Abstract
Context
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy of reproductive-aged women. In pregnancy, women with PCOS experience increased risk of miscarriage, gestational diabetes, preeclampsia, and extremes of fetal birth weight, and their offspring are predisposed to reproductive and cardiometabolic dysfunction in adulthood. Pregnancy complications, adverse fetal outcomes, and developmental programming of long-term health risks are known to have placental origins. These findings highlight the plausibility of placental compromise in pregnancies of women with PCOS.
Evidence Synthesis
A comprehensive PubMed search was performed using terms “polycystic ovary syndrome,” “placenta,” “developmental programming,” “hyperandrogenism,” “androgen excess,” “insulin resistance,” “hyperinsulinemia,” “pregnancy,” and “pregnancy complications” in both human and animal experimental models.
Conclusions
There is limited human placental research specific to pregnancy of women with PCOS. Gestational androgen excess and insulin resistance are two clinical hallmarks of PCOS that may contribute to placental dysfunction and underlie the higher rates of maternal–fetal complications observed in pregnancies of women with PCOS. Additional research is needed to prevent adverse maternal and developmental outcomes in women with PCOS and their offspring.
In this review, we summarize current knowledge of placental dysfunction in human and animal models of PCOS and propose future directions for placental research in the population with PCOS.
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder of reproductive-aged women (1, 2). With an estimated incidence of 5% to 15% (3), PCOS has reproductive, metabolic, and gynecologic implications for women throughout their lifespan. Women with PCOS can experience increased risks of abnormal uterine bleeding, infertility, depression, insulin resistance, type 2 diabetes, hyperlipidemia, hypertension, cardiovascular disease, and endometrial cancer (2). In the United States, health care expenditures related to PCOS in reproductive-aged women are estimated to exceed $4 billion annually (4).
The Rotterdam criteria are generally used for the clinical diagnosis of PCOS, requiring two of the following: (i) oligoovulation or anovulation, (ii) clinical or biochemical evidence of hyperandrogenism, and (iii) ultrasound findings of polycystic ovaries (5). Subsequently, four distinct PCOS phenotypes have been recognized, reflecting the clinical heterogeneity of the syndrome: type A (hyperandrogenism, oligo-anovulation, and polycystic ovarian morphology), type B (hyperandrogenism and oligo-anovulation), type C (hyperandrogenism and polycystic ovarian morphology), and type D (oligo-anovulation and polycystic ovarian morphology) (6). Importantly, the diagnosis of PCOS requires the exclusion of other conditions of androgen excess, including congenital adrenal hyperplasia, adrenal or ovarian neoplasm, and Cushing syndrome (5). The etiology of PCOS is largely unknown (2, 7), and there are currently no proven strategies for PCOS prevention or cure.
PCOS Is Associated With Adverse Pregnancy Outcomes
As a reproductive disorder, PCOS may be accompanied by a variety of fertility challenges. Women with PCOS may have decreased fecundity due to etiologies such as anovulation or oligoovulation, reduced oocyte competence, and impaired endometrial receptivity (8–10). Women with PCOS have a higher prevalence of overweight or obesity, both independent risk factors for subfertility (11–13). Women with oligoovulation or anovulation may require ovulation induction agents to achieve pregnancy (14, 15). When assisted reproductive technologies (ARTs) such as in vitro fertilization are used, women with PCOS may experience poorer embryo development, lower pregnancy rates, and higher risk of iatrogenic ovarian hyperstimulation syndrome, relative to women without PCOS (16). Importantly, once pregnancy is achieved, women with PCOS are also at greater risk of pregnancy complications, as summarized below.
Maternal consequences
Compared with controls without PCOS, women with PCOS can demonstrate a twofold to fourfold increased risk of pregnancy-induced hypertension, preeclampsia, gestational diabetes, spontaneous preterm labor, and increased need for cesarean delivery (17–19). Owing to the phenotypic heterogeneity of PCOS, some studies have investigated these maternal outcomes with regard to the specific phenotype expressed. However, it remains unclear whether these risks are different among women of varying PCOS phenotypes (especially hyperandrogenic vs normoandrogenic), and how the presence of additional risk factors such as obesity, metabolic disturbances, or need for assisted reproductive technology may exacerbate this risk (20–25).
Fetal and offspring consequences
Maternal PCOS is also associated with fetal and neonatal morbidity, including prematurity, intrauterine growth restriction (IUGR), extremes of birth weight (BW; small or large for gestational age), and a twofold higher rate of admission to the neonatal intensive care unit (17, 19). Epidemiologic studies have also demonstrated that prenatal exposure to excess androgens may directly predispose the offspring to develop a PCOS-like phenotype and associated reproductive, metabolic, cardiovascular, and behavioral dysfunctions in childhood and adulthood (26–28). These findings are encompassed within the developmental origins of health and disease concept arising from the Barker hypothesis, whereby in utero alterations in the nutritional, endocrine, metabolic, and inflammatory milieu may alter gene expression during fetal development and predispose to the development of noncommunicable disease (29, 30). Besides PCOS, conditions that may induce a hyperandrogenic state during pregnancy include maternal obesity, exposure to endocrine-disrupting chemicals, smoking, preeclampsia, black race, exogenous exposure to steroid hormones, and antiepileptic medications (27, 31–33).
The placenta as a mediator of adverse pregnancy outcomes
Across all species, the placenta serves a similar purpose: maintenance of pregnancy, exchange of nutrients, clearance of fetal waste products, and protection from immunologic, endocrine, and environmental insults (34). The placenta is a dynamic endocrine organ with the ability to adapt to evolving intrauterine conditions and is uniquely positioned to receive and adapt to both maternal and fetal input (35, 36). Early human placentation is characterized by the invasion of extravillous trophoblast cells into maternal decidua and myometrium, followed by remodeling of the maternal spiral uterine arteries into a low-resistance, uteroplacental vascular network (37, 38). Later in pregnancy, under optimal conditions, the placenta integrates maternal and fetal signals to optimize the well-being and growth potential of the fetus (35, 39). Nevertheless, the inner workings of the placenta remain elusive, which has reenergized research efforts into placental research (40). The US National Institute of Child Health and Human Development has recently called for expanded research into the placental pathways of pregnancy-related disorders, to ultimately develop targeted interventions and therapies to improve maternal and fetal outcomes (41).
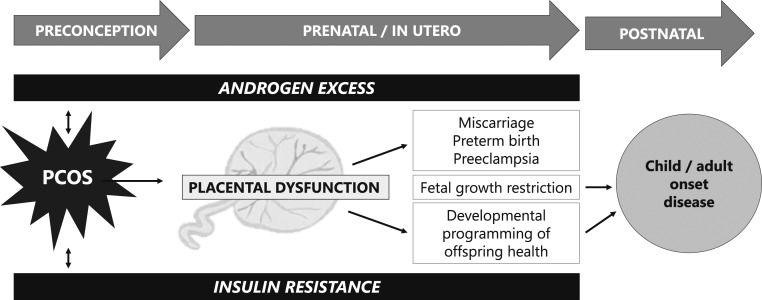
A large body of literature implicates the placenta as a mediator of both pregnancy complications, birth outcomes, and developmental programming (37, 42–46). It is intuitive, therefore, that there are placental alterations that occur in women with PCOS, which may predispose to adverse maternal, offspring, and birth outcomes. At present, our understanding of PCOS-specific placental pathologies is limited. Owing to variability in PCOS phenotypic expression, in addition to possible confounding comorbidities, including obesity, diabetes, hypertension, and/or the need for ART to conceive a pregnancy, there are significant methodological challenges in investigating placental dysfunction in PCOS. Therefore, it has been difficult to identify singular, precise mechanisms by which placental compromise may occur in women with PCOS. However, there is increasing evidence that androgen excess and insulin resistance, both of which are clinical hallmarks of PCOS, may negatively impact placental function (Fig. 1). Thus, in this review, we detail our current understanding of placental pathophysiology in the settings of (i) androgen excess and (ii) insulin resistance. We summarize current knowledge of PCOS-related placental dysfunction and identify gaps in knowledge to direct future research investigation that leads to targeted therapies for the prevention of pregnancy complications in women with PCOS.
Figure 1.
Placental dysfunction mediated by androgen excess and insulin resistance may lead to adverse pregnancy, birth, and long-term developmental outcomes in offspring.
Macroscopic and Microscopic Placental Changes Observed in Pregnancies of Women With PCOS
In routine practice, placental assessment by clinical pathologists involves an evaluation of both macroscopic and microscopic features. Here, we describe these methods and outline what is currently known in women with PCOS.
Placental assessments, as performed by clinical pathologists, routinely include placental weight (PW), diameter, thickness, shape, and location of umbilical cord insertion (47). A cross-sectional study of >11,000 healthy, singleton pregnancies delivered between 37 and 42 weeks provides reference ranges for average PW (48), although it is important to recognize that PW may be influenced by the method of placental collection, including mode of delivery, time to umbilical cord clamping, and subsequent handling of the sample (48, 49). Perhaps more clinically important than an isolated PW, is the ratio of fetal BW to PW (39). In simplified terms, the BW/PW ratio may infer how effectively the placenta has adapted to fetal growth needs. In the setting of fetal growth restriction, a diminished BW/PW ratio would indicate that the placenta was unable to functionally compensate for its small size, suggesting placental insufficiency as a mechanism for IUGR (39). Even in the absence of fetal growth restriction, impaired nutritional supply to the fetus may result in increased placental size relative to fetal size as a compensatory attempt to optimize nutritional delivery to the fetus (50). This suggests that a lower BW/PW ratio may still predict adverse pregnancy outcomes, even in the setting of BW appropriate for gestational age (51). Risk factors, including maternal diabetes and smoking, and outcomes, including lower neonatal Apgar scores and higher admission rates to the neonatal intensive care unit, have also been correlated with diminished BW/PW ratios (51–53).
Histological examinations are another important component of placental analysis (54). Broadly speaking, histologic lesions observed by placental pathologists may include either “vascular” or “villous” insults (54, 55), which may reflect a variety of immune, inflammatory, oxidative, or hypoxic conditions and provide insight into how pregnancy risk factors may translate into adverse fetal outcomes. However, limitations of placental examinations may include inherent observer bias in unblinded studies and interobserver variability among pathologists (55, 56).
In a case control study of pregnant women with PCOS (n = 30), matched with healthy pregnant controls (n = 60), the group with PCOS had a statistically significant reduction in PW, thickness, density, and volume (57). Furthermore, the derived BW/PW ratio was significantly lower in women with PCOS vs controls (5.12 ± 0.88 in women with PCOS, vs 5.90 ± 0.93 in controls). There was a trend toward decreased placental surface area in women with PCOS, although this did not reach statistical significance. Of note, the subjects with PCOS in this study only included nonobese women <35 years of age with spontaneous conceptions, uncomplicated pregnancies, and appropriate for gestational age neonates. Thus, although suggestive of an association between maternal PCOS and altered placental measures, these findings are not generalizable to a phenotypically diverse population with PCOS (57). In this same study, placentas of women with PCOS demonstrated higher rates of vascular lesions, chronic villitis, and villous immaturity than did placentas of women without PCOS, even in the absence of conditions such as maternal obesity, diabetes, prematurity, or small for gestational age neonates.
Meanwhile, Koster et al. (58) found no differences in PW or BW/PW ratio in PCOS (n = 73) vs non-PCOS controls (n = 209). In that study, subjects were not excluded for extremes of body mass index, mode of conception, or presence of pregnancy comorbidities, reflective of a more inclusive and heterogeneous sample of women with PCOS. In a subgroup analysis, the authors identified a trend toward a decreased BW/PW ratio in women who are hyperandrogenic vs normo-androgenic women (6.6 vs 7.3), although this did not reach statistical significance. Regarding histologic characteristics, women with PCOS compared with non-PCOS controls demonstrated higher rates of chorioamnionitis, funisitis, villitis, vascular thrombosis, infarction, and villous immaturity (58). These observations remained statistically significant after adjusting for confounding variables, including maternal age, ethnicity, parity, smoking status, preeclampsia, gestational diabetes, gestational age at delivery, and mode of delivery. A strength of this study is that all placental assessments were performed by a single experienced, blinded pathologist.
