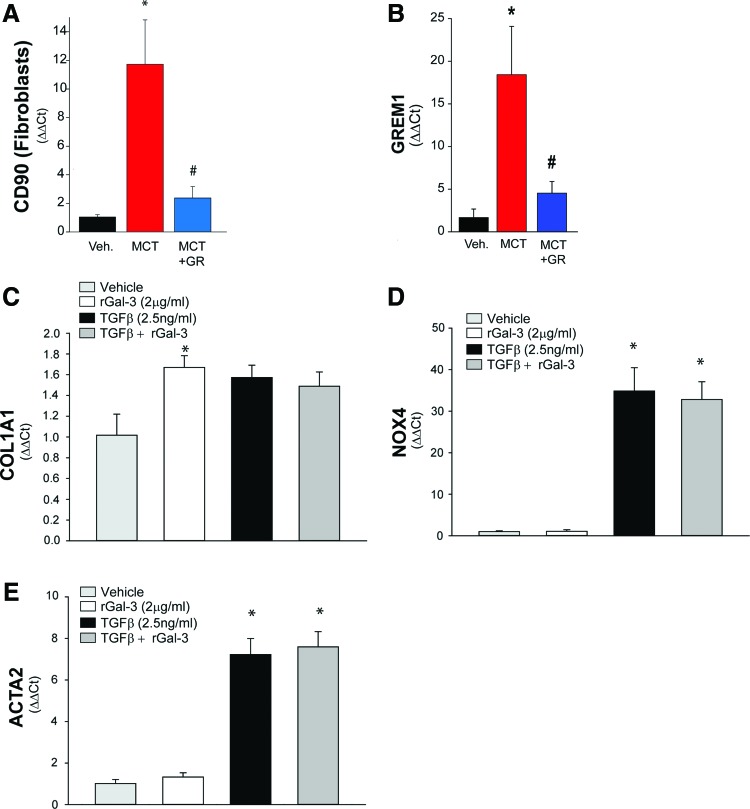
Gal-3 promotes vascular fibrosis in hypertensive PAs. The expression of profibrotic markers was determined in PAs isolated from rats treated with MCT for 4 weeks. Relative expression of (A) CD90 (Thy1, fibroblast marker) and (B) GREM1 mRNA was determined in PAs isolated from control, MCT-treated, and MCT-treated with fibroblasts. In (C), recombinant Gal-3 (2 μg/mL) increased collagen expression in fibroblasts but did not modify the ability of TGF-β1 (2.5 ng/mL). In (D), recombinant Gal-3 did not increase NOX4 expression or alter the ability of TGF-β1 to robustly increase NOX4 expression. In (E), recombinant Gal-3 did not increase the expression or smooth muscle actin or alter the ability of TGF-β1 to robustly increase expression. n = 3–4, *p < 0.05 versus vehicle, #p < 0.05 versus MCT. TGF-β1, transforming growth factor beta 1. Color images are available online.
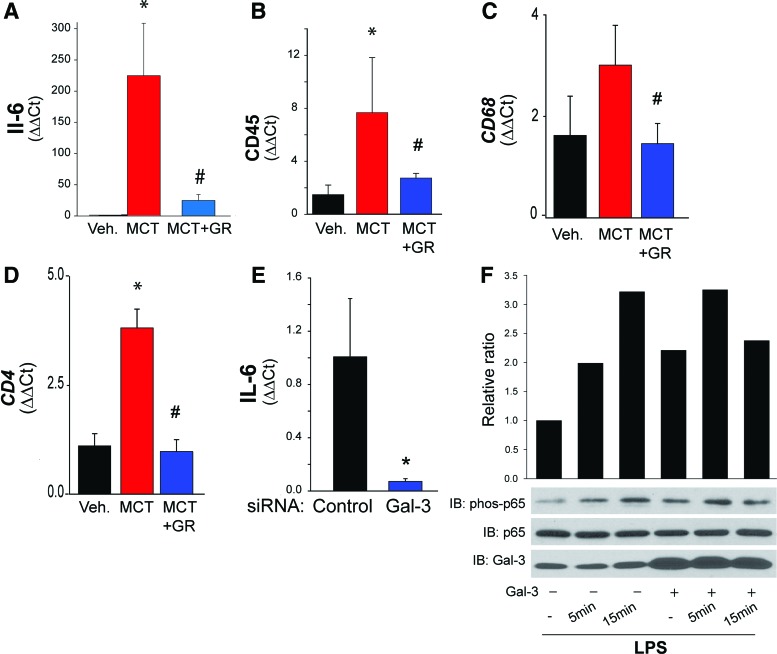
Pulmonary hypertension is accompanied by increased vascular inflammation (55, 151, 163) and recruitment of inflammatory cells (42). As discussed above, Gal-3 is intimately involved in the function of immune cells. To assess the role of Gal-3 in regulating vascular inflammation, we measured the expression level of inflammatory markers in isolated PAs from control, MCT-treated, and MCT plus Gal-3 inhibitor-treated rats. We found that MCT-induced pulmonary hypertension was associated with increased expression of IL-6 (proinflammatory cytokine), CD45 (pan leukocyte marker), CD68 (monocytic cell marker), and CD4 (T cell marker) in isolated PAs, and the inhibition of Gal-3 significantly attenuated MCT-induced vascular inflammation (Fig. 4A–D). Silencing Gal-3 resulted in reduced expression of the proinflammatory cytokine, IL-6 (Fig. 4E).
FIG. 4.
Gal-3 promotes inflammation in hypertensive PAs. The expression of proinflammatory genes was determined in PAs isolated from rats treated with MCT for 4 weeks. Relative expression of (A) IL-6 mRNA, (B) CD45 mRNA (C) CD68 mRNA, and (D) CD4 mRNA was determined in PAs isolated from control, MCT-treated, and MCT-treated with the Gal-3 inhibitor GR by real-time PCR. In (E), silencing Gal-3 in HPASMCs reduced IL-6 mRNA expression. In (F), Gal-3 regulates the NF-κB activity. HPASMCs were pretreated with recombinant Gal-3 (10 μg/mL) and then exposed to vehicle or LPS and time-dependent changes in the levels of phosphorylated p65, total p65, and Gal-3 determined by Western blot. n = 2–4, *p < 0.05 versus vehicle/control, #p < 0.05 versus MCT. HPASMCs, human pulmonary arterial vascular smooth muscle cells; IL, interleukin; LPS, lipopolysaccharide; NFκB, nuclear factor kappa light chain enhancer of activated B cells. Color images are available online.
To determine the mechanism by which Gal-3 impacts vascular inflammation, we treated human PASMC with LPS with and without recombinant Gal-3. LPS induced the phosphorylation of p65, a transcription factor that orchestrates many aspects of inflammatory signaling. In cells pretreated with recombinant Gal-3, the phosphorylation of p65 was increased in unstimulated cells, suggesting that priming and the response to LPS were enhanced (Fig. 4F).
Fibrosis contributes to the stiffening and compromised function of organs and blood vessels. Pulmonary hypertension is accompanied by increased PA stiffness (47, 173), increased deposition of matrix (160), and increased numbers of vascular fibroblasts (88). Gal-3 is a potent regulator of fibrosis and has been identified as a contributing factor to idiopathic pulmonary fibrosis (116), liver fibrosis (162), renal fibrosis (59), cardiac fibrosis (61), and vascular fibrosis (18).
