Abstract
We conducted a clinical trial of oral valganciclovir, a drug with in vitro activity against Kaposi sarcoma (KS)–associated herpesvirus (KSHV), in classic KS. Five human immunodeficiency virus–seronegative participants received valganciclovir for up to six 4-week cycles at doses used for cytomegalovirus infection. None of the study subjects showed an objective response; KS progressed in 4 subjects after 1–4 cycles and remained stable in 1 subject after 6 cycles. KS biopsies showed minimal lytic KSHV antigen and gene expression at baseline and no treatment-associated changes. Although valganciclovir was not active against KS in this setting, other appropriately targeted anti-herpesviral strategies may prove to be more effective.
The Kaposi sarcoma (KS)–associated herpesvirus (KSHV; HHV8) is associated with all forms of KS, primary effusion lymphoma (PEL), and some cases of multicentric Castleman's disease (MCD) [1–3]. KSHV is essential, but not itself sufficient, for KS development.
Although the majority of KS spindle cells are latently infected, a proportion express genes associated with lytic infection. These cells may be a source of virus to sustain ongoing cycles of KSHV infection, provide paracrine signaling molecules that modulate the inflammatory and angiogenic components of the lesion, and lead to impaired growth regulation and immune evasion (reviewed in [4]). Thus, targeting lytically-infected cells with antiviral therapy may provide an approach to KS treatment.
In preclinical studies, KSHV was inhibited by ganciclovir, foscarnet, and cidofovir, but not acyclovir [5, 6]. Although several retrospective analyses suggested a reduced risk of KS in patients with AIDS who were treated with ganciclovir or foscarnet, other studies found no evidence for a protective effect [7]. The ability of anti-herpesvirus drugs to induce regression of established AIDS-associated KS has not been well studied; anecdotal reports of KS regression have appeared, but regression has not been consistently observed [8–11].
Valganciclovir, an orally bioavailable agent used to treat cytomegalovirus infection, is converted in vivo to ganciclovir. To assess whether targeting lytic virus with valganciclovir is a potential therapeutic strategy for established KS, we designed a clinical trial (NCT00096538) to test its safety, tolerability, and efficacy in patients with classic KS (CKS). We reasoned that studying individuals with CKS without overt immunocompromise would permit evaluation of an anti-herpesvirus therapeutic strategy in the absence of confounding factors associated with human immunodeficiency virus (HIV) infection and its treatment. To assess valganciclovir's effects on KSHV replication and tumor biology, we used immunohistochemistry and real-time quantitative PCR (QPCR) to evaluate serial tumor biopsy specimens for changes in lytic and latent viral gene expression and markers of angiogenesis.
PATIENTS AND METHODS
Eligible participants were HIV-seronegative adults with biopsy-proven KS, with a minimum of 8 cutaneous lesions, and without visceral KS. Additional requirements included Karnofsky performance status ≥70, life expectancy ≥12 months, and adequate hematologic and organ function, including creatinine clearance (Ccr) ≥50 mL/min. The protocol and consent form were reviewed and approved by the Memorial Sloan-Kettering Cancer Center Institutional Review Board/Privacy Board (New York), and written informed consent was obtained from all participants.
Each planned treatment cycle lasted 28 days. Valganciclovir was provided by Roche Laboratories. Treatment commenced at a dose of 900 mg by mouth twice daily for 3 weeks (450 mg twice daily for Ccr level of ≥50 <60 mL/min), followed by 900 mg orally once daily (450 mg once daily for Ccr level of ≥50 <60 mL/min) for a total of 24 weeks (ie, 6 treatment cycles) or until tumor progression, whichever occurred first. Tumor and toxicity assessments were performed at the beginning of each cycle. Two 3-mm punch biopsies of non-marker KS lesions were performed for correlative studies at the beginning of cycles 1, 2, and 5. KS response was assessed using minor modifications of previously described criteria [12].
Toxicity was graded using National Cancer Institute Common Toxicity Criteria, version 3.0. Treatment was withheld in the presence of grade 3 or 4 nonhematologic toxicity, an absolute neutrophil count of <0.5 × 103 cells/μL, or a platelet count of <20 × 103 platelets/μL and could be resumed at a 50% dose if the grade decreased to ≤1 within 2 weeks.