Mechanisms Underlying PCOS-Associated Placental Dysfunctions
There are several challenges in conducting mechanistic human placental research. Placental samples are generally collected at the time of delivery, in the third trimester or at term (59), and additional confounding factors may include timing of onset of labor and mode of delivery (60, 61). Owing to ethical considerations and the inherent risks of invasive placental sampling, placental tissue from early gestational time points is not easily obtained for research purposes.
Capitalizing on animal models to improve our understanding of placental pathology in PCOS
Fortunately, animal models of PCOS offer translational value for understanding the mechanisms of both normal and abnormal early placentation. One major benefit offered by animal models is the ability to investigate changes at the earliest stages of placentation and throughout gestation. The advantages and disadvantages of using experimental animal models for placental investigation have been extensively reviewed elsewhere (34, 62, 63). Although there are species-specific differences in placental histology and morphology (64), animal models are necessary for understanding the normal trajectory of placental development, in addition to identifying the timing and onset of placental dysfunction (34, 40). For PCOS-related placental work, most published research involves rodent, sheep, and nonhuman primate models (65), and the benefits and limitations of these models are briefly summarized below.
Human placentas are defined as “hemochorial.” In the early stages of placental development, the invading extravillous trophoblasts are in direct contact with the maternal vasculature. This is in contrast to “epitheliochorial” and “endotheliochorial” placentation, where the trophoblasts are in contact with the uterine epithelium or maternal vessel endothelium, respectively (38, 64, 66). Rodents, similar to humans, exhibit hemochorial placentation and are valuable for studying the early gestational processes of trophoblast invasion, differentiation, and spiral artery remodeling (38, 67). Advantages of rodent models include a short gestational length and affordability. However, they differ from humans in that their offspring are altricial and born in litters, and their small physical size may create challenges for invasive and noninvasive monitoring (34, 62).
Sheep have been historically invaluable for the study of maternal and fetal physiology (34, 68). Benefits of sheep models include their large size relative to rodent species, allowing for repeated blood measures and ultrasound imaging techniques that have translational value to humans (68, 69). Sheep are a domesticated species and able to be kept in stress-free, natural flocks (70). Importantly, sheep are also a precocial species, similar to humans, and thus have translational relevance from a developmental standpoint (62, 70). In contrast to humans, sheep demonstrate epitheliochorial placentation, where chorionic villi interface with the epithelium of numerous (>50), discrete maternal placental units (placentomes). Nonetheless, there is considerable structural similarity between sheep and human placenta as reflected by the architecture of the fetal villous tree with stem, intermediate, and terminal vessels (71, 72). The terminal vessels, or capillary complex, which are the functional units of exchange, differ very little between sheep and humans. In both, the chorioallantoic villous tree builds up to fetal placental cotyledons. In sheep, each cotyledon penetrates the maternal septal system of the endometrial caruncle and, together, they form one of numerous structures called placentomes. In humans, cotyledons protrude into the extended maternal intervillous blood space of the single polycotyledonary placental disc. The presence of placentomes is an advantage, as it enables the study of small, clearly identified regions of interest.
Benefits of primate models, such as the rhesus macaque, include their large size and their close similarities to humans with regard to length of gestation, uterine anatomy, trophoblast invasion in maternal spiral artery remodeling, and parturition (34). Nonhuman primates also exhibit hemochorial placentation, with a villous structure similar to human placental villi (34, 64). Disadvantages of primate models include a long gestational period, high maintenance cost of primate colonies, and ethical concerns due to their close ancestral relationship to humans (62). Despite the limitations of these above-mentioned animal models, they provide important translational value for improving our understanding of human placental pathology.
Mediators of placental pathology in other disease states
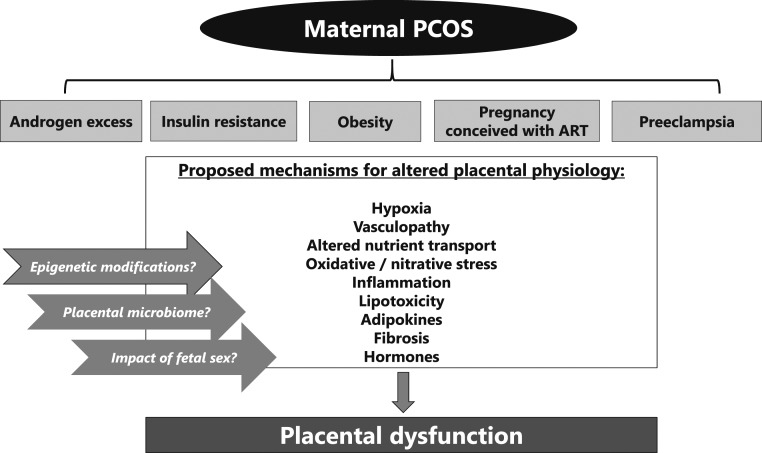
Various mediators of placental pathophysiology (Fig. 2) have been observed in maternal conditions such as obesity, preeclampsia, diabetes, and using ART to conceive, as briefly summarized below. Although these placental mechanisms have yet to be proven in pregnancies of women with PCOS, we propose that they warrant further investigation in the population with PCOS and in animal models of PCOS as potential mediators of adverse pregnancy outcomes.
Hypoxia: Early placental development, even in normal conditions, occurs in a low-oxygen environment (73) until the end of the first trimester. In animal models, excess hypoxic stress in early pregnancy may negatively affect proliferation or differentiation of trophoblast cells (74). In corollary, markers of hypoxia, such as the transcription factor hypoxia-inducible factor-1α (HIF-1α), are elevated in the placenta of women who are preeclamptic (75) and are worth investigation in PCOS models.
Vasculopathy: As a vascular organ, the placenta boasts an estimated 15 m2 of capillary surface area in healthy human pregnancies (76). Vascular growth within the placenta is complex, balanced by complementary proangiogenic and antiangiogenic factors. Miscarriage, IUGR, and preeclampsia are thought to arise from disordered angiogenesis (76–78), and PCOS may likewise be characterized by abnormal vasculogenesis or angiogenesis.
Altered nutrient transport: Optimization of fetal growth depends on the availability and transport of maternal nutrients, such as lipids, amino acids, and glucose through the placenta into the developing fetus (79). Abnormalities in nutrient transport may occur in conditions of maternal undernutrition or overnutrition and may also have long-term effects on the programming of adult-onset disease (80–83). Prior work by Maliqueo et al. (84) has demonstrated that placental STAT3 signaling, a regulator for nutrient transport and fetal growth, is enhanced in placentas of women with PCOS vs non-PCOS controls, regardless of levels of circulating sex steroids. More research is warranted on the subject of placental nutrient transport in PCOS, particularly with regard to metabolic parameters such as body mass index and insulin resistance, as well as steroidal milieu such as androgen excess, particularly because infants born from women with PCOS are paradoxically at risk for both small for gestational age or large for gestational age.
Oxidative stress: The high metabolic activity of the placenta leads to continuous production of reactive oxidative species, and thus normal pregnancy is characterized by some degree of oxidative stress (85, 86). Disease states that may alter the balance between reactive oxygen species and antioxidants, to predispose to a persistent state of placental oxidative stress and cellular damage, include spontaneous abortion, IUGR, preeclampsia, and preterm labor (87, 88), all of which are observed at higher rates in women with PCOS.
Inflammation: Normal pregnancy is characterized by some degree of systemic maternal inflammation, particularly in the third trimester (89, 90). The placenta itself is characterized by a vast network of both inflammatory cytokines and adipokines that may be involved in physiologic regulation of maternal–fetal metabolism, fetal growth, and parturition (90, 91) and adverse outcomes, including gestational diabetes and spontaneous preterm birth (92–94), also seen at higher frequency in pregnancies of women with PCOS.
Lipotoxicity: Lipotoxicity is defined as lipid accumulation in nonadipose tissue (95). Placental lipotoxicity is associated with placental inflammation and oxidative stress, and it may be observed in conditions of maternal obesity, insulin resistance, and gestational diabetes (96–98), further highlighting the possibility of placental lipotoxicity in women with PCOS.
Fibrosis: Collagen deposition within tissue is a normal, restorative response to injury. In pathologic conditions, however, excess collagen deposition can occur, leading to fibrosis, loss of tissue function, and even tissue death (99). In the obstetric literature, placental fibrotic lesions have been associated with preeclampsia (100, 101) but not yet explored in PCOS.
Endocrinopathy: The placenta is a hormonally regulated organ, demonstrating receptors to steroid hormones (including sex steroids and glucocorticoids), peptides (such as GnRH), proteins (such as insulin, prolactin, and leptin), and glycoproteins (such as human chorionic gonadotropin) (102). Hormonal dysregulation in the setting of maternal thyroid disease, insulin resistance, obesity, or PCOS may impact placental development and function via the above-mentioned mechanisms.
Figure 2.
Proposed mechanisms for placental dysfunction in women with PCOS.
Contribution of Androgen Excess and Insulin Resistance to Placental Dysfunction in PCOS
Androgen excess and insulin resistance are two clinical hallmarks of PCOS. Owing to the phenotypic heterogeneity of the condition, women with PCOS may demonstrate one, both, or neither of these features. Because placental studies are limited in women with PCOS, for purposes of this review, we additionally consider animal models of PCOS to outline the contributions of (i) androgen excess and (ii) insulin resistance on placental dysfunction.
Contribution of androgen excess
Similar to levels of estrogens and progesterone, androgen levels demonstrate a physiologic increase during normal pregnancy. They serve as substrate for placental aromatization to estrogens, and may even have a role in parturition (103, 104). Pregnant women with PCOS may demonstrate higher circulating levels of testosterone (T), dehydroepiandrosterone sulfate, and androstenedione compared with pregnant non-PCOS controls, regardless of fetal sex (105, 106). As such, maternal PCOS may confer an environment of androgen excess to the developing fetus, even if virilization of a female fetus is not observed (105). As maternal androgens are not thought to directly cross the placenta (107), and placental aromatase activity is thought to “protect” the fetus from androgen excess via conversion to estrogen, one possible mechanism is that maternal androgens may exert a programming effect on placental and/or fetal steroidogenesis to alter androgen levels within the fetal–placental unit (23, 108–110). In humans, this concept is supported by work from Maliqueo et al. (109), wherein placental tissue from women with PCOS demonstrated increased 3β-hydroxysteroid dehydrogenase 1 enzymatic activity and decreased aromatase activity compared with non-PCOS controls.
Interestingly, in studies using umbilical cord blood to estimate fetal–placental androgen levels, there is conflicting evidence as to whether, and how, maternal androgen levels are correlated to those in the fetal–placental unit in pregnancies of women with PCOS. Some studies have suggested that neonatal umbilical cord levels of T and/or androstenedione may be elevated in pregnancies of women with PCOS, irrespective of infant sex (111–113). However, other studies suggest no difference in cord blood androgen levels of infants of women with PCOS vs controls, whereas other studies demonstrate decreased androstenedione levels in cord blood of female offspring of women with PCOS (109, 114–116). These discrepancies may be due to differences in study design, patient selection, and methodologic differences of cord blood collection.
Prenatal hyperandrogenic states in animal models of PCOS (117) are associated with fetal growth restriction and low BW (118, 119), suggesting an effect on placental function and efficiency. The biologic plausibility of this notion is supported by the fact that the androgen receptor is expressed in placenta of humans and animals (120–122). In animal studies, gestational hyperandrogenism can be achieved with maternal T treatment (70, 123, 124). Rodent models of PCOS generally involve T administration relatively late in pregnancy, so these findings do not account for any potential androgen effects at early fetal–placental developmental time points. Nevertheless, studies involving prenatally androgenized rats and sheep have provided evidence for the role of androgens on placental function and pathologies, as summarized in Table 1 (107, 108, 120, 121, 125–129). In one study, T treatment in late pregnancy was linked to increased levels of circulating androgens and estrogens, in addition to increased placental gene expression of estrogen receptor (ER)-α and ER-β, androgen receptor, and 17β-hydroxysteroid dehydrogenase 2 (121). In this model, T treatment was also accompanied by decreased placental and fetal weights, but no significant change in the BW/PW ratio. Notably, the offspring of T-treated dams developed metabolic dysfunction later in life, highlighting the role of androgen excess in the developmental programming of adult metabolic disease (121). Similarly, in another experimental model, T treatment in late gestation was linked to decreased fetal weights and PWs via decreased expression of placental amino acid transporter, but no difference in the BW/PW ratio (107). T treatment has also been associated with decreased uterine artery blood flow, increased expression of HIF-1α, and decreased fetal weights and PWs in rodent models (125). In sheep, T treatment during early gestational time points also leads to intrauterine growth restriction and low BW, particularly of female offspring. Placental insufficiency may underlie these observations, as T-treated sheep demonstrated advancement of placental differentiation as early as mid-gestation (108).
