Abstract
Significance: Idiopathic pulmonary fibrosis (IPF) is a progressive age-related lung disease with a median survival of only 3 years after diagnosis. The pathogenic mechanisms behind IPF are not clearly understood, and current therapeutic approaches have not been successful in improving disease outcomes.
Recent Advances: IPF is characterized by increased production of reactive oxygen species (ROS), primarily by NADPH oxidases (NOXes) and mitochondria, as well as altered antioxidant defenses. Recent studies have identified the NOX isoform NOX4 as a key player in various important aspects of IPF pathology. In addition, mitochondrial dysfunction is thought to enhance pathological features of IPF, in part by increasing mitochondrial ROS (mtROS) production and altering cellular metabolism. Recent findings indicate reciprocal interactions between NOX enzymes and mitochondria, which affect regulation of NOX activity as well as mitochondrial function and mtROS production, and collectively promote epithelial injury and profibrotic signaling.
Critical Issues and Future Directions: The precise molecular mechanisms by which ROS from NOX or mitochondria contribute to IPF pathology are not known. This review summarizes the current knowledge with respect to the various aspects of ROS imbalance in the context of IPF and its proposed roles in disease development, with specific emphasis on the importance of inappropriate NOX activation, mitochondrial dysfunction, and the emerging evidence of NOX–mitochondria cross-talk as important drivers in IPF pathobiology.
Keywords: IPF, reactive oxygen species, aging, lung, NOX4, mitochondria
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive and irreversible lung disease of unknown etiology, and represents a specific form of chronic fibrosing interstitial pneumonia that occurs primarily in the lungs. The pathogenic mechanisms of IPF are still largely unclear, but over the last decade the paradigm of IPF pathogenesis has shifted from a generally inflammation-driven disease to an epithelial-fibroblastic one, resulting from a disrupted homeostasis of epithelial cells upon damage by various triggers (50, 75). Continuous epithelial injury results in aberrant wound healing responses, and eventually causes excessive collagen deposition in the alveolar epithelium and remodeling of the lung structure (50). IPF is generally diagnosed in the sixth decade of life, and aging is now recognized as one of the strongest risk factors for IPF (169). As the average life expectancy continues to increase worldwide, the incidence of age-related lung diseases such as IPF will also increase at a rapid pace. It is estimated that the number of people >60 years of age will increase by ∼50% between 2015 and 2050, and account for 22% of the total world population (240). As the elderly population is growing, there is a growing need to understand the underlying mechanisms of aging and age-associated phenomena and their contribution to disease pathogenesis.
Aging results in decreased resistance to multiple forms of stress and progressive loss of regenerative capacity, and thereby increases susceptibility to chronic lung diseases, including IPF, chronic obstructive pulmonary disease, and lung cancer (144). Indeed, common hallmarks of aging, including genomic instability, telomere shortening, epigenetic alterations, loss of proteostasis, dysregulated nutrient sensing, mitochondrial dysfunction, cellular senescence/apoptosis, stem cell exhaustion, and distorted intercellular communication (132), are also seen in IPF and frequently occur prematurely (4, 5, 10, 144, 147, 150, 154, 249).
Both aging and fibrotic diseases are associated with an increased oxidant burden (109, 117, 149), and lung tissues as well as breath condensates from IPF patients show increased levels of oxidative damage markers such as 8-isoprostane and carbonylated proteins (13, 126, 178). Studies in mice indicate that aging increases susceptibility to pulmonary fibrosis, likely due to decreased resistance to oxidative stress (76). The main cellular sources of reactive oxygen species (ROS) are NADPH oxidases (NOXes) and mitochondria (35), and while ROS production from both sources serves various biological roles in, for example, host defense, cell differentiation, or cellular responses to injury (207, 229), both have also been implicated in IPF pathology. For example, various lines of evidence indicate that mitochondria are dysfunctional in IPF (27), resulting in increased production of mitochondrial ROS (mtROS) (150), and that approaches to attenuate mtROS may be beneficial (254). In addition, recent studies have implicated NOX4 as an important player in the development of IPF (77), based on its ability to induce alveolar epithelial cell (AEC) death, (myo)fibroblast differentiation, and collagen deposition (88). More recently, NOX4 has also been implicated as a mediator of mitochondrial dysfunction (19), suggesting cross-talk between these two ROS-generating systems.
This review summarizes our current knowledge with respect to the presence of a redox imbalance in IPF, and the importance of ROS in the development and progression of IPF. Specifically, we highlight the importance of NOX4 and the emerging cross-talk between NOX4 and mitochondria. Finally, we discuss important gaps of knowledge and potential future approaches for development of more effective treatment strategies.
IPF—Pathology and Affected Cell Types
General pathology
IPF is the most common form of idiopathic interstitial lung diseases and is characterized by scarring of the lung tissue causing symptoms such as nonproductive cough and breathlessness. As a chronic progressive lung disease, IPF has a median survival of 2–4 years after diagnosis due to respiratory failure and hypoxemia (1). Two-thirds of IPF patients are >60 years at time of diagnosis (181), and aging is the most well-known risk factor for developing this debilitating disease. Although it is a relatively rare disease that currently affects ∼5 million people worldwide, its overall incidence increases every year probably due to the increasing number of elderly individuals (156, 158). In the United States, the incidence of IPF varies between 1.1 new cases per 100,000 person-years in 18–34 old individuals up to 19.3 in individuals >55 of age (179). Besides aging, IPF has several other risk factors, including exposure to environmental factors, for example, cigarette smoke and asbestos (151). In addition, the pathogenesis of the disease is associated with genomic instability, mitochondrial dysfunction, and altered intracellular signaling (132), all hallmarks of normal aging but also all processes compromised by environmental factors such as tobacco smoking.
Histologically, the most prominent morphological hallmark of IPF is usual interstitial pneumonia (UIP). One histologic feature of UIP is honeycombing characterized by clusters of cysts, often filled with mucus, in a subpleural location (54). An additional feature of UIP is the presence of fibroblastic foci. Fibroblastic foci contain granuloma of activated myofibroblasts producing extracellular matrix (ECM) proteins and are mainly found in dense fibrotic areas of the lungs (43). UIP is further characterized by hyperplastic type II AECs and reduced type I AECs. During IPF development, a disturbed lung homeostasis results in a profibrotic milieu, thereby affecting the survival and death of both fibroblasts and AECs. AECs become more prone to undergo apoptosis (212), while fibroblasts and myofibroblasts become more resistant against this process (203). Also, distinct alterations in immune cells are reported in IPF as the lungs of these patients exhibit chronic inflammation associated with immunosenescence (206).
Cell-specific aspects of IPF
IPF is a complex disease that involves the contribution of many different cell types. The following paragraphs summarize the specific involvement of distinct lung cell types, such as epithelial cells, fibroblasts, immune cells, and endothelial cells.
Epithelial cells
The alveolar lining of the lungs is covered by 95% type I AECs, important in facilitating gas exchange, and 5% type II AECs that are involved in surfactant production. In IPF pathogenesis, repetitive injury to the alveolar epithelium leads to abnormal re-epithelialization and repair of AECs (205). Lung tissue from IPF patients typically shows loss of integrity of the alveolar epithelium with disruption of basement membrane integrity and collapse of the alveolar structure. While type II AECs normally proliferate and differentiate into type I AECs in response to injury to promote re-epithelialization, in the context of fibrosis, AECs are more prone to undergo apoptosis, which is a prominent feature during IPF manifestation. Indeed, AEC type II cells stain positive for apoptotic markers in patients with IPF compared with controls (175). Moreover, experimental induction of epithelial cell apoptosis is sufficient to promote features of fibrosis (212), and inhibition of apoptosis attenuates bleomycin-induced pulmonary fibrosis (118, 227).
In addition to evidence for AEC type II apoptosis in IPF, numbers of type I AECs in the lung of IPF patients are reduced causing AEC type II to undergo hyperplasia, thereby replacing type I AECs and inducing ineffective re-epithelialization (95). In addition, AECs secrete a variety of profibrotic mediators, such as platelet-derived growth factor, endothelin-1, angiotensin II, connective tissue growth factor, and transforming growth factor (TGF)-β, which can induce fibroblasts to differentiate into myofibroblasts (147). Furthermore, increased epithelial senescence results in enhanced secretion of growth factors, cytokines, chemokines, and matrix metalloproteins that stimulate continuous fibroblast activity (2). In addition, these factors promote a senescence-associated secretory phenotype (SASP), which is a key event in IPF possibly leading to diminished capacity of AEC regeneration (147).
Finally, AECs have also suggested to contribute to fibroblast development in IPF due to epithelial-to-mesenchymal transition (EMT), a process in which epithelial cells lose their epithelial phenotype and acquire more mesenchymal/fibroblastic characteristics such as α-smooth muscle actin (α-SMA) and fibronectin expression. Indeed, epithelial cells isolated from IPF patients have been found to express not only epithelial but also mesenchymal markers (91). However, the overall significance of EMT to IPF development/manifestation is still not completely clear (89). Lineage tracing strategies have suggested a contribution for EMT to increased presence of fibroblasts in IPF lungs (91, 100, 238), but other studies have also disputed that epithelial cells are indeed the source of fibroblasts (192). In summary, repetitive epithelial injury and dysregulated repair are critical features in IPF development and progression as they lead to increased apoptosis, abnormal regeneration, and EMT-like features. Moreover, TGF-β, an important growth factor in IPF, can promote epithelial apoptosis as well as EMT (28), further indicating the important role of the lung epithelium in IPF.
Fibroblasts
Epithelial cells within the airways and alveoli are in proximity to and communication with mesenchymal cells such as pulmonary fibroblasts, referred to as the epithelial mesenchymal trophic unit. Consequently, epithelial damage impacts fibroblasts by promoting their differentiation into myofibroblasts, which is mediated by TGF-β1 (225). Myofibroblasts actively participate in remodeling of the lung by secreting ECM proteins into the lung alveolar structure also upon induction by TGF-β1. Various sources of myofibroblasts have been suggested to be recruited to the site of injury, including proliferation and differentiation of resident lung fibroblasts, epithelial and endothelial cells that transform into fibroblasts and transition of fibrocytes or other circulating progenitor cells (79). During normal wound repair, myofibroblasts undergo apoptosis, and clearance of the ECM occurs upon closing the wound, thus avoiding the formation of scar tissue. In IPF, however, overactivation of myofibroblasts due to persistent epithelial injury leads to aberrant wound repair (177) and increased scarring, which is characterized by the formation of fibroblastic foci. This is a major hallmark of IPF (168) and is responsible for decreased pulmonary function (107, 159). In addition, fibroblasts and myofibroblasts from IPF patients have an apoptosis-resistant phenotype, which is also associated with higher levels of senescence markers, such as p16, compared with fibroblasts from age-matched controls (5, 248). In summary, these studies suggest that fibroblasts from patients with IPF are characterized by a senescent apoptosis-resistant phenotype and are thereby actively involved in the progression of the disease by remodeling of the lung structure.
Inflammatory cells
During the last few decades, a common view has been that chronic inflammation underlies the pathogenic sequence in IPF as a main cause of lung injury and fibrogenesis. Although this “inflammatory fibrosis” hypothesis has been somewhat invalidated (61, 205), chronic inflammation is still considered a common feature of IPF, yet most likely as a secondary event instead (205). Damaged epithelial cells release a variety of chemokines and cytokines that recruit inflammatory monocytes and neutrophils to the injured site. Recruitment and activation of neutrophils to the bronchoalveolar space have been shown to correlate with disease progression (90), and are also a predictor of early mortality in IPF (106). Normally, monocytes differentiate into phagocytic macrophages that phagocytose dead cells and neutrophils. However, because of the continuous injury in IPF, neutrophils and macrophages are not eliminated quickly enough. Consequently, they can develop resistance to apoptosis, which will further exacerbate the ongoing inflammation and fibrotic cycle (28).
Macrophages can mediate antifibrotic as well as profibrotic effects depending on their phenotype. Classically activated macrophages (M1) are activated by the T-helper (TH) cell 1 cytokine interferon-γ and mainly involved in the initial inflammatory response, whereas alternatively activated macrophages (M2) are activated by TH2 cytokines, including interleukin (IL)-4 and IL-13, and involved in tissue remodeling and resolution of inflammation (243). Furthermore, studies have demonstrated that the TH1/TH2 balance is disturbed in favor of TH2 cytokines (60, 245), further contributing to a M2 phenotype. M2 polarized macrophages promote profibrotic effects through the section of profibrotic mediators, including TGF-β (14). In addition, alveolar neutrophils and macrophages undergo a respiratory burst upon phagocytosis, which in turn promotes AEC injury, thereby inducing a vicious circle (13). Taken together, studies have proven that inflammatory cells participate in the profibrotic circle by secreting profibrotic and proinflammatory cytokines, although they are not the prominent cell type involved in the development and initial manifestation of IPF.
Endothelial cells
Endothelial cells are important in maintaining the alveolar–capillary barrier for facilitating gas exchange (196). Pulmonary endothelial cells are close to the interstitium, the primary site of injury during IPF, and cover the intravascular lumen, making them vulnerable to injuries. Endothelial cells from IPF patients (134, 221), and pulmonary endothelial cells from mice with bleomycin-induced pulmonary fibrosis, display increased markers of endothelial cell injury (96) and endothelial cell apoptosis (56), which result in loss of integrity of the alveolar–capillary barrier. Moreover, endothelial progenitor cells are reduced in IPF patients (136), which impairs effective re-endothelialization and vascular repair upon injury. In addition, the damaged endothelium secretes a variety of factors that activate circulating platelets and von Willebrand factor, which are involved in angiogenesis and recruitment of inflammatory cells (122). Finally, endothelial cells have also been implicated as a source of fibroblasts through EMT during fibrogenesis (71, 174). Endothelial dysfunction in patients with IPF has also been correlated with pulmonary hypertension, a common comorbidity in IPF (127).
Taken together, AEC apoptosis and (myo)fibroblast activation are now considered the primary responsible events contributing to excessive lung remodeling in IPF, whereas the specific role of endothelial cells and inflammation is less well established and still needs to be further elucidated.
Current treatment strategies for IPF
Currently, there are only two Food and Drug Administration (FDA)-approved drugs available for patients with IPF. Both pirfenidone and nintedanib can slow down the progression of the disease (112), although they cannot reverse the existing pulmonary fibrotic injury. Pirfenidone (Esbriet®; Roche/Genentech) is thought to have antioxidative, anti-inflammatory, and antifibrotic effects, although the exact working mechanisms are not clear as confirmatory data from IPF patients are lacking (112). In vitro studies and studies on bleomycin-induced murine pulmonary fibrosis indicate that it reduces markers of oxidative stress (65, 148), decreases the secretion of proinflammatory cytokines (e.g., tumor necrosis factor-α, IL-1β, IL-6) (163), and inhibits fibroblast proliferation, myofibroblast differentiation, and TGF-β-induced collagen production (42). Nintedanib (Ofev®; Boehringer Ingelheim) is an intracellular tyrosine kinase inhibitor, which mainly inhibits the platelet-derived growth factor receptor, the fibroblast growth factor receptor, and vascular endothelial growth factor receptor as well as nonreceptor tyrosine kinases of the SRC family (112), which mediate its antifibrotic effects through its inhibitory action on fibroblasts (83).
The Disturbed Redox Balance in IPF
ROS in biology and disease
All cells in the body consume and metabolize oxygen and as a result produce ROS, which include superoxide anion (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (OH•), as well as other secondary ROS. ROS are produced as a result of aerobic metabolism within the mitochondrial electron transport chain (ETC), but can also be generated by various enzyme systems, including xanthine oxidase, lipid peroxidases, uncoupled endothelial NO synthase, cytochrome P450 enzymes, and NOXes (39). The latter group of enzymes are the only enzymes that are considered to generate ROS as their primary product, and serve essential functions as mediators of host defense against diverse pathogens, and in various other aspects of cell biology through redox-based cell signaling (15, 229). Inappropriate or uncontrolled ROS production is generally thought to contribute to disease pathology, due to overproduction of ROS by, for example, increased NOX activation or by mitochondrial dysfunction, or due to compromised metabolism of ROS by antioxidant systems (41). Such an increased oxidant burden can lead to extensive molecular damage to macromolecules, including DNA, lipids, and proteins (228), thereby causing protein dysfunction and altered proteostasis, genomic instability, or production of secondary reactive lipid-derived species (e.g., electrophiles such as malondialdehyde or 4-hydroxynonenal).
