Abstract
Multilineage-differentiating stress-enduring (Muse) cells are endogenous pluripotent stem cells that can be isolated based on stage-specific embryonic antigen-3 (SSEA-3), a pluripotent stem cell-surface marker. However, their capacities for survival, neurotrophic factor secretion, and neuronal and glial differentiation are unclear in rodents. Here we analyzed mouse adipose tissue-derived Muse cells in vitro. We collected mesenchymal stem cells (MSCs) from C57BL/6 J mouse adipose tissue and separated SSEA-3+, namely Muse cells, and SSEA-3–, non-Muse cells, to assess self-renewability; pluripotency marker expression (Nanog, Oct3/4, Sox2, and SSEA-3); spontaneous differentiation into endodermal, mesodermal, and ectodermal lineages; and neural differentiation capabilities under cytokine induction. Neurally differentiated Muse and non-Muse cell functions were assessed by calcium imaging. Antioxidant ability was measured to assess survival under oxidative stress. Brain-derived neurotrophic factor (BDNF), vascular endothelial cell growth factor (VEGF), and hepatocyte growth factor (HGF) secretion were analyzed in enzyme-linked immunosorbent assays. SSEA-3+ Muse cells (6.3 ± 1.9% of mouse adipose-MSCs), but not non-Muse cells, exhibited self-renewability, spontaneous differentiation into the three germ layers, and differentiation into cells positive for Tuj-1 (27 ± 0.9%), O4 (17 ± 3.4%), or GFAP (23 ± 1.3%) under cytokine induction. Neurally differentiated Muse cells responded to KCl depolarization with greater increases in cytoplasmic Ca2+ levels than non-Muse cells. Cell survival under oxidative stress was significantly higher in Muse cells (50 ± 2.7%) versus non-Muse cells (22 ± 2.8%). Muse cells secreted significantly more BDNF, VEGF, and HGF (273 ± 12, 1479 ± 7.5, and 6591 ± 1216 pg/mL, respectively) than non-Muse cells (133 ± 4.0, 1165 ± 20, and 2383 ± 540 pg/mL, respectively). Mouse Muse cells were isolated and characterized for the first time. Muse cells showed greater pluripotency-like characteristics, survival, neurotrophic factor secretion, and neuronal and glial-differentiation capacities than non-Muse cells, indicating that they may have better neural-regeneration potential.
Keywords: muse cell, mouse, adipose tissue, neuroregeneration, neurotrophic factor
Introduction
Mesenchymal stem cells (MSCs) derived from adipose tissue (adipose-MSCs) are currently being applied in numerous clinical studies targeting various diseases, due to their advantages in terms of easy accessibility, non-tumorigenicity, and paracrine effects1,2. Autologous adipose-MSC transplants are considered to have low safety concerns and, thus, they are currently used for regenerative therapies1.
Multilineage-differentiating stress-enduring (Muse) cells represent a subpopulation of adult human mesenchymal cells, such as bone marrow-MSCs, adipose-MSCs, and fibroblasts, and are known to exhibit pluripotent-like characteristics3. Notably, the efficiency of Muse cell differentiation in vitro (and in vivo after integration into damaged organs) into MSCs is low, possibly because the Muse cells represent a low percent of the total MSC population4. Muse cells are strongly distinguished from non-Muse cells by their pluripotency-like characteristics5.
Bone marrow-Muse cells differentiate into neural cells, extend neurites through the pyramidal tract, cross to the contralateral side, and reach the pyramidal tract in the dorsal funiculus of the spinal cord6. Skin fibroblast-Muse cells differentiate into neural cells, integrate into the sensory-motor cortex, extend their neurites into the cervical spinal cord, and display normalized hindlimb somatosensory-evoked potentials7. However, the antioxidant ability, capacity for secreting neurotrophic factors, and potential for neuronal and glial differentiation of adipose-Muse cells remain unclear5.
Muse cells have been isolated from mesenchymal tissues in humans, goats, and rabbits3,8,9. Muse cells have been transplanted as xenografts in several basic studies7,10. The isolation of mouse Muse cells would make it easier to perform allograft transplantation in these studies. Furthermore, the availability of mouse Muse cells is important for transgenic mouse studies. However, whether Muse cells can be isolated from mouse tissues is unclear. In addition, Muse cells were isolated from bone marrow in most studies6,9,11. However, adipose tissue is known to be accessible, abundant, and reliable for isolating adult stem cells suitable for tissue engineering and regenerative medicine applications12. Therefore, the isolation of Muse cells from mouse adipose tissue would constitute an important advancement in the basic study of Muse cells.
