Abstract
While limbal epithelial cells are used for treating ocular surface wounds, the therapeutic potential of mesenchymal cells cultivated from the limbal stroma (LMSC) is less clear. We have therefore examined the effects of LMSC when applied to acute ocular surface wounds. LMSC derived from male rabbits (RLMSC) were applied to the ocular surface of female rabbits immediately following removal of the corneal and limbal epithelium. Human amniotic membrane (HAM) was used as the vehicle for implanting the RLMSC. The effects of RLMSC were examined when applied alone (n = 3) and in conjunction with a stratified culture of human limbal epithelial cells (HLE) grown on the opposing surface of the HAM (n = 3). Outcomes were monitored over 3 months in comparison with animals receiving no treatment (n = 3) or treatment with HLE alone on HAM (n = 3). Animals treated with RLMSC (n = 6) displayed faster re-epithelialization (∼90% versus 70% healing after 12 weeks), with best results being observed when RLMSC were pre-cultivated and implanted in the presence of HLE (p < 0.01; 90% healing by 7 weeks). While all animals displayed conjunctival cells on the corneal surface (by presence of goblet cells and/or keratin 13 expression) and corneal neovascularization, evidence of corneal epithelial regeneration was observed in animals that received RLMSC in the presence of HLE (by staining for keratin 3 and the absence of goblet cells). Conversely, corneal neovascularization was significantly greater when RLMSC were applied in the absence of HLE (<0.05; 90% of cornea compared with 20–30% in other cohorts). Nevertheless, neither human nuclear antigen nor rabbit Y chromosome were detected within the regenerated epithelium. Our results demonstrate that while cultured LMSC encourage corneal re-epithelialization, healing is improved by the pre-cultivation and implantation of these mesenchymal cells in the presence of limbal epithelial cells.
Keywords: ocular surface, wound healing, limbal mesenchymal stromal cells, corneal neovascularization, amniotic membrane, Algerbrush II
Introduction
Autologous transplants of corneal-limbal tissue have been widely demonstrated as an effective treatment for ocular surface disease1. While the efficacy of these transplants is logically related to the presence of epithelial progenitor cells, the potential contribution of other cell types present within the transplanted tissue remains unclear. In particular, the presence of limbal mesenchymal stromal cells (LMSC) in cultures established from limbal tissue biopsies in vitro2 suggests that these cells might be exploited to improve clinical outcomes. In particular, LMSC have been shown to encourage the growth of corneal epithelial cells derived from limbal tissue biopsies3,4. Cultured LMSC may therefore be used to encourage re-epithelialization in vivo by facilitating the implantation and growth of transplanted epithelial cells, while also encouraging the growth of any healthy epithelial cells that can be retained within the host cornea5. Moreover, the immunosuppressive properties of LMSC6,7 might be exploited to improve the efficacy of epithelial cells derived from donor tissue.
In addition to the literature outlined above, our present study has been specifically designed as a direct extension of our previous studies in rabbits6,8. In the first study6, we fully characterized the phenotype of LMSC derived from the rabbit limbal stroma in comparison with cultures of LMSC derived from human tissue, and in the second study we optimized our protocol for wounding the ocular surface8. While LMSC derived from rabbit and human tissue both display a mesenchymal morphology, the limited availability of antibodies for rabbit cells prevents a more precise characterization of rabbit LMSC (RLMSC) according to accepted MSC standards. Nevertheless, RLMSC display typical patterns of mesenchymal cell differentiation when cultivated under adipogenic, chondrogenic, and osteogenic conditions and suppress proliferation of lymphocytes when tested in mixed leukocyte reaction assays6. Moreover, RLMSC encourage the growth of corneal-limbal epithelial cells in vitro6. These findings suggested to us that the rabbit would provide a suitable model for testing the impact of LMSC when applied to the ocular surface. Moreover, by implanting male RLMSC and human epithelial cells into female rabbits, we should theoretically be able to trace the fate of both cell types.
We have therefore presently investigated the effects of allogeneic RLMSC when applied alone or in conjunction with human limbal epithelial (HLE) cells cultivated on human amniotic membrane (HAM). As in our previous study8, epithelial tissue is removed from across the full width of the cornea including the limbus. A mechanical method of epithelial debridement is used in order to create a more defined wound than that caused by caustic chemicals. Since mesenchymal stromal cells are known to display anti-inflammatory effects, serum C-reactive protein (CRP) is monitored as a non-specific measure of systemic inflammation throughout the healing process. Re-epithelialization of the cornea, enabled through either the implanted HLE and/or any retained rabbit epithelial cells (including the adjacent conjunctiva), is monitored weekly for up to 12 weeks by slit lamp, with the resulting epithelial phenotype being examined using a variety of histological techniques. In particular, the relative presence of keratins 3 and 13 is used as an indicator of corneal epithelial cells9 and limbal-conjunctival epithelial cells10, respectively. Moreover, the fate of applied HLE cells and RLMSC is examined by immunohistochemistry and fluorescence in situ hybridization (FISH), respectively. Our results demonstrate that while LMSC consistently encourage re-epithelialization of the ocular surface, the phenotype of regenerated epithelium and degree of corneal neovascularization varies according to whether or not the stromal cells have been cultivated and applied in the presence of corneal-limbal epithelial cells.
Materials and Methods
Ethical Approval
The project was conducted with the approval of the University Animal Ethics Committee at the Queensland University of Technology (UAEC approval number 1200000575). Approval to work with human tissue samples was received from the Human Research Ethics Committee (HREC) of Metro South Hospital and Health Service (HREC approval number: HREC/07/QPAH/048) and the Queensland University of Technology (HREC approval number: 0800000807). The number of animals required per cohort was calculated from preliminary data for animals wounded without treatment and based upon the requirement to detect a 20% increase in re-epithelialization (by ANOVA) compared with non-treated controls by 12 weeks (for power = 0.8 and α = 0.5).
Statement of Human and Animal Rights
All studies using human tissue samples were conducted according to the National Statement on Ethical Conduct in Human Research (Australian Government, 2007). All procedures involving rabbits were conducted in accordance with the “Animal Care and Protection Act” (Queensland State Government, Australia, 2001), “Australian Code for the Care and Use of Animals for Scientific Purposes” (8th Edition, 2013), and the “ARVO Statement for Use of Animals in Ophthalmic and Vision Research.”
Statement of Informed Consent
Studies involving the use of human corneal tissue acquired from cadaveric donors were conducted with donor/next-of-kin consent.
Establishment of Cell Cultures
A working stock of male RLMSC was established and expanded to second passage (p2) as described previously6 before storage in liquid nitrogen. Culture quality was presently determined by uniform demonstration of a mesenchymal morphology (>99%) and was consistent with multiple prior cultures analyzed further by flow cytometry and tri-lineage culture experiments for determination of mesenchymal stromal cell phenotyope6. Cultures of HLE were established from discarded samples of donor corneal limbus with the aid of growth-arrested feeder cells as described previously3.
Establishment of Cultures on HAM
HAM was supplied attached to nitrocellulose backing paper and frozen in 50% glycerol/50% balanced salt solution by the New Zealand National Eye Bank (Auckland, New Zealand). With the exception of 1 piece (refer to Table 1), all pieces of HAM were procured from the same donor. Prior to seeding of cells, each piece of HAM was thawed, washed 3 times for 5 min in Hanks’ balanced salt solution and mounted within a custom-made cell culture chamber (Ludowici chamber)11. Once securely mounted within the chamber, the majority of the nitrocellulose backing paper was carefully peeled away using watchmaker forceps to facilitate visualization of HAM structure and the subsequently established cultures using phase contrast microscopy. Prior to seeding of cells, the upper HAM surface was treated with Versene followed by 0.05% trypsin/1 mM EDTA (5–7 min at 37°C) in an effort to loosen any remaining amniotic epithelial cells. After adding 1 mL of epithelial growth medium, the amniotic epithelial cells were removed by gentle trituration across the membrane surface using a 1 mL pipette. If necessary, the process was repeated until the majority of epithelial cells (approximately greater than 75%) had been removed. HLE cells were seeded onto the upper HAM surface at a density of 105/cm2. RLMSC were applied to the lower membrane surface at a density of 0.5 × 105/cm2. In the case of co-cultures, the RLMSC were seeded 48 h prior to addition of the HLE cells. All cultures were prepared in duplicate and were maintained for 10–12 days in epithelial culture medium prior to use.
Table 1.
