Abstract
Mesenchymal stromal/stem cells (MSCs) belong to the endogenous cellular reparative system, and can be used exogenously in cell-based therapy. MSCs release extracellular vesicles (EVs), including exosomes and microvesicles, which mediate some of their therapeutic activity through intercellular communication. We have previously demonstrated that metabolic syndrome (MetS) modifies the cargo packed within swine EV, but whether it influences their phenotypical characteristics remains unclear. This study tested the hypothesis that MetS shifts the size distribution of MSC-derived EVs. Adipose tissue-derived MSC-EV subpopulations from Lean (n = 6) and MetS (n = 6) pigs were characterized for number and size using nanoparticle-tracking analysis, flow cytometry, and transmission electron microscopy. Expression of exosomal genes was determined using next-generation RNA-sequencing (RNA-seq). The number of EV released from Lean and MetS pig MSCs was similar, yet MetS-MSCs yielded a higher proportion of small-size EVs (202.4 ± 17.7 nm vs. 280.3 ± 15.1 nm), consistent with exosomes. RNA-seq showed that their EVs were enriched with exosomal markers. Lysosomal activity remained unaltered in MetS-MSCs. Therefore, MetS alters the size distribution of MSC-derived EVs in favor of exosome release. These observations may reflect MSC injury and membrane recycling in MetS or increased expulsion of waste products, and may have important implications for development of adequate cell-based treatments.
Keywords: Metabolic syndrome, mesenchymal stem cells, extracellular vesicles, exosomes
Introduction
Metabolic syndrome (MetS) is characterized as a cluster of cardiometabolic risk factors that include abdominal obesity, insulin resistance, hypertension, hyperglycemia, and dyslipidemia. It affects more than one-third of American adults, and is associated with increased morbidity1. Abdominal obesity is considered the predominant instigator of MetS2. Amid the growing prevalence of obesity, the manifestation of MetS remains a health concern. The microenvironment of MetS is fraught with hyperinsulinemia, endoplasmic reticulum stress, inflammation, free radical generation, and biochemical changes related to cellular senescence3,4. Consequently, MetS accelerates the risk of type-2 diabetes, chronic kidney disease, and atherosclerotic cardiovascular diseases, ultimately leading to organ injury and dysfunction5.
Mesenchymal stromal/stem cells (MSCs) constitute an endogenous cellular repair system and are being clinically explored as a novel therapeutic strategy for a variety of diseases. MSCs exert their therapeutic properties by migrating to sites of injury, where they might temporarily engraft into damaged tissues, and at times differentiate into mature cell lineages6,7. However, more frequently, MSCs actively surveil the requirements of the microenvironment, and release soluble mediators and extracellular vesicles (EVs) that mediate their intercellular communication8. Besides apoptotic bodies, the two main types of EVs released by cells include exosomes and microvesicles9. Microvesicles (100–1000 nm) are formed by cellular membrane budding, and contain cellular cytoplasm10. Exosomes (50–130 nm) are derived by fusion of multi-vesicular bodies with the plasma membrane. In addition, multi-vesicular bodies can fuse with the lysosomes where their molecular content is degraded, and may partly serve to rid the cell of waste products11. The endosomal sorting complexes required for transport (ESCRT) is considered to be the main machinery that regulates exosome production, trafficking, anchorage at plasma membrane, and release from the cell12. Endocytic trafficking proteins such as Rho and Rab GTPases work collaboratively with the ESCRT to control vesicular trafficking13. Coordination of exosome biogenesis can also be controlled, independent of ESCRT, by Rab proteins solely or lipid rafts14.