Specific to IPF, several studies reported that IPF patients have a higher oxidant burden compared with healthy controls. Indeed, ROS production is dysregulated due to enhanced NOX expression and activation, as well as mitochondrial dysfunction and increased mtROS generation (13, 67). Pulmonary inflammatory cells obtained from epithelial lining fluid (ELF) of IPF patients generate higher levels of ROS compared with those of healthy controls (31), and IPF patients have increased levels of H2O2 within their exhaled breath condensates (EBCs) (178). IPF patients also demonstrate increased circulating markers of lipid peroxidation compared with healthy nonsmokers (182), and increased levels of the lipid oxidation product 8-isoprostane within bronchoalveolar lavage fluid (BALF) and EBC (149). BALF or lung tissue from IPF patients also contains higher amounts of irreversibly oxidized proteins (e.g., carbonylation, nitration) (126, 195, 199), and epithelial cells of patients with UIP show increased levels of DNA oxidation, illustrated by 8-hydroxy-deoxyguanosine (119). In summary, IPF patients show increased markers of oxidative damage (59). However, evidence for their causal contribution to the IPF disease is less prominent, and will be discussed in the following sections.
Pulmonary antioxidant defense systems in IPF
All organs, including the lung, contain a variety of antioxidant systems to prevent inappropriate ROS production or unwanted actions of cellular ROS. These include enzymes that metabolize ROS (superoxide dismutases [SODs], catalase, peroxiredoxins [PRXs], and glutathione peroxidases), thiol reductases that reverse oxidative cysteine modifications (thioredoxin [TRX], glutaredoxin [GRX]), phase 2 detoxifying enzymes (e.g., glutathione-S-transferases [GSTs]), metal-binding proteins (transferrin, lactoferrin), and small molecular weight antioxidants (vitamins and glutathione) (41). A number of studies have demonstrated that several of these antioxidant systems are altered or impaired in IPF (Table 1).
Table 1.
Alterations in Antioxidant Status in Idiopathic Pulmonary Fibrosis Patients
| Antioxidant | Sample | Groups (n) | Change | References |
|---|---|---|---|---|
| Nonenzymatic antioxidants | ||||
| GSH | ELF | IPF (15) vs. Ctrl (19) | Decreased | (30) |
| BALF | IPF (23) vs. Ctrl (17) (smokers excluded) | Decreased | (182) | |
| Blood | IPF (11) vs. Ctrl (9) | Decreased | (231) | |
| Blood | IPF (22) vs. Ctrl (29) | Decreased | (152) | |
| Sputum/plasma | IPF (16) vs. Ctrl (15) | Decreased | (16) | |
| ELF | IPF (17) vs. Ctrl (14) | Decreased | (146) | |
| BALF | IPF (17) vs. Ctrl (14) | No change | (146) | |
| Plasma/BALF | IPF (16) vs. Ctrl (20) | No change | (138) | |
| GSSG | BALF | IPF (23) vs. Ctrl (17) (smokers excluded) | No change | (182) |
| Blood | IPF (11) vs. Ctrl (9) | No change | (231) | |
| Blood | IPF (22) vs. Ctrl (29) | Increased | (152) | |
| BALF | IPF (16) vs. Ctrl (20) | Increased | (138) | |
| Plasma | IPF (16) vs. Ctrl (20) | No change | (138) | |
| TEAC | Plasma/BALF | IPF (23) vs. Ctrl (17) (smokers excluded) | Decreased | (182) |
| Plasma | IPF (11) vs. Ctrl (9) | No change | (231) | |
| Uric acid | Plasma | IPF (11) vs. Ctrl (9) | No change | (231) |
| Plasma/BALF | IPF (16) vs. Ctrl (20) | Increased | (138) | |
| Vitamin A | Plasma/BALF | IPF (16) vs. Ctrl (20) | Increased | (138) |
| Vitamin C | Blood | IPF (11) vs. Ctrl (9) | No change | (231) |
| Plasma/BALF | IPF (16) vs. Ctrl (20) | Increased | (138) | |
| Vitamin E | Plasma/BALF | IPF (16) vs. Ctrl (20) | Increased | (138) |
| Enzymatic antioxidants | ||||
| CAT | Lung tissue | IPF (12) vs. Ctrl (10) | Decreased | (161) |
| SOD1 | Serum | IPF (25) vs. Ctrl (40) | Increased | (22) |
| SOD3 | Lung tissue | IPF (10) (fibrotic vs. nonfibrotic tissue) | Decreased | (110) |
| GRX1 | Lung tissue | IPF (160) vs. Ctrl (132) | Decreased | (7) |
| Lung tissue | IPF (5) vs. Ctrl (5) | Decreased | (172) | |
| PRXII | Lung tissue | IPF (10) vs. Ctrl (10) | Decreased | (233) |
| TRX | Lung biopsies | UIP (15) vs. Ctrl (6) | Decreased | (226) |
| Redox-sensitive transcription factor | ||||
| Nrf2 | Lung tissue | IPF (16) vs. Ctrl (20) | Increased | (138) |
| Lung tissue | IPF (7) vs. Ctrl (7) | Increased | (143) | |
| Lung tissue (epithelium) | IPF (7) vs. Ctrl (7) | Increased | (143) | |
BALF, bronchoalveolar lavage fluid; CAT, catalase; ELF, epithelial lining fluid; IPF, idiopathic pulmonary fibrosis; GRX, glutaredoxins; GSH, glutathione; GSSG, glutathione disulfide; Nrf2, nuclear factor erythroid 2-related factor 2; PRX, peroxiredoxin; SOD, superoxide dismutase; TEAC, trolox equivalent antioxidant capacity; TRX, thioredoxin; UIP, usual interstitial pneumonia.
Among the earliest lines of evidence of altered antioxidant status in IPF are findings of reduced levels of the cellular antioxidant thiol-containing tripeptide glutathione (GSH) within the ELF of the lower respiratory tract (30, 146) as well as in blood (152, 231) and sputum (16). In some cases, these decreases were accompanied by increased levels of the oxidized form of GSH, glutathione disulfide, thus illustrating an altered redox balance in the alveolar lumen of IPF patients. Of the three known isoforms of SOD, which catalyzes the dismutation of O2•− into H2O2 and O2 (108), some studies suggest that lung tissue expression of extracellular SOD (SOD3) is reduced in IPF (Table 1) (110). The importance of IPF pathology is supported by studies using SOD3 knockout mice, which were found to have greater pulmonary fibrosis in response to bleomycin compared with wild-type littermates (57) and, conversely, overexpression of SOD3 protects mice from developing pulmonary fibrosis (24). Most likely, SOD3 prevents ROS-induced ECM degradation (111). The role of SOD1 and SOD2 in the progression of IPF is less well understood. In fact, SOD1 has been reported to be increased in IPF patients (22), and SOD1 knockout mice developed less oxidative stress and were protected from asbestos-induced pulmonary fibrosis compared with wild-type littermates (73).
The expression of catalase, an important scavenger of H2O2 that is widely expressed within the alveolar epithelium as well as the inflammatory cells in the lung, was found to be attenuated by TGF-β1 (78), and is also decreased in lungs of IPF patients (161). Since catalase is capable of inhibiting H2O2-mediated activation of fibroblasts (111), such a decrease in catalase may contribute to H2O2-mediated fibroblast activation in IPF. Additional studies also indicate alterations in other redox proteins in IPF, such as TRXs, PRXs, and GRX. For example, TRX is decreased in the alveolar epithelium of patients with UIP compared with controls but is increased in the metaplastic alveolar epithelium (226). Also, PRXII is increased in the hyperplastic epithelium of IPF patients but is decreased in IPF lung tissue compared with controls (233). Moreover, it has been shown that GRX1 mRNA expression as well as enzymatic activity is decreased in patients with IPF compared with non-IPF individuals, and recent studies indicate that GRX1 activity is lost in patients with IPF primarily by oxidative inactivation (7, 172). Moreover, consistent with the role of GRX1 in reversing protein S-glutathionylation, observed decreases in GRX are accompanied with an increase in protein S-glutathionylation, and were found to correlate significantly with reduced lung function in IPF patients (7). Taken together, while several studies highlighted reduced levels of activity of antioxidant systems in IPF, such changes appear to be highly variable due to the heterogeneous nature of IPF pathology. Hence, the specific contributions of such alterations to IPF development are not always clear.
Many antioxidant defense systems are under transcriptional control by the redox-sensitive transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) (35). This factor binds to antioxidant response element in the nucleus to induce expression of, for example, heme-oxygenase 1, GST, NADP(H) quinone oxidoreductase 1, TRX, and γ-glutamylcysteine synthetase, indicating that these systems are often induced in response to oxidative stress (37). Two clinical studies have shown increased Nrf2 expression in lungs of IPF patients (138, 143) with one of them displaying increased levels in the hyperplastic alveolar epithelium, yet not fibroblastic foci, of IPF patients compared with their normal epithelium as well as that of healthy controls (143). Animal studies support the importance of Nrf2 in IPF development, since AECs isolated from Nrf2-deficient mice are more prone to oxidant-induced cell death (187) and Nrf2-deficient mice develop more fibrosis in response to bleomycin (99). It is important to recognize, however, that Nrf2 upregulation does not necessarily lead to Nrf2 activation and induction of antioxidant gene expression. Indeed, activation of Nrf2-induced antioxidant responses is declined with increasing age (220, 256), which is associated with simultaneous upregulation of negative Nrf2 regulators such as c-MYC and BACH-1 (258). Hence, upregulation of Nrf2 in the context of IPF may not necessarily lead to enhanced activation, and therefore enhance susceptibility to oxidative stress.
Taken together, these results suggest that the activation of Nrf2 is involved in fibrosis, but its upregulation alone is not enough to counteract the increased ROS associated with the pathophysiology of this disease.
Contributions of disturbed redox homeostasis to IPF pathology
The combination of increased ROS production and compromised antioxidant mechanisms in IPF would suggest that dysregulated redox processes may contribute to the pathology of IPF. However, the molecular mechanisms by which such dysregulated redox processes contribute to IPF pathology are not fully elucidated. Over the past decade, it has become widely appreciated that biological ROS production serves a broad range of physiological functions through redox-dependent signaling processes that control proliferation, migration, differentiation, or survival, by inducing specific and reversible redox-mediated post-translational modifications on redox-sensitive proteins (49, 186). Consequently, IPF pathology may be mediated by dysregulated redox processes rather than “oxidative stress” per se (230).
One commonly accepted mechanism by which increased ROS production in IPF contributes to disease pathology is by promoting AEC death (119) and the highly aberrant wound healing response after chronic repetitive injury to the lung epithelium (224). Extracellular generation of H2O2 by lung myofibroblasts may mediate additional fibrogenic effects by inducing apoptosis of adjacent lung epithelial cells (234). One mechanism of apoptotic cell death involves activation of the death receptor Fas by Fas ligand (FasL), which contributes to cell death by caspase activation (236). Myofibroblasts derived from IPF patients are capable of inducing apoptosis of AECs through Fas-dependent mechanisms, which is enhanced by oxidative modification of the Fas receptor through S-glutathionylation on cysteine residue 294 (8, 9). The increased oxidant burden also results in myofibroblast accumulation with an apoptosis-resistant phenotype (248), which is linked with impaired induction of Nrf2 and increased H2O2 production (76).
ROS play a critical role in the activation of the profibrotic cytokine TGF-β. TGF-β1 is involved in epithelial cell apoptosis, EMT, epithelial cell migration, fibroblast proliferation, and differentiation as well as myofibroblast activation (58). TGF-β is synthesized as an inactive precursor bound to latency-binding peptide (LAP) and secreted as a latent form, which can be activated by ROS through disruption of its interaction with LAP (223). In vitro studies have shown that ROS can increase the release of TGF-β from AECs (17). In turn, TGF-β increases ROS production through mitochondria and NOXes in addition to suppressing antioxidant systems (130), introducing a vicious cycle between ROS and TGF-β, further contributing to a fibrotic milieu. ROS production also contributes to chronic inflammation through the activation of nuclear factor kappa B, which in turn induces expression of various proinflammatory cytokines (111).
In summary, the dysregulated redox balance in IPF may contribute to disease progression by diverse and inter-related mechanisms, including apoptotic AECII cell death, myofibroblast activation, and inflammation (Fig. 1). To appreciate the specific roles of ROS-based mechanisms in IPF, it is important to consider the major sources of dysregulated ROS production, which may include inappropriate activation of NOX enzymes or mtROS production (39). The remainder of this review will discuss current knowledge with respect to their specific roles in IPF pathobiology.
FIG. 1.
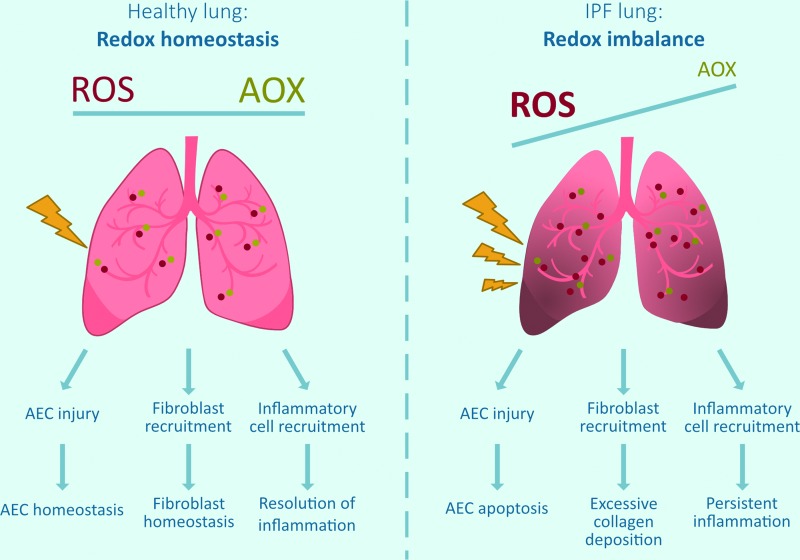
Altered lung redox homeostasis in IPF. In a healthy lung, there is a redox homeostasis, for example, ROS produced by exogenous or endogenous sources (mitochondria, NOXes, inflammatory cells) are appropriately countered by AOX. In IPF, there is a redox imbalance as ROS-generating processes are enhanced (increased NOX, mitochondrial dysfunction) and some antioxidant systems are compromised. This redox imbalance is thought to contribute to epithelial cell death, excessive collagen deposition, and persistent inflammation, resulting in pulmonary fibrosis and tissue scarring. AEC, alveolar epithelial cell; AOX, antioxidants; IPF, idiopathic pulmonary fibrosis; NOXes, NADPH oxidases; ROS, reactive oxygen species. Color images are available online.
NOX Enzymes in the Pathophysiology of IPF
The NOX family
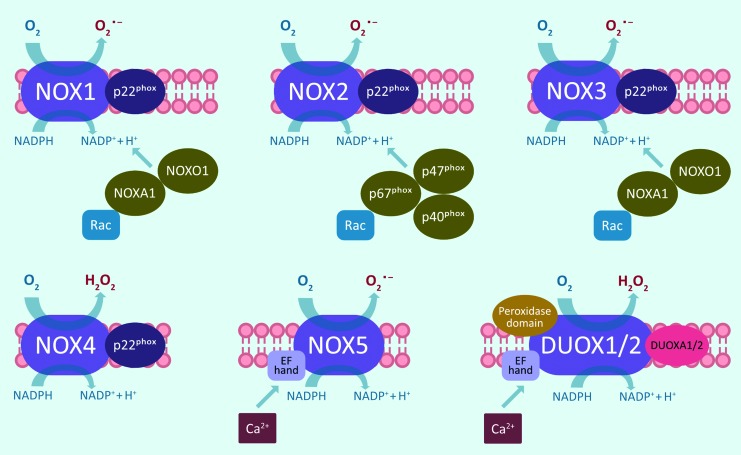
The NOX family consists of seven NOX homologs, NOX1–5 and the dual oxidases DUOX1 and 2 (Fig. 2). All NOX isoforms have six transmembrane spanning alpha helices with cytosolic N and C termini. The C-terminal flavoprotein domain contains an NADPH-binding region and a flavin adenine dinucleotide-binding region, whereas the N-terminal hydrophobic domain consists of six transmembrane alpha helices that contain two heme-binding sites (120). Through the membrane-associated flavocytochrome b558 (gp91phox) and p22phox as well as various cytosolic cofactors, active NOX enzymes promote transmembrane electron transfer from NADPH to O2, thereby reducing it to superoxide (O2•−) and H2O2 (15). Activation of NOX1–3 requires association with p22phox as well as assembly with Rac-GTPase subunits and cytosolic activation proteins, for instance p47phox and p67phox or their homologs NOX organizer 1 and NOX activator 1 (15).