To investigate a possible pathogenic role of Gal-3 in regulating vascular fibrosis in a model of PAH, we measured the indices of fibrosis in PAs from control, MCT-treated, and MCT plus a Gal-3 inhibitor-treated rats. We found that MCT-induced PAH resulted in increased expression of CD90 (a marker of fibroblasts) and Grem1 (a marker of fibrosis). Pretreatment with the Gal-3 inhibitor significantly reduced the markers of vascular fibrosis (Fig. 5A, B). In isolated lung fibroblasts, recombinant Gal-3 and TGF-β increased collagen expression. However, there was no significant interaction between Gal-3 and the actions of TGF-β (Fig. 5C). Recombinant Gal-3 failed to increase the expression of fibroblast Nox4 and ACTA2 (a marker of myofibroblasts), which were robustly increased by TGF-β. These data suggest that Gal-3 contributes to the vascular fibrosis seen in hypertensive PAs, but that its actions on fibroblasts are distinct from those of TGF-β.
Gal-3 is expressed to varying degrees in a number of cell types, and as discussed above, Gal-3 can have a variety of effects, depending on the cell type involved. Our group has shown that the majority of Gal-3 protein expression was found in the smooth muscle-rich media area of PAs where it regulates proliferation (9). Others have shown that Gal-3 is expressed in perivascular fibroblasts (94) in models of pulmonary hypertension. However, given prominent roles of macrophage Gal-3 in atherosclerosis (5, 97, 110) and fibrosis (116), it would not be surprising if this cell type also played a significant role as a source of Gal-3 in either the PA or the RV. The relative ability of Gal-3 to influence ROS, inflammation, and fibrosis (as shown in Figs. 3–5) may depend heavily on the cell type expressing Gal-3. Function delineation of the various autocrine versus paracrine actions of Gal-3 in different cell types awaits further investigation.
Summary and Clinical Perspectives
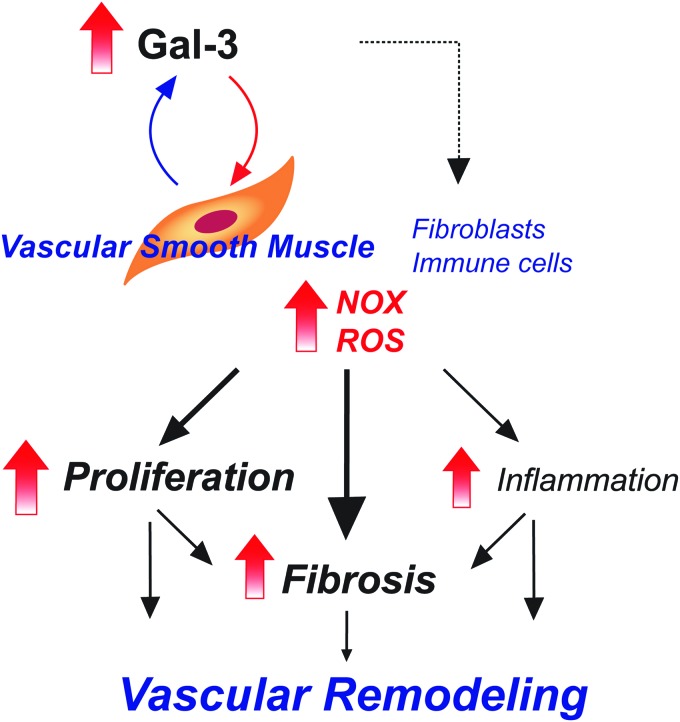
Studies thus far support the premise that Gal-3 expression is increased in both rodent and human PAH. Circulating levels of Gal-3 likely derive from increased expression in the right ventricle, but increases in expression in isolated PAs suggest local effects of Gal-3 to promote PA remodeling through changes in cell proliferation, increased ROS, inflammation, and fibrosis (Fig. 6). Gal-3 is expressed in many cell types and influences a variety of mechanisms to alter cell function. The sum of these actions contributes to the changes in cellularity and altered vascular and RV function seen in PAH.
FIG. 6.
Summary of the proposed mechanisms by which Gal-3 promotes vascular remodeling in PA to induce pulmonary hypertension. Gal-3 increases cell proliferation, inflammation, and fibrosis (matrix deposition) via paracrine and autocrine actions on different cell types. Color images are available online.
Given that PAH is a complex disease originating from diverse mechanisms in multiple cell types, it suggests that targeting Gal-3 may be a useful therapeutic approach. Recent studies suggest that Gal-3 serves as a circulating biomarker in humans that tracks PAH severity and progression. The ability of Gal-3 inhibitors to impact multiple pathways (i.e., silver shotgun as opposed to silver bullet) may be advantageous in the approach to treating complex diseases such as PAH and may also have utility in combinatorial strategies that have significantly greater potential to delay the progression of pulmonary vascular disease (147). Most of the therapies targeting Gal-3 bind in the extracellular space and whether small molecules that can suppress the actions of both extracellular and intracellular Gal-3 are more efficacious in disease states awaits further development.
Materials and Methods
Cell culture and reagents
HPASMCs were obtained from Lonza and grown in smooth muscle growth media and supplemented with fetal bovine serum to 5% final concentration (Lonza). HPASMCs stained positive for smooth muscle α-actin and negative for von Willebrand (factor VIII) as indicated by the supplier. All chemicals were purchased from Sigma, unless indicated otherwise.
Rat model of PH
The MCT model results from a single i.p. injection of MCT (60/mg/kg), which induces a severe PH 4 weeks after exposure. Adult, age-matched male SDR (250–300 g) were used as controls for both rat models of PH. The Animal Care and Use Committee at the Augusta University approved all procedures and protocols, and this study conformed to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85–23, revised 1996). All groups of rats were housed under temperature-controlled conditions (21–23°C), maintained on standard rat chow, allowed free access to food and water, and exposed to a 12:12-h light–dark cycle. Gal-3 inhibitor (Galectin Therapeutics, courtesy of Peter Traber) was administered to MCT-treated rats: (GR-MD-02; 60 mg/kg i.v.) was delivered twice weekly for 4 weeks starting at the time of the single MCT injection in SDR.
Analysis of gene expression
PAs (down to fourth order) were dissected from surrounding parenchyma, snap frozen in liquid nitrogen, pulverized, and RNA extracted using TRIZOL or proteins solubilized in 2 × Laemmli buffer. cDNA was synthesized from total RNA using the iScript cDNA Synthesis Kit (Bio-Rad) and used to assess relative PA gene expression using real-time reverse transcription polymerase chain reaction ( iQ SYBR Green; Bio-Rad). Proteins were size fractionated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and expression determined by Western blotting.
Measurement of ROS
Peritoneal macrophages were seeded into 96-well plates and transduced with Gal-3 adenovirus (100 multiplicity of infection) or treated with recombinant Gal-3 (10 μg/mL). Twenty-four hours later, the media was changed from RPMI to phenol-free Dulbecco's modified Eagle's medium (Sigma) containing L-012 (400 μM, Wako) and incubated for 30 min before the addition of agonists. Luminescence was quantified over time using a Lumistar Galaxy (BMG) luminometer. The specificity of L-012 for ROS was confirmed by co-incubation with the superoxide scavenger, SOD (100 U/mL).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics and GraphPad InStat. Data sets were assessed for normal distribution, reported as mean ± standard error of the mean, and statistical significance was determined either by the unpaired t-test (for two groups) or by two-way analysis of variance (ANOVA) (for more than three groups). In data sets analyzed by ANOVA, the Bonferroni post hoc test was employed. A value of p < 0.05 was considered statistically significant.