Both exosomes and microvesicles serve as paracrine vectors that package a broad range of proteins, lipids, and nucleic acids (messenger-RNA, miRNA, and DNA) characteristic of their parent cells, and deliver them to distant or neighboring damaged cells, therein triggering a pro-regenerative program15–18. Through their paracrine actions, EVs participate in diverse physiologic processes, including immune signaling19,20, angiogenesis16,21, as well as cellular maintenance/homeostasis22. Conversely, depending on the parent cell and the microenvironment, studies have highlighted a potential role of exosomes as mediators of disease progression, such as cancer, diabetes, cardiovascular disease, neurodegenerative pathologies, obesity, and autoimmune disorders23. Indeed, we have shown that MetS modulates MSC-derived EV packaging19, and cargo related to inflammation and insulin resistance genes and proteins24. However, the sub-populations of EVs released by MetS remain uncharacterized.
In the present study, we compared the phenotypes of MSC-derived daughter EVs harvested from Lean and MetS pigs. We tested the hypothesis that MetS differentially affects the production of exosomes compared with larger microvesicles in MSCs.
Materials and Methods
Induction of Experimental MetS
A total of 12 juvenile female domestic pigs were randomly placed on either a standard pig chow (Lean diet; 13% protein, 2% fat, 6% fiber, Purina Animal Nutrition LLC, Arden Hills, MN, USA) or MetS diet (5B4 L, protein 16.1%, ether extract fat 43.0% and carbohydrates 40.8%, Purina Test Diet, Richmond, IN, USA)25. All animals received fresh water ad libitum. After 16 weeks of diet, the pigs were euthanized by a terminal intravenous injection of sodium pentobarbital (100 mg/kg IV, Fatal Plus, Vortech Pharmaceuticals, Dearborn, MI, USA), at which time 5–10 g of subcutaneous abdominal adipose tissue was excised in order to isolate and expand MSC in culture. The Mayo Clinic Animal Care and Use Committee approved this study.
MSC Culture, Characterization, and EV Isolation
MSCs were isolated and expanded from adipose tissue, as previously described26,27. Following tissue harvest, fat was immediately processed under sterile conditions by mincing and digesting in collagenase-H at 37°C for 45 min. Serum-containing medium was added to the enzymatically digested suspension to stop the reaction. The suspension was filtered through a 100-µm cell strainer to remove remaining tissue pieces, and then centrifuged to pellet cells. Cells were resuspended in advanced minimum essential medium supplemented with 5% platelet lysate (PLTmax, Mill Creek Life Sciences, Rochester, MN, USA), and expanded in culture for three passages. MSCs were phenotyped by flow cytometry to confirm the expression of MSC-specific markers (CD90+ and CD105+; CD45– and CD34–), and RhoF (Abcam, Cat. #ab101349, Cambridge, MA, USA) protein expression evaluated using Western blot to assess vesicle trafficking activity in MSCs. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as a loading control.
MSCs were then serum-starved for 48 hr in order to stimulate release of EVs in conditioned medium. MSC supernatants were centrifuged at 2000 g for 20 min and subsequently at 37,000 rpm for 1 hr at 4°C using an ultracentrifuge to isolate EVs. EVs were collected and suspended in buffer containing 2 M sucrose and 500 mM 2-(N-morpholino)ethanesulfonic acid, 4-morpholineethanesulfonic acid (MES) at pH 6.0, and stored at –80°C until further analyses.
EV Characterization
Ultracentrifugation-based EV isolation commonly results in a heterogeneous EV preparation, containing both small and large vesicles that have co-pelleted. Nanoparticle tracking analysis (NTA) permits distinguishing the subpopulations of EVs by size, in which the most frequent size is represented by a peak17. Therefore, EV samples were diluted in filtered PBS and continuously run through a flow-cell top-plate at 50 µL/min on the NanoSight NS300 (Malvern Panalytical, Westborough, MA, USA). Ten videos (30 sec each) of Brownian motion of nanoparticles were recorded and analyzed by NTA software 3.0.
To inspect their morphology, MSC-derived EVs were also negatively stained with 2% uranyl acetate and observed under a transmission electron microscope. Images were acquired on the JEOL 1400+ transmission electron microscope (JEOL, Peabody, MA, USA)17. The diameters of 20 EVs were measured using image processing software ImageJ, and averaged.