Table 1.
Animal Models of Gestational Androgen Excess and Its Effect on Placental Function
| Study (Ref.) | Animal | Androgen Intervention | Effect of Androgen Intervention on Placental Function |
|---|---|---|---|
| Sathishkumar et al., 2011 (107) | Rodent | 0.5 mg/kg T propionate daily, gestational days 15–19 | Decreased fetal weights and PWs, but no difference in BW/PW ratio |
| Decreased placental amino acid transport | |||
| Sun et al., 2012 (121) | Rodent | 5 mg T daily, gestational days 16–19 | Decreased fetal weights and PWs, but no difference in BW/PW ratio |
| Increased placental expression of ER-α and ER-β, androgen receptor, and 17β-hydroxysteroid dehydrogenase 2 | |||
| Chinnathambi et al., 2014 (126) | Rodent | 0.5 mg/kg T propionate daily, gestational days 15–19 | Decreased fetal weights and PWs |
| Increased gene and protein expression of hypoxia-responsive genes HIF-1α, Ankrd 37, and Egln | |||
| Hu et al., 2015 (127) | Rodent | 0.5 mg/kg T daily, gestational days 15–19, with or without tamoxifen 10 µg/kg daily | Decreased PW and increased STAT3 expression |
| Gopalakrishnan et al., 2016 (125) | Rodent | 0.5 mg/kg T propionate daily, gestational days 15–19 | Decreased fetal weights and PWs, but no difference in BW/PW ratio |
| Decreased uterine artery blood flow and elevated vascular resistance | |||
| Sex-specific alterations in utero placental vasculature | |||
| Fornes et al., 2017 (128) | Rodent | 100 µL of DHT daily, gestational days 15.5–17.5 | No difference in fetal weights or PWs |
| Control diet vs high-fat-high sucrose diet | Increased placental AR expression | ||
| Fornes et al., 2019 (129) | Rodent | 100 µL of DHT daily, gestational days 15.5–18.5 | Differential expression of phosphorylated catechol-O-methyltransferase depending on maternal diet |
| Control diet vs high-fat/high-sucrose diet | |||
| Beckett et al., 2014 (108) | Sheep | 100 mg IM of T propionate biweekly, gestational days 30–90 | Placental insufficiency at gestational day 140 (decreased BW/PW ratio) |
| Advanced placental differentiation | |||
| Cleys et al., 2015 (120) | Sheep | 100 mg IM of T propionate biweekly, gestational days 30–90 | Decreased fetal weight (female lambs only) at gestational day 90 |
| Advanced placental differentiation | |||
| Increased mRNA and protein expression of VEGF |
Abbreviation: VEGF, vascular endothelial growth factor.
Prenatal androgens have also been implicated in placental vasculopathies (77, 130, 131). In pregnant rats, elevated androgens increase the vascular tone of the uterine arteries, compromising the primary blood supply to the pregnant uterus, and subsequently increase the expression of hypoxia-responsive genes (126). Additionally, in rat models, gestational hyperandrogenism has been shown to reduce gene expression of placental proangiogenic factors (107, 132, 133). In sheep, prenatal T treatment is associated with an increase in placental mRNA and protein expression of vascular endothelial growth factor (VEGF), an important regulator of placental angiogenesis (120). In this well-validated sheep model, T treatment is administered from an early gestational time point (between days 30 through 90, with lambing at gestational day 147), which provides the benefit of studying the effects of androgen excess during early, critical windows of fetal development (70, 108).
Preeclampsia, a syndrome of maternal hypertension, proteinuria, and vascular compromise, has also been linked to androgen excess (134–136). Preeclampsia is a leading cause of maternal and fetal morbidity and is thought to arise from abnormalities in early placental development. Maternal obesity, type 2 diabetes, and the use of ART, all of which are observed more frequently in women with PCOS, are established risk factors for the development of preeclampsia (137). Evidence suggests that aberrant trophoblast invasion and impaired remodeling of the maternal spiral arteries predispose to preeclampsia (42, 134). Epidemiologic studies have also demonstrated higher androstenedione and T levels in maternal serum of pregnancies of women with preeclamsia vs controls (138, 139). Interestingly, prior work by Palomba et al. (140) has demonstrated defective endovascular trophoblast invasion in late first-trimester placental samples from women with PCOS, compared with non-PCOS controls, providing support for an association between androgen excess and impairment of spiral artery remodeling.
There also exists a large body of literature related to third-trimester placental characteristics of preeclampsia. Placentas from mothers with preeclampsia at advanced gestational ages have more villous lesions (a finding that suggests maternal hypoperfusion), higher expression of inflammatory markers, and a greater proportion of antiangiogenic compared with proangiogenic factors (42). Additionally, placentas from women with preeclampsia have higher lipid content (134) and increased markers of oxidative stress (43). These investigations have highlighted several potential opportunities for the prevention of preeclampsia, including the use of antioxidants, anti-inflammatories, vasodilators, and even metformin (37, 141, 142). Although similar molecular targets have yet to be tested in PCOS-related placental pathologies, the possibility that androgen excess may underlie both PCOS and preeclampsia suggests that these two conditions may share similar preventive or therapeutic strategies.
Androgens vs estrogens
In normal pregnancy, placental production of estradiol is high (∼100 mg/d) (143). Unlike androgens, which are thought to increase uterine vascular tone and downregulate placental angiogenic genes, estrogens promote vascular relaxation and increase placental VEGF expression, particularly in early gestation (132). Estrogens also regulate genes involved in cholesterol supply to the placenta (143), important for fetal and placental steroid hormone production. The mechanisms underlying placental estrogen production are complex, but, in brief, they involve the conversion of fetal adrenal dehydroepiandrosterone into estrogens by the enzymatic activity of placental aromatase. In abnormal states, such as preeclampsia, estrogen levels are thought to be reduced, which may be linked to impaired angiogenesis through alterations in placental VEGF (144, 145).
Because androgens are precursors to estrogens, a methodical challenge is determining whether specific findings are mediated by androgenic vs estrogenic pathways (132). In animal studies, this particular challenge has been partially overcome by examining the direct effects of DHT, a nonaromatizable androgen (128, 129). In rodents, T treatment increased circulating levels of T and DHT, both of which were associated with decreased PW, suggesting that this effect may be androgen mediated (127). In this same study, cotreatment of T with tamoxifen, a selective ER modulator, also increased phosphorylated and total STAT3 protein expression, suggesting that ER is not involved in this same pathway that has been shown to be activated in women with PCOS (84, 127). In prenatally androgenized sheep models, wherein androgen excess results in IUGR through placental compromise, the distinct placental effects of T-treated vs DHT-treated pregnant sheep were investigated (108). In both experimental groups, accelerated placental aging was observed relative to controls, suggesting that the observed placental effects are more likely mediated by the androgenic, not estrogenic, actions of T. Importantly, note that DHT may be converted to 3-β-diol, which can bind ER-β and elicit downstream estrogenic effects. Thus, an alternative approach to elucidate whether placental changes are androgen or estrogen mediated is cotreatment of T with an androgen antagonist, such as flutamide. In sheep, cotreatment of T with flutamide partially prevented the observed placental advancement in this model (108), suggesting that the placental changes were mediated at least in part by androgenic action.
Contribution of insulin resistance
Normal pregnancy is characterized by decreased glucose uptake and increased insulin secretion, leading to a state of insulin resistance, which helps to provide adequate nutrition to the developing fetus (146, 147). Nevertheless, preconception insulin resistance is a clinical hallmark of PCOS (148, 149), and approximately one-third of women with obesity with PCOS demonstrate impaired glucose tolerance, independent from obesity (1). In pregnancy, women with PCOS compared with non-PCOS controls also demonstrate hyperinsulinemia and lipid abnormalities, including hypertriglyceridemia and increased low-density lipoprotein (150, 151). Maternal insulin resistance has been implicated in other complications, including miscarriage, pregnancy-induced hypertension, and growth restriction, and placental expression of insulin receptor has been widely demonstrated (152, 153), suggesting a link between insulin resistance and placental compromise (154, 155). Additionally, insulin, IGF-I, and IGF-II have been shown to inhibit the enzymatic activity of aromatase within human trophoblasts (156). These findings highlight the correlation between hyperinsulinemia and androgen excess in women with PCOS; the interaction of steroidogenic and metabolic disturbances in pregnancies of women with PCOS has been previously reviewed (157).
Insulin resistance may have negative effects on early placentation. Hyperglycemia in early pregnancy may increase rates of miscarriage, intrauterine growth restriction, and preeclampsia (158), likely due to impaired trophoblast invasion. In vitro studies of first-trimester human placental tissue have demonstrated that insulin resistance can impair IGF-1 signaling, which is important for trophoblast invasion of maternal spiral arteries. Conversely, addition of an insulin sensitizer can restore normal signaling and promote appropriate trophoblast migration and invasion (154). Relevant to PCOS, there is some evidence that women with PCOS display lower levels of IGF-1 in the first trimester of pregnancy, possibly due to insulin resistance (159, 160). Interestingly, in sheep, cotreatment of T with rosiglitazone, an insulin sensitizer, does not mitigate the observed advancement of placental aging in T-treated sheep, suggesting that insulin resistance may not be a critical mediator of placental insufficiency in this particular animal model of PCOS.
PCOS is also a known risk factor for the development of gestational diabetes mellitus (GDM) (161). GDM is generally not diagnosed until the second trimester; however, baseline insulin resistance may predispose to the development of GDM (142, 162, 163). Research on GDM-associated placental dysfunction is extensive and has highlighted several possible mechanisms, including reduced placental apoptosis, impaired vasculogenesis, increased ischemia, and villous immaturity relative to non-GDM controls (164–168). Table 2 (108, 169–174) highlights and briefly summarizes key placental findings in animal models of GDM. There is also emerging evidence that placental production and release of adipokines such as leptin, adiponectin, and resistin may be altered in pregnancies complicated by GDM, which in turn may be linked to placental oxidative stress and inflammation (91, 175–178). Because adipokines may be involved with regulating fetal growth, in addition to programming insulin sensitivity (179), their role in PCOS pregnancies warrants further investigation.
Table 2.
Animal Models of Gestational Diabetes and/or Hyperglycemia and Their Effects on Placental Function
| Study (Ref.) | Animal | Model for Gestational Diabetes | Effect of Hyperglycemia on Placental Function |
|---|---|---|---|
| Ericsson et al., 2007 (169) | Rodent | Single or multiple IP glucose injections on gestational day 10 | Increased PW and fetal weight on gestational day 21 |
| Downregulation of placental SNAT2 (system A transport) | |||
| Bobadilla et al., 2010 (170) | Rodent | Heterozygotes for defective leptin receptors (db/+) | Impaired trophoblast invasion in db/+ vs wild-type mice |
| Exogenous TNF-α treatment increases placental apoptosis in db/+ mice | |||
| Salbaum et al., 2011 (171) | Rodent | Streptozotocin injections prior to mating | Reduced junctional zone and labyrinth size |
| Altered gene expression profile (158 genes) | |||
| Li et al., 2013 (172) | Rodent | High-fat diet for 14 wk prior to mating and throughout gestation | Increased markers of hypoxia and oxidative stress |
| Increased mRNA expression of VEGF, TNF-α, and IL-6 | |||
| Cisse et al., 2013 (173) | Rodent | Single injection of nicotinamide plus streptozotocin prior to mating | Increased lipoprotein lipase-1 expression in macrosomic pups |
| Dela Justina et al., 2014 (174) | Rodent | Streptozotocin injection prior to mating | Increased PW |
| Increased nuclear factor κB activation | |||
| Increased expression of TNF-α and IL-6 | |||
| Beckett et al., 2014 (108) | Sheep | 100 mg IM of T propionate biweekly plus rosiglitazone 8 mg/kg orally daily, gestational days 30–90 | Addition of insulin sensitizer did not alter T-mediated placental advancement |
Future Directions in PCOS Placental Research
Gene environment interactions (epigenetic contributions)
Beyond efforts to understand how the human genome contributes to placental growth and development (180), there is emerging interest into the epigenetic mechanisms, including DNA methylation, histone modification, and miRNA and circular RNA expression, which may compromise placental function (Fig. 3) (181–187). For instance, a brief review of the preeclampsia literature highlights the potential role of epigenetic dysregulation, at the level of the placenta, in pregnancies with preeclampsia vs control pregnancies (188–193). Similarly, alterations in epigenetic programming have been identified in placental samples from women with GDM (194, 195).