Abbreviations Used
- ANOVA
analysis of variance
- CRD
carbohydrate recognition domain
- EGFR
epidermal growth factor receptor
- Gal-3 (or LGALS3, or Mac-2)
galectin-3
- H2O2
hydogen peroxide
- HPASMCs
human pulmonary arterial vascular smooth muscle cells
- IL
interleukin
- KO
knockout
- LMP
lysosome membrane permeabilization
- LPS
lipopolysaccharide
- LV
left ventricle
- MCT
monocrotaline
- mPAP
mean resting pulmonary artery pressure
- NFκB
nuclear factor kappa light chain enhancer of activated B cells
- NOX
NADPH oxidase
- O2−
superoxide
- PAs
pulmonary arteries
- PAH
pulmonary arterial hypertension
- PASMCs
pulmonary arterial smooth muscle cells
- PCR
polymerase chain reaction
- PDGF
platelet-derived growth factor
- PH
pulmonary hypertension
- ROS
reactive oxygen species
- RV
right ventricle
- RVSP
right ventricle systolic pressure
- SDR
Sprague-Dawley rats
- TGF-β
transforming growth factor beta
- TRIM
tripartite motif
- VEGF
vascular endothelial growth factor
- WT
wild-type
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, and Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 109: 227–233, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Agoston-Coldea L, Lupu S, Petrovai D, Mocan T, and Mousseaux E. Correlations between echocardiographic parameters of right ventricular dysfunction and Galectin-3 in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Med Ultrason 17: 487–495, 2015 [DOI] [PubMed] [Google Scholar]
- 3. Aits S, Kricker J, Liu B, Ellegaard AM, Hamalisto S, Tvingsholm S, Corcelle-Termeau E, Hogh S, Farkas T, Holm Jonassen A, Gromova I, Mortensen M, and Jaattela M. Sensitive detection of lysosomal membrane permeabilization by lysosomal galectin puncta assay. Autophagy 11: 1408–1424, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akahani S, Nangia-Makker P, Inohara H, Kim HR, and Raz A. Galectin-3: a novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res 57: 5272–5276, 1997 [PubMed] [Google Scholar]
- 5. Arar C, Gaudin JC, Capron L, and Legrand A. Galectin-3 gene (LGALS3) expression in experimental atherosclerosis and cultured smooth muscle cells. FEBS Lett 430: 307–311, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Balan V, Nangia-Makker P, Jung YS, Wang Y, and Raz A. Galectin-3: a novel substrate for c-Abl kinase. Biochim Biophys Acta 1803: 1198–1205, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Balan V, Nangia-Makker P, Kho DH, Wang Y, and Raz A. Tyrosine-phosphorylated galectin-3 protein is resistant to prostate-specific antigen (PSA) cleavage. J Biol Chem 287: 5192–5198, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Banfer S, Schneider D, Dewes J, Strauss MT, Freibert SA, Heimerl T, Maier UG, Elsasser HP, Jungmann R, and Jacob R. Molecular mechanism to recruit galectin-3 into multivesicular bodies for polarized exosomal secretion. Proc Natl Acad Sci U S A 115: E4396–e4405, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barman SA, Chen F, Li X, Haigh S, Stepp DW, Kondrikov D, Mahboubi K, Bordan Z, Traber P, Su Y, and Fulton DJR. Galectin-3 promotes vascular remodeling and contributes to pulmonary hypertension. Am J Respir Crit Care Med 197: 1488–1492, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barman SA, Chen F, Su Y, Dimitropoulou C, Wang Y, Catravas JD, Han W, Orfi L, Szantai-Kis C, Keri G, Szabadkai I, Barabutis N, Rafikova O, Rafikov R, Black SM, Jonigk D, Giannis A, Asmis R, Stepp DW, Ramesh G, and Fulton DJ. NADPH oxidase 4 is expressed in pulmonary artery adventitia and contributes to hypertensive vascular remodeling. Arterioscler Thromb Vasc Biol 34: 1704–1715, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Battegay EJ, Raines EW, Seifert RA, Bowen-Pope DF, and Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell 63: 515–524, 1990 [DOI] [PubMed] [Google Scholar]
- 12. Beltrami M, Ruocco G, Dastidar AG, Franci B, Lucani B, Aloia E, Nuti R, and Palazzuoli A. Additional value of Galectin-3 to BNP in acute heart failure patients with preserved ejection fraction. Clin Chim Acta 457: 99–105, 2016 [DOI] [PubMed] [Google Scholar]
- 13. Bennett GA. and Smith FJ. Pulmonary hypertension in rats living under compressed air conditions. J Exp Med 59: 181–193, 1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, and McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 142: 448–456, 2012 [DOI] [PubMed] [Google Scholar]
- 15. Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, and McGoon MD. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 122: 164–172, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, and Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med 169: 764–769, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Calvier L, Legchenko E, Grimm L, Sallmon H, Hatch A, Plouffe BD, Schroeder C, Bauersachs J, Murthy SK, and Hansmann G. Galectin-3 and aldosterone as potential tandem biomarkers in pulmonary arterial hypertension. Heart 102: 390–396, 2016 [DOI] [PubMed] [Google Scholar]
- 18. Calvier L, Miana M, Reboul P, Cachofeiro V, Martinez-Martinez E, de Boer RA, Poirier F, Lacolley P, Zannad F, Rossignol P, and Lopez-Andres N. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol 33: 67–75, 2013 [DOI] [PubMed] [Google Scholar]
- 19. Chauhan S, Kumar S, Jain A, Ponpuak M, Mudd MH, Kimura T, Choi SW, Peters R, Mandell M, Bruun JA, Johansen T, and Deretic V. TRIMs and galectins globally cooperate and TRIM16 and galectin-3 Co-direct autophagy in endomembrane damage homeostasis. Dev Cell 39: 13–27, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen F, Haigh S, Barman S, and Fulton DJ. From form to function: the role of Nox4 in the cardiovascular system. Front Physiol 3: 412, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cherayil BJ, Weiner SJ, and Pillai S. The Mac-2 antigen is a galactose-specific lectin that binds IgE. J Exp Med 170: 1959–1972, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Collins PM, Bum-Erdene K, Yu X, and Blanchard H. Galectin-3 interactions with glycosphingolipids. J Mol Biol 426: 1439–1451, 2014 [DOI] [PubMed] [Google Scholar]
- 23. Cowles EA, Moutsatsos IK, Wang JL, and Anderson RL. Expression of carbohydrate binding protein 35 in human fibroblasts: comparisons between cells with different proliferative capacities. Exp Gerontol 24: 577–585, 1989 [DOI] [PubMed] [Google Scholar]
