Abstract
Background. In 1999, meningococcal serogroup C conjugate (MCC) vaccines were introduced in the United Kingdom for those under 19 years of age. The impact of this intervention on asymptomatic carriage of meningococci was investigated to establish whether serogroup replacement or protection by herd immunity occurred.
Methods. Multicenter surveys of carriage were conducted during vaccine introduction and on 2 successive years, resulting in a total of 48,309 samples, from which 8599 meningococci were isolated and characterized by genotyping and phenotyping.
Results. A reduction in serogroup C carriage (rate ratio, 0.19) was observed that lasted at least 2 years with no evidence of serogroup replacement. Vaccine efficacy against carriage was 75%, and vaccination had a disproportionate impact on the carriage of sequence type (ST)-11 complex serogroup C meningococci that (rate ratio, 0.06); these meningococci also exhibited high rates of capsule expression.
Conclusions. The impact of vaccination with MCC vaccine on the prevalence of carriage of group C meningococci was consistent with herd immunity. The high impact on the carriage of ST-11 complex serogroup C could be attributed to high levels of capsule expression. High vaccine efficacy against disease in young children, who were not protected long-term by the schedule initially used, is attributed to the high vaccine efficacy against carriage in older age groups.
Bacterial meningitis and septicemia are among the diseases most feared by parents of young children worldwide. An important causative organism, Neisseria meningitidis, is a frequent commensal in the human nasopharynx, with a population carriage rate of around 10% in industrialized countries [1–3]. Meningococcal serogroup C disease has been endemic in the United Kingdom for at least 30 years, but a steady rise in the number of serogroup C meningococcal infections among adolescents during the 1990s [4] prompted the United Kingdom Departments of Health to implement the first national vaccination program with meningococcal serogroup C conjugate (MCC) vaccine. Infants received 2 doses at age 2 months, 3 months, and 4 months, and older children and teenagers up to the age of 19 years were offered a single dose; vaccinations for those aged 5 years and over were conducted through schools [5]. Clinical trials assuring the safety and immunogenicity of the vaccine had been carried out, and the vaccine was licensed on the basis of serological correlates of protection [6] without direct evidence of efficacy. As a consequence, enhanced surveillance was established to monitor any changes in disease trends following introduction of the vaccine [7, 8].
The high genetic and antigenic diversity of meningococcal populations is relevant to disease incidence; outbreaks of disease caused by this normally harmless bacterium indicate the presence in a human population of hyperinvasive meningococci that express one of the disease-associated capsules, corresponding to serogroups A, B, C, Y, W-135, or, more rarely, X. Multilocus sequence typing (MLST) [9] has demonstrated that most meningococcal disease in Europe is caused by meningococci that belong to the sequence type (ST)-8, ST-11, ST-32, ST-41/44, or ST-269 clonal complexes [10]. The increased incidence of serogroup C disease in the United Kingdom during the 1990s was the result of the spread of a serogoup C variant of the ST-11 complex (formerly ET-37 complex [11]), first identified in Canada [12].
The impact of the United Kingdom MCC vaccine program on meningococci carried by individuals aged 15–19 years in the United Kingdom was investigated. The study was powered to detect changes in the population of serogroup C ST-11 complex bacteria [13], which, although rarely isolated from carriers, are responsible for a disproportionate number of cases of invasive disease [14]. Changes in the prevalence of all clonal complexes and the proportion of meningococci expressing each of the disease-associated serogroups were also investigated. Data on the effect of vaccination 1 year after its introduction on the carriage of meningococci that were phenotypically serogroup C have been published as a research letter [13]. The definitive results of the 2-year studies showing the impact of the MMC vaccine program on carriage of meningococcal genotypes are presented here for the first time.