Summary of Clinical Data for Wounded and Treated Animals.
| Cohort | Tx | Final Assess. | Histology | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HLE Donor | HAM Donor |
RLMSC Donor |
Rabbit | mg/L CRP |
% Defect |
% CNV |
PAS | K3 | K13 | |
| No Tx | – | – | – | A | 35.8 | 35.6 | 15.5 |
– | + | + |
| – | – | – | B | 24.1 | 32.9 | 24.9 | + | – | + | |
| – | – | – | C | 37.8 | 2.25 | 14.5 | – | + | + | |
| HLE HAM |
HD1 p1 | HD7 | – | D | 20.1 | 26.9 | 37.7 | + | – | + |
| HD2 p2 | HD7 | – | E | 18.6 | 26.7 | 42.8 | + | – | + | |
| HD3 p2 | HD7 | – | F | 48.8 | 38.8 | 35.2 | – | – | + | |
| HLE HAM RLMSC |
HD4 p2 | HD7 | RD1 p3 | G | 38.2 | 0.0 | 26.2 | – | + | + |
| HD5 p2 | HD7 | RD1 p4 | H | 27.1 | 10.25 | 24.4 | – | + | + | |
| HD6 p2 | HD7 | RD1 p4 | I | 10.8 | 26.8 | 37.7 | + | – | + | |
| HAM RLMSC |
– |
HD7 | RD1 p4 | J | 17.6 | 0.0 | 95.9 | + | – | + |
| – |
HD7 | RD1 p4 | K | 13.8 |
3.9 | 89.6 | + | – | + | |
| – |
HD8 | RD1 p4 | L | 36.5 | 8.5 | 69.0 | + | – | + | |
HD = Human donor. RD = rabbit donor. CNV = corneal neovascularization. Remaining abbreviations are explained within the text.
Sourcing, Care and Clinical Assessment of Rabbits
Female New Zealand White rabbits (2.5–3.0 kg) were sourced and cared for as previously described in detail8. Likewise, anesthesia, post-operative care, and clinical assessments (including general photography, slit lamp examination, and quantification of re-epithelialization) were conducted as described previously8. Notably, post-operative pain management consisted of alternating doses of meloxicam (morning; 0.05 mg/kg) and buprenorphine (afternoon; 50 µg/kg) for up to 72 h following surgery. Animals also received topical treatment twice per day with Amacin eye ointment (5 mg/g neomycin sulfate, 5000 IU/g polymixin B sulfate, 2.5 mg/g prednisolone and 50 mg/g sulfacetamide sodium) until healed. The time course of changes in percentage defect for each animal was plotted using Prism 6 (Graph Pad) and analyzed using a two-way ANOVA followed by Tukey’s post-hoc test. Relative differences in the degree of corneal neovascularization were determined on clinical images obtained at 12 weeks. Measurements of corneal area displaying blood vessels (expressed as percentage of total corneal area) were manually traced and calculated using ImageJ. Individual values for each animal were plotted using Prism 6 and analyzed by Kruskal–Wallis test followed by Dunn’s multiple comparisons test.
Monitoring of Serum CRP Levels
Samples of whole blood were obtained from each rabbit immediately prior to wounding (day 0) and on days 1, 3, 7, and 84 (12 weeks) following wounding/treatment. Blood was obtained via 24-gauge cannula (BD Insyte, Cat. No. 381212; North Ryde, NSW, Australia) inserted into a lateral ear vein. A cream containing 25 mg/g lignocaine and 25 mg/g prilocaine (Emla; AstraZeneca, North Ryde, NSW, Australia) was applied topically to lateral ear veins 1 h prior to bleeding to anesthetize the area. During each collection, rabbits were firmly wrapped in a blanket, with eyes shielded, and placed on a warming mat. Between 2 and 3 mL of blood was collected directly into an SST II Advance blood collection tube with lid removed (BD Vacutainer, Cat. No. 367956; North Ryde, NSW, Australia) and allowed to clot for 30 min at room temperature. The resulting serum was retrieved following centrifugation and stored at –80°C until testing. Levels of CRP in each serum sample were subsequently determined using a commercial ELISA kit, according to manufacturer’s instructions (ICL Inc., Cat. No. E-15CRP; Australian Biosearch Pty Ltd., Karrinyup, WA, Australia).
Wounding of Rabbits
An experienced ophthalmic surgeon (FJL) performed all the procedures with the aid of a surgical microscope. Rabbits were prepared for surgery as described previously8 with the addition that each right eye was proptosed prior to surgery by placing a piece of sterile glove, with cross-shaped slit cut within it, across the surface of the eye, and applying light downwards pressure at the periphery with aid of a scalpel blade handle. Epithelial debridement was preceded by a 360° conjunctival peritomy, approximately 1.5 mm beyond the limbus, with dissection toward the limbus. Debridement then commenced initially with 360° superficial limbal keratectomy using an Algerbrush II fitted with 2.5 mm round-ended, diamond-dusted burr (Rumex International/Emagin Pty Ltd., Banksmeadow, NSW, Australia; Cat. No. 16-051-2.5B). The same device was subsequently applied in a circular manner with light pressure across the corneal surface. Fluorescein staining under cobalt illumination was performed in order to ensure that the majority of epithelium had been removed. If regions of poor dye penetration were noted by slit lamp examination, then further debridement was performed.
Application of Cultures to Ocular Surface
After removal from transport medium (DMEM without serum or other supplements), each culture was positioned so that the central area came into contact with the ocular surface, with the epithelial cell side (when present) facing upwards and the stromal cell side facing downwards. The periphery of each culture was then slowly and gradually released from the culture chamber by carefully cutting with iris scissors. Further trimming of the HAM was performed until a peripheral flap of approximately 3–5 mm was overlying the sclera. Eight discontinuous, superficial, and regularly spaced sutures (10.0 Vicryl) were then inserted to secure the HAM to the sclera. The peripheral edge of the HAM including sutures was subsequently covered with a circular conjunctival flap using eight additional sutures. Transport medium was applied drop-wise to the surface of the HAM every 5–10 min in an effort to reduce potential drying of the culture. The rabbit’s nictitating membrane was secured to the lower temporal side eyelid for 1 week using a 4.0 nylon suture and a central tarsorrhaphy performed. Following weekly assessments for 12 weeks, each animal was euthanized by slow intravenous injection with 325 mg/kg of sodium pentobarbital.
General Histology
Prior to retrieving eyes from deceased animals, the orientation of tissue was labeled by applying a marker pen to the superior sclera/conjunctiva. Excised tissue in the form of whole enucleated eyes was typically fixed overnight in neutral buffered formalin followed by transfer to 70% ethanol. The anterior cap from each eye was subsequently removed with the aid of iris scissors and processed into paraffin. Prior to embedding, three cuts were made along the superior–inferior axis resulting in four strips of corneal tissue. The first cut was made directly through the center of each cornea resulting in two hemi-corneas of approximately equal size. Each tissue piece was subsequently cut again resulting in a “longer central” and “shorter peripheral” segment of cornea. During embedding the opposing cut surfaces were placed face-down within the mold. After subsequent facing, each section removed off the block therefore contained four tissue sections: two spanning the entire cornea and limbus from along the central superior–inferior axis, and two similarly orientated sections from the mid-temporal and mid-nasal peripheral cornea. A dozen sections were mounted and examined for each block. Three whole sections acquired from regular spaced intervals were initially examined for general morphology after staining with Ehrlich’s hematoxylin and eosin (H&E) and adjacent sections were stained for goblet cells (GCs) using the periodic acid–Schiff reagent (PAS) method and Mayer’s hematoxylin.
Immunostaining
Immunostaining was subsequently performed using primary antibodies selective for keratin 3 (1:300 dilution; clone AE5; Millipore Pty Ltd, Cat. No. CBL218; Bayswater, VIC, Australia), keratin 13 (1:300 dilution; clone AE8, Abcam Pty Ltd, Cat. No. ab16112; Sapphire Bioscience Pty Ltd., Redfern, NSW, Australia) or human nuclear antigen (HNA; refer below). An immuno-peroxidase method was used for detection of keratins in tissue sections and an immunofluorescence method was used for detection of HNA in either cell cultures (optimization of antibody selection) and tissue sections. The immuno-peroxidase method, (including antigen retrieval protocol) was conducted as described previously8.