For an additional measure of size, EVs were labeled with Tag-it Violet™ tracking dye, visualized by the fluorescent signal, and acquired by imaging flow cytometry (FlowSight, Amnis, Seattle, WA, USA); data were analyzed using Amnis® Image Data Exploration and Analysis Software (IDEAS version 6.2)28.
MSC and EV RNA-Sequencing and Bioinformatics Analysis
Next generation RNA-sequencing (RNA-seq) of MSCs and EVs was performed as described24 using a standardized protocol. Isolated RNA from Lean and MetS pigs adipose-tissue-derived MSC and their daughter EV were sequenced on a HiSeq 2000 (Illumina, San Diego, CA, USA). Generated datasets were collected using the Tru-Seq SBS kit version 3 and HCS v2.0.12 (Illumina), and analyzed on the MAPRSeq v.1.2.1 system and Mayo Clinic’s Bioinformatics Core standard tool, which includes TopHat 2.0.6 and feature counts used for alignment and gene counts, respectively. Expression values for each gene were normalized to 1 million reads, and corrected for gene length (reads per kilobase pair per million mapped reads, RPKM).
The molecular cargo in Lean and MetS EVs was assessed using ExoCarta (http://www.exocarta.org/), an exosomal database of proteins, RNA and lipids. The GeneCards® database (http://www.genecards.org/) was used to screen genes associated with exosome biogenesis from the parent MSCs. Enriched genes in MetS-EVs and MSCs were classified as a fold-change ≥1.4 and p values ≤0.05. Morpheus (https://software.broadinstitute.org/morpheus/) was used to determine the differential expression between Lean- and MetS-EVs and MSCs.
Lysosomal Activity
Because increased lysosomal activity may intensify exosome production in parent cells, especially under conditions of oxidative and metabolic stresses11,29, we probed the MSCs for lysosomal activity. An intracellular lysosomal cell-based activity assay (BioVision, Milpitas, CA, USA) was performed, following the manufacturer’s instructions. MSCs (1 x 106) were exposed to a self-quenched substrate diluted in culture medium supplemented with 0.05% FBS. After incubation for 1 h, cells were harvested and run on the imaging flow cytometer (488 nm excitation laser).
Statistical Analysis
Data are represented as mean ± standard error and analyzed by using JMP 14.1 Software. The normality assumption was tested using the Shapiro-Wilk Test. Differences between groups were tested using a one-sided two-sample t-test with a 5% type I error rate.
Results
MSC-Derived EV Size Distribution
In our established model, following 16 weeks of high-fat diet feeding, MetS pigs developed hypertension, dyslipidemia, insulin resistance, and a significant increase in body weight, in comparison to Lean pigs (Table 1).
Table 1.
Systematic Characteristics in Experimental Groups (n = 6 pigs, each) at 16 weeks.
| Parameter | Lean | MetS |
|---|---|---|
| Body Weight (kg) | 71.1 ± 13.0 | 91.1 ± 2.5* |
| Mean blood pressure (mmHg) | 96.4 ± 12.7 | 127.2 ± 8.5* |
| Total cholesterol (mg/dl) | 81.1 ± 6.9 | 438.0 ± 81.9* |
| HDL cholesterol (mg/dl) | 46.4 ± 4.3 | 134.5 ± 27.5* |
| LDL cholesterol (mg/dl) | 32.8 ± 6.0 | 371.7 ± 143.0* |
| Triglycerides (mg/dl) | 8.0 ± 1.2 | 19.8 ± 5.8* |
| Fasting glucose (mg/dl) | 127.3 ± 13.7 | 116.5 ± 17.9 |
| Fasting insulin (µU/ml) | 0.4 ± 0.1 | 0.7 ± 0.1* |
| HOMA-IR score | 0.7 ± 0.1 | 1.8 ± 0.4* |
*p ≤ 0.05 (vs. Lean); HDL, High-density lipoprotein; LDL, Low-density lipoprotein; HOMA-IR, Homeostasis model assessment of insulin resistance.