FIG. 2.
Structural overview of NOX family enzymes. NOX enzymes consist of six transmembrane domains with a NADPH-binding cytoplasmic C-terminal. NOX1, NOX2, NOX3, and NOX4 share the same structure and require association with p22phox as well as other cytosolic factors. NOX5 has an N-terminal, which contains EF hand Ca2+-binding sites. DUOX1 and DUOX2 share the same structure as NOX5 but also contain an extracellular peroxidase domain and require the cofactor DUOXA1/2. DUOX, dual oxidase. Color images are available online.
NOX4 also needs association with p22phox but does not require any other cofactors for its activation, and is thought to be constitutively active (141). In contrast, NOX5, DUOX1, and DUOX2 are activated by calcium signaling and binding to their calcium-binding EF-hand domains, and do not need p22phox or other cofactors for their activation (121). DUOX1 and DUOX2 also contain an extracellular peroxidase domain, but its exact function in mammalian enzymes is still unclear (145). The NOX homologs are differentially expressed and regulated in various tissues, and have different subcellular localizations and even produce distinct ROS. NOX1–3 and NOX5 primarily produce O2•−, whereas NOX4, DUOX1, and DUOX2 mainly produce H2O2 (98), and through their production of ROS, the various NOX enzymes regulate not only host defense but also cell proliferation, differentiation, and migration by redox-dependent signaling pathways (18).
Specific roles of NOX enzymes in IPF
Recent studies indicate that expression or activation of several NOX enzymes is altered in the lungs of IPF patients, and may contribute to disease pathogenesis (75) (Fig. 3). Current evidence indicating alterations in NOX expression/activation in IPF and their functional contribution to pulmonary fibrosis in animal models are summarized in Table 2, and will be discussed in the following paragraphs.
FIG. 3.
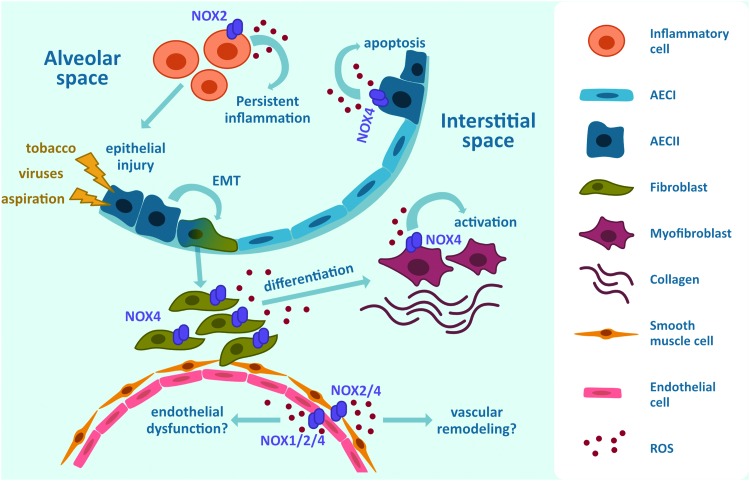
Proposed involvement of NOX enzymes in fibrotic responses. NOX-derived ROS facilitate pulmonary fibrosis by inducing apoptosis of AECII, EMT, proliferation and differentiation of fibroblasts, activation of myofibroblasts as well as proliferation of endothelial cells in response to injury to the lung epithelium. AEC, alveolar epithelial cell; EMT, epithelial-to-mesenchymal transition. Color images are available online.
Table 2.
Involvement of NADPH Oxidase Enzymes in Profibrotic Processes In Vitro, In Vivo, and in Idiopathic Pulmonary Fibrosis Patients
| NADPH oxidase | Cell/tissue type | Model | DUOX/NOX activity | Key finding | Reference |
|---|---|---|---|---|---|
| NOX1 | Human pulmonary artery endothelial cells | Radiation | Increased | NOX1 inhibition by shRNA reduces intracellular ROS and reduced phenotypic changes | (38) |
| C57BL/6 mice | Radiation | Increased | NOX1 is associated with profibrotic gene expression | (38) | |
| NOX2 | BALF mice | BLM | Increased | NOX2-deficient mice show a moderate protection from bleomycin-induced lung fibrosis | (137) |
| C57BL/6 gp91phox−/− mice | Carbon nanotubes | Increased | NOX2 deficiency is associated with the suppression of the profibrotic response, with decreased TGF-β and lower levels of collagen deposition | (210) | |
| NOX4 | Human lung fibroblasts | TGF | Increased | NOX4 regulates myofibroblast differentiation | (77) |
| Human alveolar epithelial cells | BLM | Increased | NOX4 is a key player in epithelial cell death | (32) | |
| Mouse fibroblasts | BLM | Increased | NOX4 mediates senescence and apoptosis resistance | (76) | |
| Human pulmonary smooth muscle cells | IPF patients | Increased | NOX4 is expressed in thickened pulmonary arteries | (165) | |
| Mouse fibroblasts and human fibroblasts | BLM | Increased | NOX4 is increased in senescent fibroblasts and contributes to apoptosis resistance | (76) | |
| IPF patients | |||||
| Human lung fibroblasts | IPF patients | Increased | NOX4 mediates differentiation into myofibroblasts | (6) |
BLM, bleomycin; DUOX, dual oxidase; NOX, NADPH oxidase; ROS, reactive oxygen species; TGF-β, transforming growth factor β.
NOX1
NOX1 is expressed in epithelial and endothelial cells and in smooth muscle cells (SMCs), with various described functions, but the specific contribution of NOX1 to IPF pathology is still largely unclear. Human pulmonary artery endothelial cells transfected with NOX1-targeted shRNA show decreased levels of intracellular ROS as well as reduced fibrotic markers such as α-SMA, vimentin, and CD31, and NOX inhibition using VAS2870 was found to reduce collagen deposition in mouse lungs during radiation-induced fibrosis (38), even though the latter does not necessarily implicate NOX1 since VAS2870 is a nonselective NOX inhibitor. NOX1-mediated ROS by endothelial and epithelial cells have also been implicated in the induction of cell death in response to acute lung injury (33).
NOX2
NOX2 has been studied primarily in the context of phagocytic cells as a critical component of the innate immune response (157). Although the exact role of inflammation in the development in IPF is not completely clear, studies on patients with IPF have shown that ROS production from alveolar macrophages and neutrophils contributes to AEC death (31, 216), which could be regulated through NOX2 activity. Neutrophils isolated from BALF of IPF patients have higher p47phox and p67phox expression compared with healthy controls, suggesting a specific role for NOX2 in alveolar neutrophils (235). Studies have shown that NOX2-deficient mice are protected from bleomycin (137) or carbon nanotube (210)-induced pulmonary fibrosis, but this may also involve nonimmune cells that express NOX2. However, translation to IPF patients is rather difficult since initiation of fibrosis is driven through inflammation in these animal models, which differs from the initiation process in patients where inflammation is seen as a secondary event instead (205). Conversely, it has been shown that NOX2 is important in the resolution of inflammation (211). NOX2 is also expressed in endothelial cells promoting endothelial proliferation (173); however, the specific role of NOX2 in the development of IPF still has to be elucidated.
NOX4
Among the seven members of the NOX family, NOX4 has been most commonly implicated in a variety of fibrotic diseases, including the liver, skin, kidney, heart, and lung. NOX4 is the only isoform that is highly upregulated in the lungs of IPF patients, mainly within epithelial cells (32) and (myo)fibroblasts (6), and is involved in several profibrotic processes.
NOX4 has been shown to contribute to cell death in hyperplastic type II AECs (32), and NOX4-deficient mice demonstrate significant less bleomycin-induced pulmonary fibrosis and associated AEC death (32), indicating that NOX4 contributes at least partially by inducing epithelial cell death (32, 77). As discussed above, EMT has been implicated as a feature of IPF, and the process of EMT may involve ROS-mediated mechanisms (29, 89, 92, 139). Studies on cancer cells indicate that EMT is largely driven by NOX4 (23, 80). NOX4 is highly expressed in pulmonary fibroblasts isolated from patients with IPF, and mediates fibroblast differentiation into myofibroblasts (6) and is also involved in the TGF-β1-induced activation of myofibroblasts and collagen deposition (77). IPF lung fibroblasts display a senescent phenotype, and recent studies indicate that bleomycin-induced pulmonary fibrosis in aged mice (18 months) is characterized by the accumulation of senescent myofibroblasts, which involves a role for NOX4-induced ROS (76). Furthermore, NOX4 expression can be regulated through histone modifications (200), which may be linked to the upregulation of NOX4 in senescent fibroblasts.
Patients with IPF often acquire pulmonary hypertension, which could also be mediated by increased activity of NOX4, and NOX4 is expressed in thickened arteries of IPF patients (165). Indeed, SMCs that are important in the regulation of pulmonary perfusion are activated by TGF-β1 to induce NOX4 via a SMAD2/3-dependent pathway, leading to increased SMC proliferation (217). Endothelial cells lining pulmonary vessels also express more NOX4 at sites of angiogenesis within fibrotic regions and adjacent to fibrotic foci (87). TGF-β has been implicated in endothelial cell death and linked to generation of NOX4-dependent ROS, although overexpression of NOX4 was protective against TGF-induced endothelial cell apoptosis (247), indicating that the precise role of NOX4 in endothelial cells still remains unclear. In spite of the widespread evidence for a role of NOX4 in IPF pathology, with specific actions in different cell types, the precise downstream mechanisms by which NOX4-derived H2O2 mediates these responses are not known.
Other NOXes
Almost nothing is known with respect to other NOX enzymes in IPF. Interestingly, the process of EMT might also involve DUOX2, based on studies in human colon cancer cells (93). Conversely, our group recently observed that a loss of lung epithelial DUOX1, commonly observed in various cancers, can promote EMT (129). However, the relevance for DUOX in IPF or other fibrotic diseases has to date not been addressed.
Mitochondrial Dysfunction as Driver in IPF
Mitochondrial dysfunction is a hallmark of age-related lung diseases (132), and has been associated with chronic lung diseases, including IPF (183). Mitochondrial dysfunction is characterized by a loss of efficiency in the ETC, resulting in an increased ROS generation in addition to a reduced membrane potential and altered mitochondrial function (160). IPF is also associated with changes in mitochondrial homeostasis, which is important for the maintenance of the redox balance, mitochondrial DNA (mtDNA) protection as well as AEC apoptosis and senescence, making cells more vulnerable to cellular stress (254).
Mitochondria in cell homeostasis
Mitochondria are double membrane-containing organelles that are typically between 0.75 and 3 μm in diameter, although they are approximately three times larger in type II AECs compared with other lung cells such as type I AECs (40, 131). Their main function is to produce cellular energy in the form of ATP through oxidative phosphorylation (OXPHOS), thereby generating >90% of the required metabolic energy in most cells (194). In addition, mitochondria play important roles in other cell signaling processes involved in, for example, differentiation or apoptosis, and appropriate mitochondrial function is tightly controlled by mitochondrial biogenesis, fission/fusion dynamics, and mitophagy (254). In response to cellular stress or injury, mitochondria can rapidly adapt their behavior by changing mitochondrial fusion (merging) and fission (division) dynamics, which will alter the ATP production in line with cellular demand (254).
During differentiation from type II to type I AECs, type II AECs reduce the number and size of mitochondria to adapt to cellular stress, which results in lower energy demanding type I AECs (40). Increased fusion promotes the formation of elongated mitochondria, and is a stress-resolving mechanism in which the fused mitochondria are protected from degradation to respond to the cells' higher energy demand to repair cellular damage (66). Conversely, increased fission or reduced fusion increases mitochondrial fragmentation, which under severe stress promotes mitochondrial autophagy, also called mitophagy, a process in which damaged mitochondria are removed to maintain mitochondrial homeostasis (166). Similarly, mitophagy also regulates mtROS production indirectly by removing dysfunctional mitochondria with high mtROS production (219). The intrinsic mitochondria-regulated cell apoptosis pathway is activated by various fibrotic stimuli, including hypoxia, oxidative stress, and DNA damage, which in turn stimulate the proapoptotic Bcl-2 family members in the mitochondria increasing mitochondrial membrane permeability, which causes the release of cytotoxic proteins into the cytosol such as cytochrome C, thereby activating caspase 3 and 9 (53, 115). These regulatory mechanisms are important for mitochondrial homeostasis, and if dysregulated mitochondrial dysfunction can occur predisposing to disease.
Mitochondrial dysfunction in IPF
Mitochondrial dysregulation of mitochondrial dynamics disrupts adaption to cellular stress, making lung cells more vulnerable to (oxidative) injury, thereby promoting pulmonary fibrosis. Recently, it has been identified that epithelial cells as well as fibroblasts of IPF patients show signatures of dysfunctional mitochondria and disturbed mitochondrial dynamics (150, 254).
Dysregulated fission and fusion dynamics
Mitochondrial biogenesis is regulated by the peroxisome proliferator-activated receptor γ coactivator (PGC)-1α and PGC-1β. PGC-1 is capable of activating nuclear respiratory factors (NRF-1 and NRF-2), which are essential for mitochondrial biogenesis (242). Of note, these nuclear respiratory factors are distinct from nuclear factor erythroid 2-related factors, which are commonly denoted as Nrf1 and Nrf2, a confusion made even worse by observations that PGC-1α can also upregulate Nrf2 (12). In addition, mitochondrial biogenesis requires the replication and synthesis of mtDNA, which is regulated by the mitochondrial transcription factor A (TFAM) (26). With aging, the capacity of mitochondrial biogenesis declines through reduction in upstream activators of PGC-1 (190), thereby slowing down mitochondrial turnover. In addition, expression of PGC-1α is reduced in IPF patients and in murine models of IPF (251, 254). AEC type II isolated from aged mice show an increase of mitochondrial fusion markers but a decrease in mitochondrial fission (27), resulting in an increased mitochondrial area. Increased fusion can suggest a need for more energy under stress conditions, thereby promoting cell survival (125).
Alterations in mitophagy
Mitophagy is essential to maintain normal cellular function by regulating the number of mitochondria and to prevent apoptosis through the removal of dysfunctional mitochondria by autophagy (11). Deficiency in mitophagy has been associated with the development of pulmonary fibrosis in response to injury (214). Selective mitophagy of damaged mitochondria occurs through the PTEN-induced putative kinase 1 (PINK1) that accumulates on defective mitochondria acting as a marker for mitochondrial damage (51). Subsequently, PINK1 activates the E3-ubiquitin ligase Parkin in the cytosol by phosphorylation, which labels the outer membrane of dysfunctional mitochondria for trafficking to the autophagosome (27). With aging and also in lungs of IPF patients, a reduction in autophagic activity, indicated by a reduced number of autophagosomes and expression of LC3 and p62, has been described (170). This decrease in mitophagy has been associated with a decrease in PINK1 expression in IPF lungs, thereby promoting the accumulation of damaged mitochondria in IPF (27). However, short-term stimulation with TGF-β1 induces PINK1 expression in BEAS-2B cells, thereby protecting epithelial cells by removal of damaged mitochondria and evading apoptosis, whereas long-term TGF-β1 stimulation (24 h) reduces PINK1 in lung fibroblasts (214). Interestingly, type II AECs from lungs of IPF patients express less PINK1, suggesting that PINK1 deficiency impairs mitochondrial function of AECs (27). Furthermore, PINK1 deficiency results in the accumulation of dysfunctional mitochondria and exacerbates bleomycin-induced pulmonary fibrosis, which is also associated with increased apoptosis (171). In fibroblasts, TGF-β1 inhibits autophagy leading to an increase in profibrotic gene expression such as α-SMA and fibronectin contributing to myofibroblast differentiation (170). Taken together, short-term exposure of TGF-β1 leads to the initial stabilization of PINK1, thereby inducing mitophagy, whereas long-term exposure results in impaired mitophagy through downregulation of various proteins involved in PINK1 activation promoting AEC apoptosis.
Mitochondria-mediated AEC death
Mitochondrial dysfunction is suggested to represent a key mechanism for epithelial cell apoptosis in pulmonary fibrosis (167, 183) as type II AECs are more susceptible to apoptosis when mitochondrial function is impaired (27). In addition, mtDNA damage is linked to oxidative injury and cellular stress driving apoptotic and senescence pathways, thereby contributing to pulmonary fibrosis (203). As mtDNA is more susceptible to oxidative damage compared with nuclear DNA due to its lack of histones and its proximity to the ETC (131), mtDNA repair is essential for mitochondrial dynamics. Experimental models of pulmonary fibrosis contain a higher amount of damaged mtDNA (85, 101), which is linked to insufficient mtDNA repair (254). Eight-oxoguanine DNA glycosylase 1 (OGG1) is an important base excision repair enzyme in mtDNA repair and deficiency promotes pulmonary fibrosis (102). Knockout of OGG1 has been shown to augment (36), whereas overexpression of mitochondria-targeted OGG1 prevents oxidant-induced AEC apoptosis (103) in asbestos-induced models of pulmonary fibrosis.