The purposes of this study were to isolate Muse cells from mouse adipose tissue, and to assess their neural differentiation potentials. We first tested whether cells expressing the pluripotency marker stage-specific embryonic antigen 3 (SSEA-3), which is known to be expressed in human Muse cells3, could be sorted from mouse adipose-MSCs. After successfully isolating the SSEA-3+ population, we confirmed that the sorted cells had Muse cell characteristics. Finally, we examined the neural differentiation potential of the mouse adipose-Muse cells, which was previously poorly understood for adipose-Muse cells, including their survival, neurotrophic factor secretion, and neuronal and glial differentiation. The rationale of this study was to contribute a more accessible cell source for the basic neuronal-regeneration studies of Muse cells.
Materials and Methods
Animals
Six-week-old female C57BL/6 J mice were used as a source of adipose tissue (CLEA Japan, Inc., Shizuoka, Japan). All procedures involving animals were conducted according to the guidelines of the Institutional Animal Care and Research Advisory Committee of Hirosaki University (Approval number: M14013).
Cell Harvest and Culture
Adipose-MSCs were isolated from the adipose tissue of adult mice (n = 60). Adipose tissue dissected from the inguinal subcutaneous region of each mouse was minced and digested with 10 mL of 0.2% type-I collagenase for 45 min at 37°C. The resultant cell suspensions were filtered through a 70-μm mesh, and collagenase was removed by centrifugation (1500 rpm, 4°C) for 5 min. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Thermo Fisher Scientific, Waltham, MA, USA). After 24 h, the non-adherent cells were removed, and the adherent cells were subcultured when they reached 70–80% confluence. Adipose-MSCs from passages 2 through 6 were used in the experiments. Adipose-MSCs were analyzed by flow cytometry using antibodies against the following cell-surface markers: CD29, CD44, CD90, CD45 (Thermo Fisher Scientific), Sca1, CD105, CD34 (Becton Dickinson, Franklin Lakes, NJ, USA), and CD99 (R&D Systems, Minneapolis, MN, USA).
Cell Separation
Confluent mouse MSCs were analyzed by fluorescence-activated cell sorting (FACS) as previously described5. Cells were incubated first with a monoclonal antibody against SSEA-3 (1:100; Thermo Fisher Scientific) for 1 h at 4°C and then with phycoerythrin (PE)-conjugated goat anti-rat IgM (Southern Biotech, Homewood, AL, USA), after which they were sorted for SSEA-3 expression using a FACSAriaTM II instrument (Becton Dickinson)13.
SSEA-3+ cells were also isolated by magnetic-activated cell sorting (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany), as previously described14. Briefly, Muse cells were labeled using anti-human/mouse SSEA-3 PE (1:100; Thermo Fisher Scientific) and separated by MACS using anti-PE microbeads (1:2, Miltenyi Biotec). Target cell-labeled microbeads were immobilized in a magnetic field and later collected as the positive fraction. The percentage of SSEA-3+ cells after MACS separation was assessed by flow cytometry.
Assessment of Muse Cell Characteristics
We assessed the Muse cell characteristics of the sorted SSEA-3+ cells, including the self-renewal ability, expression of pluripotency markers, and spontaneous differentiation, as previously described13.
Sorted SSEA-3+ cells were individually seeded in separate wells of 96-well plates via limiting dilution and subjected to single-cell suspension culture. Muse cell-derived cell clusters (M-clusters) were observed after 1–2 weeks of single-cell suspension culture, and the number of M-clusters was expressed as a percentage of the number of plated cells. The percentages of M-clusters formed by cells separated by FACS (n = 3) versus MACS (n = 3) was compared.
Alkaline phosphatase (ALP) staining was performed to assess self-renewal using a Leukocyte Alkaline Phosphatase Kit (Sigma-Aldrich, St. Louis, MO, USA). Cell culturing was repeated to prepare third-generation clusters.
To evaluate the expression levels of pluripotency markers, the M-clusters were assessed by immunocytochemistry. Sections of M-clusters were incubated overnight at 4°C with primary antibodies against Nanog (Millipore, Burlington, MA, USA; Alexa-488), Oct3/4 (Santa Cruz Biotechnology, Dallas, TX, USA; Alexa-647), Sox2 (Millipore; Alexa-488), and SSEA-3 (Thermo Fisher Scientific). The sections were then incubated with the corresponding secondary antibody (SSEA-3; PE). Immunofluorescence signals were observed under a fluorescence microscope (BZ-X700, Keyence, Osaka, Japan).
To assess the spontaneous differentiation of M-clusters, single M-clusters were transferred to a gelatin-coated culture dish, incubated for another 7 days, and analyzed by immunocytochemistry using antibodies against α-fetoprotein (α-FP, 1:200, Biorbyt, Cambridge, UK; Alexa-488), smooth muscle actin (SMA, 1:200, Thermo Fisher Scientific; Alexa-555), and neurofilament (NF, 1:2000, Millipore; Alexa-555).
