Abstract
Traumatic brain injury (TBI), a major cause of mortality and morbidity, affects 10 million people worldwide, with limited treatment options. We have previously shown that (-)-phenserine (Phen), an acetylcholinesterase inhibitor originally designed and tested in clinical phase III trials for Alzheimer’s disease, can reduce neurodegeneration after TBI and reduce cognitive impairments induced by mild TBI. In this study, we used a mouse model of moderate to severe TBI by controlled cortical impact to assess the effects of Phen on post-trauma histochemical and behavioral changes. Animals were treated with Phen (2.5 mg/kg, IP, BID) for 5 days started on the day of injury and the effects were evaluated by behavioral and histological examinations at 1 and 2 weeks after injury. Phen significantly attenuated TBI-induced contusion volume, enlargement of the lateral ventricle, and behavioral impairments in motor asymmetry, sensorimotor functions, motor coordination, and balance functions. The morphology of microglia was shifted to an active from a resting form after TBI, and Phen dramatically reduced the ratio of activated to resting microglia, suggesting that Phen also mitigates neuroinflammation after TBI. While Phen has potent anti-acetylcholinesterase activity, its (+) isomer Posiphen shares many neuroprotective properties but is almost completely devoid of anti-acetylcholinesterase activity. We evaluated Posiphen at a similar dose to Phen and found similar mitigation in lateral ventricular size increase, motor asymmetry, motor coordination, and balance function, suggesting the improvement of these histological and behavioral tests by Phen treatment occur via pathways other than anti-acetylcholinesterase inhibition. However, the reduction of lesion size and improvement of sensorimotor function by Posiphen were much smaller than with equivalent doses of Phen. Taken together, these results show that post-injury treatment with Phen over 5 days significantly ameliorates severity of TBI. These data suggest a potential development of this compound for clinical use in TBI therapy.
Keywords: phenserine, traumatic brain injury, controlled cortical impact, contusion volume, behavioral impairment, neuroinflammation
Introduction
The estimated annual global incidence of traumatic brain injury (TBI) is over 10 million, and the risk of subsequent morbidity, mortality, and disability is high1. Patients with TBI have been reported to develop neurodegenerative diseases, including amyotrophic lateral sclerosis, Parkinson’s disease (PD), and Alzheimer’s disease (AD)2,3. Previous studies have shown about a 2-fold increase in risk of PD for subjects who reported a TBI4,5. A meta-analysis showing that the pooled odds ratio for the association of PD and head trauma was 1.57 (95% CI, 1.35–1.83); a history of head trauma that results in concussion is thus associated with a higher risk of developing PD4.
TBI-associated brain damage can be classified into two key phases. The initial primary damage occurs at the moment of insult, and includes contusion, laceration, diffuse axonal injury, and intracranial hemorrhage that results in immediate (necrotic) cell death5. This is followed by an extended second phase that involves cascades of biological processes initiated at the time of injury that may persist for much longer times and produces ischemia, neuroinflammation, glutamate toxicity, astrocyte reactivity, and apoptosis6,7.
Several animal models for TBI have been proposed and each of them has attempted to mimic clinical TBI. Animal models of TBI that have been frequently used for research include fluid percussion injury (FPI), control cortical impact injury (CCI), weight drop impact acceleration injury, and a blast injury model8. CCI is a TBI model that provides a more specific injury in terms of velocity force, time, and depth of injury as compared with the FPI model. This model creates cortical injury, axonal injury, and subcortical injury in the thalamus and hippocampus. CCI-induced brain injuries cause long-term neurobehavioral deficits that persist for more than a year and are associated with cortical atrophy and reduced brain perfusion8.
(−)-Phenserine (Phen), initially developed for AD at the National Institute on Aging (NIA), is a low molecular weight (mw 487.5), chirally pure, lipophilic (Log D 2.2), orally bio-available agent. The compound was developed as an acetylcholinesterase (AChE) selective inhibitor with a high brain delivery9–11; importantly it can be administered in the form of its tartrate salt to support improved aqueous solubility for pharmacological actions12. Phen has a broad range of potential pharmacological benefits of relevance to the effective treatment of disorders such as TBI and AD. Such actions include anti-inflammation, reducing oxidative stress, neuroprotection from preprogrammed cell death, and neuronal stem cell augmentation13.
Studies have revealed functional impairment of the cholinergic system in experimental TBI models as well as in post mortem human TBI samples14,15. AChE inhibitors have, for example, been appraised in preclinical and clinical TBI studies, but have generated mixed results16–18. Rapid elevations in acetylcholine (ACh) levels within cerebrospinal fluid (CSF) in animal models and humans have been reported following TBI, with higher levels associated with greater injury19. This trend supported the early experimental and clinical use of anticholinergic agents, particularly muscarinic antagonists, for the mitigation of ACh-related toxicity to ameliorate TBI-induced deficits20,21.
Previous studies demonstrated that Phen enantiomers have different AChE actions. Whereas Phen has a potent AChE inhibitory action (IC50 24 nM), Posiphen ((+)-phenserine) does not (IC50 >5000 nM). Although they have different activities as AChE inhibitors, both enantiomers are equipotent in their ability to downregulate expression of amyloid precursor protein (APP) and Aβ42 protein in human neuroblastoma cell cultures and to increase neuronal differentiation of human neural stem cells22–25. Besides their potential therapeutic effects for AD, both agents have the ability to reduce α-synuclein translation26, thought to be linked to the etiology of familial PD. The beneficial effects of both enantiomers have been well documented in AD in preclinical models and in clinical trials11,24,27,28. However, their comparative efficacy in TBI remains to be evaluated. In this study, we examined the potential of Phen for repositioning as a TBI treatment in the light of its efficacy in mTBI, including its anti-AChE effects involved in histological and behavioral measures in the CCI animal model.
Materials and Methods
In Vivo Model of TBI
Animal
All animal protocols were conducted under National Institutes Health (NIH) Guidelines using the NIH handbook Animals in Research and were approved by the local Institutional Animal Care and Use Committee. Mice were housed at 25°C with a 12/12 light/dark cycle and continuous water and food supply. All efforts were made to reduce animal suffering and to minimize the number of animals used. The procedures of this study were conducted by following the Institutional Animal Care and Use Committee (IACUC) guidelines (Protocol approval number 2016-0209).
Animal studies were conducted in 8-week-old male C57/BL6 mice (25–30 g, body weight) (Jackson Laboratory, Bar Harbor, ME, USA). Fifty-nine mice were randomly assigned across five groups: sham (8 mice), CCI (8 mice), CCI-saline (15 mice), CCI-Phen (15 mice), and CCI-Posiphen (13 mice), to evaluate the effects of Phen isomers on TBI and to assess the contribution of cholinergic mechanisms to these parameters. Mice were evaluated for motor asymmetry, sensory/motor activity, motor coordination, balance function, and lesion size. Animals were subsequently assessed for cellular changes in histology and immunocytochemistry.
Animal model of TBI and drug administration
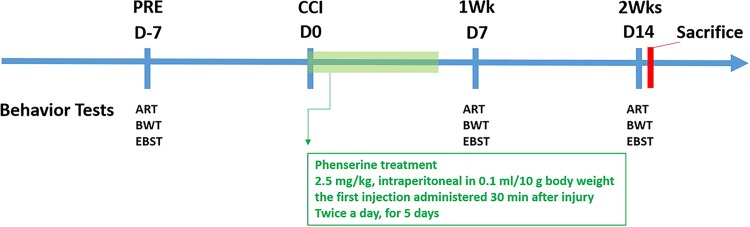
Mice were anesthetized with 2.5% tribromoethanol (Avertin: 250 mg/kg; Sigma, St. Louis, MO, USA) and placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA, USA). Using sterile procedures, the skin was retracted and a 4 mm craniotomy was performed at a point midway between the lambda and bregma sutures and laterally midway between the central suture and the temporalis muscle. The skull was carefully removed without disruption of the underlying dura. Prior to injury induction, the tip of the impactor was angled and kept perpendicular to the exposed cortical surface. The mouse CCI instrument consists of an electromagnetic impactor, Impact One (Leica Biosystems Inc., Buffalo Grove, IL, USA) that allows alteration of injury severity by controlling contact velocity and the level of cortical deformation independently. In these experiments, the contact velocity was set at 5.0 m/sec, dwell time was set at 0.2 s and deformation depth was set at 2 mm to produce moderate-severe TBI. The injury site was allowed to dry prior to suturing the wound. During surgery and recovery, a heating pad was used to maintain the core body temperature of the animals at 36–37°C. Mice were given a 5-day regimen of either Phen or Posiphen (2.5 mg/kg, intraperitoneal (i.p.) in 0.1 ml/10 g body weight) or saline injections, twice daily (every 12 h), with the first injection administered 30 min after injury (Fig. 1).