Mitochondrial damage-associated molecular patterns (mtDAMPs) have also been linked to mitochondria-mediated apoptosis (52). mtDAMPs are mitochondria-derived molecules that are important for proper cell signaling but can also behave as damage signal in response to tissue injury, thereby activating pathogen recognition receptors (40). One of the most important mtDAMPs is the release of oxidized and/or fragmented mtDNA from damaged mitochondria, which can trigger apoptosis (40) and inflammation (257). Indeed, mtDNA is increased in the lungs and blood of patients with IPF (197). In addition, it has been shown that mtDNA release can trigger TGF-β1 release from AECs in paraquat-induced pulmonary fibrosis (128) and inflammasome activation in macrophages (257). Excessive ATP release by damaged and apoptotic AECs can also act as mtDAMP, activating the purinergic receptor P2X7 (40), and is increased in bleomycin-induced pulmonary fibrosis (191).
Dysregulated mtROS production
During OXPHOS, O2 is reduced to H2O, which leads to the formation of O2•− and/or H2O2 due to electron leakage in the mitochondrial ETC, and these mtROS are thought to promote pulmonary fibrosis (86). The mitochondrial ETC comprises five large protein complexes: complex 1 (NADH-coenzyme Q oxidoreductase), complex II (succinate/coenzyme Q oxidoreductase), complex III (Q-cytochrome c oxidoreductase), complex IV (cytochrome c oxidase), and complex V (ATP synthase) (213). mtROS are generated at 11 distinct mitochondrial sites within complexes I, II, and III (239), and are the main sites of electron leakage to oxygen to produce O2•− and H2O2 (25). Complex III and mitochondrial glycerol 3-phosphate dehydrogenase generate O2•− on the cytosolic side of the mitochondrial inner membrane as well as the matrix, whereas the other sites generate O2•− and/or H2O2 exclusively in the matrix (25). Effective regulation of mtROS as well as the capacity to scavenge them by antioxidants declines with age, making the lungs more susceptible toward oxidative damage (123). Normally, mtROS production is tightly regulated, however when dysregulated they promote mitochondrial dysfunction, apoptosis, and cellular (mitochondrial) DNA damage (254).
TGF-β stimulation of fibroblasts from IPF patients results in increased mtROS compared with similar stimulation of fibroblasts from control subjects (86). Similarly, mtROS generation is also increased in bleomycin-induced pulmonary fibrosis (101). The profibrotic cytokine TGF-β1 enhances mtROS through the inhibition of mitochondrial complex IV in lung epithelial cells (250). Genetic disruption of mitochondrial complex III-generated ROS attenuates TGF-β1-induced profibrotic gene expression during myofibroblast differentiation (86), further indicating the important role of mtROS in the development of pulmonary fibrosis. Moreover, studies have suggested a key role for TGF-induced mtROS production in the induction of senescence in epithelial cells (250).
Dysregulated mtROS generation is not only a key player in profibrotic signaling but also plays a role in mtDNA damage leading to apoptosis. Persistent mtDNA damage can result in the activation of apoptosis (201). Furthermore, oxidative damage to mtDNA can lead to mutations resulting in the synthesis of defective ETC components, thereby further enhancing the production of mtROS (102). In agreement with these observations, mice that overexpress mitochondrial catalase show lower level of mtROS-induced mtDNA damage than their wild-type littermates and are protected from bleomycin-induced pulmonary fibrosis (101). Interestingly, it has been shown that mtROS can also damage nuclear DNA, which in turn causes mitochondrial dysfunction by the activation of nucleus-to-mitochondria signaling pathways (55). However, more in vivo studies are needed to elucidate the exact role of mtROS in the development of IPF. In addition to interference with normal cellular function, mitochondrial dysfunction has been implicated as a driver for EMT in cancer cells (69).
In summary, redox alterations induced by mitochondrial dysfunction contribute to IPF pathophysiology, which may also be associated with their interactions with NOX activation (86, 202), as will be discussed in the next section.
Cross-Talk Between NOX Enzymes and Mitochondria
Since most cell types express multiple NOX isoforms and all mammalian cells contain various amounts of mitochondria, it is often difficult to determine the precise cellular source of ROS, and this may in fact involve combined input from various sources. Indeed, over the past several years, various lines of evidence support the existence of cross-talk mechanisms between different NOX isoforms, and also between NOX enzymes and mitochondria, thereby potentially altering mitochondrial function or NOX activity (253). Such interactions between NOX and mitochondria are also reciprocal; that is, activation of NOX can increase mtROS production but mitochondria (via mtROS) can also contribute to activation of NOX. The significance of such NOX–mitochondria interactions and their potential relevance for IPF will be discussed in the following sections.
Stimulation of mtROS production by NOX enzymes
The first studies indicating a role for NOXes in mitochondrial dysfunction have been in the context of angiotensin II (AngII) signaling in vascular biology (241). AngII is an important hormone in the renin–angiotensin system, and induces vascular dysfunction in part by activation of vascular NOX1 and NOX2. AngII also leads to increased vascular endothelial mtROS production, which was decreased by deletion of the NOX cofactor p22phox or by preincubation with the NOX inhibitor apocynin (48, 105). In addition, AngII-induced NOX activation was associated with opening of redox-sensitive mitoKATP channels, leading to depolarization of mitochondrial membrane potential and subsequent mtROS generation (46, 48). More recent studies of vascular endothelial growth factor (VEGF)-induced angiogenesis indicated a sequential mechanism involving successive activation of NOX2, NOX4, and mtROS production (105), and VEGF-induced endothelial cell migration and proliferation could be inhibited by silencing either NOX2 or NOX4 or by mitochondrial targeting of catalase (105). Cross-talk between mitochondria and NOX has also been implicated in the development of nitrate tolerance and associated endothelial dysfunction (21, 237), which could be prevented by selective inhibition of mtROS production using rotenone or the mitochondrial pore blocker cyclosporine A, or by inhibition of NOX activity by chimeric peptide that interferes with assembly of p47phox and NOX2 (189). Taken together, these studies indicate that NOX activity increases mtROS production, although the exact mechanisms still remain to be elucidated.
Activation of NOX enzymes by mtROS
ROS production by pulmonary artery smooth muscle during acute hypoxia was found to originate from mitochondria, and subsequent NOX activation, based on inhibition of increase in NOX activity, measured by O2•− -dependent cytochrome c reduction, by the complex I inhibitor rotenone (185). A study using human leukocytes indicated that NOX2 activation depends on mtROS formation, as it was enhanced by deletion of mitochondrial SOD2 (114). Similarly, AngII-induced endothelial dysfunction, which involves NOX2, was more pronounced in SOD2 knockout mice (114). NOX activation by AngII-induced mtROS production was also demonstrated by inhibitory effects of mitochondrial-targeted antioxidant mitoTEMPO (47). Studies with the human embryonic kidney cell line 293T indicated that mtROS can also activate NOX1, under conditions of serum starvation, by promoting phosphoinositide 3-kinase and Rac1 activation, events that were inhibited by rotenone (124). Separate studies indicate that mitochondrial dysfunction (perhaps through mtROS) can also regulate NOX1 expression (241). Hence, emerging lines of evidence indicate a contribution of mtROS in activation of various NOX enzymes, including NOX1 and NOX2. More relevant to IPF, mitochondria also display cross-talk with NOX4, as will be discussed in the next section.
Cross-talk between NOX4 and mitochondria—potential role in IPF?
As mentioned earlier, NOX4 contributes to critical features of pulmonary fibrosis. Various lines of evidence indicate that NOX4 can be localized to mitochondria since NOX4 contains a 73-amino-acid long mitochondrial targeting signal in its N-terminus, allowing it to localize to the inner mitochondrial membrane (18, 68). As such, NOX4 has been reported to be expressed in the mitochondria of rat kidney cortex (20), in cardiac myocytes (116), and in cancer cells (68), and it has been suggested that mitochondrial NOX4-produced ROS are implicated in several disease pathologies through modulation of senescence, apoptosis, and carcinogenesis. Indeed, NOX4-induced mtROS production in cardiac myocytes has been implicated in cell apoptosis and cardiac hypertrophy (142). Furthermore, cardiac NOX4 contributes to cardiac failure through the generation of ROS (116), and NOX4-deficient mice have attenuated mtROS generation and mitochondrial dysfunction indicating interplay between NOX4 and mtROS in the failing heart (116). It is worth noting that inhibition of NOX4 may also lead to a more basal reduced state [based on NAD(P)+/NAD(P)H ratio], and can paradoxically enhance mtROS production under conditions of, for example, ischemia in the heart (252).
Mitochondrial activity is regulated by various extracellular and intracellular signaling pathways, including SRC kinases (162), which can be subject to NOX-dependent redox modulation (255). Conversely, the SRC family kinase member FYN was identified as a negative regulator of NOX4, by promoting NOX4 phosphorylation on tyrosine 566 (142). Studies on human endothelial cells indicate that NOX4 can also directly interact with mtROS production through inhibition of mitochondrial complex I, thereby reducing the mitochondrial respiratory capacity and contributing to mitochondrial dysfunction (113), although the specific subcellular localization of NOX4 was not determined in this study. In fact, NOX4-depleted endothelial cells showed a reduction in H2O2 production in the mitochondria as well as in the cytosol. Another study identified mitochondrial NOX4-dependent ROS production as a key mediator in apoptosis of kidney tubular cells in response to AngII (104). Most examples of connections between NOX4 and mitochondria stem from cardiovascular studies, but recent studies also highlight the presence of NOX4–mitochondria cross-talk in IPF, and suggest a role for NOX4 in mitochondrial dysfunction, associated with altered biogenesis, increased glycolysis, and increased ATP degradation (94, 254).
It was recently reported that NOX4 is able to repress mitochondrial biogenesis in lung fibroblasts through direct inhibition of Nrf2 and TFAM independent of PGC-1α (19). Indeed, inhibition of NOX4 by genetic or pharmaceutical approaches resulted in increased mitochondrial biogenesis, illustrated by increased mitochondrial-to-nuclear DNA ratio (19). Unfortunately, these studies only evaluated effects of NOX4 deletion and not of NOX4 overexpression, as it would be observed in IPF. NOX4 has been shown to influence mitochondrial biogenesis through its interaction with PDIP38, also known as DNA polymerase delta interacting protein 2, which is involved in DNA replication but is also localized to mitochondria where it associates with mitochondrial nucleotides (34, 133). This interaction could indicate that NOX4 is involved in mtDNA replication and repair via its interaction with PDIP38 or via the production of ROS, thereby modifying proteins involved in mtDNA maintenance. NOX4 can contribute to the increase of mtROS via redox signaling, thereby modulating mitochondrial functions, including the activation of PKC, mitoKATP, and TRX2 (46). Intriguingly, recent studies also implicated mitochondrial NOX4 as an energetic sensor in cancer cells (209). During normal respiration, OXPHOS-driven ATP binds NOX4 through an ATP-binding motif, thereby minimizing constitutive NOX4 activity. During transition of OXPHOS to aerobic glycolysis, as it is observed in cancer, mitochondrial ATP production is reduced and results in increased NOX4 activity (209). Metabolic alterations in IPF indicated increased ATP degradation (94), which would similarly result in enhanced NOX4 activity. Interestingly, it has been suggested that inhibition of aerobic glycolysis might be a beneficial target in IPF as well (135, 244). Administration of the mitochondria-targeted antioxidant mitoQ was found to attenuate NOX4 expression in fibroblasts from IPF patients (86), further highlighting the important role of mitochondria-produced ROS in NOX4 activation.
In summary, the various forms of cross-talk between NOX4 and mitochondria could have important consequences for IPF manifestation/progression (Fig. 4). Increased NOX4 activity in IPF promotes AEC death as well as (myo)fibroblast activation causing excessive collagen deposition (6, 32), and could be mediated by NOX4-dependent mtROS production. mtROS promotes mtDNA damage and could also directly contribute to NOX4 activation, thereby promoting a fibrotic vicious circle (86). Moreover, negative effects of NOX4 on mitochondrial biogenesis could further drive mitochondrial dysfunction. While these NOX4–mitochondria interactions may be related to localization of NOX4 in mitochondria, evidence for such localization rest primarily on detection with antibodies with unknown specificity, and it is typically not associated with costainings of p22phox, which is important for its activity. Hence, the exact role of NOX4 in mitochondria still remains unclear, and further research is needed to more firmly establish NOX4 as a mitochondrial protein, and its reciprocal interactions with mitochondrial processes.
FIG. 4.
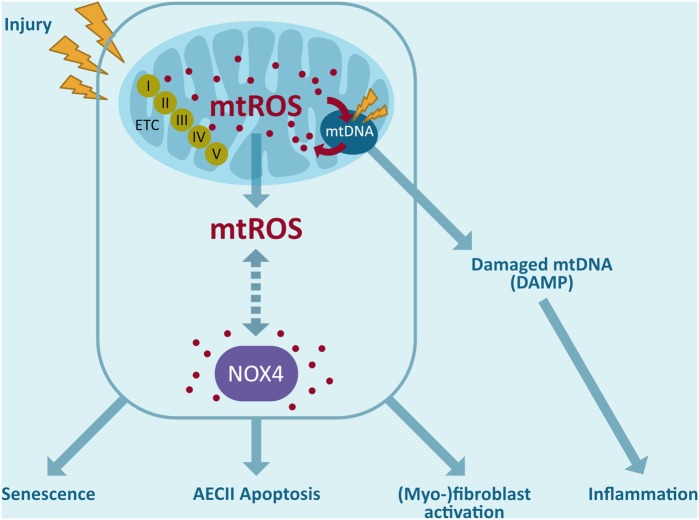
Interplay between mitochondria and NOX4. Release of ROS by mitochondria can trigger ROS production by NOX4 outside the mitochondria and possibly also within the mitochondria. mtROS leads to mtDNA damage, which can contribute to mitochondrial dysfunction leading to AEC death. In addition, mtDNA damage further increases mtROS. Oxidized mtDNA can also act as an mtDAMP outside the mitochondria and inducing inflammatory processes. These signaling cascades may further amplify NOX4-mediated effects, including fibroblast differentiation, myofibroblast activation, and AEC death. mtDAMP, mitochondrial damage-associated molecular pattern; mtDNA, mitochondrial DNA; mtROS, mitochondrial ROS. Color images are available online.
Redox-Modulatory Therapeutic Strategies
Based on the various lines of evidence implicating a redox imbalance in IPF, several redox-based therapeutic strategies have been proposed focusing at quenching ROS or restoring the disturbed redox balance. For example, several studies have addressed the ability of N-acetyl-cysteine (NAC), a precursor of GSH, to mitigate IPF. However, in spite of demonstrated encouraging results in various in vitro studies (218) as well as experimental fibrosis models (44, 70), NAC supplementation has failed to be fully effective in the clinic (140, 153, 193) because of variable effects in patients with IPF. These variable effects may be due to differences in dose of drug administration, delivery method as well as the patient population.
Several studies have suggested that inhaled NAC (352.4 mg, two times daily) improves lung function in early stage IPF (82, 152, 164), indicating that local antioxidant therapy might be more beneficial in an early stage of IPF to counteract ROS-induced damage and the underlying inflammation. Oral administration of NAC (600 mg, three times daily), on the contrary, had variable outcomes. In the PANTHER trial, no significant improvement of lung function could be observed in mild-to-moderate IPF compared with the placebo (84), whereas it was reported before in the IFIGENIA trial that the same dose of NAC in combination with prednisone and azathioprine reduced decline of lung function (45). In advanced IPF, NAC monotherapy showed no beneficial effect; however, combination therapy has been suggested to improve lung function (198). Consequently, it is important to investigate specific subgroups in IPF who could benefit from NAC as monotherapy or in combination therapy that has been suggested to be more beneficial in an advanced stage.