Figure 3.
Future directions in the field of PCOS placental research. Images used were sourced from open-source resources: www.pixabay.com, www.kisspng.com, www.wikipedia.org, and www.openclipart.org.
With regard to women with PCOS, recent studies have demonstrated that DNA methylation and miRNA expression may be altered in certain endocrine and metabolic tissues of women with PCOS, including adipose and ovaries (196–198). Meanwhile, altered epigenetic regulation of genes related to glucose homeostasis, lipid metabolism, and inflammation has been proposed based on a pilot study of umbilical cord blood in offspring of women with PCOS vs non-PCOS controls (199). However, many questions remain unanswered with regard to epigenetic patterns at the placental level, specific to women with PCOS, particularly because the placenta is a transitory organ. Because epigenetic alterations in both germ cells and placenta are thought to have an important role in developmental programming of offspring and intergenerational outcomes (200, 201), more research is needed to understand how epigenetic modifications in women with PCOS may be translated into adverse health implications for future generations.
Impact of fetal sex
A growing interest in the developmental programming of disease has expanded our awareness of the role of fetal sex on placental development. For instance, prior work has demonstrated that fetal sex impacts placental adaptations to external insults, including undernutrition or overnutrition and prenatal EDC exposure (202–206). In sheep models of PCOS, using exogenous T treatment during early developmental time points, fetal growth restriction is observed more frequently in female offspring, compared with males (108), highlighting the possibility of sex-specific placental alterations in pregnancies of women with PCOS. A recent review also highlights the potential role of the androgen receptor in mediating sex-specific fetal development (104). Additional research is needed to fully elucidate how the sex of the fetus may regulate or dysregulate placental function in pregnant women with PCOS (Fig. 3).
Capitalizing on animal models
As previously mentioned, animal models are of the utmost translational importance, as they offer the opportunity to identify both mechanisms and timing of onset of placental pathologies. Prior work in rodent and sheep models has already identified several key mechanisms that may underlie placental pathophysiology in PCOS (Tables 1 and 2). Animal models will continue to be invaluable for enhancing our understanding of, and developing intervention strategies for, PCOS-related placental compromise (Fig. 3).
Placental microbiome
The human microbiome and its role in health, disease, and developmental programming is another rapidly expanding area of research investigation (207). In pregnancy, distinct maternal microbiomes have been identified, each with a unique ability to influence pregnancy and neonatal outcomes: (i) placental, (ii) vaginal, and (iii) gastrointestinal (oral/gut) (208, 209). Characteristics of placental and vaginal microbial communities have been linked to pregnancy and birth outcomes, such as gestational diabetes and preterm birth (209–211). Additionally, bacteria are detected within newborn meconium, which may reflect exposure to the maternal gastrointestinal, placental, and/or vaginal microflora, further challenging the concept of the “sterile womb” (212). In turn, the intestinal flora of the newborn has been linked to a variety of long-term health outcomes, including immune or metabolic dysfunctions, inflammatory bowel disease, and childhood obesity (213), suggesting that in utero microbiota exposures might influence pathways for fetal developmental programming.
With regard to PCOS, research has suggested that androgen excess may contribute to altered gastrointestinal bacterial diversity in women with PCOS (214–216). Furthermore, prenatally androgenized rats have been shown to develop gut dysbiosis later in life (217). Research on the placental microbiome in women with PCOS vs non-PCOS controls is lacking. However, knowing that the diversity of the placental microbiome may influence birth outcomes such as BW, this could become an exciting avenue for future investigation in women with PCOS (Fig. 3).
“Omics” approaches: genomics, proteomics, and epigenomics
Using state-of-the-art “-omic” technologies, researchers are currently working to identify novel biomarkers of PCOS to aid in the early diagnosis and appropriate treatment of the disorder (218, 219). Identifying new, objective markers to predict the onset of maternal or fetal morbidity in pregnancy is also an exciting area of research at this time. In the context of this review, we advocate for specific research efforts to identify biomarkers, specific to each PCOS phenotype, that may predict adverse pregnancy outcomes (Fig. 3). Owing to the invasive nature of chorionic villous sampling, the identification of placental biomarkers is not practical. However, the application of -omic technologies to currently available screening tests (such as fetal cell-free DNA) to predict the onset of maternal or fetal morbidity may have relevance to women with PCOS.
Distinction of PCOS phenotypes
As previously mentioned, not all PCOS is created equal, and a woman’s particular phenotype may predispose her to greater or lesser pregnancy risk (220, 221). One must recognize the variability among PCOS phenotypes, in both the research and clinical settings, to provide evidence-based and appropriate counseling and surveillance to pregnant women with PCOS. Additionally, it is important to recognize how other individual factors, such as environment and ethnicity, may uniquely modulate a woman’s pregnancy risk (222).
Principal Points
Pregnancies of women with PCOS can be characterized by high maternal and fetal morbidity. The placenta is a key mediator of pregnancy and birth outcomes, highlighting the plausibility of placental dysfunction in pregnancies of women with PCOS. Because of the high prevalence of PCOS in reproductive-aged women, more human placental research is urgently needed to develop intervention strategies to improve outcomes for women with PCOS and their offspring.
Animal models of PCOS provide excellent translational value, especially in identifying the timing of onset of placental pathology, and they should be capitalized on for understanding placental alterations that may occur in states of gestational androgen excess or insulin resistance.
Most human placentas are discarded after delivery. Given the critical role of the placenta in supporting fetal growth and development, in addition to programming of child- and adult-onset disease, we encourage the development and expansion of human tissue biorepositories to facilitate placental research in women with PCOS.
Acknowledgments
We thank Dr. Muraly Puttabyatappa for assistance in generating Fig. 3.
Financial Support: This work was supported by National Institutes of Health Grant P01 HD44232 (to V.P.).
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Glossary
Abbreviations:
- ART
assisted reproductive technology
- BW
birth weight
- ER
estrogen receptor
- GDM
gestational diabetes mellitus
- HIF-1α
hypoxia-inducible factor-1α
- IUGR
intrauterine growth restriction
- PCOS
polycystic ovary syndrome
- PW
placental weight
- T
testosterone
- VEGF
vascular endothelial growth factor
References and Notes
- 1. Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract. 2015;21(12):1415–1426. [DOI] [PubMed] [Google Scholar]
- 2. Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS, Boivin J, Petraglia F, Wijeyeratne CN, Norman RJ, Dunaif A, Franks S, Wild RA, Dumesic D, Barnhart K. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38.e25. [DOI] [PubMed] [Google Scholar]
- 3. Kjerulff LE, Sanchez-Ramos L, Duffy D. Pregnancy outcomes in women with polycystic ovary syndrome: a metaanalysis. Am J Obstet Gynecol. 2011;204(6):558.e1–558.e6. [DOI] [PubMed] [Google Scholar]
- 4. Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. 2005;90(8):4650–4658. [DOI] [PubMed] [Google Scholar]
- 5. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. [DOI] [PubMed] [Google Scholar]
- 6. Azziz R. Polycystic ovary syndrome. Obstet Gynecol. 2018;132(2):321–336. [DOI] [PubMed] [Google Scholar]
- 7. Moore AM, Campbell RE. Polycystic ovary syndrome: understanding the role of the brain. Front Neuroendocrinol. 2017;46:1–14. [DOI] [PubMed] [Google Scholar]
- 8. Patel SS, Carr BR. Oocyte quality in adult polycystic ovary syndrome. Semin Reprod Med. 2008;26(2):196–203. [DOI] [PubMed] [Google Scholar]
- 9. Palomba S, Daolio J, La Sala GB. Oocyte competence in women with polycystic ovary syndrome. Trends Endocrinol Metab. 2017;28(3):186–198. [DOI] [PubMed] [Google Scholar]
- 10. Balen AH, Morley LC, Misso M, Franks S, Legro RS, Wijeyaratne CN, Stener-Victorin E, Fauser BC, Norman RJ, Teede H. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22(6):687–708. [DOI] [PubMed] [Google Scholar]
- 11. Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction. 2010;140(3):347–364. [DOI] [PubMed] [Google Scholar]
- 12. Mahutte N, Kamga-Ngande C, Sharma A, Sylvestre C. Obesity and reproduction. J Obstet Gynaecol Can. 2018;40(7):950–966. [DOI] [PubMed] [Google Scholar]
- 13. Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(6):618–637. [DOI] [PubMed] [Google Scholar]
- 14. Legro RS. Ovulation induction in polycystic ovary syndrome: current options. Best Pract Res Clin Obstet Gynaecol. 2016;37:152–159. [DOI] [PubMed] [Google Scholar]
- 15. Franik S, Eltrop SM, Kremer JA, Kiesel L, Farquhar C. Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2018;5:CD010287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siristatidis CS, Vrachnis N, Creatsa M, Maheshwari A, Bhattacharya S. In vitro maturation in subfertile women with polycystic ovarian syndrome undergoing assisted reproduction. Cochrane Database Syst Rev. 2013; (10):CD006606. [DOI] [PubMed] [Google Scholar]
- 17. Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12(6):673–683. [DOI] [PubMed] [Google Scholar]
- 18. Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update. 2015;21(5):575–592. [DOI] [PubMed] [Google Scholar]
- 19. Qin JZ, Pang LH, Li MJ, Fan XJ, Huang RD, Chen HY. Obstetric complications in women with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2013;11(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kollmann M, Klaritsch P, Martins WP, Guenther F, Schneider V, Herzog SA, Craciunas L, Lang U, Obermayer-Pietsch B, Lerchbaum E, Raine-Fenning N. Maternal and neonatal outcomes in pregnant women with PCOS: comparison of different diagnostic definitions. Hum Reprod. 2015;30(10):2396–2403. [DOI] [PubMed] [Google Scholar]
- 21. Yamamoto M, Feigenbaum SL, Crites Y, Escobar GJ, Yang J, Ferrara A, Lo JC. Risk of preterm delivery in non-diabetic women with polycystic ovarian syndrome. J Perinatol. 2012;32(10):770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Naver KV, Grinsted J, Larsen SO, Hedley PL, Jørgensen FS, Christiansen M, Nilas L. Increased risk of preterm delivery and pre-eclampsia in women with polycystic ovary syndrome and hyperandrogenaemia. BJOG. 2014;121(5):575–581. [DOI] [PubMed] [Google Scholar]
- 23. Palomba S, Falbo A, Russo T, Tolino A, Orio F, Zullo F. Pregnancy in women with polycystic ovary syndrome: the effect of different phenotypes and features on obstetric and neonatal outcomes. Fertil Steril. 2010;94(5):1805–1811. [DOI] [PubMed] [Google Scholar]