- 24. Cracowski JL, Cracowski C, Bessard G, Pepin JL, Bessard J, Schwebel C, Stanke-Labesque F, and Pison C. Increased lipid peroxidation in patients with pulmonary hypertension. Am J Respir Crit Care Med 164: 1038–1042, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K, and Keaney JF., Jr NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation 124: 731–740, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crittenden SL, Roff CF, and Wang JL. Carbohydrate-binding protein 35: identification of the galactose-specific lectin in various tissues of mice. Mol Cell Biol 4: 1252–1259, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. da Silva AA, Teixeira TL, Teixeira SC, Machado FC, Dos Santos MA, Tomiosso TC, Tavares PCB, Brigido R, Martins FA, Silva NSL, Rodrigues CC, Roque-Barreira MC, Mortara RA, Lopes DS, Avila VMR, and da Silva CV. Galectin-3: a friend but not a foe during trypanosoma cruzi experimental infection. Front Cell Infect Microbiol 7: 463, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dagher SF, Wang JL, and Patterson RJ. Identification of galectin-3 as a factor in pre-mRNA splicing. Proc Natl Acad Sci U S A 92: 1213–1217, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dalton P, Christian HC, Redman CW, Sargent IL, and Boyd CA. Membrane trafficking of CD98 and its ligand galectin 3 in BeWo cells—implication for placental cell fusion. FEBS J 274: 2715–2727, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Delaine T, Collins P, MacKinnon A, Sharma G, Stegmayr J, Rajput VK, Mandal S, Cumpstey I, Larumbe A, Salameh BA, Kahl-Knutsson B, van Hattum H, van Scherpenzeel M, Pieters RJ, Sethi T, Schambye H, Oredsson S, Leffler H, Blanchard H, and Nilsson UJ. Galectin-3-binding glycomimetics that strongly reduce bleomycin-induced lung fibrosis and modulate intracellular glycan recognition. Chembiochem 17: 1759–1770, 2016 [DOI] [PubMed] [Google Scholar]
- 31. Di Lella S, Sundblad V, Cerliani JP, Guardia CM, Estrin DA, Vasta GR, and Rabinovich GA. When galectins recognize glycans: from biochemistry to physiology and back again. Biochemistry 50: 7842–7857, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Diaz-Alvarez L. and Ortega E. The many roles of galectin-3, a multifaceted molecule, in innate immune responses against pathogens. Mediat Inflamm 2017: 9247574, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dong S. and Hughes RC. Macrophage surface glycoproteins binding to galectin-3 (Mac-2-antigen). Glycoconj J 14: 267–274, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Dorfmuller P, Chaumais MC, Giannakouli M, Durand-Gasselin I, Raymond N, Fadel E, Mercier O, Charlotte F, Montani D, Simonneau G, Humbert M, and Perros F. Increased oxidative stress and severe arterial remodeling induced by permanent high-flow challenge in experimental pulmonary hypertension. Respir Res 12: 119, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dumic J, Dabelic S, and Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta 1760: 616–635, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Feeley EM, Pilla-Moffett DM, Zwack EE, Piro AS, Finethy R, Kolb JP, Martinez J, Brodsky IE, and Coers J. Galectin-3 directs antimicrobial guanylate binding proteins to vacuoles furnished with bacterial secretion systems. Proc Natl Acad Sci U S A 114: E1698–e1706, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fenster BE, Lasalvia L, Schroeder JD, Smyser J, Silveira LJ, Buckner JK, and Brown KK. Galectin-3 levels are associated with right ventricular functional and morphologic changes in pulmonary arterial hypertension. Heart Vessels 31: 939–946, 2016 [DOI] [PubMed] [Google Scholar]
- 38. Fermino ML, Polli CD, Toledo KA, Liu FT, Hsu DK, Roque-Barreira MC, Pereira-da-Silva G, Bernardes ES, and Halbwachs-Mecarelli L. LPS-induced galectin-3 oligomerization results in enhancement of neutrophil activation. PLoS One 6: e26004, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fort-Gallifa I, Hernandez-Aguilera A, Garcia-Heredia A, Cabre N, Luciano-Mateo F, Simo JM, Martin-Paredero V, Camps J, and Joven J. Galectin-3 in peripheral artery disease. Relationships with markers of oxidative stress and inflammation. Int J Mol Sci 18: pii: , 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Frazziano G, Champion HC, and Pagano PJ. NADPH oxidase-derived ROS and the regulation of pulmonary vessel tone. Am J Physiol Heart Circ Physiol 302: H2166–H2177, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. French B, Wang L, Ky B, Brandimarto J, Basuray A, Fang JC, Sweitzer NK, and Cappola TP. Prognostic value of galectin-3 for adverse outcomes in chronic heart failure. J Card Fail 22: 256–262, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, and Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 168: 659–669, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Frigeri LG, Zuberi RI, and Liu FT. Epsilon BP, a beta-galactoside-binding animal lectin, recognizes IgE receptor (Fc epsilon RI) and activates mast cells. Biochemistry 32: 7644–7649, 1993 [DOI] [PubMed] [Google Scholar]
- 44. Fulton DJR, Li X, Bordan Z, Haigh S, Bentley A, Chen F, and Barman SA. Reactive oxygen and nitrogen species in the development of pulmonary hypertension. Antioxidants (Basel) 6: pii: , 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fulton RM, Hutchinson EC, and Jones AM. Ventricular weight in cardiac hypertrophy. Br Heart J 14: 413–420, 1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, and Hoeper M. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37: 67–119, 2016 [DOI] [PubMed] [Google Scholar]
- 47. Gan CT, Lankhaar JW, Westerhof N, Marcus JT, Becker A, Twisk JW, Boonstra A, Postmus PE, and Vonk-Noordegraaf A. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest 132: 1906–1912, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Gao X, Liu J, Liu X, Li L, and Zheng J. Cleavage and phosphorylation: important post-translational modifications of galectin-3. Cancer Metastasis Rev 36: 367–374, 2017 [DOI] [PubMed] [Google Scholar]
- 49. Girgis RE. Predicting long-term survival in pulmonary arterial hypertension: more than just pulmonary vascular resistance. J Am Coll Cardiol 58: 2520–2521, 2011 [DOI] [PubMed] [Google Scholar]
- 50. Green DE, Murphy TC, Kang BY, Kleinhenz JM, Szyndralewiez C, Page P, Sutliff RL, and Hart CM. The Nox4 inhibitor, GKT137831, attenuates hypoxia-induced pulmonary vascular cell proliferation. Am J Respir Cell Mol Biol 47: 718–726, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guo S. and Feng Z. Galectin-3 mediates the effect of PDGF on pulmonary arterial hypertension. Int J Clin Exp Med 8: 15302–15307, 2015 [PMC free article] [PubMed] [Google Scholar]
- 52. Halimi H, Rigato A, Byrne D, Ferracci G, Sebban-Kreuzer C, ElAntak L, and Guerlesquin F. Glycan dependence of Galectin-3 self-association properties. PLoS One 9: e111836, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hao M, Li M, and Li W. Galectin-3 inhibition ameliorates hypoxia-induced pulmonary artery hypertension. Mol Med Rep 15: 160–168, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Harrison SA, Marri SR, Chalasani N, Kohli R, Aronstein W, Thompson GA, Irish W, Miles MV, Xanthakos SA, Lawitz E, Noureddin M, Schiano TD, Siddiqui M, Sanyal A, Neuschwander-Tetri BA, and Traber PG. Randomised clinical study: GR-MD-02, a galectin-3 inhibitor, vs. placebo in patients having non-alcoholic steatohepatitis with advanced fibrosis. Aliment Pharmacol Ther 44: 1183–1198, 2016 [DOI] [PubMed] [Google Scholar]
- 55. Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, and Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 54: S10–S19, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Haudek KC, Voss PG, Locascio LE, Wang JL, and Patterson RJ. A mechanism for incorporation of galectin-3 into the spliceosome through its association with U1 snRNP. Biochemistry 48: 7705–7712, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. He J, Li X, Luo H, Li T, Zhao L, Qi Q, Liu Y, and Yu Z. Galectin-3 mediates the pulmonary arterial hypertension-induced right ventricular remodeling through interacting with NADPH oxidase 4. J Am Soc Hypertens 11: 275–289.e2, 2017 [DOI] [PubMed] [Google Scholar]