Prior to investigating the fate of implanted human cells, a preliminary study was conducted using three commercial antibodies with potential selective specificity for human versus rabbit cells; the anti-mitochondrial antibody 113 -1 (1:100 dilution; Merck Millipore Cat. No. MAB1273), the anti-HNA clone 235 -1 (1:100 dilution; Merck Millipore Cat. No. MAB1281) and the anti-HNA clone 3E1.3 (1:100 dilution; Merck Millipore Cat. No. MAB4383). These antibodies were screened using early passage (p3) cultures of corneal-limbal epithelial cells established from human and rabbit limbal tissue in 24-well culture plates. Each culture was fixed for 10 min in neutral buffered formalin, permeabilized by treatment with 0.3% Triton/PBS (2 × 5 min) and blocked by incubation for 30 min at room temperature in 2% normal goat serum/PBS. Each primary antibody was subsequently applied at a 1:100 dilution in PBS containing 1% NGS and incubated overnight at 4°C. After four washes in PBS, the secondary antibody (Alexa 488-conjugated goat-anti-mouse IgG; ThermoFisher Scientific, Cat. No. A11001) was applied at 1:100 dilution in PBS containing 1% NGS and incubated in the dark for 1 h at room temperature. The same protocol was subsequently used to stain deparaffinized tissue sections with anti-HNA antibody 235 -1. Additional controls consisted of sections obtained from normal and human tissue. Imaging of immunofluorescence was conducted using a Nikon TE-2000 equipped with a CoolSNAP ES cooled CCD camera and NIS Elements (F package).
FISH
FISH was used to investigate the potential contribution of male rabbit stromal cells to the regenerated epithelium observed in female rabbits. The procedure utilized the Dako Histology FISH Accessory kit (Cat. No., K5799; Agilent Technologies Australia Pty Ltd., Mulgrave, VIC, Australia) and a Cy3-labeled rabbit Y chromosome FISH probe purchased from Chromosome Science Labo Inc. (Cat. No., CSL OOY-10; Shiroishi-ku Sapporo, Japan). In brief, paraffinized 3 µm sections of control (male corneas) and test tissue (treated female corneas) were mounted on adhesive-coated slides and baked for 60 min at 70°C. Following removal of paraffin and rehydration in graded alcohols, the sections were immersed in the FISH Accessory kit pre-treatment solution for 10 min heated to 95°C using a water bath. The heated solution containing slides was subsequently removed from the water bath and allowed to cool for 15 min. The slides were subsequently washed twice in the kit wash buffer before application of cold pepsin solution (4°C) and incubation at room temperature for 10 min. Following removal of pepsin solution, by further treatment with wash buffer, the sections were dehydrated through graded alcohols and air dried before application of 10 µL of probe (as supplied by manufacturer without further dilution) and mounting under glass coverslip. After application of Coverslip Sealant, the slides were heated at 85°C for 10 min before being placed overnight at 37°C in a humidified chamber (cell culture incubator). Following removal of coverslips and sealant, the slides were rinsed briefly in stringent wash buffer at room temperature followed by incubation in fresh stringent wash buffer at 65°C for 10 min. After two further washes in the supplied regular wash buffer the slides were dehydrated a final time through graded alcohols before being mounted in 15 µL of fluorescence mounting medium supplied with the kit (containing blue nuclear stain). The mounted slides were stored at 4°C and imaged using a Zeiss Axio Imager.Z2 equipped with Cy3 filter set, 63x/1.4 N.A. objective lens, Zeiss Axiocam 506 mono cooled CCD camera.
Results
Construction and Analysis of Treatment Cultures
Nine pairs of duplicate cultures were established on HAM throughout this study (two cultures prepared for each animal to be treated with one to be used as a spare if required). All HAM samples were acquired from the same human donor with the exception of the last pair of cultures seeded with RLMSC alone (due to insufficient supply). Each pair of duplicate cultures containing HLE (with or without RLMSC) was prepared from a unique human tissue donor and seeded onto HAM at either passage 1 or 2. All cultures containing RLMSC were established using cells from the same donor rabbit and same passage number (p4). In the case of cultures prepared from HLE alone on HAM, one of the duplicate cultures developed a hole during the cultivation period and thus was unavailable for further analysis. For all other sets, however, a duplicate culture was available for confirmation of culture integrity by routine histology. Examination of sections after staining with H&E revealed a disorganized and stratified epithelium of approximately five layers for all HAM samples seeded with HLE (Fig. 1). In contrast, RLMSC cultures were noticeably more stratified when grown in the presence of HLE.
