Abstract
MiR-204 is expressed in vascular smooth muscle cells (VSMC). However, its role in VSMC contraction is not known. We determined if miR-204 controls VSMC contractility and blood pressure through regulation of sarcoplasmic reticulum (SR) calcium (Ca2+) release. Systolic blood pressure (SBP) and vasoreactivity to VSMC contractile agonists (phenylephrine (PE), thromboxane analogue (U46619), endothelin-1 (ET-1), angiotensin-II (Ang II) and norepinephrine (NE) were compared in aortas and mesenteric resistance arteries (MRA) from miR-204−/− mice and wildtype mice (WT). There was no difference in basal systolic blood pressure (SBP) between the two genotypes; however, hypertensive response to Ang II was significantly greater in miR-204−/− mice compared to WT mice. Aortas and MRA of miR-204−/− mice had heightened contractility to all VSMC agonists. In silico algorithms predicted the type 1 Inositol 1, 4, 5-trisphosphate receptor (IP3R1) as a target of miR-204. Aortas and MRA of miR-204−/− mice had higher expression of IP3R1 compared to WT mice. Difference in agonist-induced vasoconstriction between miR-204−/− and WT mice was abolished with pharmacologic inhibition of IP3R1. Furthermore, Ang II-induced aortic IP3R1 was greater in miR-204−/− mice compared to WT mice. In addition, difference in aortic vasoconstriction to VSMC agonists between miR-204−/− and WT mice persisted after Ang II infusion. Inhibition of miR-204 in VSMC in vitro increased IP3R1, and boosted SR Ca2+ release in response to PE, while overexpression of miR-204 downregulated IP3R1. Finally, a sequence-specific nucleotide blocker that targets the miR-204-IP3R1 interaction rescued miR-204-induced downregulation of IP3R1. We conclude that miR-204 controls VSMC contractility and blood pressure through IP3R1-dependent regulation of SR calcium release.
Keywords: Hypertension, MiR-204, IP3R1, Calcium, Vascular smooth mucsle cells contractility
1. Introduction
Hypertension is a major risk factor for heart failure, myocardial infarction, stroke, and chronic kidney disease [1]. Pathophysiological mechanisms contributing to the development of hypertension include increased vascular resistance, determined in large part by reduced vascular diameter due to increased vasoconstriction and arterial re-modelling [2]. Vascular smooth muscle (VSMC) contraction is triggered by an increase in intracellular free calcium (Ca2+) concentration, promoting actin–myosin cross-bridge formation [2]. Perturbations in VSMC signalling influence vascular reactivity and tone and is an important determinant of vascular resistance and blood pressure [2]. Accumulating data suggest that deregulation of VSMC Ca2+ is a trigger in hypertension [2,3].
Inositol 1,4,5-trisphosphate (IP3) regulates Ca2+ release from intracellular Ca2+ stores. IP3 is enzymatically synthesized in response to extracellular agonists, and acts via its cognate receptors (IP3R) to promote release of Ca2+ from the sarcoplasmic reticulum into the cytosol. The IP3-IP3R axis is critical for VSMC contractile response to vasoactive agonists [4,5]. Dysregulation in VSMC IP3R expression is associated with hypertension-related vascular complications [6]. Recent experimental evidence points to micro-RNAs (miRNAs), a class of small noncoding RNAs that target a complementary mRNA and either repress its translation or destabilize it [7], as contributing factors in vascular diseases characterized by alterations in IP3R expression and Ca2+ signalling [8,9].
MiR-204 is expressed in blood vessels and it’s downregulation in pulmonary arteriolar smooth muscle is associated with pulmonary arterial hypertension [10]. MiR-204 has also been shown to regulate vascular smooth muscle cell calcification in vitro and in vivo [11]. Recent evidence suggests that endothelial miR-204 impairs aortic endothelial function in diet-induced obesity [12], but the role of vascular miR-204 in regulating systemic blood pressure is not known. In silico algorithms predict IP3R1 as a miR-204 target. Intrigued by the possibility that vascular miR-204 is involved in pressor response, we examined if it governs VSMC contractility and blood pressure through an effect on the IP3R1/Ca2+ axis.
2. Methods
2.1. Animals
The miR-204 knockout mice were created using TALEN technology in a C57Bl/6 background. Sequences immediately flanking the miR-204 coding sequence were chosen as recognition targets, and appropriate TALEN mRNAs were generated using in vitro transcription. These TALEN mRNAs were injected into fertilized C57Bl/6 eggs which were then implanted into pseudo-pregnant dams. Several potential founder offspring were identified by PCR and DNA sequencing over the deletion site. A founder with a 10 bp deletion allele which eliminated virtually all of the miR-204 mature coding sequence was backcrossed into a C57Bl/6 background, and subsequent generations were bred to homozygosity. Quantitative PCR analyses demonstrated a significant decrease in miR-204 mRNA in heterozygous mice, while homozygous mice had no detectable miR-204 transcript.