Other studies have explored the ability of antioxidant food supplements, such as quercetin and resveratrol, to reduce oxidative stress in profibrotic responses in vitro (62, 64, 155, 231) and in animal models of pulmonary fibrosis (3, 74, 208, 232). While these compounds have reported antioxidant activity in vitro, they might act by alternative mechanisms and are also capable of activating Nrf2 (204). Intriguingly, a recent study described that quercetin reverses bleomycin-induced pulmonary fibrosis in aged mice through the reduction of various senescence markers and SASP (81). Furthermore, transcriptome analysis of pathways involved in senescence and drugs that interfere with these pathways yielded dasatinib (a SRC/ABL protein kinase inhibitor) and quercetin (a putative antioxidant but also an inhibitor of various kinases) as potent senolytic drugs (259). Indeed, the combination of dasatinib with quercetin (D + Q) was found to kill senescent fibroblasts in a mouse model of bleomycin-induced pulmonary fibrosis, and thereby improve lung function (203). While these findings do not directly indicate antioxidant-based mechanisms, such a mechanism could contribute to the observed inhibitory effects, especially in combination with other antifibrotic drugs. The combination of D + Q is currently investigated in a clinical trial (NCT02874989) to determine the effects on proinflammatory cells in IPF.
In addition to approaches using general scavengers of ROS, alternative strategies have focused on specifically modulating the activity of redox systems. For example, based on observed loss of GRX1 activity in IPF, and corresponding increases in S-glutathionylation of Fas and caspase activation, administration of recombinant GRX1 has been explored as a therapeutic strategy, and preclinical studies on mice indicate the ability of GRX1 to inhibit and even promote reversal of pulmonary fibrosis in experimental models in mice (7).
Based on the diverse and specific actions of ROS produced by specific NOX enzymes or by mitochondria, as discussed in this review, it would seem more fruitful to specifically target (enzymatic) sources of ROS that are dysregulated in IPF, rather than more generic approaches to neutralize ROS or affect redox systems, as these latter could also interfere with normal physiological functions of ROS, and thus exert unwanted effects (63, 97). Indeed, recent approaches have focused primarily on the specific roles of NOX enzymes and mitochondria in ROS production (39). Pharmacological approaches to inhibit NOX4 are being developed as potential therapeutic strategies in IPF treatment. Indeed, a small molecular inhibitor that selectively targets NOX4 as well as NOX1 (GKT137831) was found to minimize bleomycin-induced fibrosis in a mouse model, by reducing fibroblast activation and collagen deposition as well as epithelial cell death (75, 87). Moreover, GKT137831 treatment in IPF lung fibroblasts results in reduced markers of senescence and increased the susceptibility to apoptosis, suggesting that NOX4 contributes to senescence (76) but further studies with specific inhibitors are needed to establish this hypothesis.
Based on the accumulating evidence that associates mtROS with IPF development, various mitochondria-targeted therapies have been developed to improve mitochondrial biogenesis, activate mitophagy, inhibit mtROS-regulated apoptosis, and scavenge mtROS in general. The mitochondrial antioxidant mitoQ, a ubiquinone conjugated to a lipophilic triphenylphosphonium cation that accumulates within mitochondria and is reduced to the antioxidant ubiquinol, has been shown to attenuate liver fibrosis (188) and reduce TGF-induced profibrotic gene expression in human fibroblasts (86). Another mitochondrial-targeted small molecule, XBJ-5-131, was shown to improve the survival of neurons in a mouse model of Huntington's disease through inhibition of oxidative (mitochondrial) DNA damage (176, 246). Another example is the antidiabetic drug, metformin, which inhibits the activity of complex I, thereby reducing mtROS. Metformin has been suggested as antifibrotic drug as it interferes with TGF-β1 signaling, inhibits myofibroblast differentiation (222), and reduces collagen deposition in bleomycin-induced pulmonary fibrosis (184). A recent review, however, indicated no beneficial effect on lung function in patients with IPF (215).
Overall, these various findings suggest that selective targeting of specific NOX isoforms or mtROS production may be beneficial in management of IPF, especially in light of studies, indicating that redox alterations induced by mitochondrial dysfunction may also be associated with their interactions with NOX activation (86, 202).
Conclusion and Future Directions
Although IPF is well recognized for its devastating nature and poor prognosis, only minimal advances have been made in the last few years to effectively treat patients with IPF. Recent FDA-approved drugs pirfenidone and nintedanib have only elicited modest improvements in disease progression and cannot ameliorate the poor survival associated with IPF (180). Moreover, they do not work for all IPF patients, indicating the need to investigate more treatment strategies (72). As discussed in this review, IPF is characterized by a dysregulated redox homeostasis and mitochondrial dysfunction. Yet, attempts to reduce ROS with general antioxidants such as N-acetyl cysteine have largely been unsuccessful in the clinic, which is likely due to our incomplete understanding of specific redox-based mechanisms that contribute to IPF development, and the fact that such general antioxidant strategies would also interfere with beneficial homeostatic functions of ROS. However, we would caution against negative conclusions based on unsuccessful studies and dismiss the potential importance of ROS-based mechanisms in IPF pathology, because of accumulating evidence implicating specific redox-based mechanisms (increased NOX activation, mitochondrial dysfunction) that ought to be targeted more directly. In this regard, it would be prudent to explore the efficacy of selective targeting of NOX4, based on compelling preclinical studies, or approaches to specifically inhibit mtROS such as MitoQ and MitoTEMPO, which have shown some success in experimental mouse models of pulmonary fibrosis. Another common challenge with such pharmaceutical approaches is successful targeting of fibroblast foci, which may be poorly accessible to systemically administered drugs.
Another reason for our relatively limited understanding of ROS in IPF is the fact that detection of specific ROS pathways in clinical specimens is exceedingly difficult (especially reversible modifications that may be more important in dysregulated cell signaling than stable protein oxidation products). In this regard, recent studies of protein S-glutathionylation in IPF in association with clinical outcomes (7) are particularly enlightening. Moreover, addressing the causality of specific ROS pathways would require appropriate animal models, and currently used models of pulmonary fibrosis do not adequately model the pathophysiology of human IPF and its progression. Also, while IPF is well known to be age associated, most animal studies are still using young animals and thus do not recapitulate the contribution of age-associated alterations on specific redox-based pathways. Although costly, future studies should address pharmacological strategies in age-appropriate models. Another problem with studies of ROS in disease models such as IPF is that they typically address the importance of a specific NOX enzyme or antioxidant system throughout the disease model, that is, during the initiation and development of the disease, which may not necessarily translate into clinical practice if pre-existing fibrosis is not reversed. In this light, some recent studies suggesting that antioxidant-based approaches can actually reverse fibrosis, at least in animal models (7, 184), are particularly encouraging.
Although evidence is growing for a role of specific ROS pathways in IPF (e.g., NOX4, mtROS), we still know relatively little with respect to the precise molecular mechanisms involved (i.e., what are the critical cellular targets for e.g., NOX4-derived ROS that facilitate AEC apoptosis or fibroblast activation). In light of emerging evidence that NOX4 may be localized to mitochondria and that mitochondria and NOX4 exert reciprocal interactions, future studies should aim at unraveling the molecular redox mechanisms underlying such interactions, for example by using approaches to interfere with mitochondrial NOX4 signaling. Successful identification of specific cellular targets for NOX4-derived ROS or for mtROS in IPF may reveal alternative and potentially more attractive therapeutic targets for treatment of IPF. Given the rapid expansion of redox-based research in recent years, and development of novel research tools to assess site-specific redox events in subcellular compartments or in specific proteins, it is anticipated that our understanding of such NOX4–mitochondria interactions will soon increase and may indeed lead to novel redox-based therapeutic strategies that may prove to be beneficial in improving the survival and the quality of life in patients suffering from IPF.
Acknowledgments
C.V. is supported by the Nutrim Graduate Programme (Maastricht University). A.v.d.V. gratefully acknowledges research support from the National Heart Lung and Blood Institute (R01 grants HL085646 and HL138708) and the National Institute on Aging (R21 AG055325). In addition, the authors thank Birgit Hettler for her assistance in designing the figures.
Abbreviations Used
- α-SMA
α-smooth muscle actin
- AEC
alveolar epithelial cell
- AngII
angiotensin II
- BALF
bronchoalveolar lavage fluid
- DUOX
dual oxidase
- EBC
exhaled breath condensates
- ECM
extracellular matrix
- ELF
epithelial lining fluid
- EMT
epithelial-to-mesenchymal transition
- ETC
electron transport chain
- FDA
Food and Drug Administration
- GRX
glutaredoxin
- GSH
glutathione
- GST
glutathione-S-transferase
- H2O2
hydrogen peroxide
- IL
interleukin
- IPF
idiopathic pulmonary fibrosis
- LAP
latency-binding peptide
- M2
alternatively activated macrophages
- mtDAMP
mitochondrial damage-associated molecular pattern
- mtDNA
mitochondrial DNA
- mtROS
mitochondrial ROS
- NAC
N-acetyl cysteine
- NOXes
NADPH oxidases
- NRF
nuclear respiratory factor
- Nrf2
nuclear factor erythroid 2-related factor 2
- O2•−
superoxide anion
- OGG1
oxoguanine DNA glycosylase 1
- OXPHOS
oxidative phosphorylation
- PGC-1α
peroxisome proliferator-activated receptor γ coactivator 1α
- PINK1
PTEN-induced putative kinase 1
- PRX
peroxiredoxin
- ROS
reactive oxygen species
- SASP
senescence-associated secretory phenotype
- SMC
smooth muscle cell
- SOD
superoxide dismutase
- TFAM
mitochondrial transcription factor A
- TGF-β
transforming growth factor β
- TH
T-helper
- TRX
thioredoxin
- UIP
usual interstitial pneumonia
- VEGF
vascular endothelial growth factor
References
- 1. American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 161: 646–664, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Abbadie C, Pluquet O, and Pourtier A. Epithelial cell senescence: an adaptive response to pre-carcinogenic stresses? Cell Mol Life Sci 74: 4471–4509, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akgedik R, Akgedik S, Karamanli H, Uysal S, Bozkurt B, Ozol D, Armutcu F, and Yildirim Z. Effect of resveratrol on treatment of bleomycin-induced pulmonary fibrosis in rats. Inflammation 35: 1732–1741, 2012 [DOI] [PubMed] [Google Scholar]
- 4. Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, Vulto I, Xie M, Qi X, Tuder RM, Phillips JA, 3rd, Lansdorp PM, Loyd JE, and Armanios MY. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A 105: 13051–13056, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alvarez D, Cardenes N, Sellares J, Bueno M, Corey C, Hanumanthu VS, Peng Y, D'Cunha H, Sembrat J, Nouraie M, Shanker S, Caufield C, Shiva S, Armanios M, Mora AL, and Rojas M. IPF lung fibroblasts have a senescent phenotype. Am J Physiol Lung Cell Mol Physiol 313: L1164–L1173, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amara N, Goven D, Prost F, Muloway R, Crestani B, and Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax 65: 733–738, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anathy V, Lahue KG, Chapman DG, Chia SB, Casey DT, Aboushousha R, van der Velden JLJ, Elko E, Hoffman SM, McMillan DH, Jones JT, Nolin JD, Abdalla S, Schneider R, Seward DJ, Roberson EC, Liptak MD, Cousins ME, Butnor KJ, Taatjes DJ, Budd RC, Irvin CG, Ho YS, Hakem R, Brown KK, Matsui R, Bachschmid MM, Gomez JL, Kaminski N, van der Vliet A, and Janssen-Heininger YMW. Reducing protein oxidation reverses lung fibrosis. Nat Med 24: 1128–1135, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anathy V, Roberson E, Cunniff B, Nolin JD, Hoffman S, Spiess P, Guala AS, Lahue KG, Goldman D, Flemer S, van der Vliet A, Heintz NH, Budd RC, Tew KD, and Janssen-Heininger YM. Oxidative processing of latent Fas in the endoplasmic reticulum controls the strength of apoptosis. Mol Cell Biol 32: 3464–3478, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anathy V, Roberson EC, Guala AS, Godburn KE, Budd RC, and Janssen-Heininger YM. Redox-based regulation of apoptosis: S-glutathionylation as a regulatory mechanism to control cell death. Antioxid Redox Signal 16: 496–505, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Armanios M. Telomerase and idiopathic pulmonary fibrosis. Mutat Res 730: 52–58, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ashrafi G. and Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 20: 31–42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baldelli S, Aquilano K, and Ciriolo MR. Punctum on two different transcription factors regulated by PGC-1alpha: nuclear factor erythroid-derived 2-like 2 and nuclear respiratory factor 2. Biochim Biophys Acta 1830: 4137–4146, 2013 [DOI] [PubMed] [Google Scholar]
- 13. Bargagli E, Olivieri C, Bennett D, Prasse A, Muller-Quernheim J, and Rottoli P. Oxidative stress in the pathogenesis of diffuse lung diseases: a review. Respir Med 103: 1245–1256, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Barron L. and Wynn TA. Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages. Am J Physiol Gastrointest Liver Physiol 300: G723–G728, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Beeh KM, Beier J, Haas IC, Kornmann O, Micke P, and Buhl R. Glutathione deficiency of the lower respiratory tract in patients with idiopathic pulmonary fibrosis. Eur Respir J 19: 1119–1123, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Bellocq A, Azoulay E, Marullo S, Flahault A, Fouqueray B, Philippe C, Cadranel J, and Baud L. Reactive oxygen and nitrogen intermediates increase transforming growth factor-beta1 release from human epithelial alveolar cells through two different mechanisms. Am J Respir Cell Mol Biol 21: 128–136, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Bernard K, Hecker L, Luckhardt TR, Cheng G, and Thannickal VJ. NADPH oxidases in lung health and disease. Antioxid Redox Signal 20: 2838–2853, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bernard K, Logsdon NJ, Miguel V, Benavides GA, Zhang J, Carter AB, Darley-Usmar VM, and Thannickal VJ. NADPH oxidase 4 (Nox4) suppresses mitochondrial biogenesis and bioenergetics in lung fibroblasts via a nuclear factor erythroid-derived 2-like 2 (Nrf2)-dependent pathway. J Biol Chem 292: 3029–3038, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Block K, Gorin Y, and Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A 106: 14385–14390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boden WE, Padala SK, Cabral KP, Buschmann IR, and Sidhu MS. Role of short-acting nitroglycerin in the management of ischemic heart disease. Drug Des Devel Ther 9: 4793–4805, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borzi RM, Grigolo B, Meliconi R, Fasano L, Sturani C, Fabbri M, Porstmann T, and Facchini A. Elevated serum superoxide dismutase levels correlate with disease severity and neutrophil degranulation in idiopathic pulmonary fibrosis. Clin Sci 85: 353–359, 1993 [DOI] [PubMed] [Google Scholar]
- 23. Boudreau HE, Casterline BW, Rada B, Korzeniowska A, and Leto TL. Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic Biol Med 53: 1489–1499, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bowler RP, Nicks M, Warnick K, and Crapo JD. Role of extracellular superoxide dismutase in bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 282: L719–L726, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Brand MD. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic Biol Med 100: 14–31, 2016 [DOI] [PubMed] [Google Scholar]
- 26. Brenmoehl J. and Hoeflich A. Dual control of mitochondrial biogenesis by sirtuin 1 and sirtuin 3. Mitochondrion 13: 755–761, 2013 [DOI] [PubMed] [Google Scholar]