Methods
Study design. The study comprised 3 consecutive cross-sectional surveys of meningococcal carriage in 15-19-year-old students attending school or college full or part time (i.e., in further education but not attending a university). The surveys were conducted during vaccine introduction (November and December 1999) and after vaccine introduction (November and December 2000 and 2001). Subjects were recruited in 8 geographical regions throughout the United Kingdom: Bangor, Cardiff, Glasgow, London, Nottingham, Oxford, Plymouth and Stockport, as described elsewhere [13, 15]. For the later surveys, all subjects had been offered vaccination in 1999, and a small number of subjects were sampled on successive years. The age group of the study subjects was chosen because this group was the first to be vaccinated, it was readily accessible, and it exhibits high rates of meningococcal carriage [3]. The Trent Multi- Centre Research Ethics Committee approved the study.
Sample population and sampling methods. Nasopharyngeal swab samples were obtained through the mouth using cotton swabs, which were either plated on site or placed in transport medium to be plated in the laboratory within 5 hours. Samples were plated on selective plate medium that contained gonococcal agar base, antibiotics (vancomycin, colistin, nystatin, and trimethoprim), and 5% lysed or whole horse blood. The inoculated plates were incubated at 37°C in an atmosphere of5%carbon dioxide and visually inspected for putative Neisseria colonies at 24 and 48 hours. Putative Neisseria colonies were examined by Gram film (gram-negative diplococci) and examined for oxidase-positive reaction. Individual colonies were subcultured to pure culture. Where necessary, these were further discriminated by Gonocheck (EY) prior to storage in glycerol broth or with a commercial bead system (Microbank; Prolab) at ‒20°C to ‒80°C. Cultures that were positive for N. meningitidis were used to prepare lysed cell suspensions by sweeping 10–20 colonies and emulsifying them in 2mL of saline. A sample (250 µL) of this suspension was placed in a labeled tube and heated to 100°C for 5 min. Tubes were then snap-chilled at ‒20°C for 5 min, and centrifuged at 20,000 g for 10 min. Meningococcal cultures were not available for the London center for 1999, and this center was not included in the analysis.
Isolate characterization. MLST and siaD gene amplification and nucleotide sequencing were performed in accordance with published methods [16–19], employing robotic liquid handling and automated DNA analyzers. MLST determines the nucleotide sequences of 7 housekeeping gene fragments, each of which is assigned a unique allele number; the combination of the 7 numbers represents an ST. The STs were assigned to clonal complexes [11] by reference to the Neisseria pubMLST database (http://pubmlst.org/neisseria). The presence of capsule-specific genes was determined by polymerase chain reaction (PCR) amplification of the siaD gene or the capsule null locus (cnl) in the capsular region. Meningococci with the cnl locus are unable to synthesize a capsule. Where present, the nucleotide sequence of the siaD gene was determined, to establish the presence of alleles encoding the polysialyltransferases that synthesize serogroup B, C, Y, or W-135 capsules.
Data manipulation and statistical analysis. Sequences were assembled using the STARS (Sequence Typing Analysis and Retrieval System) [20] package, which is integrated into the Staden package [21] (available from http://pubmlst.org/software). Data on isolates were stored in a proprietary database based on the mlstdbNet Software [22]. Statistical analyses were performed using Intercooled Stata software for windows (version 8.0; StataCorp). Proportions were compared using a χ2 test or Fisher exact test, as appropriate. Data are presented for the 3 years of the study. All comparisons over time were made between 1999 and 2001. There was a small degree of overlap between individuals who were sampled more than once (367 of the 14,056 individuals sampled in 1999 were among the 17,770 individuals sampled in 2001 [2.1% of the 2001 sample]).
Results
In November 1999, 14,057 students were sampled at the same time as vaccine administration; of these, 2348 samples yielded meningococci, a carriage prevalence of 16.7%. In November 2000 and 2001—1 and 2 years after vaccine introduction—the prevalence of carriage was 17.7% (12,931 of 16,482) and 18.7% (3320 of 17,770) respectively. The increase in overall carriage prevalence over these 3 years (rate ratio 2001:1999, 1.12) was statistically significant (P < .001), but varied among sampling centers (rate ratios, 0.85–1.59; data not shown).