Fig 1.
Timeline of animal Phen treatment study design. Mice were first evaluated for their baseline sensorimotor, motor coordination/balance, and motor asymmetry functions by adhesive removal test (ART), beam walking test (BWT), and elevated body swing test (EBST) 1 week before CCI injury (PRE). On day 0, mice were given CCI or sham procedures, and 30 min after injury, they received a first injection of Phen (2.5mg/kg body weight, i.p.) or saline. Eight hours after the first injection, a second injection was provided. All mice received two injections of Phen or saline for five consecutive days. Behavioral tests were performed on day 7 and 14 after CCI, and thereafter, mice were euthanized for assessment of contusion volume and histology and immunocytochemistry. A similar timeline was used for Posiphen treatment.
Phen ((-)-phenylcarbamoyleseroline) and Posiphen ((+)-phenylcarbamoyleseroline) were synthesized in the form of their water-soluble tartrate salts (>99.9% chemical and 100% (-)- chiral purity) according to published procedures29. The biological half-life of (-)-phenserine is 8–10 h30, and is 4–5 h for Posiphen27. Hence, any acute effects of both drugs would be washed out well before behavioral studies.
Behavioral Assessments
Asymmetrical motor function
Body asymmetry was quantitatively analyzed by the use of the elevated body swing test (EBST), as initially described by Borlongan and co-workers31–33. Briefly, animals were examined for lateral movement/turning when their bodies were suspended 10 cm above the testing table. The animals were lifted from the table while held by the base of the tail. A left/right swing was counted when the head/torso of the animal moved more than a 10° angle from its vertical axis after elevation. The frequency of the left/right swings was scored across 20 consecutive trials. An uninjured animal shows an equal frequency to swing to either the left or right side. The number of contralateral rotations was determined and used to generate a mean number of rotations for each treatment group, which then was statistically analyzed.
Somatosensory function assessment
A tactile adhesive removal test (ART) was used to evaluate somatosensory function; this test measures the ability of the animal to perform sensitive paw-to-mouth movements and mouth-to-paw dexterity as well as sensory input. Essentially, two small adhesive stickers were used as bilateral tactile stimuli that were placed on the distal–radial region on the wrist of each forelimb. Animals were pre-trained daily for 3 days before CCI, and the time required (no longer than 2 min) for the animal to remove each sticker from the forelimb was recorded 4 days before CCI, and 1 and 2 weeks after CCI. The times taken to remove the stickers were used to generate a plot displaying the latency time of the sticker removal from each paw; the times were then used for statistical analysis.
Fine motor coordination
CCI-induced deficits in fine motor coordination were assessed by the use of a beam walk test (BWT). Mice have an inherent preference for a darkened enclosed environment, as compared with an open illuminated environment. Each animal was placed in darkened goal box for a 2 min habituation and then the trial began from another (light) end of the beam. The beam was constructed with the following dimensions: 1.2 cm (width) × 91 cm (long). The time taken for each animal to traverse the beam to reach the dark goal box and the ipsilateral and contralateral foot falls were recorded (with the caveat that total time was not to exceed 30 s). Five trials were recorded for each animal before CCI and 1 and 2 weeks after CCI. The mean times to traverse the beam were calculated, and a plot was generated to evaluate treatment effects on beam walk times and foot falls; these times were used for statistical analysis.
Biochemical Analysis
Fixation and sectioning
The animals were deeply anesthetized with 2.5% tribromoethanol, Avertin (Sigma) and perfused transcardially with 0.9% saline and 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.2). Brains were removed and post-fixed for 1 day in the same fixative and sequentially transferred to 20% and 30% sucrose in 0.1 M PB until the brain sank. The brains were cut into 25-μm sections on a cryostat (Leica Biosystems Inc.). Every seventh section was selected from a region spanning from striatum to hippocampus.
Quantification of brain lesion and lateral ventricle size in TBI animals
One set of post-TBI 2-week brain sections (25 μm) was mounted on slides. The sections were then stained in 10% Giemsa KH2PO4 buffered solution (pH 4.5) for 30 min at 40°C. After a brief rinse, slides were de-stained, differentiated, and dehydrated in absolute ethanol. Thereafter, the sections were cleared in xylene and then coverslipped. Slides were scanned in a Path Scan Enabler IV slide scanner (Meyer Instruments Inc., Houston, TX, USA), and areas of the brain images were quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The calculation formula for contusion volume size and lateral ventricle size rate was as follows: Σ(area of contralateral hemisphere – area of ipsilateral hemisphere) / Σ area of contralateral hemisphere; Σ area of ipsilateral lateral ventricle / Σ area of contralateral lateral ventricle. There were six brain sections of each mouse for counting, the region starting from bregma 0.86 mm to –1.46 mm.
Microglia, astrocyte, and neuronal cell labeling
A total of 24 brain sections per mouse were incubated with blocking buffer (4% bovine serum albumin (BSA), Sigma) for 1 h. A series of primary antibodies were prepared in the blocking buffer and the sections were incubated in the solution overnight. The antibodies used were rabbit anti-glial fibrillary acidic protein (GFAP) (1:1000; Invitrogen, Carlsbad, CA, USA), guinea pig anti-NeuN (1:1000; Millipore, Burlington, MA, USA) or mouse anti-Iba1 (1:1000; FUJIFILM Wako Pure Chemical Corporation, Richmond, VA, USA). After incubation with primary antibody, the sections were washed and incubated for 4 h at room temperature in diluted secondary antibody prepared with blocking solution (secondary antibody conjugated with Alexa 488 or 594 (1:1000; Life Technologies, Carlsbad, CA, USA). The sections were then washed with Tween tris-buffered saline, mounted, and coverslipped. Four images per mouse brain were taken using an Olympus microscope (Shinjuku Monolith, Tokyo, Japan). Omission of primary or secondary antibodies resulted in no staining and served as negative controls. Cell numbers of each image were counted using ImageJ software (National Institutes of Health).
Statistical Analysis
For statistical analysis of behavioral measurements, a two-way repeated measure analysis of variance (ANOVA) was used to test both group and time factors. Multiple within-subject comparisons were taken with the Bonferroni correction post hoc test when the main effect of time was significant. For quantification of contusion volume size and lateral ventricle size, a one-factor analysis repeated measures ANOVA was used to compare the five groups of data followed by a Bonferroni correction post hoc test on 2-weeks post lesion. Data were analyzed using SigmaPlot version 12.5 (Systat Software Inc., San Jose, CA, USA) with the significance level set at p < 0.05 for each assessment. All data are presented as the average ± standard error of the mean (SEM). The time line for the histochemical and behavioral experiments with Phen is shown in Fig. 1. Similar times were evaluated for studies with Posiphen.