- 24. de Wilde MA, Lamain-de Ruiter M, Veltman-Verhulst SM, Kwee A, Laven JS, Lambalk CB, Eijkemans MJ, Franx A, Fauser BC, Koster MP. Increased rates of complications in singleton pregnancies of women previously diagnosed with polycystic ovary syndrome predominantly in the hyperandrogenic phenotype. Fertil Steril. 2017;108(2):333–340. [DOI] [PubMed] [Google Scholar]
- 25. Mumm H, Jensen DM, Sørensen JA, Andersen LL, Ravn P, Andersen M, Glintborg D. Hyperandrogenism and phenotypes of polycystic ovary syndrome are not associated with differences in obstetric outcomes. Acta Obstet Gynecol Scand. 2015;94(2):204–211. [DOI] [PubMed] [Google Scholar]
- 26. Filippou P, Homburg R. Is foetal hyperexposure to androgens a cause of PCOS? Hum Reprod Update. 2017;23(4):421–432. [DOI] [PubMed] [Google Scholar]
- 27. Hakim C, Padmanabhan V, Vyas AK. Gestational hyperandrogenism in developmental programming. Endocrinology. 2017;158(2):199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kosidou K, Dalman C, Widman L, Arver S, Lee BK, Magnusson C, Gardner RM. Maternal polycystic ovary syndrome and the risk of autism spectrum disorders in the offspring: a population-based nationwide study in Sweden. Mol Psychiatry. 2016;21(10):1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barker DJP. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–417. [DOI] [PubMed] [Google Scholar]
- 30. Padmanabhan V, Cardoso RC, Puttabyatappa M. Developmental programming, a pathway to disease. Endocrinology. 2016;157(4):1328–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McClamrock HD, Adashi EY. Gestational hyperandrogenism. Fertil Steril. 1992;57(2):257–274. [DOI] [PubMed] [Google Scholar]
- 32. Verrotti A, D’Egidio C, Coppola G, Parisi P, Chiarelli F. Epilepsy, sex hormones and antiepileptic drugs in female patients. Expert Rev Neurother. 2009;9(12):1803–1814. [DOI] [PubMed] [Google Scholar]
- 33. Puttabyatappa M, Cardoso RC, Padmanabhan V. Effect of maternal PCOS and PCOS-like phenotype on the offspring’s health. Mol Cell Endocrinol. 2016;435:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grigsby PL. Animal models to study placental development and function throughout normal and dysfunctional human pregnancy. Semin Reprod Med. 2016;34(1):11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sandovici I, Hoelle K, Angiolini E, Constância M. Placental adaptations to the maternal–fetal environment: implications for fetal growth and developmental programming. Reprod Biomed Online. 2012;25(1):68–89. [DOI] [PubMed] [Google Scholar]
- 36. Costa MA. The endocrine function of human placenta: an overview. Reprod Biomed Online. 2016;32(1):14–43. [DOI] [PubMed] [Google Scholar]
- 37. Sibley CP. Treating the dysfunctional placenta. J Endocrinol. 2017;234(2):R81–R97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Silva JF, Serakides R. Intrauterine trophoblast migration: a comparative view of humans and rodents. Cell Adhes Migr. 2016;10(1-2):88–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hayward CE, Lean S, Sibley CP, Jones RL, Wareing M, Greenwood SL, Dilworth MR. Placental adaptation: what can we learn from birthweight:placental weight ratio? Front Physiol. 2016;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guttmacher AE, Maddox YT, Spong CY. The Human Placenta Project: placental structure, development, and function in real time. Placenta. 2014;35(5):303–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ilekis JV, Tsilou E, Fisher S, Abrahams VM, Soares MJ, Cross JC, Zamudio S, Illsley NP, Myatt L, Colvis C, Costantine MM, Haas DM, Sadovsky Y, Weiner C, Rytting E, Bidwell G. Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am J Obstet Gynecol. 2016;215(1Suppl):S1–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Soto E, Romero R, Kusanovic JP, Ogge G, Hussein Y, Yeo L, Hassan SS, Kim CJ, Chaiworapongsa T. Late-onset preeclampsia is associated with an imbalance of angiogenic and anti-angiogenic factors in patients with and without placental lesions consistent with maternal underperfusion. J Matern Fetal Neonatal Med. 2012;25(5):498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Burton GJ, Yung HW, Cindrova-Davies T, Charnock-Jones DS. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta. 2009;30(Suppl A):S43–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204(3):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jindal P, Regan L, Fourkala EO, Rai R, Moore G, Goldin RD, Sebire NJ. Placental pathology of recurrent spontaneous abortion: the role of histopathological examination of products of conception in routine clinical practice: a mini review. Hum Reprod. 2007;22(2):313–316. [DOI] [PubMed] [Google Scholar]
- 46. Longtine MS, Nelson DM. Placental dysfunction and fetal programming: the importance of placental size, shape, histopathology, and molecular composition. Semin Reprod Med. 2011;29(3):187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Haeussner E, Schmitz C, von Koch F, Frank HG. Birth weight correlates with size but not shape of the normal human placenta. Placenta. 2013;34(7):574–582. [DOI] [PubMed] [Google Scholar]
- 48. Burkhardt T, Schäffer L, Schneider C, Zimmermann R, Kurmanavicius J. Reference values for the weight of freshly delivered term placentas and for placental weight–birth weight ratios. Eur J Obstet Gynecol Reprod Biol. 2006;128(1–2):248–252. [DOI] [PubMed] [Google Scholar]
- 49. Risnes KR, Romundstad PR, Nilsen TIL, Eskild A, Vatten LJ. Placental weight relative to birth weight and long-term cardiovascular mortality: findings from a cohort of 31,307 men and women. Am J Epidemiol. 2009;170(5):622–631. [DOI] [PubMed] [Google Scholar]
- 50. Daskalakis G, Marinopoulos S, Krielesi V, Papapanagiotou A, Papantoniou N, Mesogitis S, Antsaklis A. Placental pathology in women with gestational diabetes. Acta Obstet Gynecol Scand. 2008;87(4):403–407. [DOI] [PubMed] [Google Scholar]
- 51. Shehata F, Levin I, Shrim A, Ata B, Weisz B, Gamzu R, Almog B. Placenta/birthweight ratio and perinatal outcome: a retrospective cohort analysis. BJOG. 2011;118(6):741–747. [DOI] [PubMed] [Google Scholar]
- 52. Ganer Herman H, Miremberg H, Schreiber L, Bar J, Kovo M. The association between disproportionate birth weight to placental weight ratio, clinical outcome, and placental histopathological lesions. Fetal Diagn Ther. 2017;41(4):300–306. [DOI] [PubMed] [Google Scholar]
- 53. Salavati N, Gordijn SJ, Sovio U, Zill-E-Huma R, Gebril A, Charnock-Jones DS, Scherjon SA, Smith GC. Birth weight to placenta weight ratio and its relationship to ultrasonic measurements, maternal and neonatal morbidity: a prospective cohort study of nulliparous women. Placenta. 2018;63:45–52. [DOI] [PubMed] [Google Scholar]
- 54. Redline RW. Classification of placental lesions. Am J Obstet Gynecol. 2015;213(4Suppl):S21–S28. [DOI] [PubMed] [Google Scholar]
- 55. Falco ML, Sivanathan J, Laoreti A, Thilaganathan B, Khalil A. Placental histopathology associated with pre-eclampsia: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;50(3):295–301. [DOI] [PubMed] [Google Scholar]
- 56. Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation—a workshop report. Placenta. 2005;26(Suppl A):S114–S117. [DOI] [PubMed] [Google Scholar]
- 57. Palomba S, Russo T, Falbo A, Di Cello A, Tolino A, Tucci L, La Sala GB, Zullo F. Macroscopic and microscopic findings of the placenta in women with polycystic ovary syndrome. Hum Reprod. 2013;28(10):2838–2847. [DOI] [PubMed] [Google Scholar]
- 58. Koster MP, de Wilde MA, Veltman-Verhulst SM, Houben ML, Nikkels PG, van Rijn BB, Fauser BC. Placental characteristics in women with polycystic ovary syndrome. Hum Reprod. 2015;30(12):2829–2837. [DOI] [PubMed] [Google Scholar]
- 59. Palomba S, Falbo A, Chiossi G, Tolino A, Tucci L, La Sala GB, Zullo F. Early trophoblast invasion and placentation in women with different PCOS phenotypes. Reprod Biomed Online. 2014;29(3):370–381. [DOI] [PubMed] [Google Scholar]
- 60. Hu Y, Huang K, Sun Y, Wang J, Xu Y, Yan S, Zhu P, Tao F. Placenta response of inflammation and oxidative stress in low-risk term childbirth: the implication of delivery mode. BMC Pregnancy Childbirth. 2017;17(1):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hung TH, Chen SF, Hsieh TT, Lo LM, Li MJ, Yeh YL. The associations between labor and delivery mode and maternal and placental oxidative stress. Reprod Toxicol. 2011;31(2):144–150. [DOI] [PubMed] [Google Scholar]
- 62. Carter AM. Animal models of human placentation—a review. Placenta. 2007;28(Suppl A):S41–S47. [DOI] [PubMed] [Google Scholar]
- 63. Chavatte-Palmer P, Tarrade A, Rousseau-Ralliard D. Diet before and during pregnancy and offspring health: the importance of animal models and what can be learned from them. Int J Environ Res Public Health. 2016;13(6):E586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Leiser R, Kaufmann P. Placental structure: in a comparative aspect. Exp Clin Endocrinol. 1994;102(3):122–134. [DOI] [PubMed] [Google Scholar]
- 65. Chavatte-Palmer P, Tarrade A. Placentation in different mammalian species. Ann Endocrinol (Paris). 2016;77(2):67–74. [DOI] [PubMed] [Google Scholar]
- 66. Carter AM, Enders AC. Comparative aspects of trophoblast development and placentation. Reprod Biol Endocrinol. 2004;2(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Moll W. Structure adaptation and blood flow control in the uterine arterial system after hemochorial placentation. Eur J Obstet Gynecol Reprod Biol. 2003;110(Suppl 1):S19–S27. [DOI] [PubMed] [Google Scholar]
- 68. Barry JS, Anthony RV. The pregnant sheep as a model for human pregnancy. Theriogenology. 2008;69(1):55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol. 2008;35(7):730–743. [DOI] [PubMed] [Google Scholar]
- 70. Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013;373(1–2):8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Leiser R, Krebs C, Ebert B, Dantzer V. Placental vascular corrosion cast studies: a comparison between ruminants and humans. Microsc Res Tech. 1997;38(1–2):76–87. [DOI] [PubMed] [Google Scholar]
- 72. Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta. 2004;25(2-3):114–126. [DOI] [PubMed] [Google Scholar]
- 73. Burton GJ, Jauniaux E, Murray AJ. Oxygen and placental development; parallels and differences with tumour biology. Placenta. 2017;56:14–18. [DOI] [PubMed] [Google Scholar]
- 74. Yang Y, Abdulhasan M, Awonuga A, Bolnick A, Puscheck EE, Rappolee DA. Hypoxic stress forces adaptive and maladaptive placental stress responses in early pregnancy. Birth Defects Res. 2017;109(17):1330–1344. [DOI] [PubMed] [Google Scholar]
- 75. Kimura C, Watanabe K, Iwasaki A, Mori T, Matsushita H, Shinohara K, Wakatsuki A. The severity of hypoxic changes and oxidative DNA damage in the placenta of early-onset preeclamptic women and fetal growth restriction. J Matern Fetal Neonatal Med. 2013;26(5):491–496. [DOI] [PubMed] [Google Scholar]
- 76. Chen DB, Zheng J. Regulation of placental angiogenesis. Microcirculation. 2014;21(1):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Metzler VM, de Brot S, Robinson RS, Jeyapalan JN, Rakha E, Walton T, Gardner DS, Lund EF, Whitchurch J, Haigh D, Lochray JM, Robinson BD, Allegrucci C, Fray RG, Persson JL, Ødum N, Miftakhova RR, Rizvanov AA, Hughes IA, Tadokoro-Cuccaro R, Heery DM, Rutland CS, Mongan NP. Androgen dependent mechanisms of pro-angiogenic networks in placental and tumor development. Placenta. 2017;56:79–85. [DOI] [PubMed] [Google Scholar]
