Abstract
Exposure of G protein–coupled receptors (GPCRs) to agonists can desensitize receptor signaling and lead to drug tolerance, whereas inverse agonists can sensitize signaling. For example, activation of serotonin 5-HT2C GPCRs is pharmacotherapeutic for obesity, but there is tolerance to the anorectic effect of the only approved 5-HT2C agonist, lorcaserin. We tested the hypothesis that different agonists or inverse agonists differentially desensitize or sensitize, respectively, canonical 5-HT2C-mediated activation of phospholipase C (PLC) signaling in vitro. Lorcaserin, which displays potency and efficacy equal to 5-HT, desensitized the 5-HT2C receptor significantly more than 5-HT (p < 0.05). Agonist chemotypes such as 2-aminotetralins, with similar potency but lower efficacy than 5-HT, produced little 5-HT2C desensitization. The piperazine agonist 1-(3-chlorophenyl)piperazine (mCPP), with lower potency but similar efficacy as 5-HT, elicited desensitization indistinguishable from 5-HT, while the piperazine agonist aripiprazole, with lower potency and efficacy, did not desensitize 5-HT2C-PLC signaling. Several 5-HT2C agonists also were assessed for β-arrestin recruitment—lorcaserin was a ‘super-agonist’, but a 2-aminotetralin and aripiprazole had nil activity, suggesting they are biased towards 5-HT2C-PLC signaling. We observed robust positive correlations between the magnitude of 5-HT2C desensitization and agonist efficacy to stimulate PLC or to recruit β-arrestin. In contrast, different inverse agonists caused different magnitudes of 5-HT2C sensitization that did not correlate with efficacy (or potency) to inhibit constitutive 5-HT2C-PLC signaling. Assessment of the 5-HT2C-S407A point-mutated receptor indicated this residue’s involvement in ligand-dependent desensitization, but we did not observe a role for protein kinase C.These data show that ligand structure uniquely impacts 5-HT2C desensitization and sensitization processes.
Keywords: 5-HT2C receptor, β-arrestin, Aminotetralin, Desensitization, phospholipase C, Sensitization
1. Introduction
The serotonin (5-hydroxytryptamine, 5-HT) 5-HT2C G protein-coupled receptor (GPCR) has pharmacotherapeutic relevance in obesity, substance use disorders, and other neuropsychiatric disorders (Morgan et al., 2012, 2013; Canal et al., 2013a, 2013b, 2014; Higgins et al., 2013; Kasper et al., 2013). The 5-HT2C receptor-preferring agonist lorcaserin is approved for obesity and is being evaluated as a treatment for cocaine addiction (Collins et al., 2017). Lorcaserin, however, requires daily administration for obesity (Arena, 2012; O’Neil et al., 2012; Harvey-Lewis et al., 2016), and a significant number of patients taking lorcaserin develop tolerance to its anorectic effects (Smith et al., 2010), limiting its therapeutic value. 5-HT2C receptor desensitization likely is involved in clinical tolerance that develops after prolonged lorcaserin use.
Canonical 5-HT2C receptor signaling involves agonist binding to cell membrane-embedded receptor, with stabilization of receptor conformation(s) that bind intracellular heterotrimeric G protein containing the Gαq subunit. The Gαq subunit activates by dissociating from the heterotrimer, and then stimulates phospholipase C (PLC). PLC catalyzes hydrolysis of phosphatidylinositol 4,5-bisphosphate to form membrane-bound diacylglycerol (, a protein kinase C (PKC) activator) and intracellular inositol 1,4,5-trisphosphate (IP3, that stimulates calcium release from endoplasmic reticulum). IP3 is degraded to IP2 and IP1; all three are collectively referred to as inositol phosphates (IP).
The 5-HT2C receptor shows agonist–dependent desensitization (Stout et al., 2002)—attenuated signaling after prolonged activation—and like other GPCRs, the extent of 5-HT2C receptor desensitization may depend on the structure of the agonist ligand (Gray and Roth, 2001; Van Oekelen et al., 2003). GPCR desensitization can be mediated by serine/threonine kinases, such as PKC (Benovic et al., 1985), and by G protein-coupled receptor kinases (GRKs) (Pitcher et al., 1998) that phosphorylate intracellular residues, endowing the receptor with high affinity for the polar core of β-arrestin. When bound to a GPCR, β-arrestin, creates steric hindrance, blocking (Kelly et al., 2008) that blocks heterotrimeric G proteins from coupling to the receptor, thus occluding activation of Gα proteins.