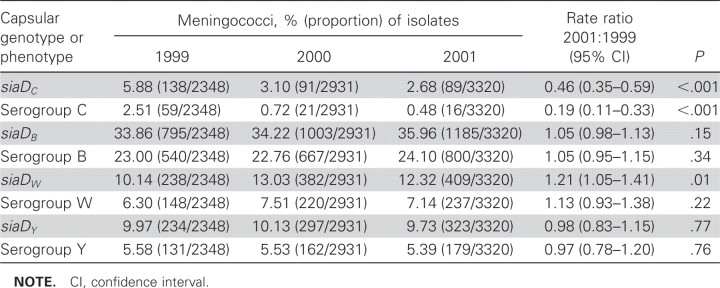
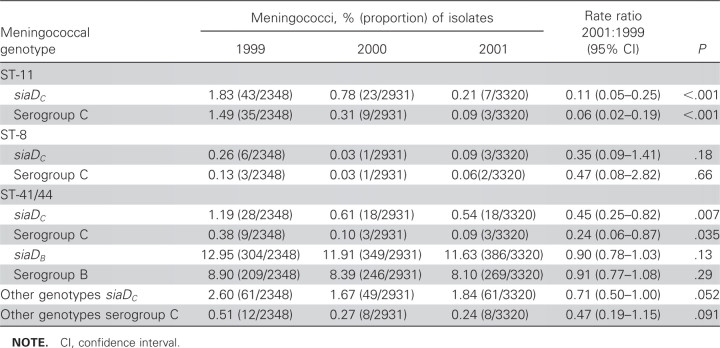
From 1999 to 2001, there was a large decrease in the prevalence of carriage of meningococci that expressed serogroup C and also in carriage of meningococci that contained the siaDC gene responsible for C capsule production (rate ratio, 0.19 and 0.46, respectively; P < .001 for both) (table 1). The prevalence of carriage of meningococci that expressed other serogroups increased slightly or remained similar over the duration of the study (rate ratios, 0.97- 1.21; table 1). A logistic regression model showed no evidence of differences between centers with respect to trends in the prevalence of carriage by serogroup, apart from serogroup Y, which decreased in some centers and increased in others. Analysis by clonal complex, siaDC gene, and serogroup expression confirmed a relatively low prevalence of carriage of ST-11 complex siaDC meningococci—the predominant epidemic strain—in 1999 (tables 1 and 2). The prevalence of carriage of this clonal complex fell sharply by 83% in 2000 and 94% in 2001 (P < .001) (table 2). There was some evidence for reduction in the prevalence of carriage of the other diseaseassociated meningococcal genotypes with the siaDC gene, but not in prevalence of carriage of the genotypes with the siaDB, siaDY, or siaDW genes.
Table 1.
Carriage of meningococci encoding disease-associated capsules and their serogroup.
Table 2.
Carriage of meningococci by clonal complex and capsule-specific genes.
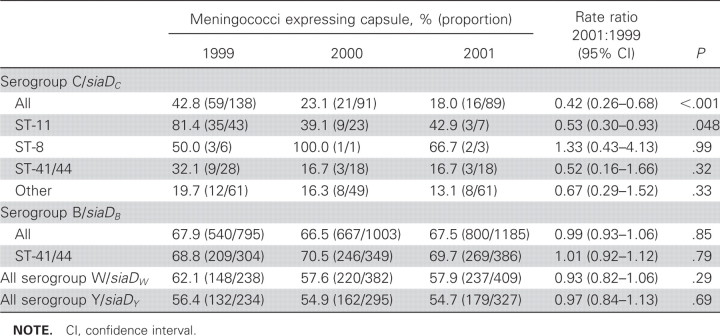
The presence of genes determining particular serogroups and their expression varied with clonal complex (table 3). In 1999, before vaccine introduction, the proportion of siaDC meningococci that expressed their capsule was significantly lower than the proportion of siaDB, siaDW, and siaDY meningococci that expressed their capsule. (P < .01, <.001, and .011, respectively). For siaDC meningococci, capsule expression varied by the clonal complex; most ST-11 siaDC meningococci expressed their capsules (35 of 43 [81%]), whereas the proportion of other clonal complexes with the siaDC gene that expressed their capsule was lower (24 of 95 [25%]; P < .001). For siaDC meningococci, there was a reduction in the proportion of meningococci that expressed their capsule from 1999 to 2001 (rate ratio, 0.42 [95% confidence interval {CI}, 0.26–0.68]) (table 3). No change was observed in levels of capsule expression among siaDB, siaDW, or siaDY meningococci.