- 27. Bueno M, Lai YC, Romero Y, Brands J, St. Croix CM, Kamga C, Corey C, Herazo-Maya JD, Sembrat J, Lee JS, Duncan SR, Rojas M, Shiva S, Chu CT, and Mora AL. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest 125: 521–538, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Camelo A, Dunmore R, Sleeman MA, and Clarke DL. The epithelium in idiopathic pulmonary fibrosis: breaking the barrier. Front Pharmacol 4: 173, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cannito S, Novo E, di Bonzo LV, Busletta C, Colombatto S, and Parola M. Epithelial-mesenchymal transition: from molecular mechanisms, redox regulation to implications in human health and disease. Antioxid Redox Signal 12: 1383–1430, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Cantin AM, Hubbard RC, and Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis 139: 370–372, 1989 [DOI] [PubMed] [Google Scholar]
- 31. Cantin AM, North SL, Fells GA, Hubbard RC, and Crystal RG. Oxidant-mediated epithelial cell injury in idiopathic pulmonary fibrosis. J Clin Invest 79: 1665–1673, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carnesecchi S, Deffert C, Donati Y, Basset O, Hinz B, Preynat-Seauve O, Guichard C, Arbiser JL, Banfi B, Pache JC, Barazzone-Argiroffo C, and Krause KH. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid Redox Signal 15: 607–619, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carnesecchi S, Deffert C, Pagano A, Garrido-Urbani S, Metrailler-Ruchonnet I, Schappi M, Donati Y, Matthay MA, Krause KH, and Barazzone Argiroffo C. NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice. Am J Respir Crit Care Med 180: 972–981, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng X, Kanki T, Fukuoh A, Ohgaki K, Takeya R, Aoki Y, Hamasaki N, and Kang D. PDIP38 associates with proteins constituting the mitochondrial DNA nucleoid. J Biochem 138: 673–678, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Cheresh P, Kim SJ, Tulasiram S, and Kamp DW. Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta 1832: 1028–1040, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cheresh P, Morales-Nebreda L, Kim SJ, Yeldandi A, Williams DB, Cheng Y, Mutlu GM, Budinger GR, Ridge K, Schumacker PT, Bohr VA, and Kamp DW. Asbestos-induced pulmonary fibrosis is augmented in 8-oxoguanine DNA glycosylase knockout mice. Am J Respir Cell Mol Biol 52: 25–36, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cho HY, Reddy SP, and Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal 8: 76–87, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Choi SH, Kim M, Lee HJ, Kim EH, Kim CH, and Lee YJ. Effects of NOX1 on fibroblastic changes of endothelial cells in radiation induced pulmonary fibrosis. Mol Med Rep 13: 4135–4142, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ciencewicki J, Trivedi S, and Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol 122: 456–468; quiz 469–470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cloonan SM. and Choi AM. Mitochondria in lung disease. J Clin Invest 126: 809–820, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Comhair SA. and Erzurum SC. Antioxidant responses to oxidant-mediated lung diseases. Am J Physiol Lung Cell Mol Physiol 283: L246–L255, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Conte E, Gili E, Fagone E, Fruciano M, Iemmolo M, and Vancheri C. Effect of pirfenidone on proliferation, TGF-beta-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur J Pharmaceut Sci 58: 13–19, 2014 [DOI] [PubMed] [Google Scholar]
- 43. Cool CD, Groshong SD, Rai PR, Henson PM, Stewart JS, and Brown KK. Fibroblast foci are not discrete sites of lung injury or repair: the fibroblast reticulum. Am J Respir Crit Care Med 174: 654–658, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cortijo J, Cerda-Nicolas M, Serrano A, Bioque G, Estrela JM, Santangelo F, Esteras A, Llombart-Bosch A, and Morcillo EJ. Attenuation by oral N-acetylcysteine of bleomycin-induced lung injury in rats. Eur Respir J 17: 1228–1235, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, Nicholson AG, Verbeken EK, Verschakelen J, Flower CD, Capron F, Petruzzelli S, De Vuyst P, van den Bosch JM, Rodriguez-Becerra E, Corvasce G, Lankhorst I, Sardina M, Montanari M, and IFEGENIA Group. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 353: 2229–2242, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51: 1289–1301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, and Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107: 106–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Doughan AK, Harrison DG, and Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Droge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 50. du Bois RM. Strategies for treating idiopathic pulmonary fibrosis. Nat Rev Drug Discov 9: 129–140, 2010 [DOI] [PubMed] [Google Scholar]
- 51. Durcan TM. and Fon EA. The three ‘P's of mitophagy: PARKIN, PINK1, and post-translational modifications. Genes Dev 29: 989–999, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ellson CD, Dunmore R, Hogaboam CM, Sleeman MA, and Murray LA. Danger-associated molecular patterns and danger signals in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 51: 163–168, 2014 [DOI] [PubMed] [Google Scholar]
- 53. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol 35: 495–516, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Evans CM, Fingerlin TE, Schwarz MI, Lynch D, Kurche J, Warg L, Yang IV, and Schwartz DA. Idiopathic pulmonary fibrosis: a genetic disease that involves mucociliary dysfunction of the peripheral airways. Physiol Rev 96: 1567–1591, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fang EF, Scheibye-Knudsen M, Chua KF, Mattson MP, Croteau DL, and Bohr VA. Nuclear DNA damage signalling to mitochondria in ageing. Nat Rev Mol Cell Biol 17: 308–321, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Farkas L, Farkas D, Ask K, Moller A, Gauldie J, Margetts P, Inman M, and Kolb M. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J Clin Invest 119: 1298–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fattman CL, Chang LY, Termin TA, Petersen L, Enghild JJ, and Oury TD. Enhanced bleomycin-induced pulmonary damage in mice lacking extracellular superoxide dismutase. Free Radic Biol Med 35: 763–771, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Fernandez IE. and Eickelberg O. The impact of TGF-beta on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc 9: 111–116, 2012 [DOI] [PubMed] [Google Scholar]
- 59. Fois AG, Paliogiannis P, Sotgia S, Mangoni AA, Zinellu E, Pirina P, Carru C, and Zinellu A. Evaluation of oxidative stress biomarkers in idiopathic pulmonary fibrosis and therapeutic applications: a systematic review. Respir Res 19: 51, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Furuie H, Yamasaki H, Suga M, and Ando M. Altered accessory cell function of alveolar macrophages: a possible mechanism for induction of Th2 secretory profile in idiopathic pulmonary fibrosis. Eur Respir J 10: 787–794, 1997 [PubMed] [Google Scholar]
- 61. Gauldie J, Kolb M, and Sime PJ. A new direction in the pathogenesis of idiopathic pulmonary fibrosis? Respir Res 3: 1, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gharaee-Kermani M, Moore BB, and Macoska JA. Resveratrol-mediated repression and reversion of prostatic myofibroblast phenoconversion. PLoS One 11: e0158357, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ghezzi P, Jaquet V, Marcucci F, and Schmidt H. The oxidative stress theory of disease: levels of evidence and epistemological aspects. Br J Pharmacol 174: 1784–1796, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Giovannelli L, Pitozzi V, Jacomelli M, Mulinacci N, Laurenzana A, Dolara P, and Mocali A. Protective effects of resveratrol against senescence-associated changes in cultured human fibroblasts. J Gerontol A Biol Sci Med Sci 66: 9–18, 2011 [DOI] [PubMed] [Google Scholar]
- 65. Giri SN, Leonard S, Shi X, Margolin SB, and Vallyathan V. Effects of pirfenidone on the generation of reactive oxygen species in vitro. J Environ Pathol Toxicol Oncol 18: 169–177, 1999 [PubMed] [Google Scholar]
- 66. Gomes LC, Di Benedetto G, and Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 13: 589–598, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gonzalez-Gonzalez FJ, Chandel NS, Jain M, and Budinger GRS. Reactive oxygen species as signaling molecules in the development of lung fibrosis. Transl Res 190: 61–68, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Graham KA, Kulawiec M, Owens KM, Li X, Desouki MM, Chandra D, and Singh KK. NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol Ther 10: 223–231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Guo Q. Changes in mitochondrial function during EMT induced by TGFbeta-1 in pancreatic cancer. Oncol Lett 13: 1575–1580, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hagiwara SI, Ishii Y, and Kitamura S. Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am J Respir Crit Care Med 162: 225–231, 2000 [DOI] [PubMed] [Google Scholar]
- 71. Hashimoto N, Phan SH, Imaizumi K, Matsuo M, Nakashima H, Kawabe T, Shimokata K, and Hasegawa Y. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 43: 161–172, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hayton C. and Chaudhuri N. Managing idiopathic pulmonary fibrosis: which drug for which patient? Drugs Aging 34: 647–653, 2017 [DOI] [PubMed] [Google Scholar]
- 73. He C, Murthy S, McCormick ML, Spitz DR, Ryan AJ, and Carter AB. Mitochondrial Cu,Zn-superoxide dismutase mediates pulmonary fibrosis by augmenting H2O2 generation. J Biol Chem 286: 15597–15607, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. He X, Wang L, Szklarz G, Bi Y, and Ma Q. Resveratrol inhibits paraquat-induced oxidative stress and fibrogenic response by activating the nuclear factor erythroid 2-related factor 2 pathway. J Pharmacol Exp Ther 342: 81–90, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hecker L, Cheng J, and Thannickal VJ. Targeting NOX enzymes in pulmonary fibrosis. Cell Mol Life Sci 69: 2365–2371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY, and Thannickal VJ. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med 6: 231ra47, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, and Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15: 1077–1081, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Herrera B, Murillo MM, Alvarez-Barrientos A, Beltran J, Fernandez M, and Fabregat I. Source of early reactive oxygen species in the apoptosis induced by transforming growth factor-beta in fetal rat hepatocytes. Free Radic Biol Med 36: 16–26, 2004 [DOI] [PubMed] [Google Scholar]
- 79. Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, and Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 170: 1807–1816, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hiraga R, Kato M, Miyagawa S, and Kamata T. Nox4-derived ROS signaling contributes to TGF-beta-induced epithelial-mesenchymal transition in pancreatic cancer cells. Anticancer Res 33: 4431–4438, 2013 [PubMed] [Google Scholar]
- 81. Hohmann MS, Habiel DM, Coelho AL, Verri WA, Jr., and Hogaboam CM. Quercetin enhances ligand-induced apoptosis in senescent IPF fibroblasts and reduces lung fibrosis in vivo. Am J Respir Cell Mol Biol 60: 28–40, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Homma S, Azuma A, Taniguchi H, Ogura T, Mochiduki Y, Sugiyama Y, Nakata K, Yoshimura K, Takeuchi M, Kudoh S, and Japan NAC Couplinical Study Group. Efficacy of inhaled N-acetylcysteine monotherapy in patients with early stage idiopathic pulmonary fibrosis. Respirology 17: 467–477, 2012 [DOI] [PubMed] [Google Scholar]
- 83. Hostettler KE, Zhong J, Papakonstantinou E, Karakiulakis G, Tamm M, Seidel P, Sun Q, Mandal J, Lardinois D, Lambers C, and Roth M. Anti-fibrotic effects of nintedanib in lung fibroblasts derived from patients with idiopathic pulmonary fibrosis. Respir Res 15: 157, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Idiopathic Pulmonary Fibrosis Clinical Research Network, Martinez FJ, de Andrade JA, Anstrom KJ, King TE, et al. , and Raghu G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 370: 2093–2101, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jablonski RP, Kim SJ, Cheresh P, Williams DB, Morales-Nebreda L, Cheng Y, Yeldandi A, Bhorade S, Pardo A, Selman M, Ridge K, Gius D, Budinger GRS, and Kamp DW. SIRT3 deficiency promotes lung fibrosis by augmenting alveolar epithelial cell mitochondrial DNA damage and apoptosis. FASEB J 31: 2520–2532, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jain M, Rivera S, Monclus EA, Synenki L, Zirk A, Eisenbart J, Feghali-Bostwick C, Mutlu GM, Budinger GR, and Chandel NS. Mitochondrial reactive oxygen species regulate transforming growth factor-beta signaling. J Biol Chem 288: 770–777, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jarman ER, Khambata VS, Cope C, Jones P, Roger J, Ye LY, Duggan N, Head D, Pearce A, Press NJ, Bellenie B, Sohal B, and Jarai G. An inhibitor of NADPH oxidase-4 attenuates established pulmonary fibrosis in a rodent disease model. Am J Respir Cell Mol Biol 50: 158–169, 2014 [DOI] [PubMed] [Google Scholar]
- 88. Jiang F, Liu GS, Dusting GJ, and Chan EC. NADPH oxidase-dependent redox signaling in TGF-beta-mediated fibrotic responses. Redox Biol 2: 267–272, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jolly MK, Ward C, Eapen MS, Myers S, Hallgren O, Levine H, and Sohal SS. Epithelial-mesenchymal transition, a spectrum of states: role in lung development, homeostasis, and disease. Dev Dyn 247: 346–358, 2018 [DOI] [PubMed] [Google Scholar]
- 90. Jones HA, Schofield JB, Krausz T, Boobis AR, and Haslett C. Pulmonary fibrosis correlates with duration of tissue neutrophil activation. Am J Respir Crit Care Med 158: 620–628, 1998 [DOI] [PubMed] [Google Scholar]
- 91. Kage H. and Borok Z. EMT and interstitial lung disease: a mysterious relationship. Curr Opin Pulm Med 18: 517–523, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kalluri R. and Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112: 1776–1784, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kang KA, Ryu YS, Piao MJ, Shilnikova K, Kang HK, Yi JM, Boulanger M, Paolillo R, Bossis G, Yoon SY, Kim SB, and Hyun JW. DUOX2-mediated production of reactive oxygen species induces epithelial mesenchymal transition in 5-fluorouracil resistant human colon cancer cells. Redox Biol 17: 224–235, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kang YP, Lee SB, Lee JM, Kim HM, Hong JY, Lee WJ, Choi CW, Shin HK, Kim DJ, Koh ES, Park CS, Kwon SW, and Park SW. Metabolic profiling regarding pathogenesis of idiopathic pulmonary fibrosis. J Proteome Res 15: 1717–1724, 2016 [DOI] [PubMed] [Google Scholar]
- 95. Kasper M. and Barth K. Potential contribution of alveolar epithelial type I cells to pulmonary fibrosis. Biosci Rep 37: pii: , 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kato S, Inui N, Hakamata A, Suzuki Y, Enomoto N, Fujisawa T, Nakamura Y, Watanabe H, and Suda T. Changes in pulmonary endothelial cell properties during bleomycin-induced pulmonary fibrosis. Respir Res 19: 127, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kawagishi H. and Finkel T. Unraveling the truth about antioxidants: ROS and disease: finding the right balance. Nat Med 20: 711–713, 2014 [DOI] [PubMed] [Google Scholar]
- 98. Kawahara T, Quinn MT, and Lambeth JD. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol 7: 109, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kikuchi N, Ishii Y, Morishima Y, Yageta Y, Haraguchi N, Itoh K, Yamamoto M, and Hizawa N. Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2 balance. Respir Res 11: 31, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, and Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A 103: 13180–13185, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kim SJ, Cheresh P, Jablonski RP, Morales-Nebreda L, Cheng Y, Hogan E, Yeldandi A, Chi M, Piseaux R, Ridge K, Michael Hart C, Chandel N, Scott Budinger GR, and Kamp DW. Mitochondrial catalase overexpressed transgenic mice are protected against lung fibrosis in part via preventing alveolar epithelial cell mitochondrial DNA damage. Free Radic Biol Med 101: 482–490, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kim SJ, Cheresh P, Jablonski RP, Williams DB, and Kamp DW. The role of mitochondrial DNA in mediating alveolar epithelial cell apoptosis and pulmonary fibrosis. Int J Mol Sci 16: 21486–21519, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kim SJ, Cheresh P, Williams D, Cheng Y, Ridge K, Schumacker PT, Weitzman S, Bohr VA, and Kamp DW. Mitochondria-targeted Ogg1 and aconitase-2 prevent oxidant-induced mitochondrial DNA damage in alveolar epithelial cells. J Biol Chem 289: 6165–6176, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kim SM, Kim YG, Jeong KH, Lee SH, Lee TW, Ihm CG, and Moon JY. Angiotensin II-induced mitochondrial Nox4 is a major endogenous source of oxidative stress in kidney tubular cells. PLoS One 7: e39739, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kim YM, Kim SJ, Tatsunami R, Yamamura H, Fukai T, and Ushio-Fukai M. ROS-induced ROS release orchestrated by Nox4, Nox2, and mitochondria in VEGF signaling and angiogenesis. Am J Physiol Cell Physiol 312: C749–C764, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kinder BW, Brown KK, Schwarz MI, Ix JH, Kervitsky A, and King TE., Jr Baseline BAL neutrophilia predicts early mortality in idiopathic pulmonary fibrosis. Chest 133: 226–232, 2008 [DOI] [PubMed] [Google Scholar]