Neural Differentiation Potentials of Muse Cells
Antioxidant abilities and cell-survival potentials were analyzed as previously described15. SSEA-3+ cells (named here as “Muse cells”) and SSEA-3− cells (named as “non-Muse cells”) were maintained in vitro under oxidative stress, which was reported to contribute to decreased intraneural cell transplantation15,16. For this analysis, 1 × 105 Muse cells or non-Muse cells were incubated in 100 µL of phosphate-buffered saline containing 200 µM hydrogen peroxide (H2O2; Sigma-Aldrich) for 24 h at 37°C. Then, 10 µL of Trypan Blue (Sigma-Aldrich) was added, and the viable (Trypan Blue-negative) cells were quantified in a hematocytometer and expressed as a percentage of all counted cells. The results from five samples were averaged.
Neurotrophic factors were analyzed as previously described17. The concentrations of brain-derived neurotrophic factor (BDNF), vascular endothelial cell growth factor (VEGF), and hepatocyte growth factor (HGF) secreted into the culture medium were measured using specific enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems). Muse cells or non-Muse cells at 1 × 105 cells/cm2 were grown in control medium for 72 h. The medium was then harvested (n = 4 per group), and growth factors were measured by performing ELISAs, according to the manufacturer’s instructions.
Neural differentiation was induced as previously described18. Muse or non-Muse cells were transferred onto a 4-well chamber slide (Thermo Fisher Scientific; 1 × 104 cells/cm2). The cells were cultured in Neurobasal Medium (Thermo Fisher Scientific) containing 1% fetal calf serum, 1× B27 supplement 1, 0.5 mM 1-methyl-3 isobutyl xanthine, 1 μM dexamethasone, 50 μM 8CPT-cAMP, 10 mM valproic acid, and 10 μM forskolin. The neural differentiation medium was changed weekly. The primary antibodies were murine-specific antibodies against the following neural cell markers: Tuj-1 (BioLegend, San Diego, CA, USA), O4 (Thermo Fisher Scientific), and GFAP (Abcam, Cambridge, UK). The cells were then incubated for 1 h at room temperature with secondary antibodies (Tuj-1, Alexa-488; O4, Alexa-488; GFAP, Alexa-594), washed, and examined under a fluorescent microscope (BZ-X700, Keyence). The cells positive for each marker were counted, and the proportion of total cells that expressed each marker was calculated. The experiments were repeated three times.
Calcium-imaging experiments were performed to assess neuronal function, as previously described19. Muse or non-Muse cells were transferred into a black-wall, clear-bottom 96-well microplate (Greiner Bio-One, Kremsmünster, Austria; 1.5 × 104 cells/well). They were cultured in neural differentiation medium for 7 days. The cells were incubated in DMEM containing 2.5 μM Fura 2-AM (Dojindo, Kumamoto, Japan), 0.04% pluronic F-127 (Dojindo), and 1.25 mM probenecid (Dojindo) for 60 min at 37°C in the dark. The 340/380 nm fluorescence ratios were analyzed with a FlexStation 3 microplate reader (Molecular Devices, San Jose, CA, USA). Fluorescence measurements were obtained at excitation wavelengths of 340 and 380 nm and an emission wavelength of 510 nm. The baseline intracellular Ca2+ levels were measured as the ratio of fluorescence (340/380 nm). Then, intracellular calcium dynamics were recorded after exposure to 50 mM KCl. The fluorescence-intensity peak above the baseline was compared between Muse cells and non-Muse cells (n = 4 per group).
Statistical Analysis
Independent-sample t tests were used to analyze the experimental data for the M-cluster-formation ratio following isolation by FACS versus MACS, and for the antioxidant ability, production of neurotrophic factors, neuronal and glial-differentiation abilities, and calcium imaging of Muse cells versus non-Muse cells. Comparison of the neuronal and glial-differentiation potentials associated with Tuj-1, O4, and GFAP expression was performed by analysis of variance (ANOVA), followed by the Tukey–Kramer test. Statistical calculations were performed with SPSS software, version 22.0 (SPSS, Chicago, IL, USA). P < 0.05 was considered to reflect a statistically significant difference. The data shown in the figures are expressed as the mean ± SEM.