- 58. Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, and Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15: 1077–1081, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Henderson NC, Mackinnon AC, Farnworth SL, Kipari T, Haslett C, Iredale JP, Liu FT, Hughes J, and Sethi T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol 172: 288–298, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, and Sethi T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci U S A 103: 5060–5065, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, Larson MG, and Levy D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol 60: 1249–1256, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Honig E, Ringer K, Dewes J, von Mach T, Kamm N, Kreitzer G, and Jacob R. Galectin-3 modulates the polarized surface delivery of beta1-integrin in epithelial cells. J Cell Sci 131: jcs213199, 2018 [DOI] [PubMed] [Google Scholar]
- 63. Hoshikawa Y, Ono S, Suzuki S, Tanita T, Chida M, Song C, Noda M, Tabata T, Voelkel NF, and Fujimura S. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. J Appl Physiol (1985) 90: 1299–1306, 2001 [DOI] [PubMed] [Google Scholar]
- 64. Houssaini A, Abid S, Mouraret N, Wan F, Rideau D, Saker M, Marcos E, Tissot CM, Dubois-Rande JL, Amsellem V, and Adnot S. Rapamycin reverses pulmonary artery smooth muscle cell proliferation in pulmonary hypertension. Am J Respir Cell Mol Biol 48: 568–577, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hsu DK, Hammes SR, Kuwabara I, Greene WC, and Liu FT. Human T lymphotropic virus-I infection of human T lymphocytes induces expression of the beta-galactoside-binding lectin, galectin-3. Am J Pathol 148: 1661–1670, 1996 [PMC free article] [PubMed] [Google Scholar]
- 66. Hsu DK, Yang RY, Pan Z, Yu L, Salomon DR, Fung-Leung WP, and Liu FT. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol 156: 1073–1083, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Huebener P, Abou-Khamis T, Zymek P, Bujak M, Ying X, Chatila K, Haudek S, Thakker G, and Frangogiannis NG. CD44 is critically involved in infarct healing by regulating the inflammatory and fibrotic response. J Immunol 180: 2625–2633, 2008 [DOI] [PubMed] [Google Scholar]
- 68. Huflejt ME, Turck CW, Lindstedt R, Barondes SH, and Leffler H. L-29, a soluble lactose-binding lectin, is phosphorylated on serine 6 and serine 12 in vivo and by casein kinase I. J Biol Chem 268: 26712–26718, 1993 [PubMed] [Google Scholar]
- 69. Hughes RC. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta 1473: 172–185, 1999 [DOI] [PubMed] [Google Scholar]
- 70. Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, and Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43: 13S–24S, 2004 [DOI] [PubMed] [Google Scholar]
- 71. Inohara H, Akahani S, Koths K, and Raz A. Interactions between galectin-3 and Mac-2-binding protein mediate cell-cell adhesion. Cancer Res 56: 4530–4534, 1996 [PubMed] [Google Scholar]
- 72. Inohara H, Akahani S, and Raz A. Galectin-3 stimulates cell proliferation. Exp Cell Res 245: 294–302, 1998 [DOI] [PubMed] [Google Scholar]
- 73. Ippel H, Miller MC, Vertesy S, Zheng Y, Canada FJ, Suylen D, Umemoto K, Romano C, Hackeng T, Tai G, Leffler H, Kopitz J, Andre S, Kubler D, Jimenez-Barbero J, Oscarson S, Gabius HJ, and Mayo KH. Intra- and intermolecular interactions of human galectin-3: assessment by full-assignment-based NMR. Glycobiology 26: 888–903, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Irodova NL, Lankin VZ, Konovalova GK, Kochetov AG, and Chazova IE. Oxidative stress in patients with primary pulmonary hypertension. Bull Exp Biol Med 133: 580–582, 2002 [DOI] [PubMed] [Google Scholar]
- 75. Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, and Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-{beta}1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol 296: L489–L499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, and Iacobelli S. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res 6: 1389–1393, 2000 [PubMed] [Google Scholar]
- 77. Jia S, Mee RP, Morford G, Agrwal N, Voss PG, Moutsatsos IK, and Wang JL. Carbohydrate-binding protein 35: molecular cloning and expression of a recombinant polypeptide with lectin activity in Escherichia coli. Gene 60: 197–204, 1987 [DOI] [PubMed] [Google Scholar]
- 78. Joo HG, Goedegebuure PS, Sadanaga N, Nagoshi M, von Bernstorff W, and Eberlein TJ. Expression and function of galectin-3, a beta-galactoside-binding protein in activated T lymphocytes. J Leukoc Biol 69: 555–564, 2001 [PubMed] [Google Scholar]
- 79. Kaltner H, Seyrek K, Heck A, Sinowatz F, and Gabius HJ. Galectin-1 and galectin-3 in fetal development of bovine respiratory and digestive tracts. Comparison of cell type-specific expression profiles and subcellular localization. Cell Tissue Res 307: 35–46, 2002 [DOI] [PubMed] [Google Scholar]
- 80. Karlsson A, Follin P, Leffler H, and Dahlgren C. Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood 91: 3430–3438, 1998 [PubMed] [Google Scholar]
- 81. Kasper M. and Hughes RC. Immunocytochemical evidence for a modulation of galectin 3 (Mac-2), a carbohydrate binding protein, in pulmonary fibrosis. J Pathol 179: 309–316, 1996 [DOI] [PubMed] [Google Scholar]
- 82. Kay JM, Harris P, and Heath D. Pulmonary hypertension produced in rats by ingestion of Crotalaria spectabilis seeds. Thorax 22: 176–179, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Knibbs RN, Agrwal N, Wang JL, and Goldstein IJ. Carbohydrate-binding protein 35. II. Analysis of the interaction of the recombinant polypeptide with saccharides. J Biol Chem 268: 14940–14947, 1993 [PubMed] [Google Scholar]
- 84. Kovacs G, Dumitrescu D, Barner A, Greiner S, Grunig E, Hager A, Kohler T, Kozlik-Feldmann R, Kruck I, Lammers AE, Mereles D, Meyer A, Meyer J, Pabst S, Seyfarth HJ, Sinning C, Sorichter S, Stahler G, Wilkens H, and Held M. Definition, clinical classification and initial diagnosis of pulmonary hypertension: updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol 272S: 11–19, 2018 [DOI] [PubMed] [Google Scholar]
- 85. Lajoie P, Partridge EA, Guay G, Goetz JG, Pawling J, Lagana A, Joshi B, Dennis JW, and Nabi IR. Plasma membrane domain organization regulates EGFR signaling in tumor cells. J Cell Biol 179: 341–356, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lee EC, Woo HJ, Korzelius CA, Steele GD, Jr, and Mercurio AM. Carbohydrate-binding protein 35 is the major cell-surface laminin-binding protein in colon carcinoma. Arch Surg 126: 1498–1502, 1991 [DOI] [PubMed] [Google Scholar]
- 87. Lepur A, Salomonsson E, Nilsson UJ, and Leffler H. Ligand induced galectin-3 protein self-association. J Biol Chem 287: 21751–21756, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li M, Riddle SR, Frid MG, El Kasmi KC, McKinsey TA, Sokol RJ, Strassheim D, Meyrick B, Yeager ME, Flockton AR, McKeon BA, Lemon DD, Horn TR, Anwar A, Barajas C, and Stenmark KR. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. J Immunol 187: 2711–2722, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Liu FT, Hsu DK, Zuberi RI, Hill PN, Shenhav A, Kuwabara I, and Chen SS. Modulation of functional properties of galectin-3 by monoclonal antibodies binding to the non-lectin domains. Biochemistry 35: 6073–6079, 1996 [DOI] [PubMed] [Google Scholar]