Table 3.
Expression of capsules by carried meningococci.
In the 2001 survey, 3235 (97.4%) of the 3320 individuals from whom meningococci were isolated reported their vaccination status; of these, 2986 (92.3%) had been vaccinated. A total of 76 (2.55%) of the vaccinated subjects carried siaDC meningococci, compared with 9 (3.61%) of the 249 unvaccinated subjects; 12 (0.40%) of the vaccinated subjects carried serogroup C meningococci, compared with 4 (1.61%) of the unvaccinated individuals. Vaccine effectiveness against carriage was calculated to be 75% (100 × [1-(0.40/1.61)]; 95% CI, 23%–92%) for serogroup C meningococci and 29% (95% CI, −39% to 64%) for siaDC meningococci. The reduction in expression of serogroup C by siaDC meningococci was not seen among the 17 unvaccinated individuals sampled in 2000 and 2001 who carried siaDC meningococci, 8 (47%) of whom carried meningococci that expressed serogroup C.
Discussion
This large-scale study was designed to account for the anticipated low prevalence of carriage of hyperinvasive ST-11 complex siaDC meningococci. This expectation was confirmed during the course of the study; this complex accounted for only 82 of the 8599 meningococci isolates obtained. Despite these low numbers, however, the study demonstrated that vaccination with MCC vaccine had a statistically significant effect on the prevalence of carriage that was specific to both serogroup and clonal complex. To our knowledge, this study represents the largest survey of meningococcal carriage reported to date. The results demonstrate the feasibility of handling and storage of large sample collections and MLST data sets, the potential application of this technology in large-scale population studies, and the relevance of this approach in studies of vaccine impact.
Over the course of the study, from just before vaccine implementation until 2 years after implementation, there was a small increase in the prevalence of meningococcal carriage (from 16.7% to 18.7%). The most likely explanation for this increase was improved sampling efficiency at some of the centers over the course of the study [23]. The composition of the population of meningococci isolates recovered from carriers remained essentially unchanged, with the exception of the serogroup C, and especially the serogroup C ST-11 complex bacteria, which declined more than any other clonal complex (P = .005). By comparison, the prevalence of carriage of the meningococci most commonly associated with serogroup B disease—siaDB members of the ST-41/44 complex—was unaffected by the introduction of vaccine (table 2). These organisms were the second most frequent cause of meningococcal disease in 1999, and they became the most common cause of meningococcal disease after MCC vaccine introduction [4, 24].
The decline in the prevalence of carriage of serogroup C meningococci between 1999 and 2000 that we reported previously [13] was confirmed, and a further decline was evident from 2000 to 2001 (table 1). This result was consistent with herd immunity, i.e., immunity among teenagers that interrupted the transmission of serogroup C meningococci, operating during the introduction of the vaccine [25]. The comparisons of data from vaccinated and unvaccinated individuals provided further evidence of a herd immunity effect. Genotyping of the siaD locus showed that the reduction in the prevalence of carriage of serogroup C organisms was the result of 2 effects that were of approximately equal magnitude: (1) the loss of organisms containing the siaDC allele from the population of carried meningococci and (2) lower rates of expression of the siaDC gene (tables 1 and 3).