Results
Separation of SSEA-3+ Cells Derived from Mouse Adipose Tissue
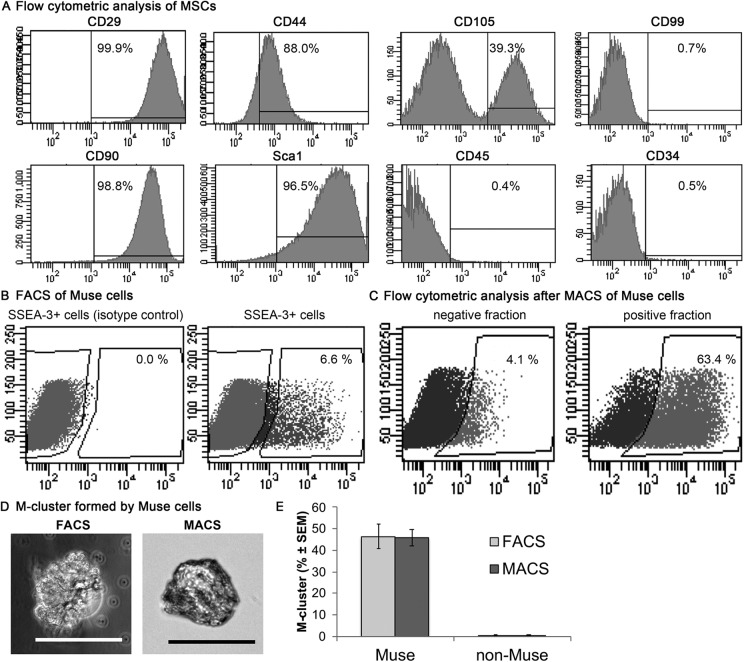
The MSC markers CD29, CD44, CD90, Sca1, and CD105 were detected in adipose-MSCs, but the adipose-MSCs were negative for CD99, CD45, and CD34 (Fig. 1A). Both FACS and MACS enabled successful isolation of SSEA-3+ cells from mouse adipose-MSCs (Fig. 1B, C). The percentage of SSEA-3+ cells in mouse adipose-MSCs was 6.3 ± 1.9%, as determined by FACS. Flow cytometric analysis of the MACS-separated cells revealed that 63.4% of them were SSEA-3+ (Fig. 1C).
Figure 1.
Separation of SSEA-3+ cells from mouse adipose-MSCs. (A) Expression of MSC surface markers were measured by flow cytometry in MSCs derived from mouse adipose tissue. (B) Representative FACS results showing the SSEA-3+ cells present in mouse adipose-MSCs. (C) Representative flow cytometry analyses after MACS, showing SSEA-3+ cell percentages. (D) Examples of M-clusters formed from a Muse cell recovered after sorting by FACS or MACS. Scale bars = 100 μm. (E) The rates of cluster formation were similar between Muse cells sorted by FACS or MACS (independent-sample t-test, P > 0.05; n = 3/group). FACS, fluorescence-activated cell sorting; MACS, magnetic-activated cell sorting; MSC, mesenchymal stem cell; SSEA-3, stage-specific embryonic antigen-3.
After single-cell suspension culture, the SSEA-3+ cells sorted by FACS or MACS formed M-clusters (Fig. 1D). However, the SSEA-3– cells did not form M-clusters (Fig. 1E). The rates of M-cluster formation were 46.4 ± 11.5% in FACS-sorted cells and 45.8 ± 9.0% in MACS-sorted cells (Fig. 1E); this difference was not statistically significant.
Muse Cell Characteristics of Sorted SSEA-3+ Cells
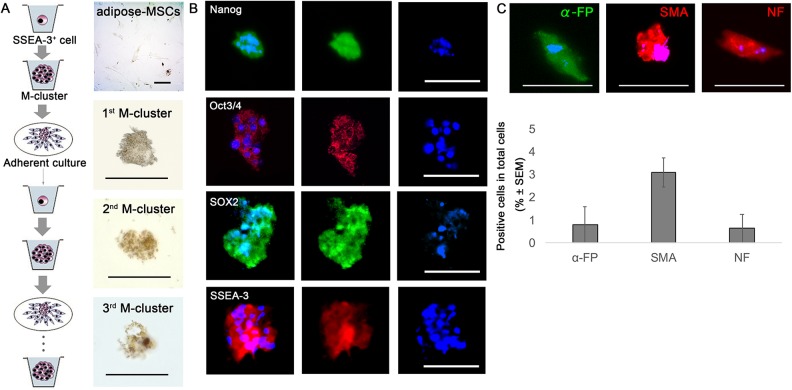
The M-clusters formed by single SSEA-3+ cells in suspension culture were positive for ALP activity; ALP positivity was detected in the first-, second-, and third-generation clusters (Fig. 2A). These M-clusters expressed pluripotency markers, including Nanog, Oct3/4, Sox2, and SSEA-3 (Fig. 2B). When the M-clusters were individually transferred onto gelatin-coated dishes and cultured for 7 days, the cells expanded from each cluster and proliferated as adherent cells. The expanded cell population contained cells that expressed the endodermal marker α-FP, the mesodermal marker SMA, and the ectodermal marker NF (Fig. 2C). The percentages of positive cells (among the total cell populations) positive for α-FP, SMA, and NF were 0.79 ± 1.4%, 3.1 ± 1.1%, 0.64 ± 1.1%, respectively (Fig. 2C).
Figure 2.