- 90. Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, and Henderson WR., Jr Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol 147: 1016–1028, 1995 [PMC free article] [PubMed] [Google Scholar]
- 91. Loimaranta V, Hepojoki J, Laaksoaho O, and Pulliainen AT. Galectin-3-binding protein: a multitask glycoprotein with innate immunity functions in viral and bacterial infections. J Leukoc Biol 104: 777–786, 2018 [DOI] [PubMed] [Google Scholar]
- 92. Lopez-Andres N, Rossignol P, Iraqi W, Fay R, Nuee J, Ghio S, Cleland JG, Zannad F, and Lacolley P. Association of galectin-3 and fibrosis markers with long-term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: insights from the CARE-HF (Cardiac Resynchronization in Heart Failure) trial. Eur J Heart Fail 14: 74–81, 2012 [DOI] [PubMed] [Google Scholar]
- 93. Lukyanov P, Furtak V, and Ochieng J. Galectin-3 interacts with membrane lipids and penetrates the lipid bilayer. Biochem Biophys Res Commun 338: 1031–1036, 2005 [DOI] [PubMed] [Google Scholar]
- 94. Luo H, Liu B, Zhao L, He J, Li T, Zha L, Li X, Qi Q, Liu Y, and Yu Z. Galectin-3 mediates pulmonary vascular remodeling in hypoxia-induced pulmonary arterial hypertension. J Am Soc Hypertens 11: 673–683 e3, 2017 [DOI] [PubMed] [Google Scholar]
- 95. Mackinnon A, Nicol L, Walker J, Ford P, Schambye H, Pederson A, Nilsson U, Leffler H, Thomas T, Francombe D, Simpson J, Gibbons M, and Maher TM. TD139, a novel inhaled galectin-3 inhibitor for the treatment of Idiopathic Pulmonary Fibrosis (IPF). Results from the First in (IPF) Patients Study. In: A24. IPF: clinical studies, therapeutics, and more I. A7560–A7560 [Google Scholar]
- 96. MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, Nilsson UJ, Haslett C, Forbes SJ, and Sethi T. Regulation of alternative macrophage activation by galectin-3. J Immunol 180: 2650–2658, 2008 [DOI] [PubMed] [Google Scholar]
- 97. MacKinnon AC, Liu X, Hadoke PW, Miller MR, Newby DE, and Sethi T. Inhibition of galectin-3 reduces atherosclerosis in apolipoprotein E-deficient mice. Glycobiology 23: 654–663, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Madrigal-Matute J, Lindholt JS, Fernandez-Garcia CE, Benito-Martin A, Burillo E, Zalba G, Beloqui O, Llamas-Granda P, Ortiz A, Egido J, Blanco-Colio LM, and Martin-Ventura JL. Galectin-3, a biomarker linking oxidative stress and inflammation with the clinical outcomes of patients with atherothrombosis. J Am Heart Assoc 3: pii, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Margadant C, van den Bout I, van Boxtel AL, Thijssen VL, and Sonnenberg A. Epigenetic regulation of galectin-3 expression by beta1 integrins promotes cell adhesion and migration. J Biol Chem 287: 44684–44693, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Markowska AI, Jefferies KC, and Panjwani N. Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J Biol Chem 286: 29913–29921, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mazurek JA, Horne BD, Saeed W, Sardar MR, and Zolty R. Galectin-3 levels are elevated and predictive of mortality in pulmonary hypertension. Heart Lung Circ 26: 1208–1215, 2017 [DOI] [PubMed] [Google Scholar]
- 102. Medarametla V, Festin S, Sugarragchaa C, Eng A, Naqwi A, Wiedmann T, and Zisman LS. PK10453, a nonselective platelet-derived growth factor receptor inhibitor, prevents the progression of pulmonary arterial hypertension. Pulm Circ 4: 82–102, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mehul B, Bawumia S, and Hughes RC. Cross-linking of galectin 3, a galactose-binding protein of mammalian cells, by tissue-type transglutaminase. FEBS Lett 360: 160–164, 1995 [DOI] [PubMed] [Google Scholar]
- 104. Milnor WR. Arterial impedance as ventricular afterload. Circ Res 36: 565–570, 1975 [DOI] [PubMed] [Google Scholar]
- 105. Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, and Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 101: 258–267, 2007 [DOI] [PubMed] [Google Scholar]
- 106. Morrell NW, Yang X, Upton PD, Jourdan KB, Morgan N, Sheares KK, and Trembath RC. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation 104: 790–795, 2001 [DOI] [PubMed] [Google Scholar]
- 107. Moutsatsos IK, Wade M, Schindler M, and Wang JL. Endogenous lectins from cultured cells: nuclear localization of carbohydrate-binding protein 35 in proliferating 3T3 fibroblasts. Proc Natl Acad Sci U S A 84: 6452–6456, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Muller SA, Sasaki T, Bork P, Wolpensinger B, Schulthess T, Timpl R, Engel A, and Engel J. Domain organization of Mac-2 binding protein and its oligomerization to linear and ring-like structures. J Mol Biol 291: 801–813, 1999 [DOI] [PubMed] [Google Scholar]
- 109. Nachtigal M, Al-Assaad Z, Mayer EP, Kim K, and Monsigny M. Galectin-3 expression in human atherosclerotic lesions. Am J Pathol 152: 1199–1208, 1998 [PMC free article] [PubMed] [Google Scholar]
- 110. Nachtigal M, Ghaffar A, and Mayer EP. Galectin-3 gene inactivation reduces atherosclerotic lesions and adventitial inflammation in ApoE-deficient mice. Am J Pathol 172: 247–255, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nangia-Makker P, Honjo Y, Sarvis R, Akahani S, Hogan V, Pienta KJ, and Raz A. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol 156: 899–909, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nathan C. and Ding A. Nonresolving inflammation. Cell 140: 871–882, 2010 [DOI] [PubMed] [Google Scholar]
- 113. Neidhart M, Zaucke F, von Knoch R, Jungel A, Michel BA, Gay RE, and Gay S. Galectin-3 is induced in rheumatoid arthritis synovial fibroblasts after adhesion to cartilage oligomeric matrix protein. Ann Rheum Dis 64: 419–424, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Newlaczyl AU. and Yu LG. Galectin-3—a jack-of-all-trades in cancer. Cancer Lett 313: 123–128, 2011 [DOI] [PubMed] [Google Scholar]
- 115. Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, Mitchell PO, Sutliff RL, and Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol 40: 601–609, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Nishi Y, Sano H, Kawashima T, Okada T, Kuroda T, Kikkawa K, Kawashima S, Tanabe M, Goto T, Matsuzawa Y, Matsumura R, Tomioka H, Liu FT, and Shirai K. Role of galectin-3 in human pulmonary fibrosis. Allergol Int 56: 57–65, 2007 [DOI] [PubMed] [Google Scholar]
- 117. Nisimoto Y, Jackson HM, Ogawa H, Kawahara T, and Lambeth JD. Constitutive NADPH-dependent electron transferase activity of the Nox4 dehydrogenase domain. Biochemistry 49: 2433–2442, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Nita-Lazar M, Banerjee A, Feng C, Amin MN, Frieman MB, Chen WH, Cross AS, Wang LX, and Vasta GR. Desialylation of airway epithelial cells during influenza virus infection enhances pneumococcal adhesion via galectin binding. Mol Immunol 65: 1–16, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ochieng J, Furtak V, and Lukyanov P. Extracellular functions of galectin-3. Glycoconj J 19: 527–535, 2004 [DOI] [PubMed] [Google Scholar]
- 120. Ochieng J, Green B, Evans S, James O, and Warfield P. Modulation of the biological functions of galectin-3 by matrix metalloproteinases. Biochim Biophys Acta 1379: 97–106, 1998 [DOI] [PubMed] [Google Scholar]