The proportion of siaDB meningococci that expressed serogroup B capsular polysaccharide was high, as observed in previous studies [26], and this proportion was consistent among clonal complexes, there being no difference between the expression rate of all serogroup B organisms and the expression rate for members of the hyperinvasive ST-41/44 complex. For serogroup C, ST-11 meningococci showed much higher levels of expression (81% of isolates) than siaDC meningococci not belonging to hyperinvasive lineages (20%). This was consistent with data obtained previously in a carriage study conducted in Bavaria, although the size of the Bavarian study was insufficient for a robust demonstration of this effect [3].
The success of the MCC vaccines in reducing disease in the United Kingdom [5] can therefore be attributed to the combined efficacy of the vaccine against disease and carriage, the high proportion of serogroup C polysaccharide expression among ST-11 complex meningococci, and the nature of the vaccine introduction. Due to a number of high-profile outbreaks in educational establishments prior to vaccine introduction, the 15—19-year-old age group was targeted for vaccination early in the campaign. As this age group is characterized by both high rates of carriage and transmission of meningococci [15], the effects of vaccination on the ST-11 siaDC meningococci were particularly marked. This was fortuitous given that the infant schedule used for MCC vaccines in the United Kingdom at the time of vaccine introduction did not confer long term protection [27]. Young children were consequently protected from infection by the virtual elimination of the disease-causing meningococci from older age groups (table 3).
It is likely that the susceptibility of ST-11 complex siaDC meningococci to MCC vaccines was a consequence of their highlevel expression of serogroup C polysaccharide, but it is unclear why this should have been so. Although the capsule is the major virulence determinant for meningococci, it cannot have evolved specifically to cause disease because meningococci gain no benefits from causing disease in terms of improved host-to-host transmission [28]. Meningococci that express a disease associated capsule must, therefore, balance any costs associated with possession of the capsule (in terms of removing hosts from the transmission system) with a benefit, for example, in improved transmission among hosts. The retention of capsule expression during colonization by siaDB meningococci can be rationalized by the relatively poor immunogenicity of this polysaccharide [29], whereas most siaDC meningococci downregulate the expression of the more immunogenic serogroup C capsule during carriage. However, it appears from these data that this strategy is not operating in the case of ST-11 complex meningococci, perhaps as a consequence of the capsule having a more important, and incompletely understood, role in the transmission of these low-prevalence bacteria.
Given the very strong effect of the MCC vaccine on ST-11 complex siaDC meningococci, it is difficult to explain the lack of emergence of ST-11 meningococci expressing other serogroups [8]. Capsular replacement by ST-11 complex serogroup B or W-135 meningococci was a particular concern because these organisms have caused disease outbreaks elsewhere and successional replacements have been reported in various countries [28, 30–32]. It is possible that the relative uniformity of the ST-11 meningococci in their subcapsular antigens [33] was at least partially responsible for this result as a consequence of naturally induced herd immunity against these antigens, but this cannot be concluded from the current data.
Previous experience with conjugate vaccines suggested that protection is not only conferred by the induction of immunological memory among vaccinated individuals [34, 35], but also by reduction of carriage and transmission among the unvaccinated population [36–40]. Indirect protection is a likely explanation for both the immediate repercussions of the massvaccination campaign and the persistence and accentuation of the effects over time. Indeed, the campaign's impact on the protection of the unvaccinated is higher by these measures than might have been expected. The prevalence of carriage of the predominant disease-causing meningococcus, ST-11 complex siaDC, showed the most dramatic decline after vaccination, although the vaccine did not specifically target these meningococci. This decline was explained by the relatively high proportion of ST-11 meningococci that expressed C capsule, compared with siaDC meningococci belonging to other clonal complexes, such that anticapsular antibody generated by vaccination had a disproportionate impact on carriage of the ST-11 complex siaDC genotype. The strong association of this genotype with the expression of capsule may be linked to its high virulence.