Muse cell characteristics of SSEA-3+ cells sorted from mouse adipose-MSCs. (A) Self-renewal capacity of mouse adipose-Muse cells, as determined by staining for ALP activity in first-, second-, and third-generation M-clusters. Adipose-MSCs were negative for ALP activity. (B) Immunocytochemistry of clusters formed from Muse cells in single-cell suspension cultures, showing that the clusters were positive for Nanog, Oct3/4, Sox2, and SSEA-3. Nuclei were visualized by staining with Hoechst dye. (C) Muse cells in single-cell suspension culture formed clusters in which the cells differentiated spontaneously, expressing endodermal (α-FP), mesodermal (SMA), and ectodermal (NF) markers. Scale bars = 100 µm. α-FP, alpha-fetoprotein; ALP, alkaline phosphatase; M-cluster, Muse cell-derived cell cluster; NF, neurofilament; SMA, smooth muscle actin.
Neural Differentiation Potential of Muse Cells
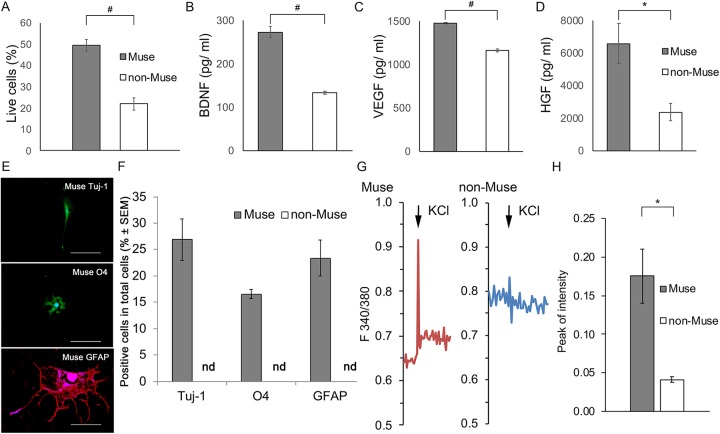
Cell survival under H2O2-induced oxidative stress was significantly higher in Muse cells (50 ± 2.7%) than in non-Muse cells (22 ± 2.8%, P < 0.005; n = 5; Fig. 3A).
Figure 3.
Neural differentiation potentials of mouse adipose-Muse cells. (A) Cell survival following H2O2-induced oxidative stress was significantly higher in Muse cells (gray) than in non-Muse cells (white) (independent-sample t-test, n = 5/group). (B–D) Muse cells secreted significantly higher levels of the neurotrophic factors BDNF (B), VEGF (C), and HGF (D) than did non-Muse cells (independent-sample t-test, n = 4/group). (E) After inducing neuronal differentiation, only Muse cells differentiated into Tuj-1+, O4+, and GFAP+ cells. (F) Percentage of Muse cells and non-Muse cells expressing the indicated markers. Differences in the percentage of Muse cells expressing Tuj-1, O4, and GFAP were not significant (ANOVA and Tukey’s test, n = 6/group). (G) Representative traces of intracellular calcium dynamics (ratiometric acquisition) showing the functional responses of differentiated Muse cells and non-Muse cells after biochemical depolarization with KCL. (H) Quantification of the peak response intensity of differentiated Muse and non-Muse cells following exposure to 50 mM KCL. The responses of differentiated Muse cells were significantly greater than those of non-Muse cells (independent-sample t-test; n = 4/group). Error bars = SEM. #P < 0.005; *P < 0.05. Scale bars = 100 µm. BDNF, brain-derived neurotrophic factor; H2O2, hydrogen peroxide; HGF, hepatocyte growth factor; nd, not detected; VEGF, vascular endothelial cell growth factor.
The concentrations of BDNF, VEGF, and HGF secreted from Muse cells (273 ± 12, 1479 ± 7.5, and 6591 ± 1216 pg/mL, respectively) were significantly higher than those secreted from non-Muse cells (133 ± 4.0, 1165 ± 20, and 2383 ± 540 pg/mL, respectively; P < 0.005 for BDNF and VEGF, P < 0.05 for HGF, independent-sample t-test; n = 4; Fig. 3B–D).
In the presence of B27 supplement 1, 0.5 mM 1-methyl-3 isobutyl xanthine, 1 μM dexamethasone, 50 μM 8CPT-cAMP, 10 mM valproic acid, and 10 μM forskolin (a known cocktail for neural differentiation), Muse cells expressed Tuj-1, GFAP, and O4 (Fig. 3E); however, non-Muse cells did not express these markers. Among the cells that differentiated from Muse cells, 26.9 ± 0.9% expressed Tuj-1, 16.6 ± 3.4% expressed O4, and 23.3 ± 1.3% expressed GFAP (Fig. 3F). The differences in the percentages of cells expressing each marker were not significant (P > 0.05 by ANOVA and Tukey’s test; n = 6).