Experiments were performed on 8–12 week-old mice lacking miR-204 (miR-204−/−) and their wild type (WT) controls. Mice were fed a solid standard diet (Na+ content 0.4%) and water. Mice were divided in 4 groups: 1) Wildtype mice infused with saline (WT); 2) Wildtype mice infused with Ang II (100 ng/kg/min) using subcutaneous mini-osmotic pumps for 4 weeks (WT Ang II); 3) miR-204−/− mice infused with saline (miR-204−/−); 4) miR-204−/− mice infused with Ang II (miR-204−/− Ang II). Terminal experiments were performed in anesthetized mice (2–5% isoflurane). The thoracic aorta and the mesenteric resistance arteries (MRA) were isolated and used for immunoblotting, real-time quantitative polymerase chain reaction (qPCR), and vascular reactivity. All protocols were approved by the Institutional Animal Care and Use Committee of the University of Iowa. All methods were performed in accordance with the guidelines and regulations of the NIH and University of Iowa.
2.2. Vascular reactivity
Male mice 8–12 weeks old were anesthetized and euthanized by rapid cardiac excision. The thoracic aorta and the MRA were carefully harvested and placed in ice-cold Krebs buffer (118.3 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 25 mM NaHCO3, 1.2 mM MgSO4, 11 mM glucose, 0.0026 mM CaNa2 EDTA). The vessels were cleaned of fat and connective tissue and cut transversely into 5–10 rings (1.8–2.0 mm wide). The rings were placed in oxygenated chambers (95% O2/5% CO2) filled with 5 mL Krebs buffer solution and maintained at 37°C and pH 7.4. Each ring was suspended between two wire stirrups in a 5 mL chamber of a four-chamber myograph system (DMT). One stirrup was connected to a three–dimensional micromanipulator and the other to a force transducer. The contractile force was recorded electronically. All rings were stretched to 2000 mg in 500 mg increments over a 1 h period to optimize the contractile response to KCl. One dose of KCl (60 mM) was added to verify vascular smooth muscle viability. Contractile response was determined by generating dose–response curves to phenylephrine (PE) (10−8 to 3.10−4 M), thromboxane analogue (U46619) (10−9 to 3.10−5 M), endothelin (ET1) (10−8 to 10−4 M), angiotensin II (Ang II) (10−10 to 10−5 M), and norepinephrine (NE) (10−8 to 10−4 M). Experiments were repeated in the presence of the IP3R blocker, 2-aminoethoxydiphenyl borate (2-APB) (50 μm).
2.3. Blood pressure
Systolic blood pressure (SBP) was measured in conscious mice with an automated multi-channel system, using the tail-cuff method with a photoelectric sensor (Kent Scientific Torrington, USA) as previously described. [13] Briefly, SBP was measured before starting the study and every week during the treatment. Arterial blood pressure measurements were performed at the same time of day (between 9 a.m. and 11 a.m.) in order to avoid the influence of the circadian cycle, and the mean of 10 measurements was used.
2.4. Western blotting
Immunoblotting for IP3R1 (Abcam, USA) was performed on aortic segments. Chemiluminescent signal was developed using the Licor Odyssey Scanner (Lincoln, NE, USA). Bands were quantified using Image J software.
2.5. Cell culture, plasmid/siRNA, and site blocker transfection
Primary vascular smooth muscle cells isolated from aortas of wildtype mice were transfected with miR-204 mimic (5′-UUC CCU UUG UCA UCC UAU GCC U-3′), miR-204 inhibitor (5′-AGG ATG ACA AAG GGA-3′), scrambled oligonucleotide (5′-ACG TCT ATA CGC CCA- 3′),IP3R1 site blocker (5′-AGT TCC CTT TAA AAA ATT-3′) and/or negative control (5′-TAA CAC GTC TAT ACG CCC A-3′)
2.6. Cytosolic calcium assay
Calcium release into the cytosol was measured as previously described [14]. Briefly, primary VSMC transfected with miR-204 Inhibitor (I), or scrambled (SC) were loaded with 20 μM Fluo-4 for 15 min. After loading, cells were washed with isotonic buffer as previously described [14]. The assay was performed in a buffer solution as previously described [14], where phenylephrine (PE) (10−3 μM) was added and fluo4 fluorescent was determined.
2.7. Quantitative real time PCR
Total RNA from cultured cells or tissues of mice was isolated by the TRIZOL (Invitrogen) method. Real-time PCR was performed using the Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) with the Super Script III Platinum SYBR Green One-Step qRT-PCR Kit (Invitrogen). The following primers purchased from Exiqon were used: Mouse IP3R1 forward 5′- TGG GCA ACT GTG GGA CCT T-3′, reverse 5′- GGA ACT CCA CAT CCA GAA CCA-3′; mouse IP3R2 forward 5′- AGG AAG GTG AGG ATG GCA TAG A-3′, reverse 5′-CAC GGT GAC AAT GCA CAT GA-3′; mouse IP3R3 forward 5′-CAG AAC GAC CGC AGG TTT GT-3′, reverse 5′- CAG GAC ATT CTG CCC ATT GTT-3′; mouse GAPDH forward 5′- GGC AAA TTC AAC GGC ACA-3′, reverse 5′-CGC TCC TGG AAG ATG GTG AT-3′. MiR-204 forward and universal reverse primers were procured from Quanta Biosciences (cDNA synthesis kit, Quanta Biosciences, Beverly, MA, USA). Mouse GAPDH and RNU6 (Quanta Biosciences, Beverly, MA, USA) were used as internal controls for mRNA and miR quantification, respectively.