Results
TBI Injury in Mice
Phenserine Treatment Reduced TBI Contusion Volume and Lateral Ventricle Size Enlargement
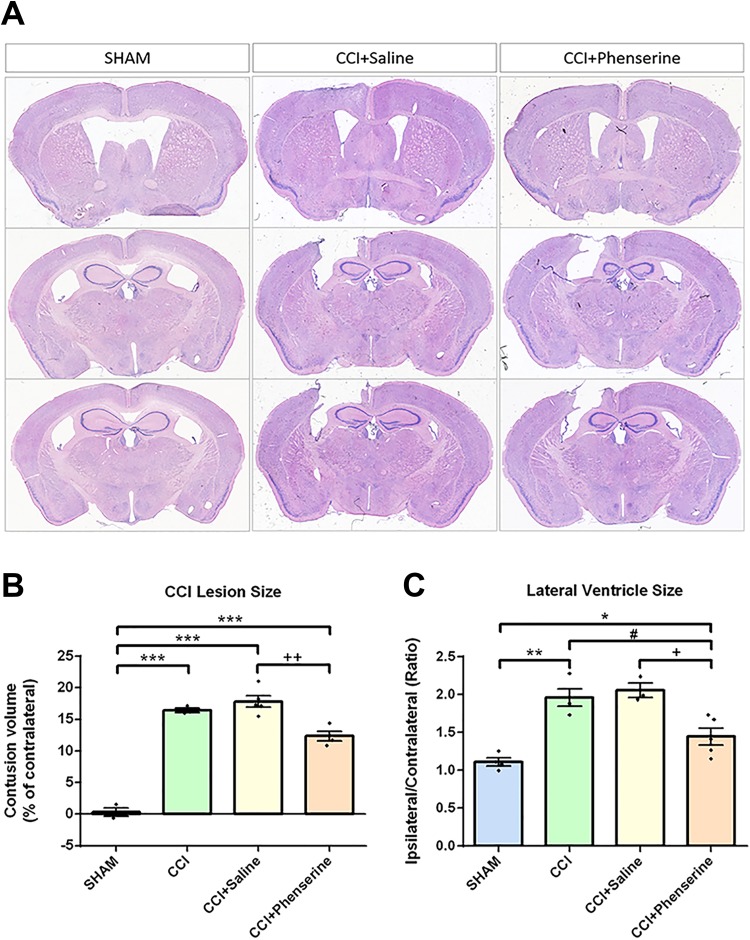
We measured the contusion volume (as % contralateral) of ipsilateral hemisphere for various groups at 2 weeks after CCI. The cortical region of the brain was injured after CCI in this TBI model, and tissue loss was observed in the ipsilateral hemisphere (Fig. 2A). The contusion volume, quantified by loss of the volume in the CCI group, was 16.44 ± 0.35% of contralateral hemisphere volume 2 weeks post-CCI. The contusion volume in CCI + Saline group was not significantly different from that in the CCI group, whereas no tissue loss was observed in the sham group (0.3 ± 0.65%). Phen treatment (2.5 mg/kg body weight, i.p., twice daily × 5 days after CCI) significantly reduced contusion volume (from 18.00 ± 0.96 to 12.93 ± 0.09%, p < 0.01, CCI + Phenserine vs. CCI+Saline) (Fig. 2B).
Fig 2.
Phen treatment (2.5 mg/kg body weight, i.p., twice a day, for 5 days after CCI) reduced contusion volume and swelling of lateral ventricle (LV) evaluated 2 weeks after CCI. Lesion volume was quantified using the IMAGE-PRO PLUS 6 software (Media Cybernetics, Inc., Rockville, MD, USA). (A) Representative Giemsa-stained coronal brain sections of the CCI-induced cavity in sham (control without CCI), CCI, CCI+Saline and CCI+phenserine rats at 2 weeks post-TBI. (B) Contusion volume. Significant reduction of lesion size was observed in the Phen-treated group. ++ p < 0.01, ***p < 0.001, compared with the saline-treated and CCI-only groups. (C) Significant differences in lateral ventricular (LV) size ratio between ipsilateral and contralateral sides were also detected between Phen-treated and saline-treated groups (+ p < 0.05) as well as CCI and CCI-Phen (#p < 0.05). Analysis by one-way repeated measure ANOVA followed by Holm–Sidak method. Data are expressed as mean ± SEM; n = 5 (SHAM, CCI), 8 (CCI+Saline, CCI+Phenserine).
In order to determine whether Phen treatment could have a correlate for clinical observations of increased intracranial pressure after TBI, we measured lateral ventricle size in our TBI model. We found the lateral ventricle enlarged after CCI (1.96 ± 0.65 fold of the contralateral ventricle size), compared with the sham group (1.11 ± 0.05 fold of the contralateral ventricle size). Moreover, the CCI-saline group also showed an increased ipsilateral lateral ventricle size (2.2 ± 0.08 fold compared with the contralateral ventricle volume), and Phen treatment reduced this change to 1.52 ± 0.09 fold (p < 0.05) (Fig. 2C).
Phenserine Treatment Reduced Neuroinflammation after CCI
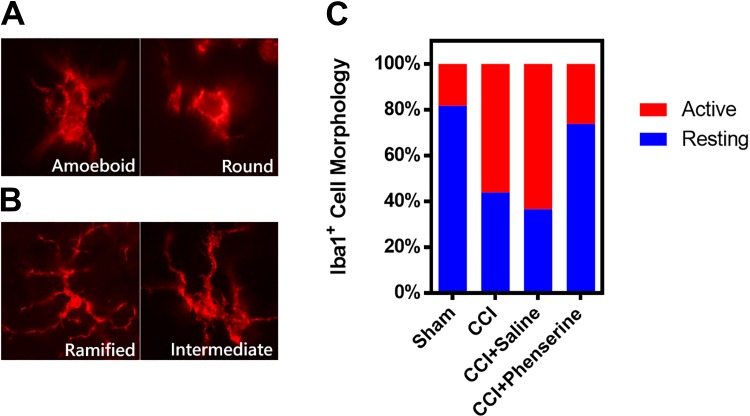
Microglia play many roles in the brain, including tissue repair and mediating the immune responses to peripheral infection. Microglia are quickly activated in response to brain injury. We assessed microglia morphology at 2 weeks after CCI (Fig. 3A, B), and represented this as percentages of morphology of the microglia (Fig. 3C). Iba1-immunofluorescence revealed microglial cells with round, amoeboid (Fig. 3A), ramified, and intermediate (Fig. 3B) morphologies. Microglial activation was assessed via morphology, with ramified and intermediate cells defined as in resting stage, whereas round and amoeboid morphologies are regarded as in activated stage. In CCI animals, the fraction of activated microglia was significantly increased in comparison with the sham group. Phen treatment significantly reduced the percentage of activated microglia from 64.3 ± 3.39% to 25.1 ± 3.59%, CCI + saline vs. CCI + phenserine (p < 0.05), at 2 weeks after CCI (Fig. 3C).
Fig 3.
The effect of Phen treatment at 2-weeks after CCI (2.5 mg/kg body weight, i.p., twice a day, for 5 days after CCI) on microglial morphology percentages in the ipsilateral (injured) cortex. Iba1 immunofluorescence staining showing microglial cells with amoeboid, round (A), ramified and intermediate (B), morphology. (C) Quantification of the proportions of microglia in activated and resting stages. The morphological stages are shown as follows: activated (A), amoeboid and round forms; resting (B), ramified long branching processes with a small cell body and intermediate transition forms. Phen treatment significantly reduced the activated forms of the microglia (p = 0.022) compared with CCI-saline group (n = 3 per group).