The differentiated Muse cells showed calcium-influx responses to 50 mM KCl (Fig. 3G). Although the differentiated non-Muse cells also showed a reduction of membrane potential, the level of reduction was much lower (Fig. 3G). The peak response intensity was significantly higher in Muse cells (0.18 ± 0.07) than in non-Muse cells (0.041 ± 0.0007, P < 0.05 by independent-sample t-test; n = 4; Fig. 3H).
Discussion
In this study, we successfully isolated Muse cells from the adipose tissue of adult mice. SSEA-3+ cells sorted from mouse adipose-MSCs formed M-clusters with the capacity for self-renewal, pluripotency marker expression (Nanog, Oct3/4, Sox2, and SSEA-3), and spontaneous generation of cells representative of all three germ layers. Compared with non-Muse cells, the Muse cells showed greater antioxidant ability and secreted significantly higher levels of neurotrophic factors. Muse cells differentiated into Tuj-1+ cells, O4+ cells, and GFAP+ cells under neural induction, whereas non-Muse cells did not.
The percentage of SSEA-3+ cells sorted from mouse adipose-MSCs by FACS was approximately 4–8% (Fig. 1B), which is similar to the percentage of SSEA-3+ cells isolated from human adipose tissue (4–9%)5. In skin fibroblasts, the percentage of SSEA-3+ cells was 1–2% in humans and 3–4% in goats3,8. Thus, our results suggest that similar percentages of SSEA-3+ cells can be isolated from different species.
Although data from one study suggested that laser irradiation used in FACS might decrease the cluster-formation ratio5, we found that cells separated by FACS and MACS formed M-clusters at comparable rates (Fig. 1E). The percentage of SSEA-3+ cells recovered after a single MACS-based collection step was 63.4% in this study (Fig. 1C), compared with 77.1% after a double-collection step in a previous study14. MACS is clinically approved for cell separation14. Thus, our results revealed the characteristics of MACS for sorting Muse cells by comparison with FACS.
SSEA-3+ cells isolated from mouse adipose tissue and placed in suspension culture formed M-clusters, and the cells in these clusters expressed pluripotency markers and spontaneously differentiated into mesodermal-, endodermal-, and ectodermal-lineage cells (Fig. 2A–C). These characteristics of cluster formation, self-renewal, pluripotency, and spontaneous differentiation are reported for Muse cells13. Thus, the SSEA-3+ cells derived from mouse adipose tissue displayed characteristics similar to Muse cells13.
In this study, the Muse cells showed better survival than non-Muse cells under oxidative stress (Fig. 3A). Previous findings showed that Muse cells were more resistant to chemical and physical genotoxic stresses than were non-Muse cells, because Muse cells were found to have an efficient and rapid DNA damage-repair system and to secrete factors needed for survival under stress20,21. Cell survival is important because the reparative effects of neuronal regeneration depend on the degree of cell survival15.
The data generated in this study also showed that Muse cells secreted significantly more BDNF, VEGF, and HGF than non-Muse cells (Fig. 3B–D). This difference between Muse cells and non-Muse cells was not reported previously. BDNF plays a crucial role in enhancing neuroprotective effects after MSC transplantation22. The effects of VEGF on endogenous gliogenesis, angiogenesis, and tissue sparing improve functional outcomes after spinal cord injury (SCI)23. HGF promotes neuron and oligodendrocyte survival, angiogenesis, axonal growth, and functional recovery after SCI24. Thus, transplanting Muse cells, which secrete higher levels of these factors, may ameliorate neural damages to a greater extent than transplanting non-Muse cells.
Our results further showed that mouse adipose-Muse cells could differentiate into astrocytes and oligodendrocytes, whereas non-Muse cells lacked this ability (Fig. 3E, F). This finding is consistent with the higher neural differentiation potential observed for human adipose-Muse cells compared with non-Muse cells in vitro5. A report of a transplantation study demonstrated that the glial-differentiation potential was more important than the neuronal-differentiation potential for promoting recovery from neurological diseases25,26. Thus, the ability of adipose-Muse cells to differentiate into glial cells makes them particularly promising for developing therapies for neurological diseases.
Neural-differentiated Muse cells exhibited active membrane properties in response to depolarization with KCl (Fig. 3G). Previous data showed that the peak response to 50 mM KCl in neural-differentiated adipose-MSCs (0.052) was lower than that in hippocampal neurons (0.13)27. In this study, the peak intensity of Muse cells (0.18) was similar to that of hippocampal neurons (Fig. 3H). Our results suggest that neural-differentiated Muse cells were more mature than neural-differentiated MSCs.
This study had some limitations. First, the regeneration potentials were only assessed in vitro. In brain infarcts, non-Muse cell transplantation also showed functional recovery compared with a control transplantation10. The ability of cells to alter their developmental fate remains difficult to explain, since normal tissue differentiation requires sequential restriction of the developmental potential. Thus, our findings will need to be confirmed by performing in vivo-transplantation studies. Second, Muse cells have other characteristics, such as the ability to home to damage sites when supplied locally or by intravenous injection11, and are non-tumorigenic5,6,13. However, these properties of the mouse adipose-Muse cells still need to be investigated in transplantation experiments.