- 107. King TE, Jr., Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA, Jr., Flint A, Thurlbeck W, and Cherniack RM. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med 164: 1025–1032, 2001 [DOI] [PubMed] [Google Scholar]
- 108. Kinnula VL. and Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med 167: 1600–1619, 2003 [DOI] [PubMed] [Google Scholar]
- 109. Kinnula VL, Fattman CL, Tan RJ, and Oury TD. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med 172: 417–422, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kinnula VL, Hodgson UA, Lakari EK, Tan RJ, Sormunen RT, Soini YM, Kakko SJ, Laitinen TH, Oury TD, and Paakko PK. Extracellular superoxide dismutase has a highly specific localization in idiopathic pulmonary fibrosis/usual interstitial pneumonia. Histopathology 49: 66–74, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kliment CR. and Oury TD. Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radic Biol Med 49: 707–717, 2010 [DOI] [PubMed] [Google Scholar]
- 112. Kolb M, Bonella F, and Wollin L. Therapeutic targets in idiopathic pulmonary fibrosis. Respir Med 131: 49–57, 2017 [DOI] [PubMed] [Google Scholar]
- 113. Koziel R, Pircher H, Kratochwil M, Lener B, Hermann M, Dencher NA, and Jansen-Durr P. Mitochondrial respiratory chain complex I is inactivated by NADPH oxidase Nox4. Biochem J 452: 231–239, 2013 [DOI] [PubMed] [Google Scholar]
- 114. Kroller-Schon S, Steven S, Kossmann S, Scholz A, Daub S, Oelze M, Xia N, Hausding M, Mikhed Y, Zinssius E, Mader M, Stamm P, Treiber N, Scharffetter-Kochanek K, Li H, Schulz E, Wenzel P, Munzel T, and Daiber A. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid Redox Signal 20: 247–266, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kubli DA. and Gustafsson AB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res 111: 1208–1221, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, and Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A 107: 15565–15570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kurundkar A. and Thannickal VJ. Redox mechanisms in age-related lung fibrosis. Redox Biol 9: 67–76, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kuwano K, Kunitake R, Maeyama T, Hagimoto N, Kawasaki M, Matsuba T, Yoshimi M, Inoshima I, Yoshida K, and Hara N. Attenuation of bleomycin-induced pneumopathy in mice by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol 280: L316–L325, 2001 [DOI] [PubMed] [Google Scholar]
- 119. Kuwano K, Nakashima N, Inoshima I, Hagimoto N, Fujita M, Yoshimi M, Maeyama T, Hamada N, Watanabe K, and Hara N. Oxidative stress in lung epithelial cells from patients with idiopathic interstitial pneumonias. Eur Respir J 21: 232–240, 2003 [DOI] [PubMed] [Google Scholar]
- 120. Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189, 2004 [DOI] [PubMed] [Google Scholar]
- 121. Lambeth JD, Kawahara T, and Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med 43: 319–331, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Leach HG, Chrobak I, Han R, and Trojanowska M. Endothelial cells recruit macrophages and contribute to a fibrotic milieu in bleomycin lung injury. Am J Respir Cell Mol Biol 49: 1093–1101, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lee HC, Yin PH, Chi CW, and Wei YH. Increase in mitochondrial mass in human fibroblasts under oxidative stress and during replicative cell senescence. J Biomed Sci 9: 517–526, 2002 [DOI] [PubMed] [Google Scholar]
- 124. Lee SB, Bae IH, Bae YS, and Um HD. Link between mitochondria and NADPH oxidase 1 isozyme for the sustained production of reactive oxygen species and cell death. J Biol Chem 281: 36228–36235, 2006 [DOI] [PubMed] [Google Scholar]
- 125. Lennon FE. and Salgia R. Mitochondrial dynamics: biology and therapy in lung cancer. Expert Opin Invest Drugs 23: 675–692, 2014 [DOI] [PubMed] [Google Scholar]
- 126. Lenz AG, Costabel U, and Maier KL. Oxidized BAL fluid proteins in patients with interstitial lung diseases. Eur Respir J 9: 307–312, 1996 [DOI] [PubMed] [Google Scholar]
- 127. Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, and Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest 129: 746–752, 2006 [DOI] [PubMed] [Google Scholar]
- 128. Li G, Yuzhen L, Yi C, Xiaoxiang C, Wei Z, Changqing Z, and Shuang Y. DNaseI protects against Paraquat-induced acute lung injury and pulmonary fibrosis mediated by mitochondrial DNA. BioMed Res Int 2015: 386952, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Little AC, Sham D, Hristova M, Danyal K, Heppner DE, Bauer RA, Sipsey LM, Habibovic A, and van der Vliet A. DUOX1 silencing in lung cancer promotes EMT, cancer stem cell characteristics and invasive properties. Oncogenesis 5: e261, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Liu RM. and Desai LP. Reciprocal regulation of TGF-beta and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol 6: 565–577, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Liu X. and Chen Z. The pathophysiological role of mitochondrial oxidative stress in lung diseases. J Transl Med 15: 207, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Lopez-Otin C, Blasco MA, Partridge L, Serrano M, and Kroemer G. The hallmarks of aging. Cell 153: 1194–1217, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, and Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res 105: 249–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Magro CM, Waldman WJ, Knight DA, Allen JN, Nadasdy T, Frambach GE, Ross P, and Marsh CB. Idiopathic pulmonary fibrosis related to endothelial injury and antiendothelial cell antibodies. Hum Immunol 67: 284–297, 2006 [DOI] [PubMed] [Google Scholar]
- 135. Maher TM. Aerobic glycolysis and the Warburg effect. An unexplored realm in the search for fibrosis therapies? Am J Respir Crit Care Med 192: 1407–1409, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Malli F, Koutsokera A, Paraskeva E, Zakynthinos E, Papagianni M, Makris D, Tsilioni I, Molyvdas PA, Gourgoulianis KI, and Daniil Z. Endothelial progenitor cells in the pathogenesis of idiopathic pulmonary fibrosis: an evolving concept. PLoS One 8: e53658, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Manoury B, Nenan S, Leclerc O, Guenon I, Boichot E, Planquois JM, Bertrand CP, and Lagente V. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir Res 6: 11, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Markart P, Luboeinski T, Korfei M, Schmidt R, Wygrecka M, Mahavadi P, Mayer K, Wilhelm J, Seeger W, Guenther A, and Ruppert C. Alveolar oxidative stress is associated with elevated levels of nonenzymatic low-molecular-weight antioxidants in patients with different forms of chronic fibrosing interstitial lung diseases. Antioxid Redox Signal 11: 227–240, 2009 [DOI] [PubMed] [Google Scholar]
- 139. Marmai C, Sutherland RE, Kim KK, Dolganov GM, Fang X, Kim SS, Jiang S, Golden JA, Hoopes CW, Matthay MA, Chapman HA, and Wolters PJ. Alveolar epithelial cells express mesenchymal proteins in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 301: L71–L78, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Martinez FJ, de Andrade JA, Anstrom KJ, King TE, Jr., and Raghu G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 370: 2093–2101, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, and Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006 [DOI] [PubMed] [Google Scholar]
- 142. Matsushima S, Kuroda J, Zhai P, Liu T, Ikeda S, Nagarajan N, Oka S, Yokota T, Kinugawa S, Hsu CP, Li H, Tsutsui H, and Sadoshima J. Tyrosine kinase FYN negatively regulates NOX4 in cardiac remodeling. J Clin Invest 126: 3403–3416, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Mazur W, Lindholm P, Vuorinen K, Myllarniemi M, Salmenkivi K, and Kinnula VL. Cell-specific elevation of NRF2 and sulfiredoxin-1 as markers of oxidative stress in the lungs of idiopathic pulmonary fibrosis and non-specific interstitial pneumonia. APMIS 118: 703–712, 2010 [DOI] [PubMed] [Google Scholar]
- 144. Meiners S, Eickelberg O, and Konigshoff M. Hallmarks of the ageing lung. Eur Respir J 45: 807–827, 2015 [DOI] [PubMed] [Google Scholar]
- 145. Meitzler JL. and Ortiz de Montellano PR. Caenorhabditis elegans and human dual oxidase 1 (DUOX1) “peroxidase” domains: insights into heme binding and catalytic activity. J Biol Chem 284: 18634–18643, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Meyer A, Buhl R, and Magnussen H. The effect of oral N-acetylcysteine on lung glutathione levels in idiopathic pulmonary fibrosis. Eur Respir J 7: 431–436, 1994 [DOI] [PubMed] [Google Scholar]
- 147. Minagawa S, Araya J, Numata T, Nojiri S, Hara H, Yumino Y, Kawaishi M, Odaka M, Morikawa T, Nishimura SL, Nakayama K, and Kuwano K. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-beta-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 300: L391–L401, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Misra HP. and Rabideau C. Pirfenidone inhibits NADPH-dependent microsomal lipid peroxidation and scavenges hydroxyl radicals. Mol Cell Biochem 204: 119–126, 2000 [DOI] [PubMed] [Google Scholar]
- 149. Montuschi P, Ciabattoni G, Paredi P, Pantelidis P, du Bois RM, Kharitonov SA, and Barnes PJ. 8-Isoprostane as a biomarker of oxidative stress in interstitial lung diseases. Am J Respir Crit Care Med 158: 1524–1527, 1998 [DOI] [PubMed] [Google Scholar]
- 150. Mora AL, Bueno M, and Rojas M. Mitochondria in the spotlight of aging and idiopathic pulmonary fibrosis. J Clin Invest 127: 405–414, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Mora AL, Rojas M, Pardo A, and Selman M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat Rev Drug Discov 16: 810, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Muramatsu Y, Sugino K, Ishida F, Tatebe J, Morita T, and Homma S. Effect of inhaled N-acetylcysteine monotherapy on lung function and redox balance in idiopathic pulmonary fibrosis. Respir Invest 54: 170–178, 2016 [DOI] [PubMed] [Google Scholar]
- 153. Myllarniemi M. and Kaarteenaho R. Pharmacological treatment of idiopathic pulmonary fibrosis—preclinical and clinical studies of pirfenidone, nintedanib, and N-acetylcysteine. Eur Clin Respir J 2, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Naikawadi RP, Disayabutr S, Mallavia B, Donne ML, Green G, La JL, Rock JR, Looney MR, and Wolters PJ. Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight 1: e86704, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Nakamura T, Matsushima M, Hayashi Y, Shibasaki M, Imaizumi K, Hashimoto N, Shimokata K, Hasegawa Y, and Kawabe T. Attenuation of transforming growth factor-beta-stimulated collagen production in fibroblasts by quercetin-induced heme oxygenase-1. Am J Respir Cell Mol Biol 44: 614–620, 2011 [DOI] [PubMed] [Google Scholar]
- 156. Nalysnyk L, Cid-Ruzafa J, Rotella P, and Esser D. Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. Eur Respir Rev 21: 355–361, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev 219: 88–102, 2007 [DOI] [PubMed] [Google Scholar]
- 158. Navaratnam V, Fleming KM, West J, Smith CJ, Jenkins RG, Fogarty A, and Hubbard RB. The rising incidence of idiopathic pulmonary fibrosis in the U.K. Thorax 66: 462–467, 2011 [DOI] [PubMed] [Google Scholar]
- 159. Nicholson AG, Fulford LG, Colby TV, du Bois RM, Hansell DM, and Wells AU. The relationship between individual histologic features and disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 166: 173–177, 2002 [DOI] [PubMed] [Google Scholar]
- 160. Nicolson GL. Mitochondrial dysfunction and chronic disease: treatment with natural supplements. Integr Med (Encinitas) 13: 35–43, 2014 [PMC free article] [PubMed] [Google Scholar]
- 161. Odajima N, Betsuyaku T, Nagai K, Moriyama C, Wang DH, Takigawa T, Ogino K, and Nishimura M. The role of catalase in pulmonary fibrosis. Respir Res 11: 183, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ogura M, Yamaki J, Homma MK, and Homma Y. Mitochondrial c-Src regulates cell survival through phosphorylation of respiratory chain components. Biochem J 447: 281–289, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Oku H, Shimizu T, Kawabata T, Nagira M, Hikita I, Ueyama A, Matsushima S, Torii M, and Arimura A. Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol 590: 400–408, 2008 [DOI] [PubMed] [Google Scholar]
- 164. Okuda R, Matsushima H, Oba T, Kawabe R, Matsubayashi M, Amano M, Nishizawa T, and Honda K. Efficacy and safety of inhaled N-acetylcysteine in idiopathic pulmonary fibrosis: a prospective, single-arm study. Respir Investig 54: 156–161, 2016 [DOI] [PubMed] [Google Scholar]
- 165. Pache JC, Carnesecchi S, Deffert C, Donati Y, Herrmann FR, Barazzone-Argiroffo C, and Krause KH. NOX-4 is expressed in thickened pulmonary arteries in idiopathic pulmonary fibrosis. Nat Med 17: 31–32; author reply 32–33, 2011 [DOI] [PubMed] [Google Scholar]
- 166. Palikaras K. and Tavernarakis N. Mitochondrial homeostasis: the interplay between mitophagy and mitochondrial biogenesis. Exp Gerontol 56: 182–188, 2014 [DOI] [PubMed] [Google Scholar]
- 167. Panduri V, Liu G, Surapureddi S, Kondapalli J, Soberanes S, de Souza-Pinto NC, Bohr VA, Budinger GR, Schumacker PT, Weitzman SA, and Kamp DW. Role of mitochondrial hOGG1 and aconitase in oxidant-induced lung epithelial cell apoptosis. Free Radic Biol Med 47: 750–759, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Pardo A. and Selman M. Idiopathic pulmonary fibrosis: new insights in its pathogenesis. Int J Biochem Cell Biol 34: 1534–1538, 2002 [DOI] [PubMed] [Google Scholar]
- 169. Pardo A. and Selman M. Lung fibroblasts, aging, and idiopathic pulmonary fibrosis. Ann Am Thorac Soc 13: S417–S421, 2016 [DOI] [PubMed] [Google Scholar]
- 170. Patel AS, Lin L, Geyer A, Haspel JA, An CH, Cao J, Rosas IO, and Morse D. Autophagy in idiopathic pulmonary fibrosis. PLoS One 7: e41394, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Patel AS, Song JW, Chu SG, Mizumura K, Osorio JC, Shi Y, El-Chemaly S, Lee CG, Rosas IO, Elias JA, Choi AM, and Morse D. Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor-beta1 in pulmonary fibrosis. PLoS One 10: e0121246, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Peltoniemi M, Kaarteenaho-Wiik R, Saily M, Sormunen R, Paakko P, Holmgren A, Soini Y, and Kinnula VL. Expression of glutaredoxin is highly cell specific in human lung and is decreased by transforming growth factor-beta in vitro and in interstitial lung diseases in vivo. Hum Pathol 35: 1000–1007, 2004 [DOI] [PubMed] [Google Scholar]
- 173. Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, and Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal 8: 1473–1484, 2006 [DOI] [PubMed] [Google Scholar]
- 174. Piera-Velazquez S, Li Z, and Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol 179: 1074–1080, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Plataki M, Koutsopoulos AV, Darivianaki K, Delides G, Siafakas NM, and Bouros D. Expression of apoptotic and antiapoptotic markers in epithelial cells in idiopathic pulmonary fibrosis. Chest 127: 266–274, 2005 [DOI] [PubMed] [Google Scholar]
- 176. Polyzos A, Holt A, Brown C, Cosme C, Wipf P, Gomez-Marin A, Castro MR, Ayala-Pena S, and McMurray CT. Mitochondrial targeting of XJB-5-131 attenuates or improves pathophysiology in HdhQ150 animals with well-developed disease phenotypes. Hum Mol Genet 25: 1792–1802, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Prasad S, Hogaboam CM, and Jarai G. Deficient repair response of IPF fibroblasts in a co-culture model of epithelial injury and repair. Fibrogenesis Tissue Repair 7: 7, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Psathakis K, Mermigkis D, Papatheodorou G, Loukides S, Panagou P, Polychronopoulos V, Siafakas NM, and Bouros D. Exhaled markers of oxidative stress in idiopathic pulmonary fibrosis. Eur J Clin Invest 36: 362–367, 2006 [DOI] [PubMed] [Google Scholar]
- 179. Raghu G, Chen SY, Hou Q, Yeh WS, and Collard HR. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18–64 years old. Eur Respir J 48: 179–186, 2016 [DOI] [PubMed] [Google Scholar]
- 180. Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, Brozek JL, Collard HR, Cunningham W, Homma S, Johkoh T, Martinez FJ, Myers J, Protzko SL, Richeldi L, Rind D, Selman M, Theodore A, Wells AU, Hoogsteden H, Schunemann HJ, American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Association. An Official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med 192: e3–e19, 2015 [DOI] [PubMed] [Google Scholar]
- 181. Raghu G, Weycker D, Edelsberg J, Bradford WZ, and Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 174: 810–816, 2006 [DOI] [PubMed] [Google Scholar]
- 182. Rahman I, Skwarska E, Henry M, Davis M, O'Connor CM, FitzGerald MX, Greening A, and MacNee W. Systemic and pulmonary oxidative stress in idiopathic pulmonary fibrosis. Free Radic Biol Med 27: 60–68, 1999 [DOI] [PubMed] [Google Scholar]