- 121. Otsuki S, Sawada H, Yodoya N, Shinohara T, Kato T, Ohashi H, Zhang E, Imanaka-Yoshida K, Shimpo H, Maruyama K, Komada Y, and Mitani Y. Potential contribution of phenotypically modulated smooth muscle cells and related inflammation in the development of experimental obstructive pulmonary vasculopathy in rats. PLoS One 10: e0118655, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Papaspyridonos M, McNeill E, de Bono JP, Smith A, Burnand KG, Channon KM, and Greaves DR. Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction. Arterioscler Thromb Vasc Biol 28: 433–440, 2008 [DOI] [PubMed] [Google Scholar]
- 123. Park AM, Hagiwara S, Hsu DK, Liu FT, and Yoshie O. Galectin-3 plays an important role in innate immunity to gastric infection by helicobacter pylori. Infect Immun 84: 1184–1193, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Parry EH. and Abrahams DG. The function of the heart in endomyocardial fibrosis of the right ventricle. Br Heart J 25: 619–629, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Pascua-Maestro R, Diez-Hermano S, Lillo C, Ganfornina MD, and Sanchez D. Protecting cells by protecting their vulnerable lysosomes: identification of a new mechanism for preserving lysosomal functional integrity upon oxidative stress. PLoS Genet 13: e1006603, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Perillo NL, Marcus ME, and Baum LG. Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med (Berl) 76: 402–412, 1998 [DOI] [PubMed] [Google Scholar]
- 127. Perros F, Dorfmuller P, Souza R, Durand-Gasselin I, Mussot S, Mazmanian M, Herve P, Emilie D, Simonneau G, and Humbert M. Dendritic cell recruitment in lesions of human and experimental pulmonary hypertension. Eur Respir J 29: 462–468, 2007 [DOI] [PubMed] [Google Scholar]
- 128. Piccolo E, Tinari N, Semeraro D, Traini S, Fichera I, Cumashi A, La Sorda R, Spinella F, Bagnato A, Lattanzio R, D'Egidio M, Di Risio A, Stampolidis P, Piantelli M, Natoli C, Ullrich A, and Iacobelli S. LGALS3BP, lectin galactoside-binding soluble 3 binding protein, induces vascular endothelial growth factor in human breast cancer cells and promotes angiogenesis. J Mol Med (Berl) 91: 83–94, 2013 [DOI] [PubMed] [Google Scholar]
- 129. Pricci F, Leto G, Amadio L, Iacobini C, Romeo G, Cordone S, Gradini R, Barsotti P, Liu FT, Di Mario U, and Pugliese G. Role of galectin-3 as a receptor for advanced glycosylation end products. Kidney Int Suppl 77: S31–S39, 2000 [DOI] [PubMed] [Google Scholar]
- 130. Pullamsetti SS, Savai R, Seeger W, and Goncharova EA. Translational advances in the field of pulmonary hypertension. From cancer biology to new pulmonary arterial hypertension therapeutics. Targeting cell growth and proliferation signaling hubs. Am J Respir Crit Care Med 195: 425–437, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Rabinovitch M. Pathobiology of pulmonary hypertension. Annu Rev Pathol 2: 369–399, 2007 [DOI] [PubMed] [Google Scholar]
- 132. Ramasamy S, Duraisamy S, Barbashov S, Kawano T, Kharbanda S, and Kufe D. The MUC1 and galectin-3 oncoproteins function in a microRNA-dependent regulatory loop. Mol Cell 27: 992–1004, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Reis GS, Augusto VS, Silveira AP, Jordao AA, Jr, Baddini-Martinez J, Poli Neto O, Rodrigues AJ, and Evora PR. Oxidative-stress biomarkers in patients with pulmonary hypertension. Pulm Circ 3: 856–861, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Roff CF. and Wang JL. Endogenous lectins from cultured cells. Isolation and characterization of carbohydrate-binding proteins from 3T3 fibroblasts. J Biol Chem 258: 10657–10663, 1983 [PubMed] [Google Scholar]
- 135. Rossez Y, Coddeville B, Elass E, Quinchon JF, Vidal O, Corfield AP, Gosset P, Lacroix JM, Michalski JC, and Robbe-Masselot C. Interaction between DMBT1 and galectin 3 is modulated by the structure of the oligosaccharides carried by DMBT1. Biochimie 93: 593–603, 2011 [DOI] [PubMed] [Google Scholar]
- 136. Runo JR. and Loyd JE. Primary pulmonary hypertension. Lancet 361: 1533–1544, 2003 [DOI] [PubMed] [Google Scholar]
- 137. Sano H, Hsu DK, Apgar JR, Yu L, Sharma BB, Kuwabara I, Izui S, and Liu FT. Critical role of galectin-3 in phagocytosis by macrophages. J Clin Invest 112: 389–397, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sano H, Hsu DK, Yu L, Apgar JR, Kuwabara I, Yamanaka T, Hirashima M, and Liu FT. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J Immunol 165: 2156–2164, 2000 [DOI] [PubMed] [Google Scholar]
- 139. Sato S, Burdett I, and Hughes RC. Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: a pathway independent of the endoplasmic reticulum-Golgi complex. Exp Cell Res 207: 8–18, 1993 [DOI] [PubMed] [Google Scholar]
- 140. Sato S, Ouellet N, Pelletier I, Simard M, Rancourt A, and Bergeron MG. Role of galectin-3 as an adhesion molecule for neutrophil extravasation during streptococcal pneumonia. J Immunol 168: 1813–1822, 2002 [DOI] [PubMed] [Google Scholar]
- 141. Schafer M, Myers C, Brown RD, Frid MG, Tan W, Hunter K, and Stenmark KR. Pulmonary arterial stiffness: toward a new paradigm in pulmonary arterial hypertension pathophysiology and assessment. Curr Hypertens Rep 18: 4, 2016 [DOI] [PubMed] [Google Scholar]
- 142. Schermuly RT, Yilmaz H, Ghofrani HA, Woyda K, Pullamsetti S, Schulz A, Gessler T, Dumitrascu R, Weissmann N, Grimminger F, and Seeger W. Inhaled iloprost reverses vascular remodeling in chronic experimental pulmonary hypertension. Am J Respir Crit Care Med 172: 358–363, 2005 [DOI] [PubMed] [Google Scholar]
- 143. Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, and Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res 110: 1217–1225, 2012 [DOI] [PubMed] [Google Scholar]
- 144. Sciacchitano S, Lavra L, Morgante A, Ulivieri A, Magi F, De Francesco GP, Bellotti C, Salehi LB, and Ricci A. Galectin-3: one molecule for an alphabet of diseases, from A to Z. Int J Mol Sci 19: pii: , 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Seczynska M. and Dikic I. Removing the waste bags: how p97 drives autophagy of lysosomes. EMBO J 36: 129–131, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, Andre S, Crijns HJ, Gabius HJ, Maessen J, and Pinto YM. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 110: 3121–3128, 2004 [DOI] [PubMed] [Google Scholar]
- 147. Sitbon O. and Gaine S. Beyond a single pathway: combination therapy in pulmonary arterial hypertension. Eur Respir Rev 25: 408–417, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Song X, Qian X, Shen M, Jiang R, Wagner MB, Ding G, Chen G, and Shen B. Protein kinase C promotes cardiac fibrosis and heart failure by modulating galectin-3 expression. Biochim Biophys Acta 1853: 513–521, 2015 [DOI] [PubMed] [Google Scholar]
- 149. Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, and Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 105: 1429–1435, 2002 [DOI] [PubMed] [Google Scholar]
- 150. Stenmark KR, Davie N, Frid M, Gerasimovskaya E, and Das M. Role of the adventitia in pulmonary vascular remodeling. Physiology (Bethesda) 21: 134–145, 2006 [DOI] [PubMed] [Google Scholar]
- 151. Stenmark KR, Fagan KA, and Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006 [DOI] [PubMed] [Google Scholar]