Figure 1.
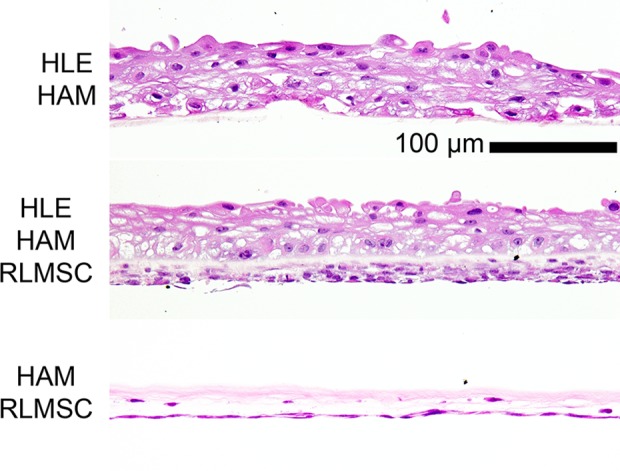
Confirmation of HLE and/or RLMSC presence in prepared cultures. Representative images of histological sections (H&E stained) obtained from spare cultures of human limbal epithelial (HLE) cells and/or rabbit limbal mesenchymal stromal cells (RLMSC) attached to human amniotic membrane (HAM). Notably, the RLMSC culture was more stratified when grown in the presence of HLE.
Baseline Response to Wounding (Epithelial Debridement Without Suturing)
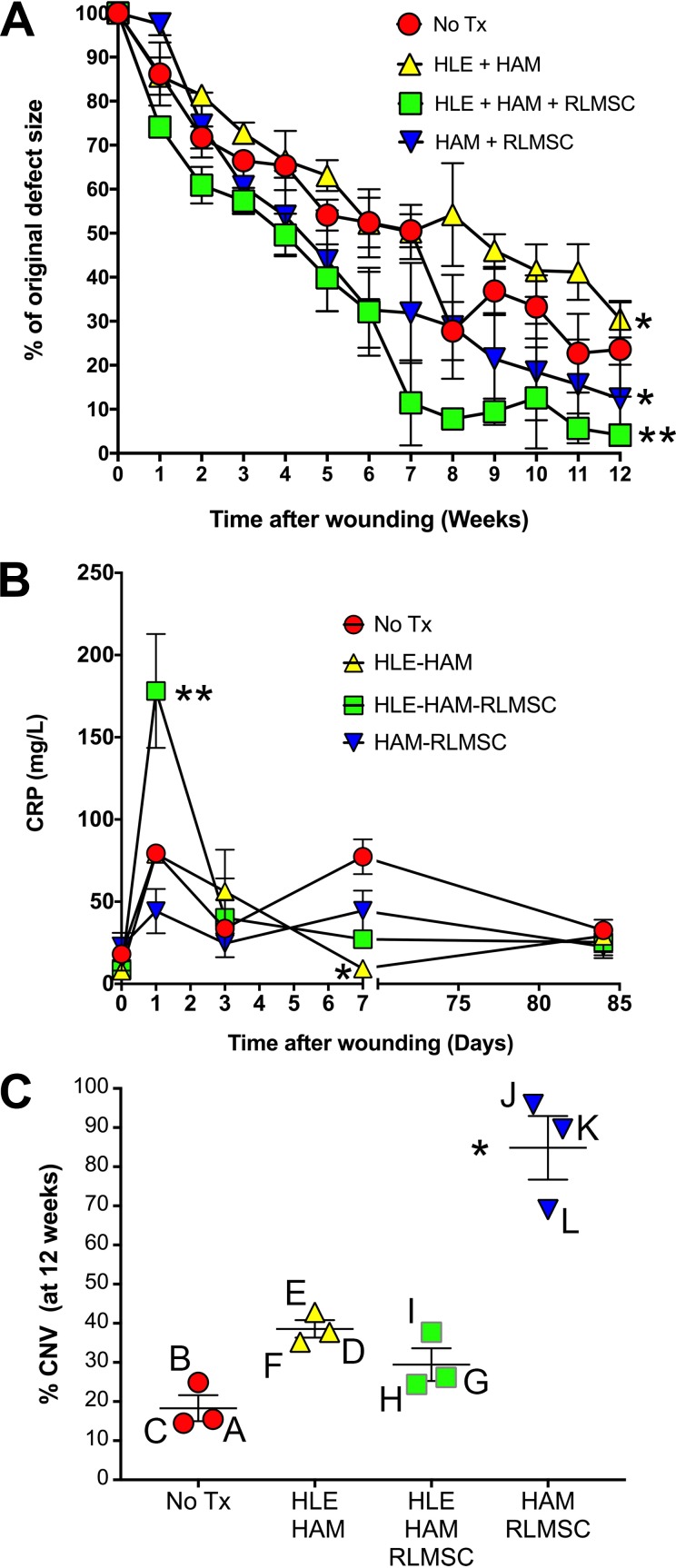
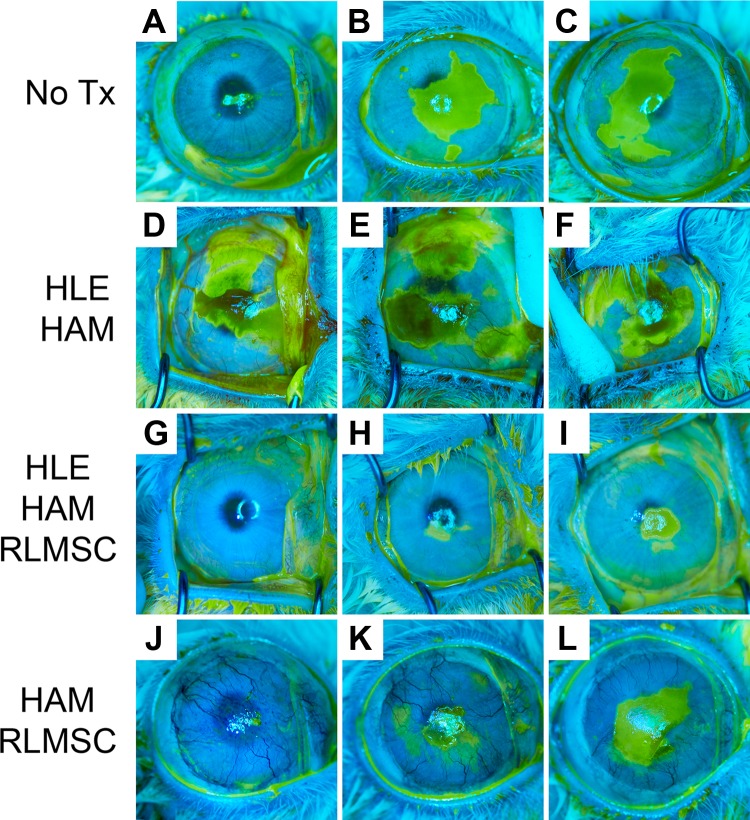
The baseline response to wounding (without subsequent treatment) was examined in a cohort of three rabbits. In the absence of treatment, the conjunctival epithelium remained resected away from the limbal margin. Examination of eyes by fluorescein staining immediately after wounding indicated that the majority of epithelial cells had been removed from the cornea and limbus (Supplementary Figure 1). Gradual re-epithelialization occurred over 12 weeks of observation, but no eyes healed completely over this time period (“No Tx” in Fig. 2A and animals A, B, and C in Fig. 3). Serum CRP levels increased within 24 h of wounding, then declined to baseline levels by 72 h (Fig. 2B). A second increase in CRP levels was observed by 7 days (following cessation of meloxicam treatment on day 3), before declining to baseline levels by 12 weeks (Fig. 2B). Corneal neovascularization was evident within 4 weeks with 3–4 quadrants becoming involved by 12 weeks (Fig. 2C and animals A, B and C in Fig. 4). Corneal opacity was evident at 12 weeks and the ocular surface remained rough. Histology at 12 weeks demonstrated a mixed phenotype of K3 and K13-positive epithelial cells in two animals, with the third displaying evidence of mature conjunctival epithelium (K13 with PAS+ goblet cells; animals A, B and C in Table 1 and Figs. 5 and 6).
Figure 2.
Graphical summary of clinical data. (A) Time course of re-epithelialization as measured under cobalt lamp illumination after fluorescein staining. Analysis of data using a two-way ANOVA (followed by Tukey’s multiple comparisons test) revealed significant differences between each pair of treatment groups (at p < 0.05 or less). Asterisks (* = p < 0.5; ** = p < 0.0001) indicate significant differences between each treatment cohort compared with the non-treated control group (No Tx). (B) Comparison of serum CRP levels. Line graphs indicate the mean +/- SEM of values (mg/L) for each cohort of three rabbits. Single asterisk indicates significant (p < 0.005) difference to animals wounded without treatment (No Tx). Double asterisk indicates a significant difference (p < 0.0001) between animals treated with co-cultures (HLE-HAM-RLMSC) compared with all other cohorts. (C) Comparison of corneal neovascularization observed between animals after 12 weeks. Line and error bars indicate the mean +/- SEM for each treatment cohort. The % corneal neovascularization (CNV) for each animal (A through L) was calculated based upon estimated measures of corneal area with blood vessels using ImageJ. Asterisk indicates a significant difference in CNV for animals receiving HAM with RLMSC cultured on the underlying surface, compared with animals that had been wounded without subsequent treatment.
Figure 3.
Healing patterns (re-epithelialization) of rabbit eyes after 12 weeks, as viewed under cobalt lamp illumination after fluorescein staining. Labels “A” through “L” indicate identity of each rabbit as summarized in Table 1. Treatment groups as described above consisted of controls (No Tx; rabbits A, B, and C), human limbal epithelial cells grown on human amniotic membrane (HLE-HAM; rabbits D, E, and F), HLE and rabbit mesenchymal stromal cells grown on HAM (HLE-HAM-RLMSC; rabbits G, H, and I), or HAM with RLMSC alone (HAM-RLMSC; rabbits J, K, and L).
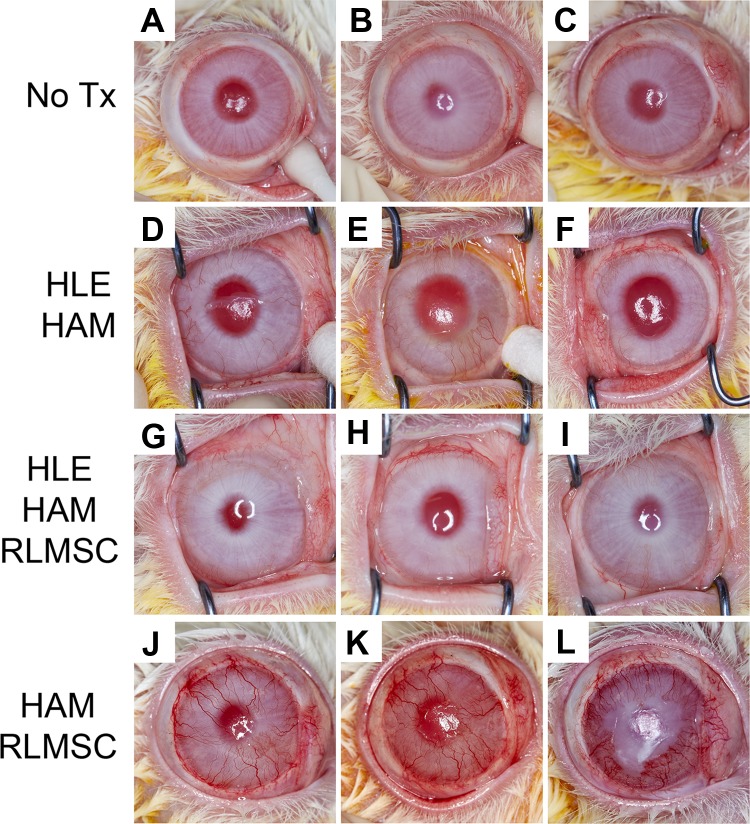
Figure 4.
Gross appearance of rabbit eyes displaying varying degrees of corneal vascularization at 12 weeks. Labels “A” through “L” indicate identity of each rabbit as summarized in Table 1. Treatment groups as described above consisted of controls (No Tx; rabbits A, B, and C), human limbal epithelial cells grown on human amniotic membrane (HLE-HAM; rabbits D, E, and F), HLE and rabbit limbal mesenchymal stromal cells grown on HAM (HLE-HAM-RLMSC; rabbits G, H, and I), or HAM with RLMSC alone (HAM-RLMSC; rabbits J, K, and L).