The planned sample was 15 subjects. If there were ≥3 objective responses, valganciclovir would be considered worthy of further study as a single agent for this indication. If there were <3 objective responders, valganciclovir could still be considered of interest as part of combination therapy regimens if there was evidence for down-regulation of KSHV lytic gene expression in lesional biopsy specimens.
Tumor Biopsy Studies
Biopsy specimens for immunohistochemistry were formalin fixed and paraffin embedded. Immunohistochemistry was performed using the BOND-MAX Autostainer (Leica Microsystems) using the Bond polymer define detection kit after antigen retrieval with Bond epitope retrieval solution 2 (Leica Microsystems). Antibodies to KSHV included LANA (clone LN3), ORF K8.1 (clone 2A3), ORF 59 (11D1), and viral interleukin-6 (v-IL6; polyclonal; Advanced Biotechnologies). Antibodies to endothelial antigens included CD34 (Biogenex), D2-40 (Covance), and podoplanin (AngioBio).
Frozen biopsy specimens were processed for KSHV viral mRNA transcriptional profiling as described elsewhere [13]. In brief, total RNA was isolated using TRI(Sigma) followed by polyA selection (Quiagen) and reverse transcribed into cDNA (Applied Biosystems), in accordance with the manufacturers’ protocols. Primer pairs specific for each of the viral open reading frames and reference controls (actin, GAPDH, myc) were employed in a SYBR-based real-time QPCR assay. Samples were assayed on an Opticon 2 unit (MJ Research). Relative levels (CT) for each viral mRNA were determined by automated threshold analysis using the manufacturer's software, normalized to the median of the 3 reference genes and subjected to unsupervised clustering.
RESULTS
Patients
Accrual was much slower than expected, leading to premature trial closure. We treated only 5 individuals during the period from October 2004 through August 2006. Several potential subjects were excluded because of low Ccr. However, the most common reasons potential subjects failed to enter were unwillingness to participate in an investigational study and logistic problems. A sixth subject consented to participate and met all study requirements, but the subject withdrew consent prior to starting, citing anxiety over taking an investigational drug.
Participant characteristics and treatment details are shown in Table 1. The study subjects were all men aged 58 to 83 years (median age, 78 years). Duration of biopsy-proven disease ranged from 6 months to almost 9 years (median duration, 54 months), although some of the subjects had undiagnosed skin lesions for long periods prior to biopsy. Three subjects had received prior systemic KS treatment, which included chemotherapy in all 3 and thalidomide in 2. Baseline Karnofsky performance status was 90 in 3 subjects and 80 and 70 in 1 subject each.
Table 1.
Baseline Characteristics and Valganciclovir Treatment
| Patient | Age, years | KPS | BaselineCCr,mL/min | Prior KS treatment(s) | No. of treatment cycles | KS response |
| 1 | 78 | 90 | 53 | None | 4 | Progression(“mixed”) |
| 2 | 81 | 70 | 87 | Liposomal doxorubicin, paclitaxel, etoposide | 1 | Progression |
| 3 | 83 | 80 | 66 | Radiation therapy,vincristine/vinblastine,liposomal doxorubicin,bleomycin,paclitaxel,thalidomide,alitretinoin gel | 3 | Progression |
| 4 | 70 | 90 | 61 | Liposomal doxorubicin,thalidomide | 6 | Stable |
| 5 | 58 | 90 | 85 | None | 3 | Progression |
NOTE. All 5 patients were men Ccr, Creatinine clearance; KPS, Karnofsky performance status; KS, Kaposi sarcoma.
Treatment and Toxicity
One participant with a baseline Ccr of 53 mL/min received reduced-dose valganciclovir throughout the study. The remaining 4 participants received the full planned dose at study entry. One of these (subject 4), whose Ccr was 61 mL/min at baseline, had a subsequent decrease in Ccr to 54 mL/min at the end of cycle 3. His dose was reduced, with no further decrease of renal function. Adverse events were generally mild, and except for the dose reduction noted above, there were no dose adjustments, treatment delays, or discontinuations related to treatment-emergent adverse events.