In conclusion, our results demonstrate that mouse adipose tissue can be used as a practical source of Muse cells in basic neuronal-regeneration studies. Compared with non-Muse cells, the adipose-Muse cells survived longer under oxidative stress, secreted more neurotrophic factors, and were better able to differentiate into neurons, oligodendrocytes, and astrocytes. Our results indicate that adipose-derived Muse cells may have better neural-regeneration potential than non-Muse cells. This is the first study where Muse cells were isolated from mouse tissue.
Acknowledgments
We thank members of the Department of Orthopedic Surgery at Hirosaki University for helpful discussions. We also thank Dr. Chikara Ohyama, Mr. Tohru Yoneyama, and Mr. Kenji Kabasawa of the Department of Urology, Hirosaki University Graduate School of Medicine, for providing technical assistance with the flow cytometric analysis. We also thank Dr. Shohei Wakao of the Department of Stem Cell Biology and Histology, Tohoku University Graduate School of Medicine, for technical advice. This study was funded by the Karoji Memorial Fund for Medical Research at Hirosaki University, Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (17K10917), and a Hirosaki University Grant for Distinguished Researchers (FY2017-2018).
Footnotes
Ethical Approval: The experimental animal protocol including the use of animal-derived tissues was approved by the Ethics Committee for Animal Experimentation of Hirosaki University, Japan (Approval number: M14013).
Statement of Human and Animals Rights: This article does not contain studies with human subjects. Animal experiments were reviewed and approved by Hirosaki university (approval number: M14013), Japan.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Gentaro Kumagai  https://orcid.org/0000-0003-0597-6603
https://orcid.org/0000-0003-0597-6603
References
- 1. Cho YB, Lee WY, Park KJ, Kim M, Yoo HW, Yu CS. Autologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: a phase I clinical study. Cell Transplant. 2013;22(2):279–285. [DOI] [PubMed] [Google Scholar]
- 2. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100(9):1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, Goda M, Akashi H, Inutsuka A, Niwa A, Shigemoto T, et al. Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci U S A. 2010;107(19):8639–8643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wakao S, Kitada M, Kuroda Y, Shigemoto T, Matsuse D, Akashi H, Tanimura Y, Tsuchiyama K, Kikuchi T, Goda M, Nakahata T, et al. Multilineage-differentiating stress-enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts. Proc Natl Acad Sci U S A. 2011;108(24):9875–9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ogura F, Wakao S, Kuroda Y, Tsuchiyama K, Bagheri M, Heneidi S, Chazenbalk G, Aiba S, Dezawa M. Human adipose tissue possesses a unique population of pluripotent stem cells with nontumorigenic and low telomerase activities: potential implications in regenerative medicine. Stem Cells Dev. 2014;23(7):717–728. [DOI] [PubMed] [Google Scholar]
- 6. Uchida H, Niizuma K, Kushida Y, Wakao S, Tominaga T, Borlongan CV, Dezawa M. Human Muse cells reconstruct neuronal circuitry in subacute lacunar stroke model. Stroke. 2017;48(2):428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uchida H, Morita T, Niizuma K, Kushida Y, Kuroda Y, Wakao S, Sakata H, Matsuzaka Y, Mushiake H, Tominaga T, Borlongan CV, et al. Transplantation of unique subpopulation of fibroblasts, Muse cells, ameliorates experimental stroke possibly via robust neuronal differentiation. Stem Cells. 2015;34(1):160–173. [DOI] [PubMed] [Google Scholar]
- 8. Yang Z, Liu J, Liu H, Qiu M, Liu Q, Zheng L, Pang M, Quan F, Zhang Y. Isolation and characterization of SSEA3+ stem cells derived from goat skin fibroblasts. Cell Reprogram. 2013;15(3):195–205. [DOI] [PubMed] [Google Scholar]
- 9. Yamada Y, Wakao S, Kushida Y, Minatoguchi S, Mikami A, Higashi K, Baba S, Shigemoto T, Kuroda Y, Kanamori H, Amin M, et al. S1P-S1PR2 axis mediates homing of Muse cells into damaged heart for long-lasting tissue repair and functional recovery after acute myocardial infarction. Circ Res. 2018;122(8):1069–1083. [DOI] [PubMed] [Google Scholar]