2.8. Statistical analysis
Statistical analysis was performed using GraphPad Prism (Version 6.0) statistical software. Significance of difference between two groups was evaluated using the t-test. For multiple comparisons, one-way analysis of variance (ANOVA) was used and post-hoc analysis was performed with Tukey’s test. Data are expressed as mean ± SEM and considered significant if P-values were ≤ 0.05. All shown data is representative of at least three independent experiments.
3. Results
3.1. Lack of miR-204 increases vascular contractility through IP3 receptor 1 (IP3R1)-dependent mechanism
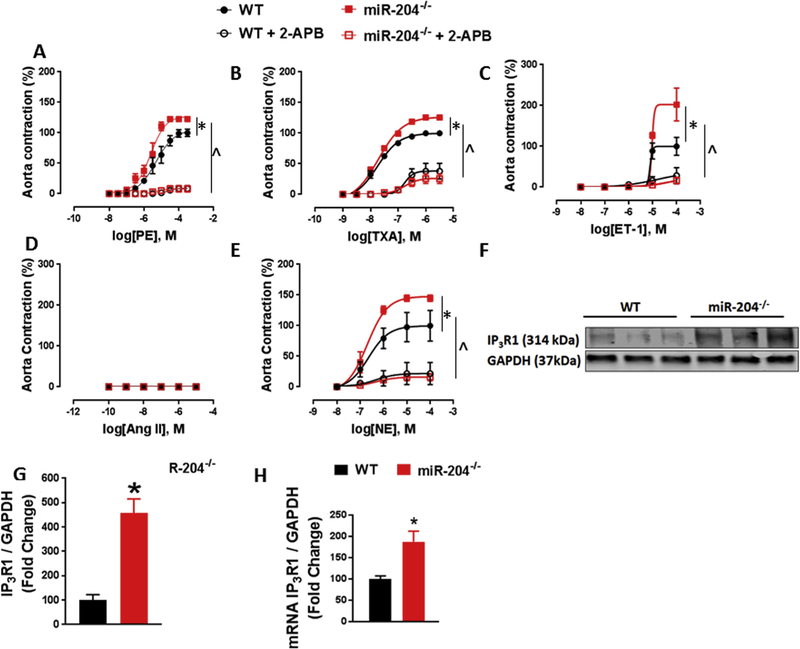
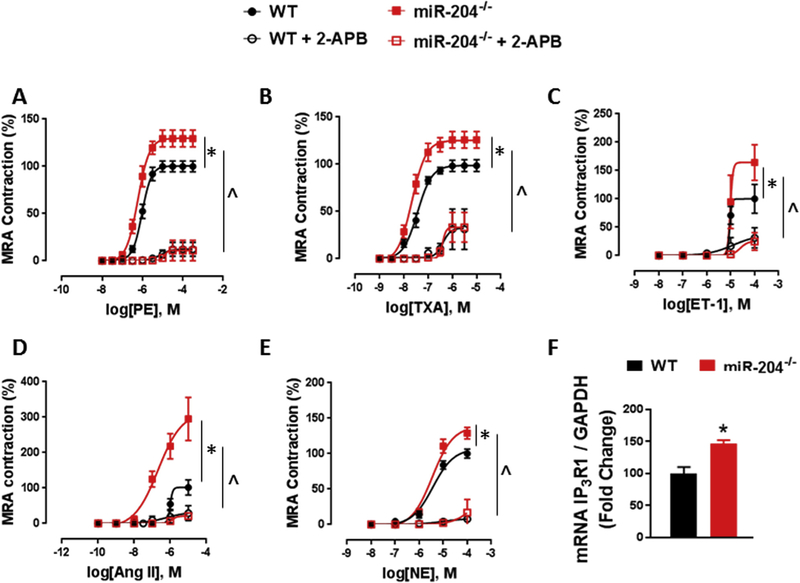
We first asked if miR-204 controls vascular contractile responses in mice. We used mice with global deletion of the miR-204 locus (miR-204−/−) and WT mice for this purpose. Aortas and mesenteric resistance arteries (MRA) from miR-204−/− and WT mice were subjected to cumulative doses of contractile agonists phenylephrine (PE), thromboxane analogue (U46619), endothelin-1 (ET-1), angiotensin-II (Ang II) and norepinephrine (NE). When compared to WT mice, miR-204−/− mice had increased contractile response to PE (Figs. 1, 2 A), U46619 (Figs. 1, 2B), ET-1 (Figs. 1, 2C), and NE (Figs. 1, 2E) in both aorta and MRA. MRA from miR-204−/− mice had higher contractile response to Ang II than WT MRA (Fig. 2D), but neither aorta from WT or miR-204−/− showed any contraction to Ang II (Fig. 1D), indicating absence of Ang II receptors in aortas. Inhibition of IP3R1 with 2-aminoethoxydiphenyl borate (2-APB) (50 μm) suppressed vasoconstriction to all agonists in both groups, negating any difference between groups (Figs. 1 and 2), suggesting that the difference is vascular contractility between WT and miR-204−/− mice is mediated by IP3R1. Western blotting (Fig. 1F) and quantification (Fig. 1G) showed that miR-204−/− mice have more IP3R1 protein in aortas compared to WT mice. Additionally, qRT-PCR showed that miR-204−/− mice express more IP3R1 mRNA in aortas and MRA compared to WT mice (Figs. 1H, 2 F).