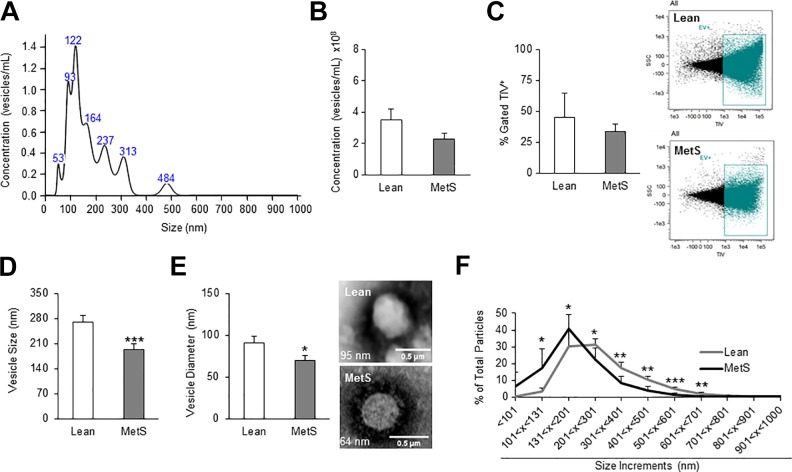
Fig. 1A illustrates representative peaks corresponding to exosomes and microvesicles obtained from NTA. NTA showed that overall MetS-MSC EV yield was not different compared with Lean-MSCs (2.9 x 108 ± 0.4 vs. 3.5 x 108 ± 0.7 vesicles/mL; p = 0.09) (Fig. 1B). Similarly, flow cytometry showed no significant difference (34% ± 8.2 vs. 45% ± 2.4, respectively; p = 0.12) in the total concentration of EV produced by Lean or MetS-MSCs (Fig. 1C). However, NTA and transmission electron microscopy both revealed that MetS-EVs were on average smaller in size (Fig. 1D) (202.4 ± 17.7 vs. 280.3 ± 15.1 nm; p = 0.005) and diameter (Fig. 1E) (69.7 ± 5.8 vs. 90.7 ± 8.1 nm; p = 0.02), respectively, as compared with Lean-EVs. EV size distribution analyses confirmed that MetS-MSCs released overall smaller vesicles, with a significantly higher percentage of EVs measuring under 130 nm, compared with EVs of Lean pigs (Fig. 1F).
Fig. 1.
Adipose-tissue-derived MSCs from MetS pigs release smaller vesicles. (A) Representative size distribution curve by NTA. (B)–(C). Overall concentration of secreted vesicles in Lean and MetS pigs using NTA and flow cytometry, with EV stained with Tag-it-Violet™, tracking dye. (D)–(F). Average vesicle size (NTA), electron microscopy images showing diameter, and percentage of vesicles separated into size increments to show size distribution in Lean- and MetS-EV. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.005 (vs. Lean).
Characterization of MSC-Derived EVs
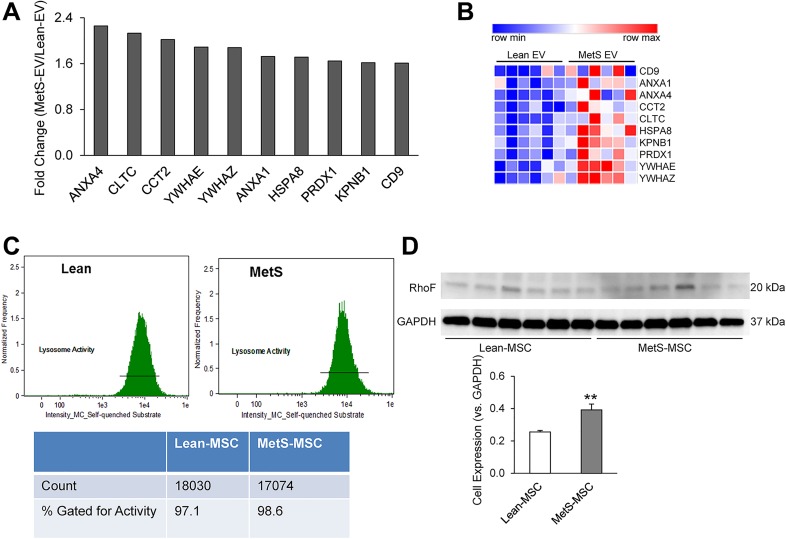
Analysis of the RNA cargo of Lean- and MetS-EVs revealed several differentially expressed exosomal genes (fold change ≥ 1.4, p ≤ 0.05). Of these, 10 commonly associated exosome genes were enriched in MetS-EVs compared with Lean-EVs (Figs. 2A and 2B), suggesting that exosomes are relatively increased in abundance in MetS-EVs.