Specific mechanisms governing desensitization of 5-HT2C canonical signaling have not been reported, however, some details are known regarding desensitization mechanisms of the 5-HT2A receptor that has ~80% transmembrane sequence homology with the 5-HT2C receptor (Julius et al., 1990). For example, PKC-mediated phosphorylation of serine 421 (S421) at the C-terminus of the 5-HT2A receptor impacts the degree of agonist–induced 5-HT2A receptor desensitization and internalization (Kagaya et al., 1990; Rahman and Neuman, 1993; Bhattacharyya et al., 2002; Gray et al., 2003). The homologue of 5-HT2A receptor residue S421 in the 5-HT2C receptor is S407, suggesting PKC may also be involved in desensitization of the 5-HT2C receptor.
The objectives of this study include reporting information on 5-HT2C receptor desensitization mechanisms and ligand chemical structural properties that impact the magnitude of 5-HT2C receptor desensitization. We also investigated 5-HT2C receptor sensitization—enhanced signaling after prolonged receptor inactivation, i.e., by inverse agonists. A long-term goal of this work is to provide information for rational drug design targeting the 5-HT2C receptor to minimize 5-HT2C receptor desensitization and clinical tolerance.
5-HT2C receptor agonists studied (Fig. 1) included indoleamines (5-HT and Ro 60–0175), piperazine derivatives (1-(3-chlorophenyl) piperazine (mCPP), aripiprazole, and WAY–161503), the benzazepine lorcaserin, the phenethylamine (±) 2,5-dimethoxy-4-iodoamphetamine (DOI) and 2-aminotetralins (2S,4R)-(−)-trans-4-phenyl-N,N-dimethyl-1,2,3,4-tetrahydronaphthalen-2-amine (4-phenyl-2-dimethylaminotetralin, PAT), (2S,4R)-(−)-trans-4-(3’[meta]-bromophenyl)-N,N-dimethyl-1,2,3,4-tetrahydronaphthalen-2-amine (meta-bromo-PAT, MBP). 5-HT2C receptor inverse agonists assessed (Fig. 2) included the diazepine clozapine, the ergoline mesulergine, the benzo-dipyrrole SB 206553, and 2-aminotetralins (2S,4R)-(−)-trans-4-cyclohexyl-N,N-dimethyl-1,2,3,4-tetrahydronaphthalen-2-amine (4-cyclohexyl-2-dimethylaminotetralin, CAT), (2S,4R)-(−)-trans-4-(3’[meta]-trifluoromethylphenyl)-N,N-dimethyl-1,2,3,4-tetrahydronaphthalene-2-amine (meta–trifluoro-PAT, MFP) (Sakhuja et al., 2015; Liu et al., 2017). We assessed both canonical Gαq signaling (via production of IP) and β-arrestin recruitment. We also used site-directed mutagenesis and PKC inhibition studies to interrogate the role of phosphorylation regarding 5-HT2C receptor desensitization.
Fig. 1.
5-HT2C receptor agonists tested for desensitization.
Fig. 2.
5-HT2C receptor inverse agonists tested for sensitization.
2. Materials and methods
2.1. Compounds
All commercial compounds were ≥ 99% pure according to the manufacturer, unless noted. 5-HT hydrochloride was purchased from Alfa Aesar (Ward Hill, MA). DOI hydrochloride (> 98%), mCPP hydrochloride, Ro 60–0175 fumarate, clozapine, SB 206553 hydrochloride hydrate, and chelerythrine chloride (≥ 95%) were purchased from Sigma Aldrich (St. Louis, MO). WAY-161503 hydrochloride and mesulergine hydrochloride were purchased from Tocris Biosciences (Bristol, U.K.). Lorcaserin hydrochloride (> 98%) was purchased from Chem Scene (Monmouth Junction, NJ). [3H]Myo–inositol (specific activity 22.5 Ci/mmol) was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). The 5-HT2C receptor agonists PAT (Booth et al., 2009) and its meta–bromophenyl substituted analog, MBP (Canal et al., 2014), as well as, the 5-HT2C receptor inverse agonists MFP (Liu et al., 2017), and CAT (Sakhuja et al., 2015) were synthesized in our laboratory; free bases were converted to hydrochloride salts (Booth et al., 2009). The novel aminotetralin derivatives were > 99% pure, according to high–resolution mass spectrum, 1H and 13C nuclear magnetic resonance, and high performance liquid chromatography data (Sakhuja et al., 2015).