This is the first time that the effects of a national vaccination campaign against bacterial disease have been monitored from its initiation by large-scale carriage studies that have included detailed phenotypic and genetic characterization of the bacterial isolates recovered. Understanding the effects of the United Kingdom'sMCC vaccine program on pharyngeal carriage of N. meningitidis has and will assist other countries in planning the introduction of conjugate or other novel meningococcal vaccines. In particular, it will be of interest to establish whether the remarkable herd immunity effect of vaccination on the ST-11 siaDC meningococci, in some ways similar to that observed with Hib vaccines [36], occurs with other lineage and/or serogroup or serotype combinations of encapsulated bacteria. Finally, the data gathered in this study will be invaluable for the development of mathematical models to enhance our understanding of, and to help predict the outcome of, large-scale public health interventions aimed at bacterial populations.
Acknowledgments
We are extremely grateful to the following organizations for providing research funding at extremely short notice after the announcement of the immunization campaign in July 1999, without which the study would have been impossible: the Wellcome Trust, the Chief Scientist Office of the Scottish Executive Health Department , and the Meningitis Trust (questionnaire). We thank the following individuals for their assistance with implementation of the study: A. D. Carr, C. Lewis, D. Casey, K. T. Dunkin, C. Roberts, R. A. Barnes, J. Murray, A. Paull, Y. K. Lau (deceased), S. Welch, P. Marks, D. Turner, D. Griffiths, M. Clacher, G. Lewendon, R. Mathews, and M. E. Ramsay. We thank Noel D. McCarthy for statistical advice. We are indebted to the student volunteers and the many other individuals who made this study possible, including nurses, laboratory staff, school principals and staff, and colleagues who supported the study in various ways.
Footnotes
Presented in part: 14th International Pathogenic Neisseria Conference, Milwaukee, WI, 6–13 September 2004 (first abstract, first session, vaccinology).
Potential conflicts of interest: none reported.
Financial support: the Wellcome Trust (grant 062057; sampling in England and Wales and molecular characterization of isolates); the Chief Scientist Office of the Scottish Executive Health Department (sampling in Scotland); and the Meningitis Trust (questionnaire).
References
- 1.Cartwright KAV, Stuart JM, Jones DM, Noah ND. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol Infect. 1987;99:591–601. doi: 10.1017/s0950268800066449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caugant DA, Høiby EA, Magnus P, et al. Asymptomatic carriage of Neisseria meningitidis in a randomly sampled population. J Clin Microbiol. 1994;32:323–30. doi: 10.1128/jcm.32.2.323-330.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claus H, Maiden MC, Wilson DJ, et al. Genetic analysis of meningococci carried by children and young adults. J Infect Dis. 2005;191:1263–71. doi: 10.1086/428590. [DOI] [PubMed] [Google Scholar]
- 4.Gray SJ, Trotter CL, Ramsay ME, et al. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J Med Microbiol. 2006;55:887–96. doi: 10.1099/jmm.0.46288-0. [DOI] [PubMed] [Google Scholar]
- 5.Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup Cdisease in the UK: a success story. Vaccine. (Suppl 1) 2001;20:S58–S67. doi: 10.1016/s0264-410x(01)00299-7. [DOI] [PubMed] [Google Scholar]