- 78. Osol G, Ko NL, Mandalà M. Altered endothelial nitric oxide signaling as a paradigm for maternal vascular maladaptation in preeclampsia. Curr Hypertens Rep. 2017;19(10):82. [DOI] [PubMed] [Google Scholar]
- 79. Brett KE, Ferraro ZM, Yockell-Lelievre J, Gruslin A, Adamo KB. Maternal–fetal nutrient transport in pregnancy pathologies: the role of the placenta. Int J Mol Sci. 2014;15(9):16153–16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vaughan OR, Rosario FJ, Powell TL, Jansson T. Regulation of placental amino acid transport and fetal growth. Prog Mol Biol Transl Sci. 2017;145:217–251. [DOI] [PubMed] [Google Scholar]
- 81. Lewis RM, Desoye G. Placental Lipid and Fatty Acid Transfer in Maternal Overnutrition. Ann Nutr Metab. 2017;70(3):228–231. [DOI] [PubMed] [Google Scholar]
- 82. Hart B, Morgan E, Alejandro E. Nutrient sensor signaling pathways and cellular stress in fetal growth restriction. J Mol Endocrinol. 2019;62(2):R155–R165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cetin I, Parisi F, Berti C, Mandò C, Desoye G. Placental fatty acid transport in maternal obesity. J Dev Orig Health Dis. 2012;3(6):409–414. [DOI] [PubMed] [Google Scholar]
- 84. Maliqueo M, Sundström Poromaa I, Vanky E, Fornes R, Benrick A, Åkerud H, Stridsklev S, Labrie F, Jansson T, Stener-Victorin E. Placental STAT3 signaling is activated in women with polycystic ovary syndrome. Hum Reprod. 2015;30(3):692–700. [DOI] [PubMed] [Google Scholar]
- 85. Wu F, Tian FJ, Lin Y, Xu WM. Oxidative stress: placenta function and dysfunction. Am J Reprod Immunol. 2016;76(4):258–271. [DOI] [PubMed] [Google Scholar]
- 86. Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 2010;42(10):1634–1650. [DOI] [PubMed] [Google Scholar]
- 88. Pereira AC, Martel F. Oxidative stress in pregnancy and fertility pathologies. Cell Biol Toxicol. 2014;30(5):301–312. [DOI] [PubMed] [Google Scholar]
- 89. Bowen JM, Chamley L, Keelan JA, Mitchell MD. Cytokines of the placenta and extra-placental membranes: roles and regulation during human pregnancy and parturition. Placenta. 2002;23(4):257–273. [DOI] [PubMed] [Google Scholar]
- 90. Hauguel-de Mouzon S, Guerre-Millo M. The placenta cytokine network and inflammatory signals. Placenta. 2006;27(8):794–798. [DOI] [PubMed] [Google Scholar]
- 91. Lacroix M, Kina E, Hivert MF. Maternal/fetal determinants of insulin resistance in women during pregnancy and in offspring over life. Curr Diab Rep. 2013;13(2):238–244. [DOI] [PubMed] [Google Scholar]
- 92. Rodrigo N, Glastras SJ. The emerging role of biomarkers in the diagnosis of gestational diabetes mellitus. J Clin Med. 2018;7(6):E120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wei SQ, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol. 2010;116(2 Pt 1):393–401. [DOI] [PubMed] [Google Scholar]
- 94. Boyle AK, Rinaldi SF, Norman JE, Stock SJ. Preterm birth: inflammation, fetal injury and treatment strategies. J Reprod Immunol. 2017;119:62–66. [DOI] [PubMed] [Google Scholar]
- 95. Engin AB. What is lipotoxicity? Adv Exp Med Biol. 2017;960:197–220. [DOI] [PubMed] [Google Scholar]
- 96. Saben J, Lindsey F, Zhong Y, Thakali K, Badger TM, Andres A, Gomez-Acevedo H, Shankar K. Maternal obesity is associated with a lipotoxic placental environment. Placenta. 2014;35(3):171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Diamant YZ, Metzger BE, Freinkel N, Shafrir E. Placental lipid and glycogen content in human and experimental diabetes mellitus. Am J Obstet Gynecol. 1982;144(1):5–11. [DOI] [PubMed] [Google Scholar]
- 98. Visiedo F, Bugatto F, Sánchez V, Cózar-Castellano I, Bartha JL, Perdomo G. High glucose levels reduce fatty acid oxidation and increase triglyceride accumulation in human placenta. Am J Physiol Endocrinol Metab. 2013;305(2):E205–E212. [DOI] [PubMed] [Google Scholar]
- 99. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fedorova OV, Ishkaraeva VV, Grigorova YN, Reznik VA, Kolodkin NI, Zazerskaya IE, Zernetkina V, Agalakova NI, Tapilskaya NI, Adair CD, Lakatta EG, Bagrov AY. Antibody to marinobufagenin reverses placenta-induced fibrosis of umbilical arteries in preeclampsia. Int J Mol Sci. 2018;19(8):E2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ohmaru-Nakanishi T, Asanoma K, Fujikawa M, Fujita Y, Yagi H, Onoyama I, Hidaka N, Sonoda K, Kato K. Fibrosis in preeclamptic placentas is associated with stromal fibroblasts activated by the transforming growth factor-β1 signaling pathway. Am J Pathol. 2018;188(3):683–695. [DOI] [PubMed] [Google Scholar]
- 102. Fowden AL, Forhead AJ, Sferruzzi-Perri AN, Burton GJ, Vaughan OR. Review: endocrine regulation of placental phenotype. Placenta. 2015;36(Suppl 1):S50–S59. [DOI] [PubMed] [Google Scholar]
- 103. Makieva S, Saunders PT, Norman JE. Androgens in pregnancy: roles in parturition. Hum Reprod Update. 2014;20(4):542–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Meakin AS, Clifton VL. Review: understanding the role of androgens and placental AR variants: Insight into steroid-dependent fetal-placental growth and development. Placenta. 2019;84:63–68. [DOI] [PubMed] [Google Scholar]
- 105. Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Pérez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17(10):2573–2579. [DOI] [PubMed] [Google Scholar]
- 106. Glintborg D, Jensen RC, Bentsen K, Schmedes AV, Brandslund I, Kyhl HB, Bilenberg N, Andersen MS. Testosterone levels in third trimester in polycystic ovary syndrome. Odense Child Cohort. J Clin Endocrinol Metab. 2018;103(10):3819–3827. [DOI] [PubMed] [Google Scholar]
- 107. Sathishkumar K, Elkins R, Chinnathambi V, Gao H, Hankins GD, Yallampalli C. Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport. Reprod Biol Endocrinol. 2011;9(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Beckett EM, Astapova O, Steckler TL, Veiga-Lopez A, Padmanabhan V. Developmental programing: impact of testosterone on placental differentiation. Reproduction. 2014;148(2):199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Maliqueo M, Lara HE, Sánchez F, Echiburú B, Crisosto N, Sir-Petermann T. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;166(2):151–155. [DOI] [PubMed] [Google Scholar]
- 110. Barry JA, Hardiman PJ, Siddiqui MR, Thomas M. Meta-analysis of sex difference in testosterone levels in umbilical cord blood. J Obstet Gynaecol. 2011;31(8):697–702. [DOI] [PubMed] [Google Scholar]
- 111. Daan NM, Koster MP, Steegers-Theunissen RP, Eijkemans MJ, Fauser BC. Endocrine and cardiometabolic cord blood characteristics of offspring born to mothers with and without polycystic ovary syndrome. Fertil Steril. 2017;107(1):261–268.e3. [DOI] [PubMed] [Google Scholar]
- 112. Barry JA, Kay AR, Navaratnarajah R, Iqbal S, Bamfo JE, David AL, Hines M, Hardiman PJ. Umbilical vein testosterone in female infants born to mothers with polycystic ovary syndrome is elevated to male levels. J Obstet Gynaecol. 2010;30(5):444–446. [DOI] [PubMed] [Google Scholar]
- 113. Mehrabian F, Kelishadi R. Comparison of the metabolic parameters and androgen level of umbilical cord blood in newborns of mothers with polycystic ovary syndrome and controls. J Res Med Sci. 2012;17(3):207–211. [PMC free article] [PubMed] [Google Scholar]
- 114. Caanen MR, Kuijper EA, Hompes PG, Kushnir MM, Rockwood AL, Meikle WA, Homburg R, Lambalk CB. Mass spectrometry methods measured androgen and estrogen concentrations during pregnancy and in newborns of mothers with polycystic ovary syndrome. Eur J Endocrinol. 2016;174(1):25–32. [DOI] [PubMed] [Google Scholar]
- 115. Hickey M, Sloboda DM, Atkinson HC, Doherty DA, Franks S, Norman RJ, Newnham JP, Hart R. The relationship between maternal and umbilical cord androgen levels and polycystic ovary syndrome in adolescence: a prospective cohort study. J Clin Endocrinol Metab. 2009;94(10):3714–3720. [DOI] [PubMed] [Google Scholar]
- 116. Anderson H, Fogel N, Grebe SK, Singh RJ, Taylor RL, Dunaif A. Infants of women with polycystic ovary syndrome have lower cord blood androstenedione and estradiol levels. J Clin Endocrinol Metab. 2010;95(5):2180–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Padmanabhan V, Veiga-Lopez A. Animal models of the polycystic ovary syndrome phenotype. Steroids. 2013;78(8):734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Barrett ES, Swan SH. Stress and androgen activity during fetal development. Endocrinology. 2015;156(10):3435–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Recabarren SE, Sir-Petermann T, Maliqueo M, Lobos A, Rojas-García P. Prenatal exposure to androgens as a factor of fetal programming [in Spanish]. Rev Med Chil. 2006;134(1):101–108. [DOI] [PubMed] [Google Scholar]
- 120. Cleys ER, Halleran JL, Enriquez VA, da Silveira JC, West RC, Winger QA, Anthony RV, Bruemmer JE, Clay CM, Bouma GJ. Androgen receptor and histone lysine demethylases in ovine placenta. PLoS One. 2015;10(2):e0117472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sun M, Maliqueo M, Benrick A, Johansson J, Shao R, Hou L, Jansson T, Wu X, Stener-Victorin E. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. Am J Physiol Endocrinol Metab. 2012;303(11):E1373–E1385. [DOI] [PubMed] [Google Scholar]
- 122. Hsu TY, Lan KC, Tsai CC, Ou CY, Cheng BH, Tsai MY, Kang HY, Tung YH, Wong YH, Huang KE. Expression of androgen receptor in human placentas from normal and preeclamptic pregnancies. Taiwan J Obstet Gynecol. 2009;48(3):262–267. [DOI] [PubMed] [Google Scholar]
- 123. Shah AB, Nivar I, Speelman DL. Elevated androstenedione in young adult but not early adolescent prenatally androgenized female rats. PLoS One. 2018;13(5):e0196862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Domonkos E, Borbélyová V, Kolátorová L, Chlupáčová T, Ostatníková D, Hodosy J, Stárka L, Celec P. Sex differences in the effect of prenatal testosterone exposure on steroid hormone production in adult rats. Physiol Res. 2017;66(Suppl 3):S367–S374. [DOI] [PubMed] [Google Scholar]
- 125. Gopalakrishnan K, Mishra JS, Chinnathambi V, Vincent KL, Patrikeev I, Motamedi M, Saade GR, Hankins GD, Sathishkumar K. Elevated testosterone reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant rats. Hypertension. 2016;67(3):630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chinnathambi V, Blesson CS, Vincent KL, Saade GR, Hankins GD, Yallampalli C, Sathishkumar K. Elevated testosterone levels during rat pregnancy cause hypersensitivity to angiotensin II and attenuation of endothelium-dependent vasodilation in uterine arteries. Hypertension. 2014;64(2):405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hu M, Richard JE, Maliqueo M, Kokosar M, Fornes R, Benrick A, Jansson T, Ohlsson C, Wu X, Skibicka KP, Stener-Victorin E. Maternal testosterone exposure increases anxiety-like behavior and impacts the limbic system in the offspring. Proc Natl Acad Sci USA. 2015;112(46):14348–14353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Fornes R, Maliqueo M, Hu M, Hadi L, Jimenez-Andrade JM, Ebefors K, Nyström J, Labrie F, Jansson T, Benrick A, Stener-Victorin E. The effect of androgen excess on maternal metabolism, placental function and fetal growth in obese dams. Sci Rep. 2017;7(1):8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Fornes R, Manti M, Qi X, Vorontsov E, Sihlbom C, Nyström J, Jerlhag E, Maliqueo M, Hirschberg AL, Carlström M, Benrick A, Stener-Victorin E. Mice exposed to maternal androgen excess and diet-induced obesity have altered phosphorylation of catechol-O-methyltransferase in the placenta and fetal liver [published online ahead of print 22 January 2019]. Int J Obes. doi: 10.1038/s41366-018-0314-8. [DOI] [PubMed] [Google Scholar]