KS Response
The total number of treatment cycles ranged from 1 to 6 (median, 3). There were no objective responses. Four participants were removed prematurely because KS progressed after 1, 2, 3, and 4 cycles, respectively. One of these (subject 1) showed a “mixed response,” with flattening of some lesions present at baseline at the same time that other lesions progressed. One subject showed stable KS throughout 6 cycles of treatment.
Biologic Endpoints/Correlative Studies
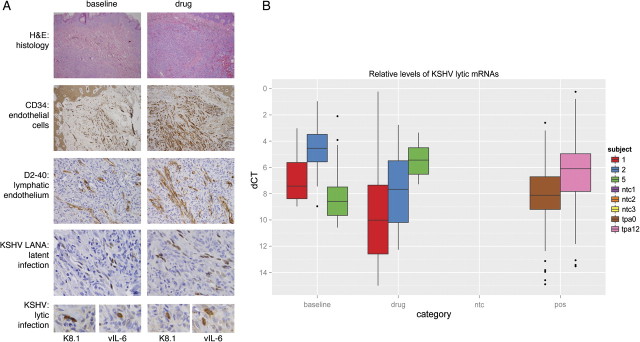
A total of 12 biopsy samples from 5 participants were evaluated by hematoxylin and eosin staining and immunochemistry for latent and lytic KSHV antigen expression and endothelial cell markers (Figure 1). Specifically, KSHV latency-associated nuclear antigen (LANA) is a latent antigen expressed in all infected tumor cells; vIL-6 is considered a lytic antigen but is expressed in a substantial proportion of latently infected cells in PEL and MCD; and both K8.1 and ORF59 protein (also called processivity factor 8) are early lytic viral antigens. We also evaluated endothelial cell density, as determined by expression of CD34 and, specifically, that of lymphatic endothelium, with antibodies against podoplanin and the M2A oncofetal antigen (D2-40).
Figure 1.
Pathologic and Virologic Comparison of Patients at Baseline and After Treatment. A, Histologic and immunophenotypic characterization of patient 1 shown at baseline and at day 113 after initiation of therapy. Biopsy specimens from both lesions showed numerous spindle cells in the dermis. Immunohistochemical staining for all endothelial (CD34) and, specifically, lymphatic endothelial cells (D2-40) highlight the vascular spaces. No appreciable difference was found in terms of quantity and quality of the blood vessels in biopsy specimens taken at baseline or after treatment. Kaposi sarcoma–associated herpesvirus (KSHV) latency was demonstrated with antibodies to LANA. Immunohistochemistry with antibodies to KSHV K8.1 and vIL-6 showed scattered positive cells, indicating lytic replication in a few cells at baseline as well as after treatment (original magnification for hematoxylin and eosin staining and CD34 is 10×; for D2-40, LANA, K8.1 and vIL-6, it was 40×). B, Shown on the category axis are baseline; drug (ie, ≥29 days of treatment); ntc (ie, nontemplate control); and pos (ie, the positive control). The positive controls are BCBL-1 cells before (tpa0) or 12 h after treatment with phorbol ester (tpa12). Phorbol esters have been shown to reactivate KSHV and uniformly increase the expression of all lytic viral genes. The vertical axis represents the KSHV mRNA level relative to gapdh mRNA on a -log2 scale (eg, a level of 4 corresponds to 1/16 the level of gapdh mRNA). The colors indicate subjects 1, 2, and 5 for which we had good RNA (RIN value, >7) at the before- and on-treatment time points. Finally, the box and whisker plots represent the median, upper and lower quartile, minimum and maximum of 93 viral and 3 human mRNAs. Dots indicate outliers, which are greater than or less than than 3/2 × quartile.