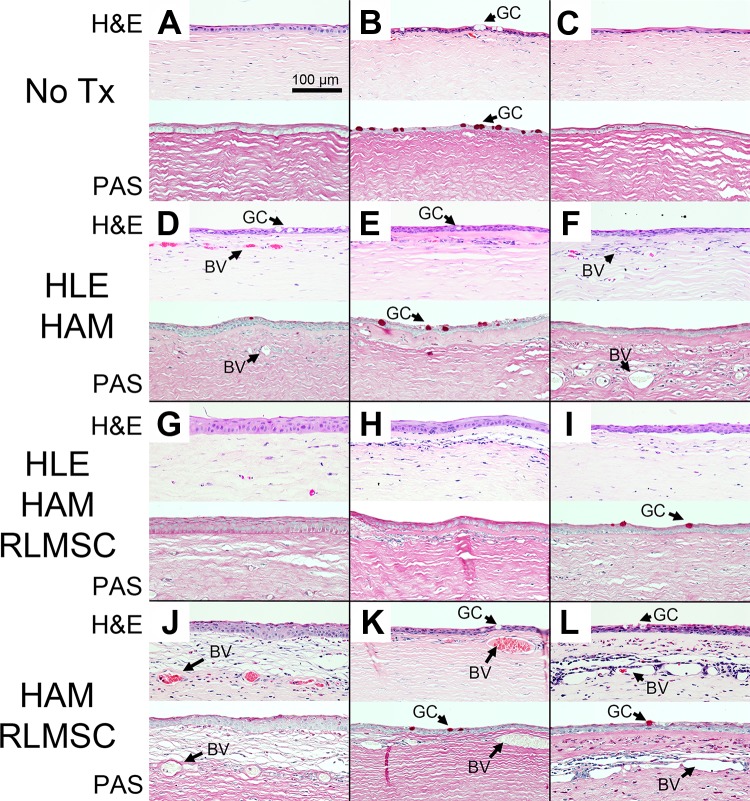
Figure 5.
Basic histology of rabbit corneas at 12 weeks as revealed by staining of sections with hematoxylin and eosin (H&E) and periodic acid–Schiff stain (PAS). Labels “A” through “L” indicate identity of each rabbit as summarized in Table 1. Treatment groups consisted of controls (No Tx), human limbal epithelial cells grown on human amniotic membrane (HLE-HAM), HLE and rabbit mesenchymal stromal cells grown on HAM (HLE-HAM-RLMSC), or HAM with RLMSC alone (HAM-RLMSC). Arrows highlight the location of goblet cells (GC) and blood vessels (BV).
Figure 6.
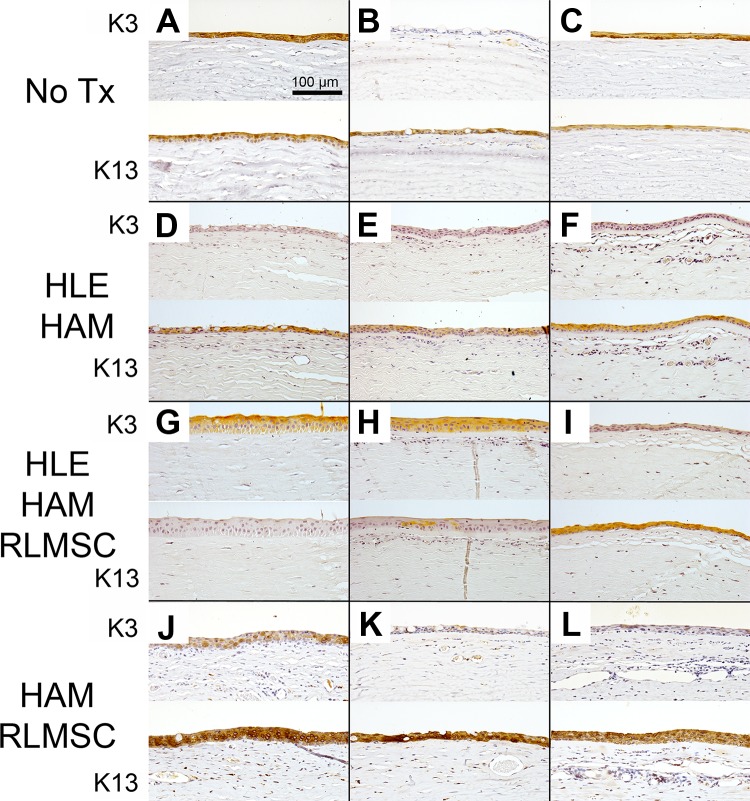
LMSC affect the phenotype of healed epithelium. Immunohistochemical staining of rabbit corneas at 12 weeks to demonstrate typical presence of corneal (K3) and conjunctival (K13) epithelium. Labels “A” through “L” indicate identity of each rabbit as summarized in Table 1. Treatment groups as described above consisted of controls (No Tx), human limbal epithelial cells grown on human amniotic membrane (HLE-HAM), HLE and rabbit mesenchymal stromal cells grown on HAM (HLE-HAM-RLMSC), or HAM with RLMSC alone (HAM-RLMSC). Notably, the best healing outcomes were achieved for two animals receiving RLMSC in the presence of HLE (parts G and H).
Effects of Treatments on Re-epithelialization
All treated cohorts displayed a gradual increase in re-epithelialization over the 12 weeks of observation as monitored by fluorescein staining under cobalt illumination (Figs. 2A and 3). The fastest rates of re-epithelialization, however, were observed in cohorts treated with RLMSC, with the greatest overall healing being observed in animals receiving both HLE and RLMSC on HAM (90% healed by 7 weeks compared with 50% healed at this same time point for the non-treated control; p < 0.0001; assessed by two-way ANOVA followed by Tukey’s multiple comparison test).
Effects of Treatments on Serum CRP Levels
The majority of treated animals (8 out of 9) displayed a similar profile of changes in serum CRP levels to the non-treated cohort, with an initial peak being observed at 24 h after wounding, followed by a decline within 3–7 days (Fig. 2B). Animals that received co-cultures of RLMSC and HLE on HAM, however, displayed a 3–4-fold greater increase in serum CRP levels at 24 h compared with all other cohorts (p < 0.0001 by two-way ANOVA followed by Tukey’s multiple comparisons test). In addition, animals that received HLE alone on HAM displayed 90% lower serum CRP levels after 1 week when compared with non-treated animals (p < 0.005). Similar levels were seen for all cohorts by 12 weeks.
Effects of Treatments on Neovascularization
All animals developed varying degrees of corneal neovascularization over the 12 weeks of observation (Figs. 2C and 4). The greatest level of neovascularization was observed in animals receiving cultures of RLMSC alone on HAM, which was 4-fold higher than for animals wounded without treatment (p < 0.05 by Kruskal–Wallis test followed by Dunn’s multiple comparisons test).
Histological Analyses
Examination of control (non-wounded) tissue demonstrated the expected normal structure for corneal and conjunctival tissue (Supplementary Figure 2). In brief, the cornea displayed a stratified epithelium that was devoid of GCs and stromal blood vessels. Moreover, the corneal epithelium displayed positive immunostaining for K3 and was negative for K13. Conversely, the conjunctival epithelium displayed positive staining for K13, but was negative for K3. The supra-basal layers of the limbal epithelium, however, stained positively for both K3 and K13.
Examination of H&E-stained sections of wounded eyes confirmed the development of corneal vascularization, with the largest and best developed vessels being observed in animals that received treatment with HAM seeded with RLMSC alone (animals J, K, and L in Table 1 and Fig. 5). The presence of GCs (as confirmed by PAS staining) was also most consistently observed in animals treated with RLMSC alone. Strongest immunostaining for K13 (a marker for superior limbal and conjunctival epithelial cells) was also observed in this cohort (animals J, K, and L in Table 1 and Fig. 6). In contrast, the clearest example of immunostaining for K3 (a marker for superior limbal and corneal epithelial cells) was observed in two animals treated with both HLE and RLMSC on HAM (animals G and H in Table 1 and Fig. 6). Conversely, no staining for K3 was observed for animals treated with HLE alone on AM (animals D, E, and F in Table 1 and Fig. 6). The epithelia that had partially regenerated in animals wounded without treatment expressed either both K3 and K13, or K13 alone in the presence of PAS-stained GCs (animals A, B, and C in Table 1 and Figs. 5 and 6).