2.2. Human 5-HT2C receptor S407A construct
The human 5-HT2C–INI (wild-type, WT) complementary deoxyribonucleic acid (cDNA) cloned in the pcDNA3.1 + vector was obtained from Missouri S&T cDNA Resource Center (Rolla, MO). Point mutation of 5-HT2C receptor residue 407 from serine (WT) to alanine was made by polymerase chain reaction using the QuikChange II Site–Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA) according to the manufacturer’s protocol. Mutagenesis and sequencing primers were obtained from Life Technologies (Carlsbad, CA). The primers used to make the 5-HT2C–INI S407A point mutant were 5’–cgccactgctttggctgggagggagct–3’ (sense) and 5’–agctccctcccagccaaagcagtggcg–3’ (anti-sense). Polymerase chain reaction was performed as previously described, with optimization (Canal et al., 2011). Parental DNA in the reaction mixture was digested using restriction endonuclease DpnI (from diplococcus pneumoniae) at 37 °C for one hour. Digestion mixture (2 μl) was transformed into XL1–Blue supercompetent cells by heat pulse at 42 °C for 45 s. The transformed reaction was incubated in Super Optimal broth with Catabolite repression medium (Sigma–Aldrich) and then plated onto Luria–Bertani agar plates containing 100 μg/ml ampicillin. A single colony was selected for sequencing, and the point mutation was confirmed using the Sanger sequencing method by Genewiz (South Plainfield, NJ).
2.3. Cell culture and transfections
Chinese hamster ovarian K1 (CHO-K1) cells (ATCC, CCL–61) were used for 5-HT2C receptor–Gαq–IP assays. They were grown in a humidified incubator at 37 °C with 5% carbon dioxide in Dulbecco’s modified Eagle’s medium (10–013–CV, Mediatech, Manassas) with 10% fetal bovine serum and 1% penicillin/streptomycin (SV30079.01, Thermo Scientific, Waltham, MA). Cells from pass 4–20 were used in functional assays (counting pass one as first pass from a stock purchased from ATCC). CHO-K1 cells were grown to 70–80% confluency in 10 cm plates, washed with phosphate buffered saline (PBS), and then transiently transfected for 20 h overnight in an incubator with 15 μg 5-HT2C–INI pcDNA (5-HT2C WT or 5-HT2C S407A) and 30 μl Turbofect reagent (Thermo-Fisher), in 6 ml Dulbecco’s modified Eagle’s medium containing 5% dialyzed fetal bovine serum and 4 ml Opti-MEM. Initial, pilot results from [3H]mesulergine saturation binding experiments indicated that transiently transfected CHO-K1 cells expressed 5-HT2C receptor at a density of 1.5 ± 0.2 pmol/mg protein.
2.4. Desensitization and sensitization of 5-HT2C receptor canonical signaling
To assess 5-HT2C receptor desensitization, or sensitization, we measured activity at the canonical 5-HT2C–PLC/IP signaling pathway. For the control (not desensitized or sensitized) condition, 24 h post–-transfection, medium was removed from 10 cm plates containing cells at ~ 90% confluency and was replaced with 14 ml of inositol–free Dulbecco’s modified Eagle’s medium containing 5% dialyzed fetal bovine serum. Cells then were detached via scraping, and 2 μCi/ml [3H] Myo–inositol was added. Cells then were mixed by vortexing, and 270 μl of cell suspension per well was seeded into 48–well CellBind® plates (Corning, Lowell, MA) and placed in an incubator at 37 °C with 5% carbon dioxide. After 2–4 h, cells adhered to the plate. Then, 30 μl of inositol–free Dulbecco’s modified Eagle’s medium vehicle (basal condition) was added under culture conditions for 20 h. For desensitization and sensitization conditions, the same procedure as above was used except that 20–4 h after 5-HT2C–expressing cells adhered to plates, 30 μl agonist or inverse agonist test ligand at a final concentration of 1 μM was added to cells under culture conditions for 20 h. The concentration of test ligands (except aripiprazole) was > 25–fold higher than EC/IC50 values for 5-HT2C–mediated PLC/IP signaling in the control condition, i.e. treatments were at saturating concentrations, curtailing the possibility of differences in desensitization or sensitization based on divergent ligand potencies. Aripiprazole had very low potency (EC50 ~ 1 μM; Table 1) as a 5-HT2C partial agonist and it had low solubility in the desensitization assay, thus, it was used at same concentration (1 μM) as for other agonists. At 1 μM, tested ligands do not have relevant affinity for Gαq-coupled receptors endogenously expressed in CHO-K1 cells (i.e., muscarinic, purinogenic, thrombin, and calcitonin GPCRs), minimizing the possibility of off target receptor cross-talk in these studies.
Table 1.
5-HT2C agonists pEC50 and Emax values, relative to 5-HT: Inositol phosphate production.
| Agonist | pEC50 | Emax (% 5-HT) |
|---|---|---|
| 5-HT | 8.12 ± 0.10 | 100 ± 6 |
| WAY-161503 | 8.07 ± 0.08 | 100 ± 2 |
| lorcaserin | 8.20 ± 0.15 | 100 ± 1 |
| Ro 60–0175 | 8.30 ± 0.05 | 99 ± 6 |
| mCPP | 7.41 ± 0.21a | 88 ± 10 |
| DOI | 8.32 ± 0.10 | 82 ± 4 |
| MBP | 7.76 ± 0.04 | 73 ± 4a |
| PAT | 8.01 ± 0.14 | 50 ± 1a |
| aripiprazole | 6.28 ± 0.30a | 40 ± 4a |
n = 3 or 4.