Fig. 1.
Deletion of mir-204 increases contractile response and IP3R1 expression in aorta. (A–E) Contractile response to phenylephrine (PE) (A), thromboxane analogue (U46619) (B), endothelin-1 (ET-1) (C), angiotensin-II (Ang II) (D) and norepinephrine (NE) (E) in aortas of miR-204−/− and WT mice. *p < 0.05 vs WT. Inhibition of IP3R1 with 2-aminoethoxydiphenyl borate (2-APB) (50 μm) suppresses vasoconstriction to all agonists in both groups, without any difference between them (Fig. 1A–E). ^p < 0.05 vs WT + 2-APB. (F–G) Immunoblots for IP3R1 (F) with quantification (G) in aortas of miR-204−/− and WT mice. *p < 0.05 vs WT. (H) qRT-PCR for IP3R1 in aortas of miR-204−/− mice and WT mice. *p < 0.05 vs WT.
Fig. 2.
Deletion of mir-204 increases contractile response and IP3R1 expression mesenteric resistance arteries (MRA). (A–E) Contractile response to phenylephrine (PE) (A), thromboxane analogue (U46619) (B), endothelin-1 (ET-1) (C), angiotensin-II (Ang II) (D) and norepinephrine (NE) (E) in MRA of miR-204−/− and WT mice. *p < 0.05 vs WT. Inhibition of IP3R1 with 2-aminoethoxydiphenyl borate (2-APB) (50 μm) suppresses vasoconstriction to all agonists in both groups, without any difference between them (Fig. 2A–E). ^p < 0.05 vs WT + 2-APB. (F) qRT-PCR for IP3R1 in MRA of miR-204−/− and WT mice. *p < 0.05 vs WT.
3.2. MiR-204−/− mice have an exaggerated pressor response
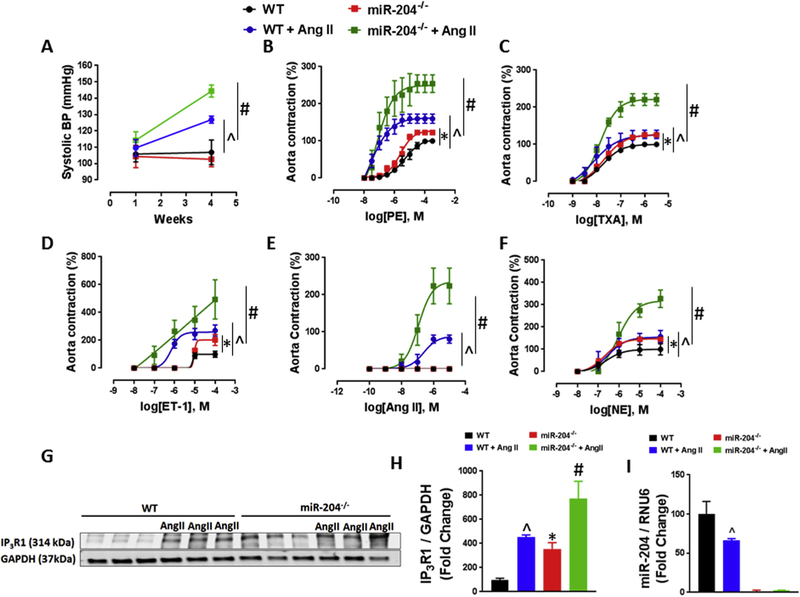
We then looked for differences in blood pressure between miR-204−/− and WT mice. Resting systolic blood pressure (SBP) was not different in miR-204−/− mice and WT mice (Fig. 3A). However, pressor response to Ang II (100 ng/kg/min) was markedly higher in miR-204−/− compared to WT mice (Fig. 3A). In addition, contractile responses of aortas to phenylephrine, U46619, endothelin-1, angiotensin-II and norepinephrine were significantly greater in AngII-treated miR-204−/− compared to WT mice (Fig. 3B–F). Immunoblotting showed that Ang II infusion upregulated IP3R1 expression in aortas, and this increase was potentiated in miR-204−/− mice (Fig. 3G, H). Moreover, Ang II infusion itself decreased aortic miR-204 expression in aortas of WT mice (Fig. 3I).
Fig. 3.
Deletion of miR-204 accentuates increases systolic hypertension. (A) Pressor response to Ang II infusion (100 ng/kg/min) in miR-204−/− and WT mice. ^p < 0.05 vs WT + Ang II; #p < 0.05 vs miR-204−/− + Ang II. (B–F) Contractile response to phenylephrine (PE) (B), thromboxane analogue (U46619) (C), endothelin-1 (ET-1) (D), angiotensin-II (Ang II) (E) and norepinephrine (NE) (F) in aorta of miR-204−/− and WT mice infused with Ang II. *p < 0.05 vs WT; ^p < 0.05 vs WT + Ang II; #p < 0.05 vs miR-204−/− + Ang II. (G–H) Immunoblots for IP3R1 (G) with quantification (H) in aortas of WT and miR-204−/− mice infused with Ang II. *p < 0.05 vs WT; ^p < 0.05 vs WT + Ang II; #p < 0.05 vs miR-204−/− + Ang II. (I) qRT-PCR for miR-204 in aortas of miR-204−/− and WT mice infused with Ang II. *p < 0.05 vs WT; ^p < 0.05 vs WT + Ang II; #p < 0.05 vs Mir-204−/− + Ang II.