Given the remarkable staining for K3 in two animals receiving HLE in conjunction with RLMSC (animals G and H), the potential presence of human cells was investigated by immunostaining using an antibody to HNA. Fixed cultures of rabbit and human corneal epithelial cells were initially screened by immunofluorescence to confirm the specificity of this antibody (Supplementary Figures 3 and 4). Control sections of human tissue were also reactive toward this antibody (Fig. 7). No staining for HNA, however, was subsequently detected when sections of wounded/treated tissue were examined by immunohistochemistry (as shown for rabbit G in Fig. 7). Likewise, the rabbit Y chromosome was not detected within the regenerated epithelium when examined by FISH (as shown for rabbit G in Fig. 8). Thus, neither HLE nor male RLMSC were detected within the regenerated corneal epithelium.
Figure 7.
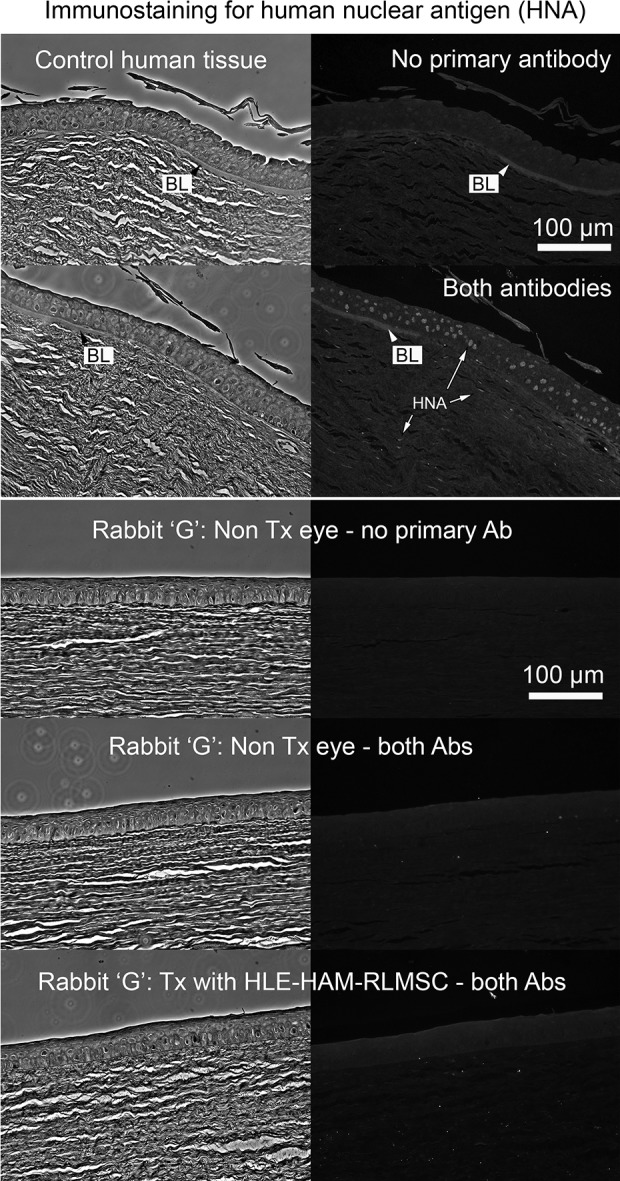
The regenerated epithelium does not contain HLE. Upper panel: Confirmation of immunoreactivity of control human tissue toward antibody to human nuclear antigen (mab 235 -1). The terminal end of Bowman’s layer (BL) is visible by phase contrast images (left) and via background fluorescence in stained sections (right) indicating that the images are acquired at the corneal limbus. Lower panel: Demonstrates example of staining outcomes when sections of non-wounded control (No Tx eye) and treated rabbit tissue (Rabbit “G”) are stained for human nuclear antigen. The absence of staining suggests that no human epithelial cells (HLE) have been retained by 12 weeks. NB: BL is not present in the rabbit cornea.
Figure 8.
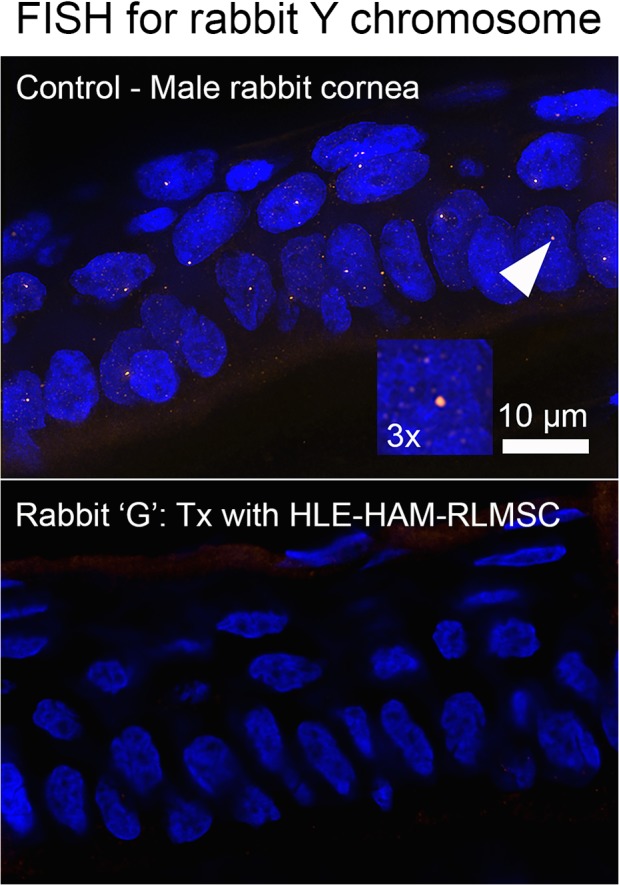
The regenerated epithelium does not contain RLMSC. Results of FISH staining for the rabbit Y chromosome within sections of control male corneal epithelium (A; with arrow denoting positive labeling and magnified 3-fold within insert) compared with negative reactivity with the regenerated epithelium observed in rabbit “G.”
Discussion
While the therapeutic benefits of cultivated limbal epithelial cells in human subjects are well accepted12,13, prior studies of the therapeutic properties of LMSC are limited to a handful of studies in rodents and rabbits (as summarized in Table 2). Significantly, three out of the five studies have examined the effects of LMSC on wounds caused by methods typically used to induce limbal stem cell deficiency (LSCD)14–16. Despite significant variations in wound models and methods of administration, a consistent pattern of improved stromal healing has been observed as indicated by increased corneal transparency and reductions in edema, and/or corneal neovascularization. The effects of LMSC on the ocular surface, however, are less clear with only two studies having examined the effects of LMSC on re-epithelialization14,15 and the resulting epithelial phenotype only having been examined in one case15. Moreover, the effects of LMSC have yet to be examined in conjunction with a cultured limbal epithelial cell transplant, which is surprising given the severity of wounds examined. We therefore sought presently to examine the effects of LMSC when applied to the wounded ocular surface of rabbits. Moreover, we examined the impact of LMSC cultures that had been cultivated and implanted in the presence of HLE cells, compared with those that had been cultivated and implanted in the absence of HLE.
Table 2.
Prior Studies of Corneal Tissue Response to LMSC when Applied in Vivo.
| Study | Species | Wound model |
Treatment | Key Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LMSC Donor | Host | Agent | Area | Age | Formulation | Route | Epi. | Trans. | Edema | Fibrosis | CNV | |
| 2 | Hum. | M | Mechanical Algerbrush II |
Central cornea including basement membrane | <1 h | Suspended in fibrin glue. | Topical | NE | ↑ |
NE | ↓ | ↓ |
| 14 | Rat | Rat | Chemical Alkali burn |
Cornea & limbus | 24 h | Suspended in medium. | - Topical - Conj. inj. - i.p. inj. |
↑ ↑ ↑ Ph: NE |
↑ ↑ ↑ |
NE | NE | ↓ ↓ ↓ |
| 15 | Rab. | Rab. | Chemical Alkali burn |
Cornea & limbus | <1 h | Attached to fibrous scaffold prepared from PLA. | Topical | ↑ Ph: K3+ |
↑ | ↓ | NE | ↓ |
| 16 | Hum. M |
M | Mechanical Algerbrush II |
Cornea & limbus | <1 h | Suspended in fibrin glue. | Topical | NE | NE | NE | NE | ↓ |
| 17 | Hum. | M | Mechanical 27G needle |
Stromal pocket | <1 h | Cultivated sheet. | Stromal implant | NE | 1-wk: ↓ 5-wk: Norm. |
NE | NE | No |
Abbreviations: Species, Hum. = Human, M = mouse, Rab. = rabbit. Treatment, Conj. Inj = sub-conjunctival injection, i.p. = intraperitoneal injection. Key Outcomes, Epi. = epithelialization, Trans = transparency, CNV = corneal neovascularization, NE = not examined, Ph = cell phenotype, K3 = keratin 3, Norm. = normal., ↑ = significant increase, ↓ = significant decrease, No = not present.