- 10. Yamauchi T, Kuroda Y, Morita T, Shichinohe H, Houkin K, Dezawa M, Kuroda S. Therapeutic effects of human multilineage-differentiating stress enduring (MUSE) cell transplantation into infarct brain of mice. PloS One. 2015;10(3):e0116009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iseki M, Kushida Y, Wakao S, Akimoto T, Mizuma M, Motoi F, Asada R, Shimizu S, Unno M, Chazenbalk G, Dezawa M. Muse cells, nontumorigenic pluripotent-like stem cells, have liver regeneration capacity through specific homing and cell replacement in a mouse model of liver fibrosis. Cell Transplant. 2017;26(5):821–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kokai LE, Marra K, Rubin JP. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl Res. 2014;163(4):399–408. [DOI] [PubMed] [Google Scholar]
- 13. Kuroda Y, Wakao S, Kitada M, Murakami T, Nojima M, Dezawa M. Isolation, culture and evaluation of multilineage-differentiating stress-enduring (Muse) cells. Nat Protoc. 2013;8(7):1391–1415. [DOI] [PubMed] [Google Scholar]
- 14. Kinoshita K, Kuno S, Ishimine H, Aoi N, Mineda K, Kato H, Doi K, Kanayama K, Feng J, Mashiko T, Kurisaki A, et al. Therapeutic potential of adipose-derived SSEA-3-positive Muse cells for treating diabetic skin ulcers. Stem Cells Transl Med. 2015;4(2):146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ritfeld GJ, Rauck BM, Novosat TL, Park D, Patel P, Roos RA, Wang Y, Oudega M. The effect of a polyurethane-based reverse thermal gel on bone marrow stromal cell transplant survival and spinal cord repair. Biomaterials. 2014;35(6):1924–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siriphorn A, Chompoopong S, Floyd CL. 17β-estradiol protects schwann cells against H2O2-induced cytotoxicity and increases transplanted Schwann cell survival in a cervical hemicontusion spinal cord injury model. J Neurochem. 2010;115(4):864–872. [DOI] [PubMed] [Google Scholar]
- 17. Ikegame Y, Yamashita K, Hayashi S, Mizuno H, Tawada M, You F, Yamada K, Tanaka Y, Egashira Y, Nakashima S, Yoshimura S, et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy. 2011;13(6):675–685. [DOI] [PubMed] [Google Scholar]
- 18. Boulland JL, Mastrangelopoulou M, Boquest AC, Jakobsen R, Noer A, Glover JC, Collas P. Epigenetic regulation of nestin expression during neurogenic differentiation of adipose tissue stem cells. Stem Cells Dev. 2013;22(7):1042–1052. [DOI] [PubMed] [Google Scholar]
- 19. Fox LE, Shen J, Ma K, Liu Q, Shi G, Pappas GD, Qu T, Cheng J. Membrane properties of neuron-like cells generated from adult human bone-marrow-derived mesenchymal stem cells. Stem Cells Dev. 2010;19(12):1831–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alessio N, Ozcan S, Tatsumi K, Murat A, Peluso G, Dezawa M, Galderisi U. The secretome of MUSE cells contains factors that may play a role in regulation of stemness, apoptosis and immunomodulation. Cell Cycle. 2017;16(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alessio N, Squillaro T, Ozcan S, Di Bernardo G, Venditti M, Melone M, Peluso G, Galderisi U. Stress and stem cells: adult Muse cells tolerate extensive genotoxic stimuli better than mesenchymal stromal cells. Oncotarget. 2018;9(27):19328–19341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ritfeld GJ, Patel A, Chou A, Novosat TL, Castillo DG, Roos RA, Oudega M. The role of brain-derived neurotrophic factor in bone marrow stromal cell-mediated spinal cord repair. Cell Transplant. 2015;24(11):2209–2220. [DOI] [PubMed] [Google Scholar]
- 23. Kim HM, Hwang DH, Lee JE, Kim SU, Kim BG. Ex vivo VEGF delivery by neural stem cells enhances proliferation of glial progenitors, angiogenesis, and tissue sparing after spinal cord injury. PloS One. 2009;4(3):e4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kitamura K, Fujiyoshi K, Yamane J, Toyota F, Hikishima K, Nomura T, Funakoshi H, Nakamura T, Aoki M, Toyama Y, Okano H, et al. Human hepatocyte growth factor promotes functional recovery in primates after spinal cord injury. PloS One. 2011;6(11):e27706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumagai G, Okada Y, Yamane J, Nagoshi N, Kitamura K, Mukaino M, Tsuji O, Fujiyoshi K, Katoh H, Okada S, Shibata S, et al. Roles of ES cell-derived gliogenic neural stem/progenitor cells in functional recovery after spinal cord injury. PloS One. 2009;4(11):e7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsuji O, Miura K, Okada Y, Fujiyoshi K, Mukaino M, Nagoshi N, Kitamura K, Kumagai G, Nishino M, Tomisato S, Higashi H, et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A. 2010;107(28):12704–12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Wang D, Guo D. MiR-124 promote neurogenic transdifferentiation of adipose derived mesenchymal stromal cells partly through RhoA/ROCK1, but not ROCK2 signaling pathway. PLoS One. 2016;11(1):e0146646. [DOI] [PMC free article] [PubMed] [Google Scholar]