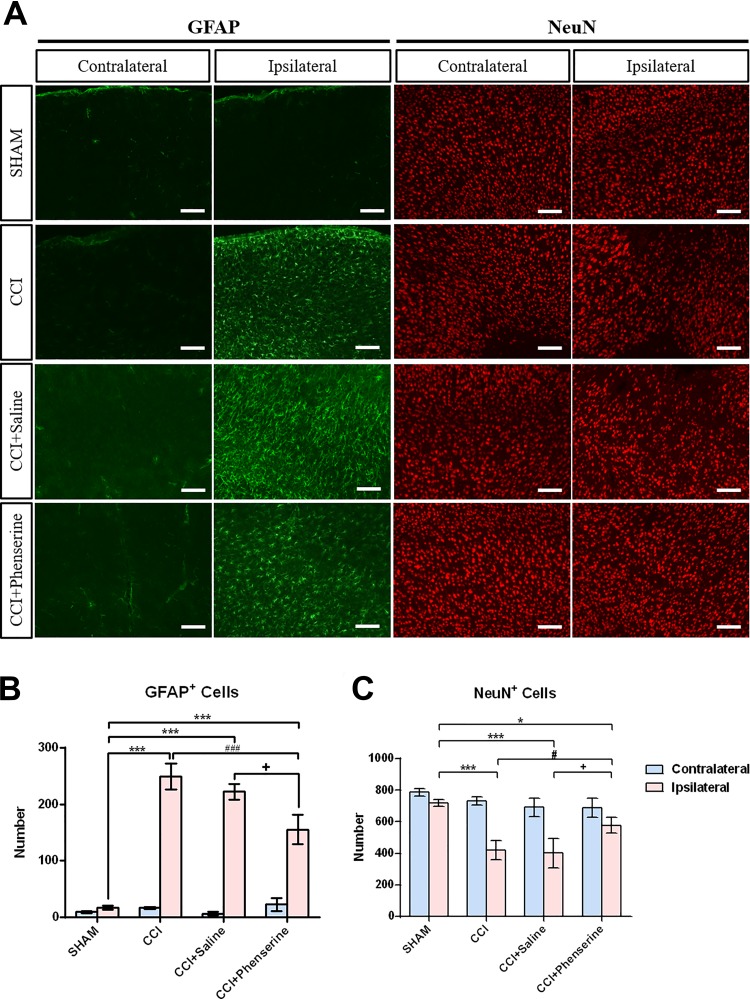
The reactive gliosis that is known to occur after brain injury is associated with upregulation of GFAP protein34. We observed that astrocyte activation persisted in tissue adjacent to the lesion area at 2 weeks after CCI and GFAP immunoreactivity was significantly elevated in the ipsilateral cortex of CCI mice, compared with sham animals. The numbers of GFAP-positive cells in the ipsilateral brain of CCI, CCI + saline, and CCI + phenserine groups were greater than in the ipsilateral brain of sham group, indicating reactive gliosis in the ipsilateral (injured) site in all CCI animals. (Fig. 4A, B; p < 0.001, ipsilateral CCI vs. ipsilateral sham; p < 0.001 vs. contralateral side; n = 4 per group). Treatment with Phen decreased the gliosis caused by CCI (Fig. 4B; p < 0.05, ipsilateral CCI+Saline vs. ipsilateral CCI+Phen; p < 0.001 vs. contralateral side; n = 4 per group). The number of ipsilateral cortical astrocytes in the CCI group was 249 ± 23 cells/image field, (p < 0.001 vs. sham group, 17 ± 3 cells/image field; n = 4 per group) (Fig. 4B). Phen treatment decreased the number of ipsilateral cortical astrocytes to 155 ± 26 cells/field, compared with the CCI + saline group, 222 ± 13 cells/image field (p < 0.05; n = 4 per group) (Fig. 4B).
Fig 4.
Phen post-injury treatment (2.5 mg/kg body weight, i.p., twice a day, for 5 days after CCI) decreased GFAP-positive astrocyte and increased NeuN-positive neuron numbers at 2 weeks after CCI. (A) Immunofluorescence of GFAP and NeuN in cortical brain sections. GFAP, a marker for astrocytes, is showed in green. NeuN, a marker for neurons, is shown in red. (B) CCI injury significantly increased the number of astrocytes in the ipsilateral cortex. ***p < 0.001, ipsilateral sham vs. ipsilateral CCI. Treatment with Phen significantly reduced astrocytic increments caused by CCI. Mean ± SEM (n = 4 per group). + p < 0.05, ipsilateral CCI+Saline vs. ipsilateral CCI+Phenserine; ### p < 0.001 ipsilateral CCI vs. ipsilateral CCI+Phenserine; two-way ANOVA with Bonferroni t-test for multiple comparisons. (C) CCI injury significantly decreased the number of neurons in the ipsilateral cortex. ***p < 0.001, ipsilateral sham vs. ipsilateral CCI, CCI+Saline, CCI+Phenserine. Treatment with Phen significantly reduced neuronal loss caused by CCI. Mean ± SEM (n = 4 per group). # p < 0.05, ipsilateral CCI, CCI+Saline vs. ipsilateral CCI+Phenserine; two-way ANOVA with Bonferroni t-test for multiple comparisons.
Phenserine Treatment Reduced Neuronal Loss after CCI
We counted the number of neuronal cells at 2 weeks after CCI (Fig. 4A, 4C) in each group, shown by representative cortical sections (Fig. 4A). Significant levels of neuronal loss occur in the injured cortex after CCI, the number of ipsilateral cortical neurons in the CCI group was 421 ± 60 cells/image field, (p < 0.001 vs. sham group, 719 ± 19 cells/image field; n = 4 per group) (Fig. 4A and Fig. 4C). In CCI + Phen animals, the number of neuronal cells was significantly increased at 2 weeks after CCI, compared with the CCI and the CCI + Saline animals (Fig. 4C; p < 0.05, ipsilateral CCI+Saline vs. ipsilateral CCI+Phen; n = 4 per group). Phen treatment increased the number of ipsilateral cortical neurons to 578 ± 51 cells/field, compared with the CCI + saline group, 402 ± 92 cells/image field (p < 0.05; n = 4 per group) (Fig. 4C).
Phenserine Improved Multiple Behavioral Outcomes as Shown by Behavioral Assessment at 1Wk and 2Wks after CCI
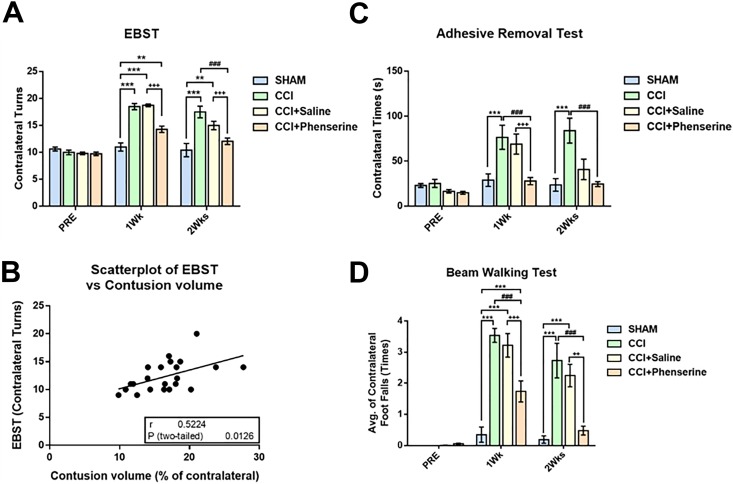
Groups were assigned to behavioral evaluations 4 days before CCI then weekly after injury (Figs. 1 and 5). When asymmetrical motor function was evaluated by the EBST, a significant difference was detected after injury. Asymmetry was increased in the CCI group compared with the sham group with elevated body swings toward the contralateral side. Phen treatment significantly improved behavioral asymmetry by reducing the contralateral swing turns from 18.73 ± 0.25 (CCI+Saline) to 14.29 ± 0.6 (CCI+phenserine) at 1 week post-CCI (p < 0.001), and from 15.00 ± 0.78 (CCI+Saline) to 12.07 ± 0.61 (CCI+phenserine) at 2 weeks post-CCI (p < 0.001) (Fig. 5A). Moreover, the results of the EBST evaluation had a significant positive correlation with contusion volume, as evident by scatterplot analysis (r = 0.7727, p = 0.0012) (Fig. 5B).
Fig 5.
Phen treatment improved functional recovery as revealed by behavioral measurements. (A) Motor asymmetry evaluated by elevated body swing test (EBST). TBI-induced deficits were attenuated by Phen treatment (2.5 mg/kg body weight, i.p., twice a day, for 5 days after CCI) 1 and 2 weeks after CCI. (B) Pearson correlation coefficient (r) and p-value (p) showed a positive correlation between EBST and lesioned area. Scatter plot illustrating that there is a significant correlation between EBST and size of lesioned area. r = 0.5224, p = 0.0126. (C) Sensory/motor function was evaluated by adhesive removal test. Mice will spend more time to remove an adhesive sticker from their contralateral front foot paw than ipsilateral after CCI injury. Treatment with Phen significantly reduced this behavioral deficit. (D) CCI-induced abnormalities in motor coordination and balance were measured by a beam walking test. Mice with CCI tended to have more contralateral foot faults compared with the ipsilateral side. Mice treated with Phen showed significantly fewer behavioral abnormalities. ++ p < 0.01, ### p < 0.001, compared with the saline-treated and CCI-only groups. Analysis by two-way ANOVA followed by Bonferroni t-test. Data are expressed as mean ± SEM; n = 8 (SHAM, CCI), n = 15 (CCI+Saline, CCI+Phenserine).