Fig. 2.
Exosome genes are upregulated in MetS-EVs. (A) Statistically significant exosome genes with a fold-change ≥1.4, compared with Lean EV. (B) Heat map depicting the differentially expressed exosome genes in Lean- vs. MetS-EVs. (C) Lysosomal activity assay performed using imaging flow cytometer, showing the intensity of intracellular uptake of the self-quenched substrate and percentage of positively stained cells. (D) Western blot analysis showed increased protein expression of RhoF in MetS-MSC. **p ≤ 0.01, (vs. Lean); BF, Brightfield; SQS, Self-quenched substrate; SSC, side scatter.
We therefore evaluated potential mechanisms of exosome generation in the parent MSCs. There was no significant difference in lysosomal activity between Lean and MetS parent MSCs, as shown by the percentage of cells that take up the substrate (Fig. 2C). We then investigated MSC expression of RhoF – a small GTPase that modulates trafficking in the cell. As displayed in Fig. 2D, MetS-MSCs exhibited increased protein expression of RhoF.
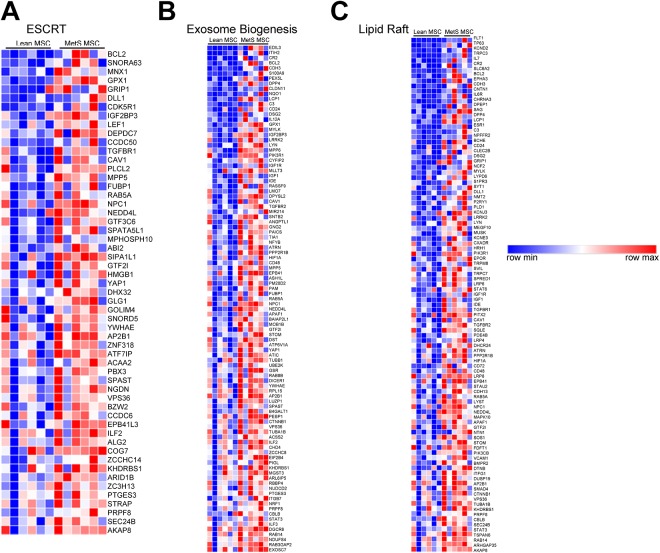
Furthermore, RNA-seq analysis of the parent MSCs showed in MetS enrichment of genes associated with ESCRT, such as VSP36 – a component necessary for multi-vesicular body formation (Fig. 3A). Additionally, MetS-MSCs showed upregulated expression of 99 and 101 genes involved in the exosome biogenesis and the lipid raft pathways, respectively (Fig. 3B and 3C). These included several Rab family members (e.g., RAB5A, RAB14, RAB5, and RAB3GAP) that were enriched in MetS-MSCs, as was NPC1, a mediator of intracellular lipoprotein trafficking, as well as TSPAN6, a gene involved in exosomal release.
Fig. 3.
Pathways associated with exosome production are enriched in MetS-MSCs. (A)–(C) RNA-seq has shown that genes involved in ESCRT machinery, exosome biogenesis, and the lipid raft are enriched in MetS-MSCs, consistent with increased exosome release activity in these cells.