- 6.Borrow R, Andrews N, Goldblatt D, Miller E. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect Immun. 2001;69:1568–73. doi: 10.1128/IAI.69.3.1568-1573.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shigematsu M, Davison KL, Charlett A, Crowcroft NS. National enhanced surveillance of meningococcal disease in England, Wales and Northern Ireland, January 1999–June 2001. Epidemiol Infect. 2002;129:459–70. doi: 10.1017/s0950268802007549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maiden MCJ, Spratt BG. Meningococcal conjugate vaccines: new opportunities and new challenges. Lancet. 1999;354:615–6. doi: 10.1016/s0140-6736(99)00252-4. [DOI] [PubMed] [Google Scholar]
- 9.Maiden MC. Multilocus sequence typing of bacteria. Annu Rev Microbiol. 2006;60:561–88. doi: 10.1146/annurev.micro.59.030804.121325. [DOI] [PubMed] [Google Scholar]
- 10.Brehony C, Jolley KA, Maiden MC. Multilocus sequence typing for global surveillance of meningococcal disease. FEMS Microbiol Rev. 2007;31:15–26. doi: 10.1111/j.1574-6976.2006.00056.x. [DOI] [PubMed] [Google Scholar]
- 11.Caugant DA. Population genetics and molecular epidemiology of Neisseria meningitidis. Apmis. 1998;106:505–25. [PubMed] [Google Scholar]
- 12.Jelfs J, Munro R, Ashton F, Rawlinson W, Caugant DA. Program and abstracts of the 11th International Pathogenic Neisseria Conference. Nice, France: Editions E.D.K.; 1998. Global study of variation in a new variant of the ET-37 complex of Neisseria meningitidis; p. 5. [Google Scholar]
- 13.Maiden MC, Stuart JM, UK Meningococcal Carriage Group Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet. 2002;359:1829–31. doi: 10.1016/S0140-6736(02)08679-8. [DOI] [PubMed] [Google Scholar]
- 14.Yazdankhah SP, Kriz P, Tzanakaki G, et al. Distribution of serogroups and genotypes among disease-associated and carried isolates of Neisseria meningitidis from the Czech Republic, Greece, and Norway. J Clin Microbiol. 2004;42:5146–53. doi: 10.1128/JCM.42.11.5146-5153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacLennan J, Kafatos G, Neal K, et al. Social behavior and meningococcal carriage in British teenagers. Emerg Infect Dis. 2006;12:950–7. doi: 10.3201/eid1206.051297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jolley KA, Kalmusova J, Feil EJ, et al. Carried meningococci in the Czech Republic: a diverse recombining population. Journal of Clinical Microbiology. 2000;38:4492–8. doi: 10.1128/jcm.38.12.4492-4498.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borrow R, Claus H, Guiver M, et al. Non-culture diagnosis and serogroup determination of meningococcal B and C infection by a sialyltransferase (siaD) PCR ELISA. Epidemiol Infect. 1997;118:111–117. doi: 10.1017/s0950268896007261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borrow R, Claus H, Chaudhry U, et al. siaD PCR ELISA for confirmation and identification of serogroup Y and W135 meningococcal infections. FEMS Microbiol Lett. 1998;159:209–14. doi: 10.1111/j.1574-6968.1998.tb12862.x. [DOI] [PubMed] [Google Scholar]
- 19.Claus H, Maiden MC, Maag R, Frosch M, Vogel U. Many carried meningococci lack the genes required for capsule synthesis and transport. Microbiology. 2002;148:1813–9. doi: 10.1099/00221287-148-6-1813. [DOI] [PubMed] [Google Scholar]
- 20.Jolley KA, Feil EJ, Chan MS, Maiden MC. Sequence type analysis and recombinational tests (START) Bioinformatics. 2001;17:1230–1. doi: 10.1093/bioinformatics/17.12.1230. [DOI] [PubMed] [Google Scholar]
- 21.Staden R. The Staden sequence analysis package. Mol Biotechnol. 1996;5:233–41. doi: 10.1007/BF02900361. [DOI] [PubMed] [Google Scholar]
- 22.Jolley KA, Chan MS, Maiden MC. mlstdbNet-distributed multi-locus sequence typing (MLST) databases. BMC Bioinformatics. 2004;5:86. doi: 10.1186/1471-2105-5-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham R, Matthews R, Lewendon G, Harrison S, Stuart JM. Improved rate of isolation of Neisseria meningitidis by direct plating of pharyngeal swabs. J Clin Microbiol. 2001;39:4575–6. doi: 10.1128/JCM.39.12.4575-4576.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mooney JD, Christie P, Robertson C, Clarke SC. The impact of meningococcal serogroup C conjugate vaccine in Scotland. Clin Infect Dis. 2004;39:349–56. doi: 10.1086/421947. [DOI] [PubMed] [Google Scholar]