Somatosensory function was evaluated by the ART, assessed by latency to remove adhesive stickers from the front paws. Sensory and motor functions were impaired on the contralateral paw of mice after CCI (Fig. 5C). There was no difference in time spent removing the sticker from the contralateral paw in the sham group at all time points. However, the CCI and CCI+Saline groups showed functional deficits, and had significantly increased times for removing stickers from their contralateral paw, compared with the sham group (40.83 ± 11.34 s, CCI+Saline; 83.96 ± 13.91 s, CCI; 23.66 ± 6.98 s, sham, p < 0.001) at the 2-week time point. Importantly, CCI+Phen animals were less impaired than the CCI+Saline group, requiring a significantly shorter time to remove the contralateral sticker (27.89 ± 4.03 s, p < 0.001 vs. CCI+Saline group (69.01 ± 11.18 s)) at 1 week after injury. However, there was no significant difference between the CCI-saline (40.83 ± 11.34 s) and CCI-Phen (24.55 ± 2.79 s) groups at 2 weeks after injury (Fig. 5C).
When motor coordination was evaluated with the BWT, we found that CCI significantly impaired function in the injured mice; the contralateral foot faults in CCI and CCI+Saline mice were significantly increased from 0.19 ± 0.12 in sham to 2.73 ± 0.56 (CCI) and 2.25 ± 0.36 (CCI+Saline) 2 weeks after injury (Fig. 5D, p < 0.001). However, the CCI+Phen group demonstrated significantly better performance with the average foot faults in the contralateral side (0.48 ± 0.14, p < 0.001 vs. CCI or CCI+Saline group).
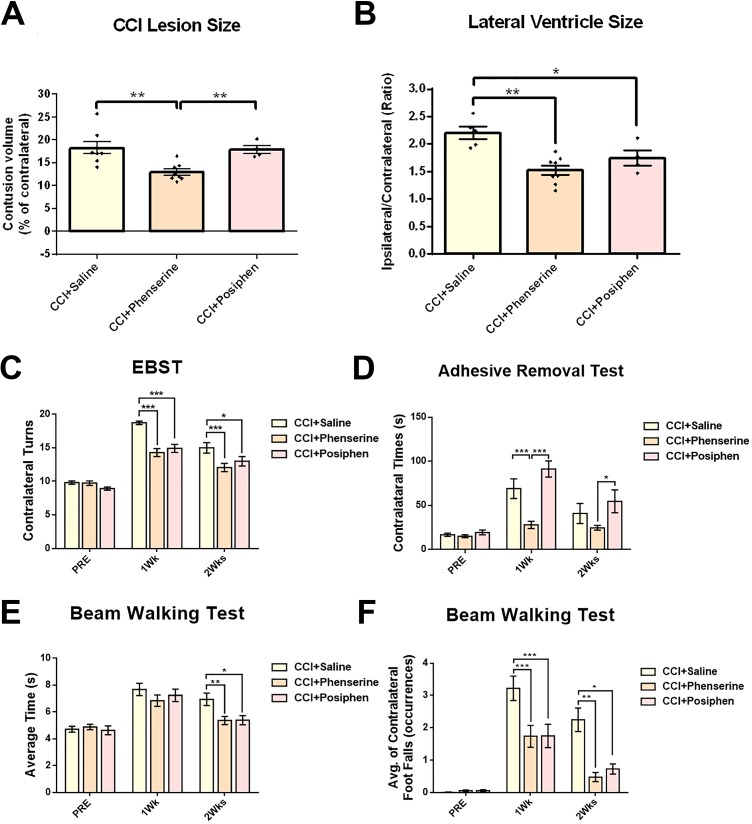
Posiphen Shared not all Effects on Tissue Loss, Lateral Ventricle Size, EBST, BWT at 1Wk and 2Wks after CCI
In order to ascertain whether AChE activity is responsible for the behavioral and histological improvements of CCI animals, we compared the effectiveness of Phen and its non-cholinergic (+) chiral enantiomer (Posiphen) in our study. Unlike the Phen treatment group (Fig. 2A, 6A), we did not observe a reduction of tissue loss in the ipsilateral hemisphere in the CCI+Posiphen group, in which loss was 17.88 ± 0.86% of the contralateral hemisphere volume compared with 12.35 ± 0.75% in the CCI-Phen group and 17.82 ± 0.92% in the CCI-saline group (both p < 0.01 vs. CCI+Phen) (Fig. 6A). On the other hand, the enlargement of lateral ventricle size after CCI that was reduced by Phen treatment was also seen in the Posiphen treatment group (1.75 ± 0.14 fold more than contralateral ventricle volume), compared with the CCI+Saline group (2.2 ± 0.08 fold) (p < 0.05). Moreover, there was no difference between Posiphen and Phen groups in lateral ventricle size (1.52 ± 0.09 fold, Fig. 6B).
Fig 6.
Comparison of the treatment effects between Phen and Posiphen measured by CCI contusion volume size, lateral ventricle size, and behavioral tests. (A) Significant reduction of lesion size was observed in the Phen-treated group. **p < 0.01, compared with the CCI+Saline group. Posiphen showed no difference in lesion size compared with CCI+Saline group. (B) The LV size ratio between ipsilateral and contralateral sides of CCI+Phenserine and CCI+Posiphen groups were significantly different from the saline-treated group (*p < 0.05, **p < 0.01). Analysis by one-way repeated measure ANOVA followed by Holm–Sidak method. Data are expressed as mean ± SEM; n = 8 (CCI+Saline, CCI+Phenserine), n=4 (CCI+Posiphen). (C) Motor asymmetry evaluated by elevated body swing test (EBST). TBI-induced deficits were attenuated by both Phen and Posiphen treatment (2.5 mg/kg body weight, i.p., twice a day, for 5 days after CCI) 1 and 2 weeks after CCI. (D) Sensory/motor function was evaluated by adhesive removal test. Mice will spend more time to remove an adhesive sticker from their contralateral front foot paw than ipsilateral after CCI injury. Treatment with Phen significantly reduced this behavioral deficit. However, Posiphen had no effect. (E, F) CCI-induced abnormalities in motor coordination and balance were measured by a beam walking test. Mice with CCI tended to spend more traveling time (E) and have more contralateral foot faults compared with the ipsilateral side (F). Mice treated with both Phen and Posiphen showed significantly less behavioral abnormalities. *p < 0.05, **p < 0.01, ***p < 0.001, compared with the saline-treated group. Analysis by two-way ANOVA followed by Bonferroni t-test. Data are expressed as mean ± SEM; n = 15 (CCI+Saline, CCI+Phenserine), n=13 (CCI+Posiphen).
In the EBST test, unilateral CCI-lesioned mice exhibited significant biased swing activity with the direction contralateral to the lesioned side, and Phen effectively improved this behavioral deficit by reducing the contralateral swing numbers (p < 0.001) (Fig. 5A, 6C), as noted above. Posiphen had a similar effect to Phen on EBST (14.92 ± 0.61 1-week post-CCI (p < 0.001), and 13.00 ± 0.71 2 weeks post-CCI (p < 0.05)) (Fig. 6C).
Somatosensory function was evaluated by the ART, assessed by latency to remove adhesive stickers from their front paws. Sensory and motor functions were impaired on the contralateral paws of mice after CCI (Fig. 5C). CCI+Phen animals were less impaired than the CCI+Saline group, requiring significantly less time to remove the contralateral sticker at 1 week after injury (Fig. 5C, 6D), whereas Posiphen showed no positive effect on this behavioral deficit at both 1 week (91.22 ± 9.2 s) and 2 weeks (54.62 ± 12.92 s) after CCI.
Motor coordination was evaluated with the BWT. We found that CCI significantly impaired this function in the injured mice; the average transit time of both CCI+Phen (5.36 ± 0.3 s) and CCI+Posiphen (5.39 ± 0.34 s) groups were significantly decreased at 2 weeks after injury compared with CCI+Saline group (6.94 ± 0.46 s) (p < 0.01 to CCI+Phen; p < 0.05 to CCI+Posiphen) (Fig. 6E). The contralateral foot faults in CCI+Phen and CCI+Posiphen mice were also significantly decreased from 3.22 ± 0.38 in CCI+Saline to 1.74 ± 0.34 (CCI+Phen) and 1.75 ± 0.36 (CCI+Posiphen) 1 week after injury (Fig. 6F, p < 0.001), and from 2.25 ± 0.36 in CCI+Saline to 0.48 ± 0.14 (CCI+Phen) (p < 0.01) and 0.73 ± 0.16 (CCI+Posiphen) 2 weeks after injury (Fig. 6F, p < 0.05). Thus there were both similarities and differences in behavioral improvements after CCI between Phen and Posiphen.