Discussion
This study reveals distinct differences among EV populations released by Lean and MetS pig MSCs. We employed quantitative analyses to characterize the phenotypic differences in Lean- and MetS-EVs released from adipose-tissue-derived MSCs, and assessed gene expression and the activity of the lysosome intracellularly in the parent MSCs. EVs released from Lean pig MSCs were larger in size and relatively depleted of exosomal genes. In contrast, MetS pig MSCs released smaller vesicles that were packed with genes commonly associated with exosomes. The exosomal nature of MetS-EVs was supported by evident upregulation of genes involved in the exosome production and release machinery in the parent MSCs. Overall, these observations demonstrate that MetS alters the size distribution of MSC-derived EVs, and might provide insight into the modifications of MSC-EVs in MetS and the associated mechanisms leading to EV release.
MSCs release EVs, which acts to mediate their communication with other cells, directly delivering a unique signature of biological molecules from the parent (MSC) to the target cell17,18. Through their paracrine effects, EVs also contribute to the repair process15–18. We previously demonstrated that intrarenal delivery of MSC-derived EVs attenuates inflammation and fibrosis in a porcine model of renal artery stenosis, signifying that EVs directly boost the regenerative capacity of the injured kidney16. Conversely, we have shown that, in a diseased microenvironment, MetS interferes with the protein30, miRNA31, and mRNA19 content packaged within porcine EVs. The primary goal of this study was to extend our previous observations, and to compare the phenotypic characteristics of EVs derived from a healthy (Lean) versus MetS-MSCs, which may impose aberrant reparative capacities.
Ultracentrifugation remains one of the most common methods of EV isolation from cell culture supernatants. Cells can release at least three different types of extracellular vesicles, including apoptotic bodies, exosomes, and microvesicles, with the latter two constituting the most studied EV populations. Exosomes and microvesicles can be distinguished based on their size/morphology, associated protein markers, and intracellular origin. Exosomes originate from the endosomal multi-vesicular compartment of the cell, and are released extracellularly following fusion of multi-vesicular bodies with the plasma membrane. In contrast, microvesicles, which are larger, are formed by cellular membrane protrusions that bleb from the cell, and express proteins reflective of the parent cell10. In this study, we observed distinct differences in the size of EVs released by Lean and MetS pig MSCs. Although we detected no significant difference in overall EV number released by Lean- and MetS-MSCs, assessment of their size distribution disclosed that Lean-EVs contained larger-size vesicles in comparison to MetS-EVs, and that classical genes associated with exosomes were enriched in MetS-EVs. In agreement with our current findings, we previously found that MetS-EVs have intensified expression of CD9, a commonly reported exosomal marker, while proteins specific to the parent cell (CD73) remained unchanged between Lean- and MetS-EVs31, supporting the notion that MetS pigs may release a higher proportion of exosomes. Interestingly, not only were MetS-EVs on average smaller than Lean-EVs, but transmission electron microscopy demonstrated that the exosome diameters were also smaller.
Therefore, our findings highlight that smaller EVs, likely exosomes, are released into the extracellular environment by Lean- and MetS-MSCs. Possibly, amplified exosome release by MSCs might facilitate disposal or recycling of waste products engulfed or produced by the cell in the noxious microenvironment involving hyperlipidemia and insulin resistance, but these may, in turn, harm neighboring or target cells. The precise mechanisms responsible for these processes can be speculated, based on the origin of the different populations. Intraluminal vesicles destined for exosome release after fusion with the multi-vesicular bodies can be regulated by the ESCRT machinery, and by various Rab GTPases, which act to coordinate the essential stages of vesicle biogenesis and trafficking. Indeed, RNA-seq analyses demonstrated in MetS-MSCs a higher abundance of genes known to influence exosome trafficking and fusion to the plasma membrane, consistent with increased exosome release activity in MetS-MSCs. For example, the ESCRT contain more than 20 proteins that assemble to form four different complexes that interact with the endosomal membrane, and ultimately exosomes are extruded from the cell. In addition, members of the Rab superfamily (Rab5, Rab7, Rab9, and Rab27) aggregate with the ESCRT to modulate different steps of exosome release and trafficking13. Furthermore, Rab11 and -35 work independently of this machinery, and can elicit degradation of molecular content or recycling of the cargo. Alvarez-Erviti et al.11 demonstrated that alpha-synuclein-expressing exosomes are increased following changes to lysosomal function, and can influence the transmission of this cargo to stimulate disease pathology. Thus, lysosome activity levels can regulate exosome secretion. However, in our study, we found no differences in intracellular lysosomal activity between Lean- and MetS-MSCs, arguing against its effect on differences in exosome production, and suggesting alternative production routes.