In order to provide accurate context, it is first necessary to discuss the nature of wounds created in our study. We have presently wounded the ocular surface of rabbits using a rotating burr tool (Algerbrush II). Our prior analyses of wounds created using this method indicate that this is an efficient way to remove epithelial cells from both the cornea and limbus with minimal damage to the underlying stroma8. While the level of epithelial debridement was checked by fluorescein staining (Supplementary Figure 1), it remains possible that small islands of epithelial cells are retained, such as has been observed for some patients with eye injuries5. Indeed, this could explain the unexpected pattern of re-epithelialization for rabbit A. The epithelial wounds created in this study should therefore be regarded as extensive, but by no means should be considered as a model of total LSCD. The goal of the study was therefore to investigate the impact of cultured LMSC when applied to extensive, freshly created epithelial wounds, rather than chronic wounds with an established LSCD phenotype. Notably, the outcomes observed were found to be highly dependent upon whether or not the RLMSC had been cultivated and implanted in the presence of corneal-limbal epithelial cells.
Overall, the results from our study illustrate that when allogeneic cultures of rabbit LMSC are applied to the ocular surface in the absence of cultivated epithelial cells, the rate of re-epithelialization is significantly improved, but the epithelium originates from the peripheral conjunctival tissue. Moreover, the enhanced conjunctivalization is associated with a significant increase in corneal neovascularization. In contrast, when the LMSC are supplied in the presence of cultivated HLE cells, there is less conjunctivalization of the ocular surface and an associated decrease in corneal neovascularization. The marked improvement in epithelial phenotype observed for two animals (G and H, as judged by K3 expression in the absence of GCs) initially suggested that the rabbit LMSC may have encouraged the implantation and retention of human epithelial cells. This would have been a remarkable result, since it would have encouraged the use of LMSC as tool for facilitating the retention of donor HLE in the treatment of patients with bilateral ocular surface disease. The failure to detect retained human epithelial cells in our study, however, indicates that an alternative mechanism of action is likely to be involved.
In the absence of staining for HNA, we conclude that the regenerated epithelium was derived from rabbit cells; either from remnants of corneal-limbal epithelium or surrounding conjunctiva tissue. The improved outcomes for animals G and H might, therefore, have arisen through two processes. Firstly, factors secreted by the co-cultures may provide a more potent trigger for stimulating remnants of intact corneal-limbal epithelium. Alternatively, pre-cultivation of LMSC in the presence of epithelial cells may have conditioned these cells to enable an enhanced healing response when subsequently applied to the ocular surface. While these two theories are not mutually exclusive, the enhanced stratification of stromal cultures observed in the presence of epithelial cells suggests an effect of HLE on LMSC biology. Trans-differentiation of stromal cells into epithelium is unlikely, however, given the absence of rabbit Y chromosome in regenerated epithelium when examined by FISH.
It is also possible that the LMSC may have in turn altered the biology of the applied HLE. Notably, application of co-cultures was associated with a significantly higher level of serum CRP at 24 h after wounding. While CRP is a rather non-specific marker of acute inflammation associated with tissue damage, its production by the liver is signaled by interleukin-6 (IL-6), which has itself been shown to be upregulated in co-cultures of HLE and limbal fibroblasts compared with HLE cultured under control conditions18. Nevertheless, it is unlikely that the application of cultured HLE to the ocular surface would have been responsible for altering systemic CRP levels. Indeed, it is more likely that the significantly elevated CRP levels observed after 24 h was simply in response to the greater quantity of cellular material applied to the wound.
The present findings agree with those of Acar et al.14 and Holan et al.15 in so far as both prior studies reported increased re-epithelialization of acute wounds when treated with LMSC. Nevertheless, the majority of previous studies have demonstrated a decrease in corneal neovascularization when LMSC are applied to the ocular surface (Table 2). Differences in methodology including wounding method, treatment method, and animal model may well account for this. In particular, the use of HAM as a carrier in conjunction with HLE is novel to the present study. With respect to the fate of applied epithelial cells, our inability to detect HLE (by staining for HNA) 12 weeks after application to the injured rabbit ocular surface is consistent with findings from clinical studies when the fate of allogeneic cultures of HLE has been investigated19. It could therefore be argued that healing of the ocular surface is ultimately dependent upon the so-called hidden epithelial cells retained following injury5, rather than the long-term engraftment of cultivated epithelial cells. Nevertheless, as highlighted in a recent review20, this remains a controversial issue since some clinical data are consistent with donor cells being retained for several years before being rejected. More research is therefore clearly required into the factors that determine cell fate following transplantation to the ocular surface.
The use of conventional histological methods to study the resulting epithelial phenotype of healed corneas was both a strength and limitation of our study. The advantage of this strategy is that it supported the use of multiple staining methods (H&E, PAS, and immunohistochemistry for cytokeratins 3 and 13) as opposed to probing a whole flat-mounted cornea for a single marker. The disadvantage of this approach, however, is that we were unable to accurately measure the relative presence of corneal and conjunctival epithelial cells within the healed tissues. Ideally, a transgenic mouse model containing endogenous fluorescent labeling for corneal epithelial cells (e.g., red K12) and conjunctival epithelial cells (e.g., green K13) should be used and would have the additional advantage of enabling longitudinal monitoring of healing patterns. Nevertheless, the co-expression of K13 with K3, both within the normal corneal limbus and subsequently on healing corneas, encourages the search for additional markers. Interestingly, an apparent translocation of limbal phenotype onto the corneal surface has also been observed in murine models based upon shifts in K14 expression21. In any case, based upon the results of the present study, we conclude that the presence of PAS-reactive GCs is a more definitive marker for mature conjunctival epithelium and especially since positive staining was never observed in the tissue that expressed K3.
Conclusions
In conclusion, these results provide further evidence of a potential clinical application for LMSC. In particular, the greatest benefits were observed when the stromal cells were applied in conjunction with a culture of human corneal-limbal epithelial cells. Nevertheless, since no human epithelial cells could be detected following treatment, it appears that the effects of LMSC might be mediated in part by pre-conditioning of the stromal cells in culture by the epithelial cells prior to their application to the ocular surface. Nevertheless, the regenerated epithelium in all cases appears to be primarily derived from endogenous cells (corneal and/or conjunctival). These findings encourage deeper consideration of the potential role for stromal cells as contaminants of HLE cultures when applied to the ocular surface. Moreover, we propose that donor LMSC when applied in conjunction with donor HLE could provide an effective tool for the management of bilateral ocular surface disease in cases where islands of healthy corneal limbus can be detected by in vivo scanning confocal microscopy.
Supplemental Material
Supplemental Material, Supplementary_Figures for The Impact of Limbal Mesenchymal Stromal Cells on Healing of Acute Ocular Surface Wounds Is Improved by Pre-cultivation and Implantation in the Presence of Limbal Epithelial Cells by Elham Nili, Fiona J. Li, Rebecca A. Dawson, Cora Lau, Blair McEwan, Nigel L. Barnett, Steven Weier, Jennifer Walshe, Neil A. Richardson and Damien G. Harkin in Cell Transplantation
Acknowledgements
The idea for this project originated during discussions between the senior author (DGH) and former members of the research team (Dr Zeke Barnard, Dr Louise Ainscough and Professor Ivan Schwab). The authors also wish to acknowledge the contributions made by Professor Lawrie Hirst, Professor Traian Chirila, Professor Kerry Atkinson and Professor Dietmar Hutmacher during submission of grant applications to fund this work. Helpful discussions were also had with Dr Allison Sutherland and Dr Brendan Cronin during the planning phase of this project. Guidance on selection of an antibody to human nuclear antigen was provided by Dr Louise Morgan and Professor Julie Daniels (University College London). Assistance with imaging of FISH data was provided by Dr Christina Theodoropoulos (Queensland University of Technology). Samples of human amniotic membrane were generously donated by the New Zealand Eye Bank (Auckland, NZ). We also acknowledge the support of staff from the Queensland Eye Bank and Queensland Eye Hospital for facilitating access to cadaveric human eye tissue.