- 25.Ramsay ME, Andrews NJ, Trotter CL, Kaczmarski EB, Miller E. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. BMJ. 2003;326:365–6. doi: 10.1136/bmj.326.7385.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ala'Aldeen DAA, Neal KR, Ait-Tahar K, et al. Dynamics of meningococcal long-term carriage among university students and their implications for mass vaccination. J Clin Microbiol. 2000;38:2311–6. doi: 10.1128/jcm.38.6.2311-2316.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364:365–7. doi: 10.1016/S0140-6736(04)16725-1. [DOI] [PubMed] [Google Scholar]
- 28.Maiden MC. Dynamics of bacterial carriage and disease: lessons from the meningococcus. Adv Exp Med Biol. 2004;549:23–9. doi: 10.1007/978-1-4419-8993-2_5. [DOI] [PubMed] [Google Scholar]
- 29.Wyle FA, Artenstein MS, Brandt BL, et al. Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis. 1972;126:514–521. doi: 10.1093/infdis/126.5.514. [DOI] [PubMed] [Google Scholar]
- 30.Stefanelli P, Fazio C, Neri A, Sofia T, Mastrantonio P. First report of capsule replacement among electrophoretic type 37 Neisseria meningitidis strains in Italy. J Clin Microbiol. 2003;41:5783–6. doi: 10.1128/JCM.41.12.5783-5786.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alcala B, Arreaza L, Salcedo C, Uria MJ, De La Fuente L, Vazquez JA. Capsule switching among C:2b:P1.2,5 meningococcal epidemic strains after mass immunization campaign, Spain. Emerg Infect Dis. 2002;8:1512–4. doi: 10.3201/eid0812.020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Trallero E, Vicente D, Montes M, Cisterna R. Positive effect of meningococcal C vaccination on serogroup replacement in Neisseria meningitidis. Lancet. 2002;360:953. doi: 10.1016/S0140-6736(02)11061-0. [DOI] [PubMed] [Google Scholar]
- 33.Wang J-F, Caugant DA, Morelli G, Koumaré B, Achtman M. Antigenic and epidemiological properties of the ET-37 complex of Neisseria meningitidis. J Infect Dis. 1993;167:1320–9. doi: 10.1093/infdis/167.6.1320. [DOI] [PubMed] [Google Scholar]
- 34.Heath PT, McVernon J. The UK Hib vaccine experience. Arch Dis Child. 2002;86:396–9. doi: 10.1136/adc.86.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly DF, Moxon ER, Pollard AJ. Haemophilus influenzae type B conjugate vaccines. Immunology. 2004;113:163–74. doi: 10.1111/j.1365-2567.2004.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eskola J, Takala AK, Kayhty H. Haemophilus influenzae type B polysaccharide-protein conjugate vaccines in children. Curr Opin Pediatr. 1993;5:55–9. doi: 10.1097/00008480-199302000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Cohen R, Levy C, de La Rocque F, et al. Impact of pneumococcal conjugate vaccine and of reduction of antibiotic use on nasopharyngeal carriage of nonsusceptible pneumococci in children with acute otitis media. Pediatr Infect Dis J. 2006;25:1001–7. doi: 10.1097/01.inf.0000243163.85163.a8. [DOI] [PubMed] [Google Scholar]
- 38.Dagan R, Givon-Lavi N, Zamir O, et al. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J Infect Dis. 2002;185:927–36. doi: 10.1086/339525. [DOI] [PubMed] [Google Scholar]
- 39.Dagan R, Muallem M, Melamed R, Leroy O, Yagupsky P. Reduction of pneumococcal nasopharyngeal carriage in early infancy after immunization with tetravalent pneumococcal vaccines conjugated to either tetanus toxoid or diphtheria toxoid. Pediatr Infect Dis J. 1997;16:1060–4. doi: 10.1097/00006454-199711000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Dagan R, Melamed R, Muallem M, et al. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J Infect Dis. 1996;174:1271–8. doi: 10.1093/infdis/174.6.1271. [DOI] [PubMed] [Google Scholar]