- 152. Stenmark KR, Meyrick B, Galie N, Mooi WJ, and McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297: L1013–L1032, 2009 [DOI] [PubMed] [Google Scholar]
- 153. Stewart SE, Menzies SA, Popa SJ, Savinykh N, Petrunkina Harrison A, Lehner PJ, and Moreau K. A genome-wide CRISPR screen reconciles the role of N-linked glycosylation in galectin-3 transport to the cell surface. J Cell Sci 130: 3234–3247, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, and Baum LG. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol 176: 778–789, 2006 [DOI] [PubMed] [Google Scholar]
- 155. Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, and Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 290: L661–L673, 2006 [DOI] [PubMed] [Google Scholar]
- 156. Sundqvist M, Welin A, Elmwall J, Osla V, Nilsson UJ, Leffler H, Bylund J, and Karlsson A. Galectin-3 type-C self-association on neutrophil surfaces; the carbohydrate recognition domain regulates cell function. J Leukoc Biol 103: 341–353, 2018 [DOI] [PubMed] [Google Scholar]
- 157. Suzuki Y, Inoue T, Yoshimaru T, and Ra C. Galectin-3 but not galectin-1 induces mast cell death by oxidative stress and mitochondrial permeability transition. Biochim Biophys Acta 1783: 924–934, 2008 [DOI] [PubMed] [Google Scholar]
- 158. Takenaka Y, Fukumori T, Yoshii T, Oka N, Inohara H, Kim HR, Bresalier RS, and Raz A. Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs. Mol Cell Biol 24: 4395–4406, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, and Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 15: 427–438, 2001 [DOI] [PubMed] [Google Scholar]
- 160. Todorovich-Hunter L, Johnson DJ, Ranger P, Keeley FW, and Rabinovitch M. Altered elastin and collagen synthesis associated with progressive pulmonary hypertension induced by monocrotaline. A biochemical and ultrastructural study. Lab Invest 58: 184–195, 1988 [PubMed] [Google Scholar]
- 161. Traber PG, Chou H, Zomer E, Hong F, Klyosov A, Fiel MI, and Friedman SL. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS One 8: e75361, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Traber PG. and Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One 8: e83481, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Tuder RM, Groves B, Badesch DB, and Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 144: 275–285, 1994 [PMC free article] [PubMed] [Google Scholar]
- 164. van den Brule FA, Liu FT, and Castronovo V. Transglutaminase-mediated oligomerization of galectin-3 modulates human melanoma cell interactions with laminin. Cell Adhes Commun 5: 425–435, 1998 [PubMed] [Google Scholar]
- 165. van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, Postmus PE, and Vonk-Noordegraaf A. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J 28: 1250–1257, 2007 [DOI] [PubMed] [Google Scholar]
- 166. Veith C, Kraut S, Wilhelm J, Sommer N, Quanz K, Seeger W, Brandes RP, Weissmann N, and Schroder K. NADPH oxidase 4 is not involved in hypoxia-induced pulmonary hypertension. Pulm Circ 6: 397–400, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Vlassara H, Li YM, Imani F, Wojciechowicz D, Yang Z, Liu FT, and Cerami A. Identification of galectin-3 as a high-affinity binding protein for advanced glycation end products (AGE): a new member of the AGE-receptor complex. Mol Med 1: 634–646, 1995 [PMC free article] [PubMed] [Google Scholar]
- 168. Voelkel NF. and Tuder RM. Cellular and molecular mechanisms in the pathogenesis of severe pulmonary hypertension. Eur Respir J 8: 2129–2138, 1995 [DOI] [PubMed] [Google Scholar]
- 169. Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, Naeije R, Newman J, Oudiz RJ, Provencher S, Torbicki A, Voelkel NF, and Hassoun PM. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 62: D22–D33, 2013 [DOI] [PubMed] [Google Scholar]
- 170. Waldenstrom A, Martinussen HJ, Gerdin B, and Hallgren R. Accumulation of hyaluronan and tissue edema in experimental myocardial infarction. J Clin Invest 88: 1622–1628, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Wang X, Wang Y, Zhang J, Guan X, Chen M, Li Y, and Zhang L. Galectin-3 contributes to vascular fibrosis in monocrotaline-induced pulmonary arterial hypertension rat model. J Biochem Mol Toxicol 2017. [Epub ahead of print]; DOI: 10.1002/jbt.21879 [DOI] [PubMed] [Google Scholar]
- 172. Wang Y, Balan V, Kho D, Hogan V, Nangia-Makker P, and Raz A. Galectin-3 regulates p21 stability in human prostate cancer cells. Oncogene 32: 5058–5065, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Wang Z. and Chesler NC. Pulmonary vascular wall stiffness: an important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulm Circ 1: 212–223, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Wilson DW, Segall HJ, Pan LC, Lame MW, Estep JE, and Morin D. Mechanisms and pathology of monocrotaline pulmonary toxicity. Crit Rev Toxicol 22: 307–325, 1992 [DOI] [PubMed] [Google Scholar]
- 175. Woo HJ, Lotz MM, Jung JU, and Mercurio AM. Carbohydrate-binding protein 35 (Mac-2), a laminin-binding lectin, forms functional dimers using cysteine 186. J Biol Chem 266: 18419–18422, 1991 [PubMed] [Google Scholar]
- 176. Woo HJ, Shaw LM, Messier JM, and Mercurio AM. The major non-integrin laminin binding protein of macrophages is identical to carbohydrate binding protein 35 (Mac-2). J Biol Chem 265: 7097–7099, 1990 [PubMed] [Google Scholar]
- 177. Xue J, Gao X, Fu C, Cong Z, Jiang H, Wang W, Chen T, Wei Q, and Qin C. Regulation of galectin-3-induced apoptosis of Jurkat cells by both O-glycans and N-glycans on CD45. FEBS Lett 587: 3986–3994, 2013 [DOI] [PubMed] [Google Scholar]
- 178. Yamaoka A, Kuwabara I, Frigeri LG, and Liu FT. A human lectin, galectin-3 (epsilon bp/Mac-2), stimulates superoxide production by neutrophils. J Immunol 154: 3479–3487, 1995 [PubMed] [Google Scholar]
- 179. Yan YP, Lang BT, Vemuganti R, and Dempsey RJ. Galectin-3 mediates post-ischemic tissue remodeling. Brain Res 1288: 116–124, 2009 [DOI] [PubMed] [Google Scholar]
- 180. Yang RY, Rabinovich GA, and Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med 10: e17, 2008 [DOI] [PubMed] [Google Scholar]
- 181. Yoshii T, Fukumori T, Honjo Y, Inohara H, Kim HR, and Raz A. Galectin-3 phosphorylation is required for its anti-apoptotic function and cell cycle arrest. J Biol Chem 277: 6852–6857, 2002 [DOI] [PubMed] [Google Scholar]
- 182. Yu L, Ruifrok WP, Meissner M, Bos EM, van Goor H, Sanjabi B, van der Harst P, Pitt B, Goldstein IJ, Koerts JA, van Veldhuisen DJ, Bank RA, van Gilst WH, Sillje HH, and de Boer RA. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail 6: 107–117, 2013 [DOI] [PubMed] [Google Scholar]
- 183. Yu LG. Circulating galectin-3 in the bloodstream: an emerging promoter of cancer metastasis. World J Gastrointest Oncol 2: 177–180, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Zhang S, Yang T, Xu X, Wang M, Zhong L, Yang Y, Zhai Z, Xiao F, and Wang C. Oxidative stress and nitric oxide signaling related biomarkers in patients with pulmonary hypertension: a case control study. BMC Pulm Med 15: 50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Zhao QD, Viswanadhapalli S, Williams P, Shi Q, Tan C, Yi X, Bhandari B, and Abboud HE. NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NFkappaB signaling pathways. Circulation 131: 643–655, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]