All biopsy specimens showed a heterogeneous cell population with vascular spaces admixed with inflammatory cells, including lymphocytes and macrophages. None of them exhibited extensive areas composed of spindle cells, but the density of vascular spaces was greatest in the biopsy specimen from subject 1, in which extensive hemosiderin deposits were also identified. Antibodies to CD34, podoplanin, and M2A highlighted vascular spaces, but cellular areas outside vascular spaces were usually negative. The biopsy samples from subject 2 also showed perivascular cells expressing endothelial cell antigens.
All biopsy specimens showed immunoreactivity with antibodies to KSHV-LANA in the cells lining the vascular spaces, which coincided with cells expressing lymphatic endothelial cell antigens. The proportion of KSHV-infected cells ranged from 5% to 40% and varied between subjects but remained consistent at different times after treatment in individual subjects. The biopsy specimens with the highest proportion of LANA-positive cells were from subject 2. Most pre- and posttreatment biopsies were negative for KSHV lytic antigen expression; only rare cells within some lesions expressed vIL-6 or K8.1. This was most evident in biopsy specimens from subject 3, in which 6–7 cells were found with K8.1 positivity. In addition, 1–2 cells were positive for K8.1 in patient 1 at the 2 on-treatment time points but not at baseline. No ORF59 expression was seen in any of the specimens. We observed no reduction of lytic antigen expression in any of the posttreatment biopsy specimens, compared with the baseline biopsy specimen.
QPCR detected KSHV latent mRNA encoding orf73/LANA in each of the biopsy specimens but showed low levels of viral lytic gene expression at baseline. There was intersubject variation in viral mRNA levels at baseline and subsequent time points. However, no significant changes in KSHV transcription were observed in response to valganciclovir administration.
DISCUSSION
In this small group of individuals with CKS, oral valganciclovir at doses recommended for treatment of cytomegalovirus infection had no detectable effect on tumor growth or KSHV antigen or gene expression. Although valganciclovir has been shown to reduce oropharyngeal KSHV shedding [14] (an effect not studied in this trial), we found no evidence that it influenced the course of established CKS. This is consistent with the observation by Little et al [11] that cidofovir failed to induce regression of HIV-associated or CKS, and there is no current evidence for the utility of this approach. The possibility exists, however, that people who develop KS while relatively immunocompetent do not express the viral target proteins of valganciclovir, which are products of late lytic genes, whereas a subset of patients who develop KS while severely immunosuppressed (ie, those with late-stage AIDS) do. Indeed, the quantity of KSHV lytic mRNA varies in lesions from different individuals [13], and a recent study showed that KS biopsy specimens from treatment-naive, HIV-positive Ugandans expressed a preponderance of lytic KSHV gene products [15]. Similar individuals might benefit from oral valganciclovir, possibly as an adjunct to systemic chemotherapy.
Although in most lesions lytic viral genes are expressed in only a small proportion of KSHV-positive cells, they have important pro-inflammatory and pro-angiogenic activities and are thought to be important in KS pathogenesis. These include vIL6, viral chemokines, and the viral G protein-coupled receptor [4]. A widely accepted model of KSHV-induced sarcomagenesis is that by expressing lytic viral products in a subset of cells, the majority population of adjacent inflammatory and latently infected endothelial cells is influenced in a paracrine fashion to proliferate [4]. It is likely that some latently infected cells switch periodically to the lytic program of gene expression, perpetuating these paracrine angiogenic effects. The lack of observed clinical responses may reflect the inability of valganciclovir to prevent the lytic switch and impede lytic gene expression, as evidenced by our immunohistochemistry and viral gene expression results. Our study was not, however, designed to evaluate whether valganciclovir prevents completion of the lytic program, resulting in an abortive lytic cycle.
At the doses and schedule used, valganciclovir was well tolerated in this group of older individuals. Although some potential participants were excluded because of moderately impaired renal function, a more significant accrual barrier was the reluctance of elderly people to enroll in an investigational drug study. Potential participants who declined enrollment cited fear of trying new and unproven treatments, dependence on others to bring them to clinic visits, and self-imposed ageism. Thus, although we expected that studying KS without confounding by HIV-associated or iatrogenic posttransplantation immunosuppression or HIV treatment would simplify evaluation of the effects of valganciclovir on KS, the target population proved difficult to accrue. Nonetheless, these findings, coupled with the negative findings reported for cidofovir [11], provide little support for the use of currently available anti-herpesviral drugs as single-agent treatments for KS, but leave open the possibility that other appropriately targeted anti-herpesviral strategies may prove more effective.