3.3. MiR-204 controls IP3R1 expression
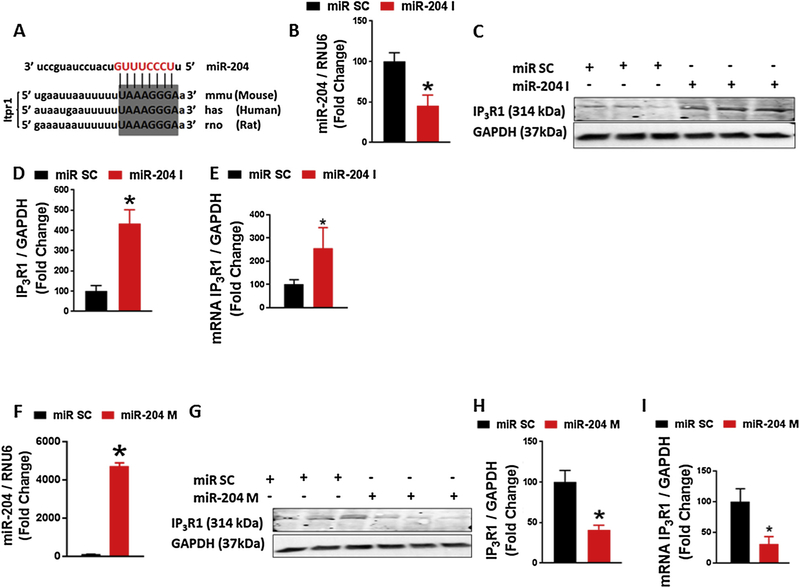
In silico algorithms predict the type 1 Inositol 1, 4, 5-trisphosphate receptor (IP3R1) as a target of miR-204 in human, rat and mouse species (Fig. 4A). IP3R1 is the most abundantly expressed IP3R in VSMC. Given this, we asked whether miR-204 controls IP3R1 expression. Primary VSMC were isolated from WT mice and transfected with miR-204 nucleotide inhibitor (I) or mimic (M) or scrambled control (SC). Downregulation of miR-204 with the inhibitor (Fig. 4B) was associated with increase of IP3R1 protein and mRNA expression (Fig. 4C–E). Conversely, increase of miR-204 using miR-204 mimic (Fig. 4F) was associated with downregulation of IP3R1 mRNA and protein (Fig. 4G–I).
Fig. 4.
Mir-204 regulates IP3R1 expression in aortic VSMC. (A) In silico analysis predicting conserved sequence in 3′-UTR of IP3R1 as a target of miR-204. (B) qRT-PCR of miR-204 in VSMC transfected with miR-204 inhibitor (miR-204 I 50 nM for 48 h). *p < 0.05 vs miR SC (C) Immunoblots for IP3R1 (C) with quantification (D) in VSMC transfected with miR-204 I. *p < 0.05 vs miR SC (E) qRT-PCR for IP3R1 VSMC transfected with miR-204 I. *p < 0.05 vs miR SC. (F) qRT-PCR for miR-204 in VSMC transfected with miR-204 mimic (miR-204 M 50 nM for 48 h). *p < 0.05 vs miR SC. (G) Immunoblots for IP3R1 (G) with quantification (H) in VSMC transfected with miR-204 M. *p < 0.05 vs miR SC. (I) qRT-PCR for IP3R1 in VSMC transfected with miR-204 M. *p < 0.05 vs miR SC.
3.4. IP3R1 mRNA is a direct target of miR-204
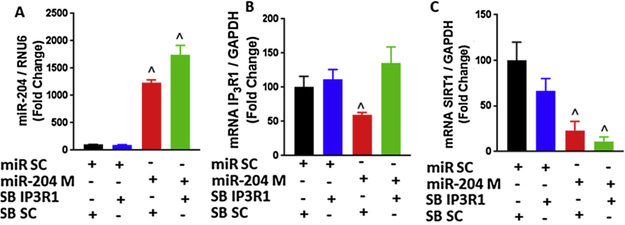
To determine whether IP3R1 is a direct target for miR-204, we designed a sequence-specific antisense oligonucleotide that binds to the miR-204 target site on IP3R1 mRNA, shielding it from miR-204. Using this blocking oligonucleotide, we asked if miR-204 directly targets IP3R1 mRNA in VSMC. Overexpression of miR-204 in VSMC using miR-204 mimic (Fig. 5A) led to reduction of IP3R1 mRNA (Fig. 5B). This decline in expression was blocked by the sequence-specific IP3R1 blocking oligonucleotide (Fig. 5B). Importantly, the IP3R1 blocking oligonucleotide did not negate downregulation of sirtuin 1 (SIRT1) by miR-204 (Fig. 5C), a validated target of miR-204. [12]. Thus, miR-204 directly targets IP3R1 in VSMC. MRNA of IP3R2 and IP3R3 was not different in aortas of WT and miR-204−/− mice, and was not downregulated by miR-204 mimic in aortic VSMC (Fig. Supp 1) indicating the miR-204 selectively targets IP3R1.