The present study is limited by several factors. A brief induction of MetS in our experimental model was sufficient to observe increases in cardiometabolic risk factors, but does not fully mimic the lengthy duration of human disease or the heterogeneous population. Additionally, the reparative function of MetS-EVs in this context will need to be interrogated in future studies. Furthermore, the exact mechanism initiating the abundant release of exosomes in MetS-MSCs remains to be resolved.
Conclusions
In summary, this study shows that adipose-tissue-derived MSCs obtained from MetS pigs release smaller size EVs consistent with exosomes, compared with those obtained from Lean pigs. These findings extend our understanding of EVs release from Lean and MetS-MSCs under pathological conditions. Further studies are needed to determine the functional differences among those EVs.
Acknowledgments
We thank the Mayo Clinic Microscopy and Cell Analysis Core Facility for assistance and acquisition of electron images.
Footnotes
Ethical Approval: This study was approved by our institutional animal care and use committee (IACUC) (approval number: A00002903-17).
Statement of Human and Animal Rights: All of the experimental animal procedures were approved by IACUC Mayo Clinic, Rochester, MN, USA (approval number: A00002903-17).
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This study was partly supported by National Institutes of Health (NIH) grants: T32-DK07013, DK120292 HL123160, DK104273, DK102325, DK109134, and UL1-TR000135.
ORCID iD: Amrutesh S. Puranik  https://orcid.org/0000-0001-7249-5602
https://orcid.org/0000-0001-7249-5602
Lilach O. Lerman  https://orcid.org/0000-0002-3271-3887
https://orcid.org/0000-0002-3271-3887
References
- 1. Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA. 2015;313(19):1973–1974. [DOI] [PubMed] [Google Scholar]
- 2. Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the third national health and nutrition examination survey, 1988-1994. Arch Intern Med. 2003;163(4):427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Banuls C, Rovira-Llopis S, Martinez de Maranon A, Veses S, Jover A, Gomez M, Rocha M, Hernandez-Mijares A, Victor VM. Metabolic syndrome enhances endoplasmic reticulum, oxidative stress and leukocyte-endothelium interactions in PCOS. Metabolism. 2017;71:153–162. [DOI] [PubMed] [Google Scholar]
- 4. Zhu XY, Ma S, Eirin A, Woollard JR, Hickson LJ, Sun D, Lerman A, Lerman LO. Functional plasticity of adipose-derived stromal cells during development of obesity. Stem Cells Transl Med. 2016;5(7):893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo Y, Musani SK, Sims M, Pearson TA, DeBoer MD, Gurka MJ. Assessing the added predictive ability of a metabolic syndrome severity score in predicting incident cardiovascular disease and type 2 diabetes: the atherosclerosis risk in communities study and Jackson heart study. Diabetol Metab Syndr. 2018;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Becker A, Riet IV. Homing and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy? World J Stem Cells. 2016;8(3):73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kean TJ, Lin P, Caplan AI, Dennis JE. MSCs: delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. 2013;2013:732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. [DOI] [PubMed] [Google Scholar]
- 9. Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. [DOI] [PubMed] [Google Scholar]
- 10. Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Thery C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113(8):E968–E977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ, Cooper JM. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011;42(3):360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Thery C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–5565. [DOI] [PubMed] [Google Scholar]
- 13. Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30; sup pp 1–13. [DOI] [PubMed] [Google Scholar]