- 130. Kumar S, Gordon GH, Abbott DH, Mishra JS. Androgens in maternal vascular and placental function: implications for preeclampsia pathogenesis. Reproduction. 2018;156(5):R155–R167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Williams CJ, Chu A, Jefferson WN, Casero D, Sudhakar D, Khurana N, Hogue CP, Aryasomayajula C, Patel P, Sullivan P, Padilla-Banks E, Mohandessi S, Janzen C, Wadehra M. Epithelial membrane protein 2 (EMP2) deficiency alters placental angiogenesis, mimicking features of human placental insufficiency. J Pathol. 2017;242(2):246–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Maliqueo M, Echiburú B, Crisosto N. Sex steroids modulate uterine-placental vasculature: implications for obstetrics and neonatal outcomes. Front Physiol. 2016;7:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Fornes R, Hu M, Maliqueo M, Kokosar M, Benrick A, Carr D, Billig H, Jansson T, Manni L, Stener-Victorin E. Maternal testosterone and placental function: effect of electroacupuncture on placental expression of angiogenic markers and fetal growth. Mol Cell Endocrinol. 2016;433:1–11. [DOI] [PubMed] [Google Scholar]
- 134. Brown SH, Eather SR, Freeman DJ, Meyer BJ, Mitchell TW. A lipidomic analysis of placenta in preeclampsia: evidence for lipid storage. PLoS One. 2016;11(9):e0163972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Mastrogiannis DS, Spiliopoulos M, Mulla W, Homko CJ. Insulin resistance: the possible link between gestational diabetes mellitus and hypertensive disorders of pregnancy. Curr Diab Rep. 2009;9(4):296–302. [DOI] [PubMed] [Google Scholar]
- 136. Scioscia M, Gumaa K, Rademacher TW. The link between insulin resistance and preeclampsia: new perspectives. J Reprod Immunol. 2009;82(2):100–105. [DOI] [PubMed] [Google Scholar]
- 137. Wright D, Syngelaki A, Akolekar R, Poon LC, Nicolaides KH. Competing risks model in screening for preeclampsia by maternal characteristics and medical history. Am J Obstet Gynecol. 2015;213(1):62.e1–62.e10. [DOI] [PubMed] [Google Scholar]
- 138. Troisi R, Potischman N, Roberts JM, Ness R, Crombleholme W, Lykins D, Siiteri P, Hoover RN. Maternal serum oestrogen and androgen concentrations in preeclamptic and uncomplicated pregnancies. Int J Epidemiol. 2003;32(3):455–460. [DOI] [PubMed] [Google Scholar]
- 139. Sharifzadeh F, Kashanian M, Fatemi F. A comparison of serum androgens in pre-eclamptic and normotensive pregnant women during the third trimester of pregnancy. Gynecol Endocrinol. 2012;28(10):834–836. [DOI] [PubMed] [Google Scholar]
- 140. Palomba S, Russo T, Falbo A, Di Cello A, Amendola G, Mazza R, Tolino A, Zullo F, Tucci L, La Sala GB. Decidual endovascular trophoblast invasion in women with polycystic ovary syndrome: an experimental case-control study. J Clin Endocrinol Metab. 2012;97(7):2441–2449. [DOI] [PubMed] [Google Scholar]
- 141. Romero R, Erez O, Hüttemann M, Maymon E, Panaitescu B, Conde-Agudelo A, Pacora P, Yoon BH, Grossman LI. Metformin, the aspirin of the 21st century: its role in gestational diabetes mellitus, prevention of preeclampsia and cancer, and the promotion of longevity. Am J Obstet Gynecol. 2017;217(3):282–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tran HT, Liong S, Lim R, Barker G, Lappas M. Resveratrol ameliorates the chemical and microbial induction of inflammation and insulin resistance in human placenta, adipose tissue and skeletal muscle. PLoS One. 2017;12(3):e0173373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Chatuphonprasert W, Jarukamjorn K, Ellinger I. Physiology and pathophysiology of steroid biosynthesis, transport and metabolism in the human placenta. Front Pharmacol. 2018;9:1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Hertig A, Liere P, Chabbert-Buffet N, Fort J, Pianos A, Eychenne B, Cambourg A, Schumacher M, Berkane N, Lefevre G, Uzan S, Rondeau E, Rozenberg P, Rafestin-Oblin ME. Steroid profiling in preeclamptic women: evidence for aromatase deficiency. Am J Obstet Gynecol. 2010;203(5):477.e1–477.e9. [DOI] [PubMed] [Google Scholar]
- 145. Bussen S, Bussen D. Influence of the vascular endothelial growth factor on the development of severe pre-eclampsia or HELLP syndrome. Arch Gynecol Obstet. 2011;284(3):551–557. [DOI] [PubMed] [Google Scholar]
- 146. Sonagra AD, Biradar SM, Dattatreya K, Jayaprakash Murthy DS. Normal pregnancy—a state of insulin resistance. J Clin Diagn Res. 2014;8(11):CC01–CC03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Vejrazkova D, Vcelak J, Vankova M, Lukasova P, Bradnova O, Halkova T, Kancheva R, Bendlova B. Steroids and insulin resistance in pregnancy. J Steroid Biochem Mol Biol. 2014;139:122–129. [DOI] [PubMed] [Google Scholar]
- 148. Naderpoor N, Shorakae S, de Courten B, Misso ML, Moran LJ, Teede HJ. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21(5):560–574. [DOI] [PubMed] [Google Scholar]
- 149. Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, Teede HJ. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod. 2013;28(3):777–784. [DOI] [PubMed] [Google Scholar]
- 150. Sir-Petermann T, Echiburú B, Maliqueo MM, Crisosto N, Sánchez F, Hitschfeld C, Cárcamo M, Amigo P, Pérez-Bravo F. Serum adiponectin and lipid concentrations in pregnant women with polycystic ovary syndrome. Hum Reprod. 2007;22(7):1830–1836. [DOI] [PubMed] [Google Scholar]
- 151. Palomba S, Falbo A, Chiossi G, Muscogiuri G, Fornaciari E, Orio F, Tolino A, Colao A, La Sala GB, Zullo F. Lipid profile in nonobese pregnant women with polycystic ovary syndrome: a prospective controlled clinical study. Steroids. 2014;88:36–43. [DOI] [PubMed] [Google Scholar]
- 152. Frasca F, Pandini G, Sciacca L, Pezzino V, Squatrito S, Belfiore A, Vigneri R. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem. 2008;114(1):23–37. [DOI] [PubMed] [Google Scholar]
- 153. Westermeier F, Sáez T, Arroyo P, Toledo F, Gutiérrez J, Sanhueza C, Pardo F, Leiva A, Sobrevia L. Insulin receptor isoforms: an integrated view focused on gestational diabetes mellitus. Diabetes Metab Res Rev. 2016;32(4):350–365. [DOI] [PubMed] [Google Scholar]
- 154. Mayama R, Izawa T, Sakai K, Suciu N, Iwashita M. Improvement of insulin sensitivity promotes extravillous trophoblast cell migration stimulated by insulin-like growth factor-I. Endocr J. 2013;60(3):359–368. [DOI] [PubMed] [Google Scholar]
- 155. Solomon CG, Seely EW. Brief review: hypertension in pregnancy: a manifestation of the insulin resistance syndrome? Hypertension. 2001;37(2):232–239. [DOI] [PubMed] [Google Scholar]
- 156. Nestler JE. Regulation of the aromatase activity of human placental cytotrophoblasts by insulin, insulin-like growth factor-I, and -II. J Steroid Biochem Mol Biol. 1993;44(4–6):449–457. [DOI] [PubMed] [Google Scholar]
- 157. Cardoso RC, Puttabyatappa M, Padmanabhan V. Steroidogenic versus metabolic programming of reproductive neuroendocrine, ovarian and metabolic dysfunctions. Neuroendocrinology. 2015;102(3):226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Gabbay-Benziv R, Baschat AA. Gestational diabetes as one of the “great obstetrical syndromes”—the maternal, placental, and fetal dialog. Best Pract Res Clin Obstet Gynaecol. 2015;29(2):150–155. [DOI] [PubMed] [Google Scholar]
- 159. Jakubowicz DJ, Essah PA, Seppälä M, Jakubowicz S, Baillargeon JP, Koistinen R, Nestler JE. Reduced serum glycodelin and insulin-like growth factor-binding protein-1 in women with polycystic ovary syndrome during first trimester of pregnancy. J Clin Endocrinol Metab. 2004;89(2):833–839. [DOI] [PubMed] [Google Scholar]
- 160. Morris DV, Falcone T. The relationship between insulin sensitivity and insulin-like growth factor-binding protein-1. Gynecol Endocrinol. 1996;10(6):407–412. [DOI] [PubMed] [Google Scholar]
- 161. de Wilde MA, Veltman-Verhulst SM, Goverde AJ, Lambalk CB, Laven JS, Franx A, Koster MP, Eijkemans MJ, Fauser BC. Preconception predictors of gestational diabetes: a multicentre prospective cohort study on the predominant complication of pregnancy in polycystic ovary syndrome. Hum Reprod. 2014;29(6):1327–1336. [DOI] [PubMed] [Google Scholar]
- 162. Gözükara YM, Aytan H, Ertunc D, Tok EC, Demirtürk F, Şahin Ş, Aytan P. Role of first trimester total testosterone in prediction of subsequent gestational diabetes mellitus. J Obstet Gynaecol Res. 2015;41(2):193–198. [DOI] [PubMed] [Google Scholar]
- 163. Powe CE. Early pregnancy biochemical predictors of gestational diabetes mellitus. Curr Diab Rep. 2017;17(2):12. [DOI] [PubMed] [Google Scholar]
- 164. Belkacemi L, Kjos S, Nelson DM, Desai M, Ross MG. Reduced apoptosis in term placentas from gestational diabetic pregnancies. J Dev Orig Health Dis. 2013;4(3):256–265. [DOI] [PubMed] [Google Scholar]
- 165. Madazli R, Tuten A, Calay Z, Uzun H, Uludag S, Ocak V. The incidence of placental abnormalities, maternal and cord plasma malondialdehyde and vascular endothelial growth factor levels in women with gestational diabetes mellitus and nondiabetic controls. Gynecol Obstet Invest. 2008;65(4):227–232. [DOI] [PubMed] [Google Scholar]
- 166. Meng Q, Shao L, Luo X, Mu Y, Xu W, Gao L, Xu H, Cui Y. Expressions of VEGF-A and VEGFR-2 in placentae from GDM pregnancies. Reprod Biol Endocrinol. 2016;14(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Wang K, Zheng J. Signaling regulation of fetoplacental angiogenesis. J Endocrinol. 2012;212(3):243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Zhou J, Ni X, Huang X, Yao J, He Q, Wang K, Duan T. Potential role of hyperglycemia in fetoplacental endothelial dysfunction in gestational diabetes mellitus. Cell Physiol Biochem. 2016;39(4):1317–1328. [DOI] [PubMed] [Google Scholar]
- 169. Ericsson A, Säljö K, Sjöstrand E, Jansson N, Prasad PD, Powell TL, Jansson T. Brief hyperglycaemia in the early pregnant rat increases fetal weight at term by stimulating placental growth and affecting placental nutrient transport. J Physiol. 2007;581(Pt 3):1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Bobadilla RA, van Bree R, Vercruysse L, Pijnenborg R, Verhaeghe J. Placental effects of systemic tumour necrosis factor-α in an animal model of gestational diabetes mellitus. Placenta. 2010;31(12):1057–1063. [DOI] [PubMed] [Google Scholar]
- 171. Salbaum JM, Kruger C, Zhang X, Delahaye NA, Pavlinkova G, Burk DH, Kappen C. Altered gene expression and spongiotrophoblast differentiation in placenta from a mouse model of diabetes in pregnancy. Diabetologia. 2011;54(7):1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Li HP, Chen X, Li MQ. Gestational diabetes induces chronic hypoxia stress and excessive inflammatory response in murine placenta. Int J Clin Exp Pathol. 2013;6(4):650–659. [PMC free article] [PubMed] [Google Scholar]
- 173. Cisse O, Fajardy I, Dickes-Coopman A, Moitrot E, Montel V, Deloof S, Rousseaux J, Vieau D, Laborie C. Mild gestational hyperglycemia in rat induces fetal overgrowth and modulates placental growth factors and nutrient transporters expression. PLoS One. 2013. 14;8(5):e64251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Dela Justina V, Gonçalves JS, de Freitas RA, Fonseca AD, Volpato GT, Tostes RC, Carneiro FS, Lima VV, Giachini FR. Increased O-Linked N-Acetylglucosamine Modification of NF-ΚB and Augmented Cytokine Production in the Placentas from Hyperglycemic Rats. Inflammation. 2017;40(5):1773–1781. [DOI] [PubMed] [Google Scholar]