Fig. 5.
IP3R1 is a direct target of miR-204. (A–C) qRT-PCR for miR-204 (A), IP3R1 (B) and SIRT1 (C) in VSMC transfected with miR-204 mimic (miR-204 M 50 nM for 48 h) in the presence and absence of sequence-specific site blocker that targets IP3R1-miR-204 binding (SB IP3R1, 50 nM). Controls were transfected with a scrambled oligonucleotide miR (miR SC) and scrambled site blocker (SB SC). ^p < 0.05 compared to SB SC + miR SC.
3.5. MiR-204 controls SR Ca2+ release in VSMC
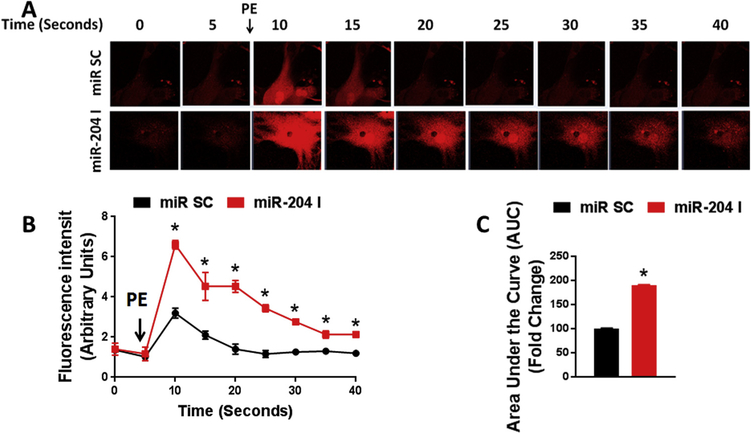
We next determined if miR-204 regulates agonist-induced Ca2+ release from the sarcoplasmic reticulum (SR) into the cytosol. Cytosolic Ca2+ in response to phenylephrine was significantly higher in VSMC transfected with miR-204 inhibitor compared to scrambled control (Fig. 6A–C). Thus, inhibition of endogenous miR-204 boosts agonist-stimulated SR Ca2+ release into the cytosol.
Fig. 6.
MiR-204 inhibition augments agonist-induced time-dependent SR Ca+2 release into the cytosol. Confocal images (A) with fluorescence intensity (B) and quantification (C) of phenylephrine (PE)-induced cytosolic Ca+2 release in aortic VSMC transfected with miR-204 inhibitor (miR-204 I 50 nM for 48 h). Controls were transfected with a scrambled miR (miR SC). *p < 0.05 compared to miR SC.
4. Discussion
Regulation of IP3 receptors by microRNAs has been reported in the heart and other cell types. Drawnel et al showed that miR-133a regulates IP3R2 leading to hypertrophy in cardiomyocytes [15]. Another study identified miR-506 as a regulator of IP3R3 and Ca2+ signaling in HEK293 cells [16]. Additionally, miR-25 regulates IP3R1 in the cardiac myocyte cell line HL-1 [8]. Although these studies showed a correlation between microRNAs and IP3R, the nature of this relationship in VSMC is unknown. Our data showed the miR-204 deletion was associated with an increased level of IP3R1 in the vasculature.
All three IP3R isoforms are present at the mRNA and protein levels in cultured VSMC and intact aorta. However, among the three receptors, IP3R1 is best expressed and plays the dominant role in VSMC function [17,18]. IP3R1 is vital in receptor-regulated Ca2+ signaling in response to a variety of vasoactive agonists. Our findings that exaggerated vasoconstriction in bloods vessels of miR-204−/− mice is due to upregulation of in IP3R1are consistent with a study by Zhou et al showing that PE-induced aortic contraction is reduced in IP3R1-deficient mice [17]. Another study highlighted the roles of all three isoforms of IP3R by showing that vascular contractility is reduced in IP3R1,2, 3-deficient aortas [18]. Additionally our data are in accordance with studies showing that pharmacological inhibition of IP3R using IP3R blockers such as heparin, 2-APB or Xestospongin leads to a reduction in the contractility of vessels [19–21]. All together, these data demonstrate that IP3R1-mediated Ca2+ release plays an important role in regulating vasoconstriction following activation of G-protein-coupled receptors. However, 2-APB is a broad inhibitor and has also been shown to inhibit calcium release-activated calcium channel protein 1 (ORAI1) and transient receptor potential cation channels (TRPC) [22], which are activated downstream of IP3R [23]. Therefore, additional studies using mice with conditional deletion of IP3R1 in vascular smooth muscle will clarify the relative contribution of IP3R1 in miR-204-mediated changes in vasoconstriction.
Intracellular Ca2+ is a crucial regulator of VSMC contractility. Our findings demonstrate the important part miR-204 plays in intracellular Ca2+ homeostasis, and the mediating role of IP3R1 in this phenomenon. However, we cannot rule out the contribution of IP3R1-independent mechanisms in miR-204-mediated control of VSMC Ca2+. MiR-204 regulates Ca2+ by targeting Caveolin-1 and Transient Receptor Potential Cation Channel Subfamily M Member 3 (TRPM3) in renal cell carcinoma [24]. This TRPM3-dependent mechanism may also be operative in VSMC. A recent study by Koyama et al suggests that miR-204 also regulates low voltage-activated Ca2+ currents in ventricular cardiomyocytes, and accelerates ventricular cell spontaneous beatings through this mechanism [25].