Discussion
TBI is typically considered as a time-dependent process, consisting of an initial primary injury that involves a focal deformation of the brain followed by a series of secondary processes that include neuroinflammation, oxidative stress, and excitotoxicity responses35. To date, there is no approved drug for the treatment of TBI, despite the evaluation of a large number of drug classes focused on a range of different specific mechanisms pertinent to TBI. Therefore, an effective pharmacological treatment for TBI is urgently needed. In this study, we used the well-characterized CCI as a TBI model in mice. The primary injury typically leads to the formation of a necrotic core that is not amenable to pharmacological treatment35,36. Previous studies have demonstrated that the experimental AD drug Phen has neuroprotective effects in cortical cell cultures challenged with oxidative stress and glutamate excitotoxicity, two insults implicated in the pathogenesis of a wide number of acute and chronic neurological disorders, including TBI37–39. Importantly, these neuroprotective effects translated significantly into the amelioration of motor and sensory-motor impairments in our mouse model of TBI.
The lateral ventricles contain CSF that provides cushioning for the brain while also helping to circulate nutrients and remove waste. Previous clinical studies reveal that ventricular enlargement is a frequent finding in patients with TBI and is regarded as an early sign of asymmetric intracranial pathology. In TBI patients, increased ventricular volume may be related to an atrophic process resulting from diffuse axonal injury and other mechanisms, a secondary CSF absorptive deficit, or a combination of both phenomena40–42. In our study, we also discovered the same phenomenon in the mouse model of TBI, and Phen reduced the enlargement caused by CCI.
The effects of Phen were assessed by this well-known mouse TBI model following clinically translatable doses of the drug (2.5 mg/kg, BID × 5 days) initiated 30 min after injury. This dose is approximately equivalent to 12 mg in a 60 kg human, following body surface area normalization43. The dose is similar to that previously used in clinical AD trials.
TBI occurs when the brain structure is disrupted due to mechanical insult to the cranium, resulting in neuronal, axonal, and vascular damage. In response to TBI, the brain undergoes a complex immunological tissue reaction similar to that in ischemic reperfusion injury35. It has been suggested that macrophages and microglia migrate to the site of the injury to establish a protective environment that can mitigate deleterious consequences of the injury44. The acute function of microglia in response to TBI is to eliminate cellular and molecular debris. Injured cells release Danger-Associated Molecular Patterns (DAMPs), which can become potent inflammatory stimuli, resulting in further tissue damage45,46. The vast majority of the ionized calcium-binding adapter molecule 1 (Iba1)-immunostained microglia normally exhibit a ramified phenotype, followed by an intermediate form with shorter processes, and larger soma. In response to tissue damage or pathogen invasion, microglia change into an amoeboid morphology to act in a phagocytic fashion and are difficult to differentiate from infiltrating macrophages47. The stages of microglia changes could therefore be relevant to the progression of TBI into other neurological disorders such as AD and PD, and could interfere with recovery of the patients and the effectiveness of particular anti-inflammatory treatments48. It should be noted that both microglia and macrophages are Iba1 positive and both elements are present at the injury site. Prior studies have demonstrated that Phen possesses anti-inflammatory actions49, and the finding that it normalizes the microglial signature response to TBI in the present study may prove valuable to ensure that the short-term benefits of TBI-induced microglial activation to initiate reparative actions are not lost to a prolonged inflammatory phase that drives oxidative stress and, ultimately, pathological processes50–52. This is important for consideration of treatment of long-term post-TBI deficits since potentiated neuroinflammation, particularly in the hippocampus, leads to memory impairments53.
The evidence for the beneficial effect of AChE inhibitors in TBI remains controversial18,54. There is a sound basis for a cholinergic involvement in TBI-mediated cognitive impairments, as reviewed by Arciniegas and colleagues55,56. Cholinergic neurons and their ascending projections appear to be especially susceptible to TBI-induced damage. Acutely, central cholinergic neurons are triggered by mechanical trauma57,58 and, similar to other neurotransmitters like glutamate, release excess neurotransmitter. Such acute cholinergic overload is shortly followed by a chronic decrease in brain ACh levels, whereas initial excesses in other neurotransmitters return to normal over time55,59. Consequent to ACh’s key role in attention, memory consolidation, and other critical features of cognition56,60,61, central cholinergic loss and dysfunction may significantly promote TBI-induced cognitive impairments and explain, in part, the differential and superior actions of Phen versus Posiphen in our equimolar comparison here.
Although opposite enantiomers and clearly structurally related, Phen and Posiphen are wholly separate and discrete drugs both pharmacologically and chemically, and there is no chiral switching in the 3a chiral position of either compound. Whereas their molecular weight and physicochemical characteristics are alike in that both have a balanced lipophilicity (cLogP value 2.22) to support a similarly high brain penetration28, the pharmacokinetic profile of each is unique, generating different metabolite profiles in a different time-dependent manner, and hence the toxicokinetics of the two agents are different10,27. Both agents appear to lower APP and α-synuclein levels, and demonstrate potent neurotrophic and protective actions at equimolar concentrations24,26,62. However, only Phen, but not Posiphen, has anticholinesterase actions28; it is thus possible that Phen and its (-)-enantiomeric metabolites have other actions as well that differentiate the final pharmacological profiles of these two drugs in animals and humans. In the current TBI study, Phen demonstrated an additional range of pharmacological properties, only some of which are related to cholinergic activity, that provided greater efficacy in specific evaluations, as was also evident in a different model of neuronal apoptosis involving soman-induced toxicity63.
In a recent study, we used neuronal culture and a mild weight drop TBI animal model to address the effects of Phen treatment in TBI64. We found that Phen effectively protected neurons from oxidative stress and glutamate excitotoxicity, and also ameliorated mild TBI-induced cognitive deficits. In the current study, using CCI-induced focal injury, we demonstrate Phen efficacy across a more severe TBI animal model, which is notable since no single model mimics the human condition65. Comparison of our results with Phen and Posiphen suggest that Phen-mediated effects on reduction of contusion volume and sensorimotor function may involve cholinergic mechanisms, whereas effects on lateral ventricle size, motor asymmetry, and motor coordination may involve other mechanisms. A previous study has shown that AChE activity is elevated after TBI in the basal forebrain66, which contains numerous cholinergic neurons, and projects to the hippocampus and cortex. Reports further showed that the basal forebrain and hippocampus have cholinergic neuron loss after TBI in rodents and humans67,68. Hence the multiple combined cholinergic and non-cholinergic actions of Phen may provide this drug with a broad range of favorable actions to mitigate the scope of impairments that are manifested in humans following TBI.
In summary, post-injury treatment with a clinically translatable dose of Phen significantly alleviated behavioral impairments in a well-defined mouse model of controlled cortical impact TBI. Phen reduced the injury contusion volume, lateral ventricle size, and ameliorated neuroinflammation. These findings support further appraisal and optimization of Phen as a new treatment strategy for clinical TBI.
Footnotes
Author Contributions: SCH, JYW and DL carried out these experiments. BJH, JPM, NG, WS, and YHC planned the studies and wrote/edited this paper. NG supplied Phen and Posiphen compounds tested for purity.
Ethical Approval: This study was approved by the local Institutional Animal Care and Use Committee (Protocol approval number 2016-0209).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the National Institutes Health (NIH) Guidelines using the NIH handbook Animals in Research and were approved by the local Institutional Animal Care and Use Committee.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research was supported in part by (i) NIH grant R56 AG057028 (JM and BJH), (ii) the Intramural Research Program of the National Institute on Aging, National Institutes of Health, USA (NG), and (iii) the Ministry of Science and Technology, Taiwan (MOST104-2923-B-038-001-MY3, MOST 107-2314-B-038-042) and DP2-107-21121-01-N-05, DP2-108-21121-01-N-05-01, Taipei Medical University, Taipei, Taiwan (JYW and YHC).