- 14. Valapala M, Vishwanatha JK. Lipid raft endocytosis and exosomal transport facilitate extracellular trafficking of annexin A2. J Biol Chem. 2011;286(35):30911–30925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eirin A, Riester SM, Zhu XY, Tang H, Evans JM, O’Brien D, van Wijnen AJ, Lerman LO. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eirin A, Zhu XY, Jonnada S, Lerman A, van Wijnen AJ, Lerman LO. Mesenchymal stem cell-derived extracellular vesicles improve the renal microvasculature in metabolic renovascular disease in swine. Cell Transplant. 2018;27(7):1080–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eirin A, Zhu XY, Puranik AS, Woollard JR, Tang H, Dasari S, Lerman A, van Wijnen AJ, Lerman LO. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci Rep. 2016;6:36120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eirin A, Zhu XY, Puranik AS, Woollard JR, Tang H, Dasari S, Lerman A, van Wijnen AJ, Lerman LO. Integrated transcriptomic and proteomic analysis of the molecular cargo of extracellular vesicles derived from porcine adipose tissue-derived mesenchymal stem cells. PLoS One. 2017;12(3):e0174303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meng Y, Eirin A, Zhu XY, O’Brien DR, Lerman A, van Wijnen AJ, Lerman LO. The metabolic syndrome modifies the mRNA expression profile of extracellular vesicles derived from porcine mesenchymal stem cells. Diabetol Metab Syndr. 2018;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23(11):1233–1244. [DOI] [PubMed] [Google Scholar]
- 21. van Balkom BW, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, van Eijndhoven MA, Pegtel DM, Stoorvogel W, Wurdinger T, Verhaar MC. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121(19):3997–4006, S1–15. [DOI] [PubMed] [Google Scholar]
- 22. Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S, Takasugi M, Watanabe S, Kanemaki MT, Obuse C, Hara E. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun. 2017;8:15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Toro J, Herschlik L, Waldner C, Mongini C. Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front Immunol. 2015;6:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Conley SM, Zhu XY, Eirin A, Tang H, Lerman A, van Wijnen AJ, Lerman LO. Metabolic syndrome alters expression of insulin signaling-related genes in swine mesenchymal stem cells. Gene. 2018;644:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pawar AS, Zhu XY, Eirin A, Tang H, Jordan KL, Woollard JR, Lerman A, Lerman LO. Adipose tissue remodeling in a novel domestic porcine model of diet-induced obesity. Obesity (Silver Spring). 2015;23(2):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ebrahimi B, Eirin A, Li Z, Zhu XY, Zhang X, Lerman A, Textor SC, Lerman LO. Mesenchymal stem cells improve medullary inflammation and fibrosis after revascularization of swine atherosclerotic renal artery stenosis. PLoS One. 2013;8(7):e67474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eirin A, Zhu XY, Krier JD, Tang H, Jordan KL, Grande JP, Lerman A, Textor SC, Lerman LO. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells. 2012;30(5):1030–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun IO, Santelli A, Abumoawad A, Eirin A, Ferguson CM, Woollard JR, Lerman A, Textor SC, Puranik AS, Lerman LO. Loss of renal peritubular capillaries in hypertensive patients is detectable by urinary endothelial microparticle levels. Hypertension. 2018;72(5):1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miao Y, Li G, Zhang X, Xu H, Abraham SN. A TRP channel senses lysosome neutralization by pathogens to trigger their expulsion. Cell. 2015;161(6):1306–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eirin A, Zhu XY, Woollard JR, Tang H, Dasari S, Lerman A, Lerman LO. Metabolic syndrome interferes with packaging of proteins within porcine mesenchymal stem cell-derived extracellular vesicles. Stem Cells Transl Med. 2019;8(5):430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meng Y, Eirin A, Zhu XY, Tang H, Chanana P, Lerman A, Van Wijnen AJ, Lerman LO. The metabolic syndrome alters the miRNA signature of porcine adipose tissue-derived mesenchymal stem cells. Cytometry A. 2018;93(1):93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]