Importantly, miR-204−/− mice were not spontaneously hypertensive, indicating that upregulation of IP3R1 in these mice is not sufficient to lead to a hypertensive phenotype. That the miR-204−/− mice have an exaggerated increase in blood pressure with Ang II infusion compared to WT mice, indicated that an additional hypertensive stimulus is required to bring out the phenotypic difference between the two genotypes. This suggests that miR-204-dependent of IP3R-mediated Ca2+ release might not be required for the maintenance of physiological blood pressure. In contrast, a recent report shows that inducible and specific deletion of G protein-coupled receptors (Gq) in VSMC results in sustained decrease of basal blood pressure [26], suggesting that Gq may control basal blood pressure through additional IP3R-independent mechanisms.
We used a low pressor dose of Ang II in our studies. A high dose of Ang II (400 ng/kg/min) did not elicit difference in blood pressure between miR-204−/− and WT mice (data not shown). This indicates that miR-204−/− mice are sensitized to Ang II-induced pressor response. The role of miR-204 in blood pressure regulation is corroborated by a recent study showing that a miR-204 agomir reduces blood pressure in the spontaneously hypertensive rat [27]. It is interesting that we also observed a decrease in aortic miR-204 expression with Ang II, consistent with a recent publication showing downregulation of miR-204 in hypertension [27]. Studies show that Ang II downregulates specific miRNAs through Gq- and ERK-dependent mechanisms [28–30]. While we did not explore how AngII downregulates miR-204 in vascular smooth muscle cells, these mechanisms may be functional. Although we did not pursue the mechanism responsible for this downregulation, it suggests that dynamic control of vascular miR-204 may play a part in blood pressure regulation in physiological and pathophysiological states.
In summary, our findings show that miR-204 regulates constriction of systemic vasculature to a wide range of physiologic agonists and systemic pressor response to Angiotensin II by governing IP3R1-mediated SR Ca2+ release into the cytosol. These findings may provide impetus for development of miR-204-directed RNA-based therapies to treat hypertension.
Supplementary Material
Acknowledgements
K. Irani was supported by VA Merit Award I01 BX002940–04 and the University of Iowa Endowed Professorship in Cardiovascular Medicine; M. Kassan by NIH grants T32 HL007121 and AHA-18CDA34030155.
Footnotes
Competing financial interests
None.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.ceca.2019.03.006.
References
- [1].Drozdz D, Kawecka-Jaszcz K, Cardiovascular changes during chronic hypertensive states, Pediatr. Nephrol 29 (2014) 1507–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Touyz RM, Alves-Lopes R, Rios FJ, Camargo LL, Anagnostopoulou A, Arner A, Montezano AC, Vascular smooth muscle contraction in hypertension, Cardiovasc. Res 114 (2018) 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Potier M, Trebak M, New developments in the signaling mechanisms of the store-operated calcium entry pathway, Pflügers Archiv-Eur. J. Physiol 457 (2008) 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Eid AH, El-Yazbi AF, Zouein F, Arredouani A, Ouhtit A, Rahman M, Zayed H,Pintus G, Abou-Saleh H, Inositol 1, 4, 5-trisphosphate Receptors in Hypertension, Front. Physiol 9 (2018) 1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Berridge MJ, The inositol trisphosphate/calcium signaling pathway in health and disease, Physiol. Rev 96 (2016) 1261–1296. [DOI] [PubMed] [Google Scholar]
- [6].Abou-Saleh H, Pathan AR, Daalis A, Hubrack S, Abou-Jassoum H, Al-Naeimi H, Rusch NJ, Machaca K, Inositol 1, 4, 5-trisphosphate (IP3) receptor up-regulation in hypertension is associated with sensitization of Ca2+ release and vascular smooth muscle contractility, J. Biol. Chem 288 (2013) 32941–32951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Song Z, Li G, Role of specific microRNAs in regulation of vascular smooth muscle cell differentiation and the response to injury, J. Cardiovasc. Transl. Res 3 (2010) 246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wahlquist C, Jeong D, Rojas-Muñoz A, Kho C, Lee A, Mitsuyama S, van Mil A, Park WJ, Sluijter JP, Doevendans PA, Inhibition of miR-25 improves cardiac contractility in the failing heart, Nature 508 (2014) 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ha T-Y, MicroRNAs in human diseases: from cancer to cardiovascular disease, Immune Netw 11 (2011) 135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Courboulin A, Paulin R, Giguère NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Côté J, Role for miR-204 in human pulmonary arterial hypertension, J. Exp. Med 208 (2011) 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cui R-R, Li S-J, Liu L-J, Yi L, Liang Q-H, Zhu X, Liu G-Y, Liu Y, Wu S-S, Cr X-BJ. Liao, MicroRNA-204 regulates vascular smooth muscle cell calcification in vitro and in vivo, Cardiovasc. Res 96 (2012) 320–329. [DOI] [PubMed] [Google Scholar]