Funding
Roche Laboratories.
Acknowledgments
We thank Suzan Aird for data management and Andrea Martelli for research nursing support.
References
- 1.Moore PS, Chang Y. Detection of herpesvirus-like DNA sequences in KS in patients with and without HIV infection. N Engl J Med. 1995;332:1181–5. doi: 10.1056/NEJM199505043321801. [DOI] [PubMed] [Google Scholar]
- 2.Soulier J, Grollet L, Oksenhendler E, et al. Kaposi's Sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276–80. [PubMed] [Google Scholar]
- 3.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–91. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 4.Ganem D. KSHV and the pathogenesis of Kaposi sarcoma: listening to human biology and medicine. J Clin Invest. 2010;120:939–49. doi: 10.1172/JCI40567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medveczky MM, Horvath E, Lund T, Medveczky PG. In vitro antiviral drug sensitivity of the Kaposi's sarcoma-associated herpesvirus. AIDS. 1997;11:1327–2. doi: 10.1097/00002030-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Kedes DH, Ganem D. Sensitivity of Kaposi's sarcoma-associated herpesvirus replication to antiviral drugs. Implications for potential therapy. J Clin Invest. 1997;99:2082–6. doi: 10.1172/JCI119380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones JL, Hanson DL, Dworkin MS, Jaffe HW. Incidence and trends in Kaposi's sarcoma in the era of effective antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;24:270–4. doi: 10.1097/00126334-200007010-00013. [DOI] [PubMed] [Google Scholar]
- 8.Morfeldt L, Torssander J. Long-term remission of Kaposi's sarcoma following foscarnet treatment in HIV-infected patients. Scand J Infect Dis. 1994;26:749–52. doi: 10.3109/00365549409008645. [DOI] [PubMed] [Google Scholar]
- 9.Robles R, Lugo D, Gee L, Jacobson MA. Effect of antiviral drugs used to treat cytomegalovirus end-organ disease on subsequent course of previously diagnosed Kaposi's sarcoma in patients with AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:34–8. doi: 10.1097/00042560-199901010-00005. [DOI] [PubMed] [Google Scholar]
- 10.Cordero E, Lopez-Cortes LF, Viciana P, Alarcon A, Pachon J. Foscarnet and AIDS-associated Kaposi's sarcoma. AIDS. 1997;11:1787–8. [PubMed] [Google Scholar]
- 11.Little RF, Merced-Galindez F, Staskus K, et al. A pilot study of cidofovir in patients with Kaposi sarcoma. J Infect Dis. 2003;187:149–53. doi: 10.1086/346159. [DOI] [PubMed] [Google Scholar]
- 12.Cianfrocca M, Cooley TP, Lee JY, et al. Matrix metalloproteinase inhibitor COL-3 in the treatment of AIDS-related Kaposi's sarcoma: a phase I AIDS malignancy consortium study. J Clin Oncol. 2002;20:153–9. doi: 10.1200/JCO.2002.20.1.153. [DOI] [PubMed] [Google Scholar]
- 13.Dittmer DP. Transcription profile of Kaposi's sarcoma-associated herpesvirus in primary Kaposi's sarcoma lesions as determined by real-time PCR arrays. Cancer Res. 2003;63:2010–5. [PubMed] [Google Scholar]
- 14.Casper C, Krantz E, Corey L, et al. Valganciclovir for suppression of human herpesvirus–8 replication: a randomized, double-blind, placebo-controlled, crossover trial. J Infect Dis. 2008;198:23–30. doi: 10.1086/588820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phipps W, Orem J, Mutyaba I, et al. Characterization of lytic human herpesvirus-8 gene expression in Kaposi sarcoma tumor tissue and its clinical correlates. [abstract 4] 12th International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies (ICMAOI). Bethesda, MD: 2010:66. [Google Scholar]