- 175. Lappas M, Yee K, Permezel M, Rice GE. Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies. J Endocrinol. 2005;186(3):457–465. [DOI] [PubMed] [Google Scholar]
- 176. Shang M, Dong X, Hou L. Correlation of adipokines and markers of oxidative stress in women with gestational diabetes mellitus and their newborns. J Obstet Gynaecol Res. 2018;44(4):637–646. [DOI] [PubMed] [Google Scholar]
- 177. Lappas M, Permezel M, Rice GE. Release of proinflammatory cytokines and 8-isoprostane from placenta, adipose tissue, and skeletal muscle from normal pregnant women and women with gestational diabetes mellitus. J Clin Endocrinol Metab. 2004;89(11):5627–5633. [DOI] [PubMed] [Google Scholar]
- 178. Schanton M, Maymó JL, Pérez-Pérez A, Sánchez-Margalet V, Varone CL. Involvement of leptin in the molecular physiology of the placenta. Reproduction. 2018;155(1):R1–R12. [DOI] [PubMed] [Google Scholar]
- 179. Sartori C, Lazzeroni P, Merli S, Patianna VD, Viaroli F, Cirillo F, Amarri S, Street ME. From placenta to polycystic ovarian syndrome: the role of adipokines. Mediators Inflamm. 2016;2016:4981916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Woods L, Perez-Garcia V, Hemberger M. Regulation of placental development and its impact on fetal growth—new insights from mouse models. Front Endocrinol (Lausanne). 2018;9:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Cox B, Leavey K, Nosi U, Wong F, Kingdom J. Placental transcriptome in development and pathology: expression, function, and methods of analysis. Am J Obstet Gynecol. 2015;213(4Suppl):S138–S151. [DOI] [PubMed] [Google Scholar]
- 182. Ahmed MS, Aleksunes LM, Boeuf P, Chung MK, Daoud G, Desoye G, Díaz P, Golos TG, Illsley NP, Kikuchi K, Komatsu R, Lao T, Morales-Prieto DM, Nanovskaya T, Nobuzane T, Roberts CT, Saffery R, Tamura I, Tamura K, Than NG, Tomi M, Umbers A, Wang B, Weedon-Fekjaer MS, Yamada S, Yamazaki K, Yoshie M, Lash GE. IFPA Meeting 2012 Workshop Report II: epigenetics and imprinting in the placenta, growth factors and villous trophoblast differentiation, role of the placenta in regulating fetal exposure to xenobiotics during pregnancy, infection and the placenta. Placenta. 2013;34(Suppl):S6–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Leeuwerke M, Eilander MS, Pruis MGM, Lendvai Á, Erwich JJ, Scherjon SA, Plösch T, Eijsink JJ. DNA methylation and expression patterns of selected genes in first-trimester placental tissue from pregnancies with small-for-gestational-age infants at birth. Biol Reprod. 2016;94(2):37. [DOI] [PubMed] [Google Scholar]
- 184. Gheorghe CP, Goyal R, Mittal A, Longo LD. Gene expression in the placenta: maternal stress and epigenetic responses. Int J Dev Biol. 2010;54(2–3):507–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Hiura H, Hattori H, Kobayashi N, Okae H, Chiba H, Miyauchi N, Kitamura A, Kikuchi H, Yoshida H, Arima T. Genome-wide microRNA expression profiling in placentae from frozen-thawed blastocyst transfer. Clin Epigenetics. 2017;9(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Cai M, Kolluru GK, Ahmed A. Small molecule, big prospects: microRNA in pregnancy and its complications. J Pregnancy. 2017;2017:6972732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Qian Y, Lu Y, Rui C, Qian Y, Cai M, Jia R. Potential significance of circular RNA in human placental tissue for patients with preeclampsia. Cell Physiol Biochem. 2016;39(4):1380–1390. [DOI] [PubMed] [Google Scholar]
- 188. Li X, Wu C, Shen Y, Wang K, Tang L, Zhou M, Yang M, Pan T, Liu X, Xu W. Ten-eleven translocation 2 demethylates the MMP9 promoter, and its down-regulation in preeclampsia impairs trophoblast migration and invasion. J Biol Chem. 2018;293(26):10059–10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Leavey K, Wilson SL, Bainbridge SA, Robinson WP, Cox BJ. Epigenetic regulation of placental gene expression in transcriptional subtypes of preeclampsia. Clin Epigenetics. 2018;10(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Peixoto AB, Rolo LC, Nardozza LM, Araujo Júnior E. Epigenetics and preeclampsia: programming of future outcomes. Methods Mol Biol. 2018;1710:73–83. [DOI] [PubMed] [Google Scholar]
- 191. Laganà AS, Vitale SG, Sapia F, Valenti G, Corrado F, Padula F, Rapisarda AMC, D’Anna R. miRNA expression for early diagnosis of preeclampsia onset: hope or hype? J Matern Fetal Neonatal Med. 2018;31(6):817–821. [DOI] [PubMed] [Google Scholar]
- 192. Mayne BT, Leemaqz SY, Smith AK, Breen J, Roberts CT, Bianco-Miotto T. Accelerated placental aging in early onset preeclampsia pregnancies identified by DNA methylation. Epigenomics. 2017;9(3):279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Zhou W, Wang H, Wu X, Long W, Zheng F, Kong J, Yu B. The profile analysis of circular RNAs in human placenta of preeclampsia. Exp Biol Med (Maywood). 2018;243(14):1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Yan J, Yang H. Gestational diabetes mellitus, programing and epigenetics. J Matern Fetal Neonatal Med. 2014;27(12):1266–1269. [DOI] [PubMed] [Google Scholar]
- 195. Reichetzeder C, Dwi Putra SE, Pfab T, Slowinski T, Neuber C, Kleuser B, Hocher B. Increased global placental DNA methylation levels are associated with gestational diabetes. Clin Epigenetics. 2016;8(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Concha CF, Sir PT, Recabarren SE, Pérez BF. Epigenetics of polycystic ovary syndrome [in Spanish]. Rev Med Chil. 2017;145(7):907–915. [DOI] [PubMed] [Google Scholar]
- 197. Ilie IR, Georgescu CE. Polycystic ovary syndrome—epigenetic mechanisms and aberrant microRNA. Adv Clin Chem. 2015;71:25–45. [DOI] [PubMed] [Google Scholar]
- 198. Yu YY, Sun CX, Liu YK, Li Y, Wang L, Zhang W. Genome-wide screen of ovary-specific DNA methylation in polycystic ovary syndrome. Fertil Steril. 2015;104(1):145–153.e6. [DOI] [PubMed] [Google Scholar]
- 199. Lambertini L, Saul SR, Copperman AB, Hammerstad SS, Yi Z, Zhang W, Tomer Y, Kase N. Intrauterine reprogramming of the polycystic ovary syndrome: evidence from a pilot study of cord blood global methylation analysis. Front Endocrinol (Lausanne). 2017;8:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Barouki R, Melén E, Herceg Z, Beckers J, Chen J, Karagas M, Puga A, Xia Y, Chadwick L, Yan W, Audouze K, Slama R, Heindel J, Grandjean P, Kawamoto T, Nohara K. Epigenetics as a mechanism linking developmental exposures to long-term toxicity. Environ Int. 2018;114:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Yamada L, Chong S. Epigenetic studies in developmental origins of health and disease: pitfalls and key considerations for study design and interpretation. J Dev Orig Health Dis. 2017;8(1):30–43. [DOI] [PubMed] [Google Scholar]
- 202. Dearden L, Bouret SG, Ozanne SE. Sex and gender differences in developmental programming of metabolism. Mol Metab. 2018;15:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. McCabe C, Anderson OS, Montrose L, Neier K, Dolinoy DC. Sexually dimorphic effects of early-life exposures to endocrine disruptors: sex-specific epigenetic reprogramming as a potential mechanism. Curr Environ Health Rep. 2017;4(4):426–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Nugent BM, O’Donnell CM, Epperson CN, Bale TL. Placental H3K27me3 establishes female resilience to prenatal insults. Nat Commun. 2018;9(1):2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Carpenter T, Grecian SM, Reynolds RM. Sex differences in early-life programming of the hypothalamic–pituitary–adrenal axis in humans suggest increased vulnerability in females: a systematic review. J Dev Orig Health Dis. 2017;8(2):244–255. [DOI] [PubMed] [Google Scholar]
- 206. Banu SK, Stanley JA, Taylor RJ, Sivakumar KK, Arosh JA, Zeng L, Pennathur S, Padmanabhan V. Sexually dimorphic impact of chromium accumulation on human placental oxidative stress and apoptosis. Toxicol Sci. 2018;161(2):375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Friedrich MJ. Microbiome project seeks to understand human body’s microscopic residents. JAMA. 2008;300(7):777–778. [DOI] [PubMed] [Google Scholar]
- 208. Fox C, Eichelberger K. Maternal microbiome and pregnancy outcomes. Fertil Steril. 2015;104(6):1358–1363. [DOI] [PubMed] [Google Scholar]
- 209. Vinturache AE, Gyamfi-Bannerman C, Hwang J, Mysorekar IU, Jacobsson B; Preterm Birth International Collaborative (PREBIC). Maternal microbiome—a pathway to preterm birth. Semin Fetal Neonatal Med. 2016;21(2):94–99. [DOI] [PubMed] [Google Scholar]
- 210. Taddei CR, Cortez RV, Mattar R, Torloni MR, Daher S. Microbiome in normal and pathological pregnancies: a literature overview. Am J Reprod Immunol. 2018;80(2):e12993. [DOI] [PubMed] [Google Scholar]
- 211. Zheng J, Xiao X, Zhang Q, Mao L, Yu M, Xu J, Wang T. the placental microbiota is altered among subjects with gestational diabetes mellitus: a pilot study. Front Physiol. 2017;8:675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Stinson LF, Payne MS, Keelan JA. Planting the seed: origins, composition, and postnatal health significance of the fetal gastrointestinal microbiota. Crit Rev Microbiol. 2017;43(3):352–369. [DOI] [PubMed] [Google Scholar]
- 213. Stanislawski MA, Dabelea D, Wagner BD, Sontag MK, Lozupone CA, Eggesbø M. Pre-pregnancy weight, gestational weight gain, and the gut microbiota of mothers and their infants. Microbiome. 2017;5(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Thackray VG. Sex, microbes, and polycystic ovary syndrome. Trends Endocrinol Metab. 2019;30(1):54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Torres PJ, Siakowska M, Banaszewska B, Pawelczyk L, Duleba AJ, Kelley ST, Thackray VG. Gut microbial diversity in women with polycystic ovary syndrome correlates with hyperandrogenism. J Clin Endocrinol Metab. 2018;103(4):1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Insenser M, Murri M, Del Campo R, Martínez-García MÁ, Fernández-Durán E, Escobar-Morreale HF. Gut microbiota and the polycystic ovary syndrome: influence of sex, sex hormones, and obesity. J Clin Endocrinol Metab. 2018;103(7):2552–2562. [DOI] [PubMed] [Google Scholar]
- 217. Sherman SB, Sarsour N, Salehi M, Schroering A, Mell B, Joe B, Hill JW. Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes. 2018;9(5):400–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Insenser M, Escobar-Morreale HF. Proteomics and polycystic ovary syndrome. Expert Rev Proteomics. 2013;10(5):435–447. [DOI] [PubMed] [Google Scholar]
- 219. Li S, Zhu D, Duan H, Tan Q. The epigenomics of polycystic ovarian syndrome: from pathogenesis to clinical manifestations. Gynecol Endocrinol. 2016;32(12):942–946. [DOI] [PubMed] [Google Scholar]
- 220. Jovanovic VP, Carmina E, Lobo RA. Not all women diagnosed with PCOS share the same cardiovascular risk profiles. Fertil Steril. 2010;94(3):826–832. [DOI] [PubMed] [Google Scholar]
- 221. Zhao Y, Qiao J. Ethnic differences in the phenotypic expression of polycystic ovary syndrome. Steroids. 2013;78(8):755–760. [DOI] [PubMed] [Google Scholar]
- 222. Fux-Otta C, Maliqueo M, Echiburú B, Rosato O, Crisosto N, Iraci GS, Fiol de Cuneo M, Szafryk de Mereshian P, Sir-Petermann T. Pregnancy outcomes in women with polycystic ovary syndrome in two Latin American populations. J Obstet Gynaecol. 2018;38(6):750–755. [DOI] [PubMed] [Google Scholar]