- [12].Vikram A, Kim Y-R, Kumar S, Li Q, Kassan M, Jacobs JS, Irani K.J.Nc., Vascular microRNA-204 is remotely governed by the microbiome and impairs endothelium-dependent vasorelaxation by downregulating Sirtuin1, Nat. Commun 7 (2016) 12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kassan M, Galán M, Partyka M, Saifudeen Z, Henrion D, Trebak M, Matrougui K, Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice, Arterioscler. Thromb. Vasc. Biol 32 (2012) 1652–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter, Nat. Cell Biol 15 (2013) 1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Drawnel FM, Wachten D, Molkentin JD, Maillet M, Aronsen JM, Swift F, Sjaastad I, Liu N, Catalucci D, Mikoshiba K, Mutual antagonism between IP3RII and miRNA-133a regulates calcium signals and cardiac hypertrophy, J. Cell Biol (2012) 201111095jcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ananthanarayanan M, Banales JM, Guerra MT, Spirli C, Munoz-Garrido P, Mitchell-Richards K, Tafur D, Saez E, Nathanson MH, Post-translational regulation of the type III inositol 1, 4, 5-trisphosphate receptor by miRNA-506, J. Biol. Chem 290 (2015) 184–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhou H, Nakamura T, Matsumoto N, Hisatsune C, Mizutani A, Iesaki T, Daida H, Mikoshiba K, Predominant role of type 1 IP 3 receptor in aortic vascular muscle contraction, Biochem. Biophys. Res. Commun 369 (2008) 213–219. [DOI] [PubMed] [Google Scholar]
- [18].Lin Q, Zhao G, Fang X, Peng X, Tang H, Wang H, Jing R, Liu J, Lederer WJ, Chen J, IP3 receptors regulate vascular smooth muscle contractility and hypertension, JCI Insight 1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hashimoto T, Kihara M, Sato K, Imai N, Tanaka Y, Sakai M, Tamura K, Hirawa N, Toya Y, Kitamura H, Heparin recovers AT1 receptor and its intracellular signal transduction in cultured vascular smooth muscle cells, FEBS Lett 579 (2005) 281–284. [DOI] [PubMed] [Google Scholar]
- [20].Kobayashi S, Kitazawa T, Somlyo A, Somlyo A, Cytosolic heparin inhibits muscarinic and alpha-adrenergic Ca2+ release in smooth muscle. Physiological role of inositol 1, 4, 5-trisphosphate in pharmacomechanical coupling, J. Biol. Chem 264 (1989) 17997–18004. [PubMed] [Google Scholar]
- [21].Lamont C, Gill Wier W, Different roles of ryanodine receptors and inositol (1, 4, 5)- trisphosphate receptors in adrenergically stimulated contractions of small arteries, American journal of physiology, Heart Circ. Physiol 287 (2004) H617–H625. [DOI] [PubMed] [Google Scholar]
- [22].Trebak M, Bird GSJ, McKay RR, Putney JW Jr, Comparison of human TRPC3 channels in receptor-activated and store-operated modes Differential sensitivity to channel blockers suggests fundamental differences in channel composition, J. Biol. Chem 277 (2002) 21617–21623. [DOI] [PubMed] [Google Scholar]
- [23].Lefièvre L, Nash K, Mansell S, Costello S, Punt E, Correia J, Morris J, Kirkman-Brown J, Wilson SM, Barratt CLJBJ, 2-APB-potentiated channels amplify CatSper-induced Ca2+ signals in human sperm, Biochem. J 448 (2012) 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cost NG, Czyzyk-Krzeska MFJM, Regulation of autophagy by two products of one gene: TRPM3 and miR-204, Mol. Cell. Oncol 2 (2015) e1002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Koyama R, Mannic T, Ito J, Amar L, Zennaro M-C, Rossier M, Ijoms Maturana AJ, MicroRNA-204 is necessary for aldosterone-stimulated T-type calcium channel expression in cardiomyocytes, Int. J. Mol. Sci 19 (2018) 2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wirth A, Benyó Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Örsy P,Horváth B, Maser-Gluth C, Nm EJ. Greiner, G 12-G 13–LARG–mediated signaling in vascular smooth muscle is required for salt-induced hypertension, Nat. Med 14 (2008) 64. [DOI] [PubMed] [Google Scholar]
- [27].Yu Z, Zhan X, Li Xia, MiR-204 inhibits hypertension by regulating proliferation and apoptosis of vascular smooth muscle cells, Int. J. Clin. Exp. Med 11 (2018) 8214–8222. [Google Scholar]
- [28].Shyu K-G, Cheng W-P, Wang B-W, Angiotensin II downregulates MicroRNA-145 to regulate kruppel-like factor 4 and myocardin expression in human coronary arterial smooth muscle cells under high glucose conditions, Mol. Med 21 (2015) 616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jeppesen PL, Christensen GL, Schneider M, Nossent AY, Jensen HB, Andersen DC, Eskildsen T, Gammeltoft S, Hansen JL, Sheikh SP, Angiotensin II type 1 receptor signalling regulates microRNA differentially in cardiac fibroblasts and myocytes, Br. J. Pharmacol 164 (2011) 394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kemp JR, Unal H, Desnoyer R, Yue H, Bhatnagar A, Karnik SS, Angiotensin II-regulated microRNA 483–3p directly targets multiple components of the renin–angiotensin system, J. Mol. Cell. Cardiol 75 (2014) 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.