ORCID iD: Yung-Hsiao Chiang  https://orcid.org/0000-0002-8426-4016
https://orcid.org/0000-0002-8426-4016
References
- 1. Ruff RL, Riechers RG, 2nd, Wang XF, Piero T, Ruff SS. A case-control study examining whether neurological deficits and PTSD in combat veterans are related to episodes of mild TBI. BMJ Open. 2012;2(2):e000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nemetz PN, Leibson C, Naessens JM, Beard M, Kokmen E, Annegers JF, Kurland LT. Traumatic brain injury and time to onset of Alzheimer’s disease: a population-based study. Am J Epidemiol. 1999;149(1):32–40. [DOI] [PubMed] [Google Scholar]
- 3. Rugbjerg K, Ritz B, Korbo L, Martinussen N, Olsen JH. Risk of Parkinson’s disease after hospital contact for head injury: population based case-control study. BMJ. 2008;337:a2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jafari S, Etminan M, Aminzadeh F, Samii A. Head injury and risk of Parkinson disease: a systematic review and meta-analysis. Mov Disord. 2013;28(9):1222–1229. [DOI] [PubMed] [Google Scholar]
- 5. Greig NH, Tweedie D, Rachmany L, Li Y, Rubovitch V, Schreiber S, Chiang YH, Hoffer BJ, Miller J, Lahiri DK, Sambamurti K, et al. Incretin mimetics as pharmacologic tools to elucidate and as a new drug strategy to treat traumatic brain injury. Alzheimers Dement. 2014;10(suppl 1):S62–S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clin Sports Med. 2011;30(1):33–48, vii–iii. [DOI] [PubMed] [Google Scholar]
- 7. Greve MW, Zink BJ. Pathophysiology of traumatic brain injury. Mt Sinai J Med. 2009;76(2):97–104. [DOI] [PubMed] [Google Scholar]
- 8. Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14(2):128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greig NH, Pei XF, Soncrant TT, Ingram DK, Brossi A. Phenserine and ring c hetero-analogues: drug candidates for the treatment of Alzheimer’s disease. Med Res Rev. 1995;15(1):3–31. [DOI] [PubMed] [Google Scholar]
- 10. Greig NH, Ruckle J, Comer P, Brownell L, Holloway HW, Flanagan DR, Jr., Canfield CJ, Burford RG. Anticholinesterase and pharmacokinetic profile of phenserine in healthy elderly human subjects. Curr Alzheimer Res. 2005;2(4):483–492. [DOI] [PubMed] [Google Scholar]
- 11. Winblad B, Giacobini E, Frolich L, Friedhoff LT, Bruinsma G, Becker RE, Greig NH. Phenserine efficacy in Alzheimer’s disease. J Alzheimers Dis. 2010;22(4):1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greig NH, Sambamurti K, Lahiri DK, Becker RE. Amyloid-beta precursor protein synthesis inhibitors for Alzheimer’s disease treatment. Ann Neurol. 2014;76(4):629–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoffer BJ, Pick CG, Hoffer ME, Becker RE, Chiang YH, Greig NH. Repositioning drugs for traumatic brain injury - n-acetyl cysteine and phenserine. J Biomed Sci. 2017;24(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verbois SL, Scheff SW, Pauly JR. Time-dependent changes in rat brain cholinergic receptor expression after experimental brain injury. J Neurotrauma. 2002;19(12):1569–1585. [DOI] [PubMed] [Google Scholar]
- 15. Murdoch I, Perry EK, Court JA, Graham DI, Dewar D. Cortical cholinergic dysfunction after human head injury. J Neurotrauma. 1998;15(5):295–305. [DOI] [PubMed] [Google Scholar]
- 16. Poole NA, Agrawal N. Cholinomimetic agents and neurocognitive impairment following head injury: a systematic review. Brain Inj. 2008;22(7–8):519–534. [DOI] [PubMed] [Google Scholar]
- 17. Holschneider DP, Guo Y, Roch M, Norman KM, Scremin OU. Acetylcholinesterase inhibition and locomotor function after motor-sensory cortex impact injury. J Neurotrauma. 2011;28(9):1909–1919. [DOI] [PubMed] [Google Scholar]
- 18. Shaw KE, Bondi CO, Light SH, Massimino LA, McAloon RL, Monaco CM, Kline AE. Donepezil is ineffective in promoting motor and cognitive benefits after controlled cortical impact injury in male rats. J Neurotrauma. 2013;30(7):557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lyeth BG, Jiang JY, Robinson SE, Guo H, Jenkins LW. Hypothermia blunts acetylcholine increase in CSF of traumatically brain injured rats. Mol Chem Neuropathol. 1993;18(3):247–256. [DOI] [PubMed] [Google Scholar]
- 20. Cox CD, West EJ, Liu MC, Wang KK, Hayes RL, Lyeth BG. Dicyclomine, an m1 muscarinic antagonist, reduces biomarker levels, but not neuronal degeneration, in fluid percussion brain injury. J Neurotrauma. 2008;25(11):1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lyeth BG, Liu S, Hamm RJ. Combined scopolamine and morphine treatment of traumatic brain injury in the rat. Brain Res. 1993;617(1):69–75. [DOI] [PubMed] [Google Scholar]
- 22. Klein J. Phenserine. Expert Opin Investig Drugs. 2007;16(7):1087–1097. [DOI] [PubMed] [Google Scholar]
- 23. Teich AF, Sharma E, Barnwell E, Zhang H, Staniszewski A, Utsuki T, Padmaraju V, Mazell C, Tzekou A, Sambamurti K, Arancio O, et al. Translational inhibition of app by posiphen: efficacy, pharmacodynamics, and pharmacokinetics in the app/ps1 mouse. Alzheimers Dement (N Y). 2018;4:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lahiri DK, Chen D, Maloney B, Holloway HW, Yu QS, Utsuki T, Giordano T, Sambamurti K, Greig NH. The experimental Alzheimer’s disease drug posiphen [(+)-phenserine] lowers amyloid-beta peptide levels in cell culture and mice. J Pharmacol Exp Ther. 2007;320(1):386–396. [DOI] [PubMed] [Google Scholar]
- 25. Marutle A, Ohmitsu M, Nilbratt M, Greig NH, Nordberg A, Sugaya K. Modulation of human neural stem cell differentiation in Alzheimer (app23) transgenic mice by phenserine. Proc Natl Acad Sci U S A. 2007;104(30):12506–12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mikkilineni S, Cantuti-Castelvetri I, Cahill CM, Balliedier A, Greig NH, Rogers JT. The anticholinesterase phenserine and its enantiomer posiphen as 5’untranslated-region-directed translation blockers of the Parkinson’s alpha synuclein expression. Parkinsons Dis. 2012;2012:142372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maccecchini ML, Chang MY, Pan C, John V, Zetterberg H, Greig NH. Posiphen as a candidate drug to lower csf amyloid precursor protein, amyloid-beta peptide and tau levels: target engagement, tolerability and pharmacokinetics in humans. J Neurol Neurosurg Psychiatry. 2012;83(9):894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Greig NH, Sambamurti K, Yu QS, Brossi A, Bruinsma GB, Lahiri DK. An overview of phenserine tartrate, a novel acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Curr Alzheimer Res. 2005;2(3):281–290. [DOI] [PubMed] [Google Scholar]
- 29. Yu Q, Holloway HW, Flippen-Anderson JL, Hoffman B, Brossi A, Greig NH. Methyl analogues of the experimental Alzheimer drug phenserine: synthesis and structure/activity relationships for acetyl- and butyrylcholinesterase inhibitory action. J Med Chem. 2001;44(24):4062–4071. [DOI] [PubMed] [Google Scholar]
- 30. Greig NH, De Micheli E, Holloway HW, Yu QS, Utsuki T, Perry TA, Brossi A, Ingram DK, Deutsch J, Lahiri DK, Soncrant TT. The experimental Alzheimer drug phenserine: preclinical pharmacokinetics and pharmacodynamics. Acta Neurol Scand Suppl. 2000;176:74–84. [DOI] [PubMed] [Google Scholar]
- 31. Borlongan CV, Sanberg PR. Elevated body swing test: a new behavioral parameter for rats with 6-hydroxydopamine-induced hemiparkinsonism. J Neurosci. 1995;15(7 Pt 2):5372–5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Borlongan CV, Randall TS, Cahill DW, Sanberg PR. Asymmetrical motor behavior in rats with unilateral striatal excitotoxic lesions as revealed by the elevated body swing test. Brain Res. 1995;676(1):231–234. [DOI] [PubMed] [Google Scholar]