Authors' Note: Nigel L. Barnett is now with the Clem Jones Centre for Regenerative Medicine, Faculty of Health Sciences and Medicine, Bond University, Gold Coast, Queensland, Australia.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions: EN and FJL contributed equally to this study. EN contributed to the planning and/or execution of all experiments and made a leading contribution to drafting the manuscript. FJL contributed to the planning and execution of animal studies and was responsible for performing all surgical procedures and clinical assessments. RAD contributed to the planning of all experiments and was responsible for coordinating the supply of cultured cells. CL and BM contributed to the planning and execution of animal studies. NB optimized parameters for photography and was responsible for processing and archiving of clinical images. SW conducted measurements of C-reactive protein levels in rabbit serum. JW assisted with live animal studies and optimized protocols for detection of rabbit Y chromosome in tissue sections via FISH. NAR contributed to studies involving histology and conducted FISH studies. DGH contributed to the planning and execution of all experiments and was responsible for coordinating the drafting and submission of the final manuscript. All authors contributed to the editing and approval of the final manuscript.
Ethical Approval: The project was conducted with the approval of the University Animal Ethics Committee at the Queensland University of Technology (UAEC approval number 1200000575). Approval to work with human tissue samples was received from the Human Research Ethics Committee (HREC) of Metro South Hospital and Health Service (HREC approval number: HREC/07/QPAH/048) and the Queensland University of Technology (HREC approval number: 0800000807).
Statement of Human and Animal Rights: All studies using human tissue samples were conducted according to the National Statement on Ethical Conduct in Human Research (Australian Government, 2007). All procedures involving rabbits were conducted in accordance with the “Animal Care and Protection Act” (Queensland State Government, Australia, 2001), “Australian Code for the Care and Use of Animals for Scientific Purposes” (8th Edition, 2013), and the “ARVO Statement for Use of Animals in Ophthalmic and Vision Research.”
Statement of Informed Consent: Studies involving the use of human corneal tissue acquired from cadaveric donors were conducted with donor/next-of-kin consent.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The project was primarily funded by a Project Grant (Grant No. 1049050) received from the National Health & Medical Research Council (NH&MRC) of Australia. EN was supported by an Australian Postgraduate Research Award administered by the Queensland University of Technology. Supplementary funds were provided by the Queensland Eye Institute Foundation.
ORCID iD: Damien G. Harkin  https://orcid.org/0000-0002-7358-7987
https://orcid.org/0000-0002-7358-7987
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Holland EJ. Management of limbal stem cell deficiency: a historical perspective, past, present, and future. Cornea. 2015;34(Suppl 10):S9–S15. [DOI] [PubMed] [Google Scholar]
- 2. Basu S, Hertsenberg AJ, Funderburgh ML, Burrow MK, Mann MM, Du Y, Lathrop KL, Syed-Picard FN, Adams SM, Birk DE, Funderburgh JL. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci Transl Med. 2014;6(266):266ra172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ainscough SL, Linn ML, Barnard Z, Schwab IR, Harkin DG. Effects of fibroblast origin and phenotype on the proliferative potential of limbal epithelial progenitor cells. Exp Eye Res. 2011;92(1):10–19. [DOI] [PubMed] [Google Scholar]
- 4. O’Callaghan AR, Morgan L, Daniels JT, Lewis MP. Human-derived feeder fibroblasts for the culture of epithelial cells for clinical use. Regen Med. 2016;11(6):529–543. [DOI] [PubMed] [Google Scholar]
- 5. Chan E, Le Q, Codriansky A, Hong J, Xu J, Deng SX. Existence of normal limbal epithelium in eyes with clinical signs of total limbal stem cell deficiency. Cornea. 2016;35(11):1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bray LJ, Heazlewood CF, Munster DJ, Hutmacher DW, Atkinson K, Harkin DG. Immunosuppressive properties of mesenchymal stromal cell cultures derived from the limbus of human and rabbit corneas. Cytotherapy. 2014;16(1):64–73. [DOI] [PubMed] [Google Scholar]
- 7. Garfias Y, Nieves-Hernandez J, Garcia-Mejia M, Estrada-Reyes C, Jimenez-Martinez MC. Stem cells isolated from the human stromal limbus possess immunosuppressant properties. Mol Vis. 2012;18:2087–2095. [PMC free article] [PubMed] [Google Scholar]
- 8. Li FJ, Nili E, Lau C, Richardson NA, Walshe J, Barnett NL, Cronin BG, Hirst LW, Schwab IR, Chirila TV, Harkin DG. Evaluation of the AlgerBrush II rotating burr as a tool for inducing ocular surface failure in the New Zealand White rabbit. Exp Eye Res. 2016;147:1–11. [DOI] [PubMed] [Google Scholar]
- 9. Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64 K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103(1):49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramirez-Miranda A, Nakatsu MN, Zarei-Ghanavati S, Nguyen CV, Deng SX. Keratin 13 is a more specific marker of conjunctival epithelium than keratin 19. Mol Vis. 2011;17:1652–1661. [PMC free article] [PubMed] [Google Scholar]
- 11. Harkin DG, Dunphy SE, Shadforth AMA, Dawson RA, Walshe J, Zakaria N. Mounting of biomaterials for use in ophthalmic cell therapies. Cell Transplant. 2017;26(11):1717–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernandez-Buenaga R, Aiello F, Zaher SS, Grixti A, Ahmad S. Twenty years of limbal epithelial therapy: an update on managing limbal stem cell deficiency. BMJ Open Ophthalmol. 2018;3(1):e000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rama P, Ferrari G, Pellegrini G. Cultivated limbal epithelial transplantation. Curr Opin Ophthalmol. 2017;28(4):387–389. [DOI] [PubMed] [Google Scholar]
- 14. Acar U, Pinarli FA, Acar DE, Beyazyildiz E, Sobaci G, Ozgermen BB, Sonmez AA, Delibasi T. Effect of allogeneic limbal mesenchymal stem cell therapy in corneal healing: role of administration route. Ophthalmic Res. 2015;53(2):82–89. [DOI] [PubMed] [Google Scholar]
- 15. Holan V, Trosan P, Cejka C, Javorkova E, Zajicova A, Hermankova B, Chudickova M, Cejkova J. A comparative study of the therapeutic potential of mesenchymal stem cells and limbal epithelial stem cells for ocular surface reconstruction. Stem Cells Transl Med. 2015;4(9):1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eslani M, Putra I, Shen X, Hamouie J, Afsharkhamseh N, Besharat S, Rosenblatt MI, Dana R, Hematti P, Djalilian AR. Corneal mesenchymal stromal cells are directly antiangiogenic via PEDF and sFLT-1. Invest Ophthalmol Vis Sci. 2017;58(12):5507–5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Syed-Picard FN, Du Y, Hertsenberg AJ, Palchesko R, Funderburgh ML, Feinberg AW, Funderburgh JL. Scaffold-free tissue engineering of functional corneal stromal tissue. J Tissue Eng Regen Med. 2018;12(1):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Notara M, Shortt AJ, Galatowicz G, Calder V, Daniels JT. IL6 and the human limbal stem cell niche: a mediator of epithelial-stromal interaction. Stem Cell Res. 2010;5(3):188–200. [DOI] [PubMed] [Google Scholar]
- 19. Chen P, Zhou Q, Wang J, Zhao X, Duan H, Wang Y, Liu T, Xie L. Characterization of the corneal surface in limbal stem cell deficiency and after transplantation of cultured allogeneic limbal epithelial cells. Graefes Arch Clin Exp Ophthalmol. 2016;254(9):1765–1777. [DOI] [PubMed] [Google Scholar]
- 20. Saghizadeh M, Kramerov AA, Svendsen CN, Ljubimov AV. Concise review: stem cells for corneal wound healing. Stem Cells. 2017;35(10):2105–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pajoohesh-Ganji A, Pal-Ghosh S, Tadvalkar G, Stepp MA. K14 + compound niches are present on the mouse cornea early after birth and expand after debridement wounds. Dev Dyn. 2016;245(2):132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Supplementary_Figures for The Impact of Limbal Mesenchymal Stromal Cells on Healing of Acute Ocular Surface Wounds Is Improved by Pre-cultivation and Implantation in the Presence of Limbal Epithelial Cells by Elham Nili, Fiona J. Li, Rebecca A. Dawson, Cora Lau, Blair McEwan, Nigel L. Barnett, Steven Weier, Jennifer Walshe, Neil A. Richardson and Damien G. Harkin in Cell Transplantation