- 183. Rangarajan S, Bernard K, and Thannickal VJ. Mitochondrial dysfunction in pulmonary fibrosis. Ann Am Thorac Soc 14: S383–S388, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Rangarajan S, Bone NB, Zmijewska AA, Jiang S, Park DW, Bernard K, Locy ML, Ravi S, Deshane J, Mannon RB, Abraham E, Darley-Usmar V, Thannickal VJ, and Zmijewski JW. Metformin reverses established lung fibrosis in a bleomycin model. Nat Med 24: 1121–1127, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, and Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 45: 1223–1231, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Ray PD, Huang BW, and Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24: 981–990, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Reddy NM, Kleeberger SR, Cho HY, Yamamoto M, Kensler TW, Biswal S, and Reddy SP. Deficiency in Nrf2-GSH signaling impairs type II cell growth and enhances sensitivity to oxidants. Am J Respir Cell Mol Biol 37: 3–8, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Rehman H, Liu Q, Krishnasamy Y, Shi Z, Ramshesh VK, Haque K, Schnellmann RG, Murphy MP, Lemasters JJ, Rockey DC, and Zhong Z. The mitochondria-targeted antioxidant MitoQ attenuates liver fibrosis in mice. Int J Physiol Pathophysiol Pharmacol 8: 14–27, 2016 [PMC free article] [PubMed] [Google Scholar]
- 189. Rey FE, Cifuentes ME, Kiarash A, Quinn MT, and Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(−) and systolic blood pressure in mice. Circ Res 89: 408–414, 2001 [DOI] [PubMed] [Google Scholar]
- 190. Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, and Shulman GI. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab 5: 151–156, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, Kanellopoulos J, Quesniaux VF, Marchand-Adam S, Crestani B, Ryffel B, and Couillin I. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med 182: 774–783, 2010 [DOI] [PubMed] [Google Scholar]
- 192. Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, and Hogan BL. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A 108: E1475–E1483, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Rogliani P, Calzetta L, Cavalli F, Matera MG, and Cazzola M. Pirfenidone, nintedanib and N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Pulm Pharmacol Ther 40: 95–103, 2016 [DOI] [PubMed] [Google Scholar]
- 194. Romano AD, Greco E, Vendemiale G, and Serviddio G. Bioenergetics and mitochondrial dysfunction in aging: recent insights for a therapeutical approach. Curr Pharm Des 20: 2978–2992, 2014 [DOI] [PubMed] [Google Scholar]
- 195. Rottoli P, Magi B, Cianti R, Bargagli E, Vagaggini C, Nikiforakis N, Pallini V, and Bini L. Carbonylated proteins in bronchoalveolar lavage of patients with sarcoidosis, pulmonary fibrosis associated with systemic sclerosis and idiopathic pulmonary fibrosis. Proteomics 5: 2612–2618, 2005 [DOI] [PubMed] [Google Scholar]
- 196. Ryan US. and Ryan JW. Cell biology of pulmonary endothelium. Circulation 70: III46–III62, 1984 [PubMed] [Google Scholar]
- 197. Ryu C, Sun H, Gulati M, Herazo-Maya JD, Chen Y, Osafo-Addo A, Brandsdorfer C, Winkler J, Blaul C, Faunce J, Pan H, Woolard T, Tzouvelekis A, Antin-Ozerkis DE, Puchalski JT, Slade M, Gonzalez AL, Bogenhagen DF, Kirillov V, Feghali-Bostwick C, Gibson K, Lindell K, Herzog RI, Dela Cruz CS, Mehal W, Kaminski N, Herzog EL, and Trujillo G. Extracellular mitochondrial DNA is generated by fibroblasts and predicts death in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 196: 1571–1581, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Sakamoto S, Itoh T, Muramatsu Y, Satoh K, Ishida F, Sugino K, Isobe K, and Homma S. Efficacy of pirfenidone in patients with advanced-stage idiopathic pulmonary fibrosis. Intern Med 52: 2495–2501, 2013 [DOI] [PubMed] [Google Scholar]
- 199. Saleh D, Barnes PJ, and Giaid A. Increased production of the potent oxidant peroxynitrite in the lungs of patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 155: 1763–1769, 1997 [DOI] [PubMed] [Google Scholar]
- 200. Sanders YY, Liu H, Liu G, and Thannickal VJ. Epigenetic mechanisms regulate NADPH oxidase-4 expression in cellular senescence. Free Radic Biol Med 79: 197–205, 2015 [DOI] [PubMed] [Google Scholar]
- 201. Santos JH, Hunakova L, Chen Y, Bortner C, and Van Houten B. Cell sorting experiments link persistent mitochondrial DNA damage with loss of mitochondrial membrane potential and apoptotic cell death. J Biol Chem 278: 1728–1734, 2003 [DOI] [PubMed] [Google Scholar]
- 202. Sato N, Takasaka N, Yoshida M, Tsubouchi K, Minagawa S, Araya J, Saito N, Fujita Y, Kurita Y, Kobayashi K, Ito S, Hara H, Kadota T, Yanagisawa H, Hashimoto M, Utsumi H, Wakui H, Kojima J, Numata T, Kaneko Y, Odaka M, Morikawa T, Nakayama K, Kohrogi H, and Kuwano K. Metformin attenuates lung fibrosis development via NOX4 suppression. Respir Res 17: 107, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y, Mazula DL, Brooks RW, Fuhrmann-Stroissnigg H, Pirtskhalava T, Prakash YS, Tchkonia T, Robbins PD, Aubry MC, Passos JF, Kirkland JL, Tschumperlin DJ, Kita H, and LeBrasseur NK. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 8: 14532, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Schmidt HH, Stocker R, Vollbracht C, Paulsen G, Riley D, Daiber A, and Cuadrado A. Antioxidants in translational medicine. Antioxid Redox Signal 23: 1130–1143, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Selman M, King TE, Pardo A, American Thoracic S, European Respiratory Society, and American College of Chest Physicians. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 134: 136–151, 2001 [DOI] [PubMed] [Google Scholar]
- 206. Selman M. and Pardo A. Revealing the pathogenic and aging-related mechanisms of the enigmatic idiopathic pulmonary fibrosis. An integral model. Am J Respir Crit Care Med 189: 1161–1172, 2014 [DOI] [PubMed] [Google Scholar]
- 207. Sena LA. and Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48: 158–167, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Sener G, Topaloglu N, Sehirli AO, Ercan F, and Gedik N. Resveratrol alleviates bleomycin-induced lung injury in rats. Pulm Pharmacol Ther 20: 642–649, 2007 [DOI] [PubMed] [Google Scholar]
- 209. Shanmugasundaram K, Nayak BK, Friedrichs WE, Kaushik D, Rodriguez R, and Block K. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nat Commun 8: 997, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Shvedova AA, Kisin ER, Murray AR, Kommineni C, Castranova V, Fadeel B, and Kagan VE. Increased accumulation of neutrophils and decreased fibrosis in the lung of NADPH oxidase-deficient C57BL/6 mice exposed to carbon nanotubes. Toxicol Appl Pharmacol 231: 235–240, 2008 [DOI] [PubMed] [Google Scholar]
- 211. Singel KL. and Segal BH. NOX2-dependent regulation of inflammation. Clin Sci 130: 479–490, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, Dave A, Engelhardt JF, Liu X, White ES, Thannickal VJ, Moore BB, Christensen PJ, and Simon RH. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med 181: 254–263, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Smeitink J, van den Heuvel L, and DiMauro S. The genetics and pathology of oxidative phosphorylation. Nat Rev Genet 2: 342–352, 2001 [DOI] [PubMed] [Google Scholar]
- 214. Sosulski ML, Gongora R, Danchuk S, Dong C, Luo F, and Sanchez CG. Deregulation of selective autophagy during aging and pulmonary fibrosis: the role of TGFbeta1. Aging Cell 14: 774–783, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Spagnolo P, Kreuter M, Maher TM, Wuyts W, Bonella F, Corte TJ, Kopf S, Weycker D, Kirchgaessler KU, and Ryerson CJ. Metformin does not affect clinically relevant outcomes in patients with idiopathic pulmonary fibrosis. Respiration 96: 314–322, 2018 [DOI] [PubMed] [Google Scholar]
- 216. Strausz J, Muller-Quernheim J, Steppling H, and Ferlinz R. Oxygen radical production by alveolar inflammatory cells in idiopathic pulmonary fibrosis. Am Rev Respir Dis 141: 124–128, 1990 [DOI] [PubMed] [Google Scholar]
- 217. Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, and Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 290: L661–L673, 2006 [DOI] [PubMed] [Google Scholar]
- 218. Sugiura H, Ichikawa T, Liu X, Kobayashi T, Wang XQ, Kawasaki S, Togo S, Kamio K, Mao L, Ann Y, Ichinose M, and Rennard SI. N-acetyl-l-cysteine inhibits TGF-beta1-induced profibrotic responses in fibroblasts. Pulm Pharmacol Ther 22: 487–491, 2009 [DOI] [PubMed] [Google Scholar]
- 219. Sureshbabu A. and Bhandari V. Targeting mitochondrial dysfunction in lung diseases: emphasis on mitophagy. Front Physiol 4: 384, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Swamy SM, Rajasekaran NS, and Thannickal VJ. Nuclear factor-erythroid-2-related factor 2 in aging and lung fibrosis. Am J Pathol 186: 1712–1723, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Takabatake N, Arao T, Sata M, Abe S, Inoue S, Shibata Y, Takeishi Y, and Kubota I. Involvement of pulmonary endothelial cell injury in the pathogenesis of pulmonary fibrosis: clinical assessment by 123I-MIBG lung scintigraphy. Eur J Nucl Med Mol Imaging 32: 221–228, 2005 [DOI] [PubMed] [Google Scholar]
- 222. Thakur S, Viswanadhapalli S, Kopp JB, Shi Q, Barnes JL, Block K, Gorin Y, and Abboud HE. Activation of AMP-activated protein kinase prevents TGF-beta1-induced epithelial-mesenchymal transition and myofibroblast activation. Am J Pathol 185: 2168–2180, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Thannickal VJ. and Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem 270: 30334–30338, 1995 [DOI] [PubMed] [Google Scholar]
- 224. Thannickal VJ. and Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc 3: 350–356, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, and Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem 278: 12384–12389, 2003 [DOI] [PubMed] [Google Scholar]
- 226. Tiitto L, Kaarteenaho-Wiik R, Sormunen R, Holmgren A, Paakko P, Soini Y, and Kinnula VL. Expression of the thioredoxin system in interstitial lung disease. J Pathol 201: 363–370, 2003 [DOI] [PubMed] [Google Scholar]
- 227. Uhal BD, Joshi I, Hughes WF, Ramos C, Pardo A, and Selman M. Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung. Am J Physiol 275: L1192–L1199, 1998 [DOI] [PubMed] [Google Scholar]
- 228. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, and Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84, 2007 [DOI] [PubMed] [Google Scholar]
- 229. van der Vliet A. NADPH oxidases in lung biology and pathology: host defense enzymes, and more. Free Radic Biol Med 44: 938–955, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. van der Vliet A, Janssen-Heininger YMW, and Anathy V. Oxidative stress in chronic lung disease: from mitochondrial dysfunction to dysregulated redox signaling. Mol Aspects Med 63: 59–69, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Veith C, Drent M, Bast A, van Schooten FJ, and Boots AW. The disturbed redox-balance in pulmonary fibrosis is modulated by the plant flavonoid quercetin. Toxicol Appl Pharmacol 336: 40–48, 2017 [DOI] [PubMed] [Google Scholar]
- 232. Verma R, Kushwah L, Gohel D, Patel M, Marvania T, and Balakrishnan S. Evaluating the ameliorative potential of quercetin against the bleomycin-induced pulmonary fibrosis in wistar rats. Pulm Med 2013: 921724, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Vuorinen K, Ohlmeier S, Lepparanta O, Salmenkivi K, Myllarniemi M, and Kinnula VL. Peroxiredoxin II expression and its association with oxidative stress and cell proliferation in human idiopathic pulmonary fibrosis. J Histochem Cytochem 56: 951–959, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Waghray M, Cui Z, Horowitz JC, Subramanian IM, Martinez FJ, Toews GB, and Thannickal VJ. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J 19: 854–856, 2005 [DOI] [PubMed] [Google Scholar]
- 235. Wang CL, Kang J, and Li ZH. [Increased expression of NADPH oxidase p47-PHOX and p67-PHOX factor in idiopathic pulmonary fibrosis]. Zhonghua Jie He He Hu Xi Za Zhi 30: 265–268, 2007. (Article in Chinese) [PubMed] [Google Scholar]
- 236. Waring P. and Mullbacher A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol Cell Biol 77: 312–317, 1999 [DOI] [PubMed] [Google Scholar]
- 237. Wenzel P, Mollnau H, Oelze M, Schulz E, Wickramanayake JM, Muller J, Schuhmacher S, Hortmann M, Baldus S, Gori T, Brandes RP, Munzel T, and Daiber A. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid Redox Signal 10: 1435–1447, 2008 [DOI] [PubMed] [Google Scholar]
- 238. Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, and Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol 166: 1321–1332, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Wong HS, Dighe PA, Mezera V, Monternier PA, and Brand MD. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J Biol Chem 292: 16804–16809, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. World health organization. Ageing and health. 2018. http://who.int/news-room/fact-sheets/detail/ageing-and-health (accessed November26, 2018)
- 241. Wosniak J, Jr., Santos CX, Kowaltowski AJ, and Laurindo FR. Cross-talk between mitochondria and NADPH oxidase: effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid Redox Signal 11: 1265–1278, 2009 [DOI] [PubMed] [Google Scholar]
- 242. Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, and Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999 [DOI] [PubMed] [Google Scholar]
- 243. Wynn TA. and Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 44: 450–462, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Xie N, Tan Z, Banerjee S, Cui H, Ge J, Liu RM, Bernard K, Thannickal VJ, and Liu G. Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am J Respir Crit Care Med 192: 1462–1474, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Xu X, Dai H, and Wang C. Epithelium-dependent profibrotic milieu in the pathogenesis of idiopathic pulmonary fibrosis: current status and future directions. Clin Respir J 10: 133–141, 2016 [DOI] [PubMed] [Google Scholar]
- 246. Xun Z, Rivera-Sanchez S, Ayala-Pena S, Lim J, Budworth H, Skoda EM, Robbins PD, Niedernhofer LJ, Wipf P, and McMurray CT. Targeting of XJB-5-131 to mitochondria suppresses oxidative DNA damage and motor decline in a mouse model of Huntington's disease. Cell Rep 2: 1137–1142, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Yan F, Wang Y, Wu X, Peshavariya HM, Dusting GJ, Zhang M, and Jiang F. Nox4 and redox signaling mediate TGF-beta-induced endothelial cell apoptosis and phenotypic switch. Cell Death Dis 5: e1010, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Yanai H, Shteinberg A, Porat Z, Budovsky A, Braiman A, Ziesche R, and Fraifeld VE. Cellular senescence-like features of lung fibroblasts derived from idiopathic pulmonary fibrosis patients. Aging (Albany NY) 7: 664–672, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Yang IV. and Schwartz DA. Epigenetics of idiopathic pulmonary fibrosis. Transl Res 165: 48–60, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Yoon YS, Lee JH, Hwang SC, Choi KS, and Yoon G. TGF beta1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in Mv1Lu cells. Oncogene 24: 1895–1903, 2005 [DOI] [PubMed] [Google Scholar]
- 251. Yu G, Tzouvelekis A, Wang R, Herazo-Maya JD, Ibarra GH, Srivastava A, de Castro JPW, DeIuliis G, Ahangari F, Woolard T, Aurelien N, Arrojo EDR, Gan Y, Graham M, Liu X, Homer RJ, Scanlan TS, Mannam P, Lee PJ, Herzog EL, Bianco AC, and Kaminski N. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med 24: 39–49, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Yu Q, Lee CF, Wang W, Karamanlidis G, Kuroda J, Matsushima S, Sadoshima J, and Tian R. Elimination of NADPH oxidase activity promotes reductive stress and sensitizes the heart to ischemic injury. J Am Heart Assoc 3: e000555, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Zandalinas SI. and Mittler R. ROS-induced ROS release in plant and animal cells. Free Radic Biol Med 122: 21–27, 2018 [DOI] [PubMed] [Google Scholar]
- 254. Zank DC, Bueno M, Mora AL, and Rojas M. Idiopathic Pulmonary Fibrosis: aging, Mitochondrial Dysfunction, and Cellular Bioenergetics. Front Med (Lausanne) 5: 10, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Zhang H, Davies KJ, and Forman HJ. TGFbeta1 rapidly activates Src through a non-canonical redox signaling mechanism. Arch Biochem Biophys 568: 1–7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Zhang H, Davies KJA, and Forman HJ. Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med 88: 314–336, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Zhong Z, Liang S, Sanchez-Lopez E, He F, Shalapour S, Lin XJ, Wong J, Ding S, Seki E, Schnabl B, Hevener AL, Greenberg HB, Kisseleva T, and Karin M. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 560: 198–203, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Zhou L, Zhang H, Davies KJA, and Forman HJ. Aging-related decline in the induction of Nrf2-regulated antioxidant genes in human bronchial epithelial cells. Redox Biol 14: 35–40, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O'Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, and Kirkland JL. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14: 644–658, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]