- 33. Roghani M, Behzadi G, Baluchnejadmojarad T. Efficacy of elevated body swing test in the early model of Parkinson’s disease in rat. Physiol Behav. 2002;76(4–5):507–510. [DOI] [PubMed] [Google Scholar]
- 34. Schiff L, Hadker N, Weiser S, Rausch C. A literature review of the feasibility of glial fibrillary acidic protein as a biomarker for stroke and traumatic brain injury. Mol Diagn Ther. 2012;16(2):79–92. [DOI] [PubMed] [Google Scholar]
- 35. Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4–9. [DOI] [PubMed] [Google Scholar]
- 36. Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39(3):253–262. [DOI] [PubMed] [Google Scholar]
- 37. Barger SW. An unconventional hypothesis of oxidation in Alzheimer’s disease: intersections with excitotoxicity. Front Biosci. 2004;9:3286–3295. [DOI] [PubMed] [Google Scholar]
- 38. Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Doyle KM, Kennedy D, Gorman AM, Gupta S, Healy SJ, Samali A. Unfolded proteins and endoplasmic reticulum stress in neurodegenerative disorders. J Cell Mol Med. 2011;15(10):2025–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Toth A, Schmalfuss I, Heaton SC, Gabrielli A, Hannay HJ, Papa L, Brophy GM, Wang KK, Buki A, Schwarcz A, Hayes RL, et al. Lateral ventricle volume asymmetry predicts midline shift in severe traumatic brain injury. J Neurotrauma. 2015;32(17):1307–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poca MA, Sahuquillo J, Mataro M, Benejam B, Arikan F, Baguena M. Ventricular enlargement after moderate or severe head injury: a frequent and neglected problem. J Neurotrauma. 2005;22(11):1303–1310. [DOI] [PubMed] [Google Scholar]
- 42. Levin HS, Meyers CA, Grossman RG, Sarwar M. Ventricular enlargement after closed head injury. Arch Neurol. 1981;38(10):623–629. [DOI] [PubMed] [Google Scholar]
- 43. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. [DOI] [PubMed] [Google Scholar]
- 44. Faden AI, Wu J, Stoica BA, Loane DJ. Progressive inflammation-mediated neurodegeneration after traumatic brain or spinal cord injury. Br J Pharmacol. 2016;173(4):681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Solito E, Sastre M. Microglia function in Alzheimer’s disease. Front Pharmacol. 2012;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang Z, Zhang ZY, Wu Y, Schluesener HJ. Immunolocalization of toll-like receptors 2 and 4 as well as their endogenous ligand, heat shock protein 70, in rat traumatic brain injury. Neuroimmunomodulation. 2012;19(1):10–19. [DOI] [PubMed] [Google Scholar]
- 47. Thored P, Heldmann U, Gomes-Leal W, Gisler R, Darsalia V, Taneera J, Nygren JM, Jacobsen SE, Ekdahl CT, Kokaia Z, Lindvall O. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57(8):835–849. [DOI] [PubMed] [Google Scholar]
- 48. Donat CK, Scott G, Gentleman SM, Sastre M. Microglial activation in traumatic brain injury. Front Aging Neurosci. 2017;9:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reale M, Di Nicola M, Velluto L, D’Angelo C, Costantini E, Lahiri DK, Kamal MA, Yu QS, Greig NH. Selective acetyl- and butyrylcholinesterase inhibitors reduce amyloid-beta ex vivo activation of peripheral chemo-cytokines from Alzheimer’s disease subjects: exploring the cholinergic anti-inflammatory pathway. Curr Alzheimer Res. 2014;11(6):608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Krukowski K, Chou A, Feng X, Tiret B, Paladini MS, Riparip LK, Chaumeil MM, Lemere C, Rosi S. Traumatic brain injury in aged mice induces chronic microglia activation, synapse loss, and complement-dependent memory deficits. Int J Mol Sci. 2018;19(12):E3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marcet P, Santos N, Borlongan CV. When friend turns foe: central and peripheral neuroinflammation in central nervous system injury. Neuroimmunol Neuroinflamm. 2017;4:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lozano D, Gonzales-Portillo GS, Acosta S, de la Pena I, Tajiri N, Kaneko Y, Borlongan CV. Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr Dis Treat. 2015;11:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barrientos RM, Kitt MM, Watkins LR, Maier SF. Neuroinflammation in the normal aging hippocampus. Neuroscience. 2015;309:84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bengtsson M, Godbolt AK. Effects of acetylcholinesterase inhibitors on cognitive function in patients with chronic traumatic brain injury: a systematic review. J Rehabil Med. 2016;48(1):1–5. [DOI] [PubMed] [Google Scholar]
- 55. Arciniegas DB. The cholinergic hypothesis of cognitive impairment caused by traumatic brain injury. Curr Psychiatry Rep. 2003;5(5):391–399. [DOI] [PubMed] [Google Scholar]
- 56. Arciniegas D, Adler L, Topkoff J, Cawthra E, Filley CM, Reite M. Attention and memory dysfunction after traumatic brain injury: cholinergic mechanisms, sensory gating, and a hypothesis for further investigation. Brain Inj. 1999;13(1):1–13. [DOI] [PubMed] [Google Scholar]
- 57. Saija A, Hayes RL, Lyeth BG, Dixon CE, Yamamoto T, Robinson SE. The effect of concussive head injury on central cholinergic neurons. Brain Res. 1988;452(1–2):303–311. [DOI] [PubMed] [Google Scholar]
- 58. Dixon CE, Bao J, Johnson KM, Yang K, Whitson J, Clifton GL, Hayes RL. Basal and scopolamine-evoked release of hippocampal acetylcholine following traumatic brain injury in rats. Neurosci Lett. 1995;198(2):111–114. [DOI] [PubMed] [Google Scholar]
- 59. Whitlock JA., Jr Brain injury, cognitive impairment, and donepezil. J Head Trauma Rehabil. 1999;14(4):424–427. [DOI] [PubMed] [Google Scholar]
- 60. Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16(6):710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Haam J, Yakel JL. Cholinergic modulation of the hippocampal region and memory function. J Neurochem. 2017;142(suppl 2):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lilja AM, Luo Y, Yu QS, Rojdner J, Li Y, Marini AM, Marutle A, Nordberg A, Greig NH. Neurotrophic and neuroprotective actions of (-)- and (+)-phenserine, candidate drugs for Alzheimer’s disease. Plos One. 2013;8(1):e54887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen J, Pan H, Chen C, Wu W, Iskandar K, He J, Piermartiri T, Jacobowitz DM, Yu QS, McDonough JH, Greig NH, et al. (-)-phenserine attenuates soman-induced neuropathology. Plos One. 2014;9(6):e99818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tweedie D, Fukui K, Li Y, Yu QS, Barak S, Tamargo IA, Rubovitch V, Holloway HW, Lehrmann E, Wood WH, 3rd, Zhang Y, et al. Cognitive impairments induced by concussive mild traumatic brain injury in mouse are ameliorated by treatment with phenserine via multiple non-cholinergic and cholinergic mechanisms. Plos One. 2016;11(6):e0156493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Moppett IK. Traumatic brain injury: assessment, resuscitation and early management. Br J Anaesth. 2007;99(1):18–31. [DOI] [PubMed] [Google Scholar]
- 66. Donat CK, Schuhmann MU, Voigt C, Nieber K, Schliebs R, Brust P. Alterations of acetylcholinesterase activity after traumatic brain injury in rats. Brain Inj. 2007;21(10):1031–1037. [DOI] [PubMed] [Google Scholar]
- 67. Dixon CE, Flinn P, Bao J, Venya R, Hayes RL. Nerve growth factor attenuates cholinergic deficits following traumatic brain injury in rats. Exp Neurol. 1997;146(2):479–490. [DOI] [PubMed] [Google Scholar]
- 68. Murdoch I, Nicoll JA, Graham DI, Dewar D. Nucleus basalis of meynert pathology in the human brain after fatal head injury. J Neurotrauma. 2002;19(2):279–284. [DOI] [PubMed] [Google Scholar]