= P < 0.05 different from 5-HT via one-way ANOVA.
To assess the role of PKC in ligand-induced 5-HT2C receptor desensitization, chelerythrine, a selective inhibitor of PKC (Ki = 700 nM), was included at 10 μM (i.e., > 10-times Ki to ensure interaction with PKC over the course of the assay) (Herbert et al., 1990) (Absolinova et al., 2010).
After 20 h, medium was removed, and then cells were washed with PBS three times for 5 min each. For agonist–induced desensitization, the same agonist (1–10,000 nM in inositol–free Dulbecco’s modified Eagle’s medium containing 100 mM lithium chloride (LiCl) and 10 μM pargyline) was added to cells to re–stimulate the 5-HT2C receptor; for inverse agonist sensitization, 5-HT (1–10,000 nM in inositol–free Dulbecco’s modified Eagle’s medium containing 100 mM (LiCl) and 10 μM pargyline) was added to re–stimulate the receptor. Medium was discarded 45 min later, and 400 μl of 50 mM formic acid was added to each well to stop reactions. The acid was neutralized one hour later by addition of 200 μl 150 mM ammonium hydroxide, and the culture plates were frozen at −40 °C overnight. After thawing and centrifugation at 1000g for 5 min, supernatant from the cell mixture was added to individual BioRad anion–exchange columns containing AG 1–X8 formate–form resin. After washing each loaded column with 10 ml deionized water, [3H]IP were eluted with 4 ml of 800 mM ammonium formate into vials. An aliquot of 1 ml was mixed vigorously with 10 ml scintillation cocktail (ScintiVerse Cocktail, Fisher), and [3H] scintillations were counted on a PerkinElmer Tri–Carb 2190TR liquid scintillation counter. The data presented are from three or four independent experiments that included quadruplicate samples for each data point.
2.5. 5-HT2C receptor β-arrestin recruitment
The DiscoverX PathHunter β-arrestin kit, containing human osteosarcoma U2OS cells stably expressing the 5-HT2C-INI receptor, was utilized to quantify agonist–induced β-arrestin recruitment to the 5-HT2C receptor per the manufacturer’s protocol with no deviations (DiscoverX, Fremont, CA). Cells were plated at a density of 10,000 cells/well into a 96–well plate using cell plating 19 media (DiscoverX), and treated with agonist for 2 h, followed by a 1 h enzyme complementation incubation at room temperature. Luminescence readings were obtained using a Synergy H1 reader (Biotek, Winooski, VT). The data presented are from three independent experiments that included quadruplicate samples for each data point, except for 5-HT positive control, which included two samples for each data point.
DiscoverRX does not provide information on the expression level of 5-HT2C receptor in the assay kit, however, Dr. John Allen (University of Texas Medical Branch–Galveston) kindly reported to us the cells in the DiscoverRX kit express 5-HT2C-INI at about 1.2 pmol/mg protein, measured by [3H]mesulergine saturation binding (n = 3). This receptor binding site density closely matched what we observed following transient transfections of CHO-K1 cells.
2.6. Data transformations and statistical analyses
Functional pharmacology data were analyzed using nonlinear regression curve–fitting algorithms in GraphPad Prism, 6.0 for Mac (San Diego, CA), and were fitted using the “log(agonist) vs. response (three parameters)” model. The degree of desensitization or sensitization was defined by the following equation:
Calculations of one–way analysis of variance (ANOVA) with Tukey’s multiple–comparison post hoc tests were performed to compare the effects of ligands on 5-HT2C receptor desensitization or sensitization. Calculations of two–way ANOVA with Sidak’s multiple–comparison post hoc test were performed to compare the differential effects of ligand–induced desensitization between WT and S407A 5-HT2C receptor and the effects of PKC inhibition. Pearson correlation coefficients were calculated to determine the relationship between second messenger signaling outputs (IP signaling Emax, β-arrestin recruitment Emax, IP signaling pEC50, β-arrestin recruitment pEC50) and the degree of receptor desensitization and sensitization. Ligand efficacies and potencies are presented as mean values (± standard error of the mean [S.E.M]), with the adjusted P values from aforementioned statistical analyses. Unless otherwise stipulated, P values are noted with asterisks in figures and are defined as *, P < 0.05; **, P < 0.005; ***, P < 0.001; and ****, P < 0.0001. All asterisks in figures represent differences from vehicle group unless otherwise indicated.
3. Results
3.1. 5-HT2C receptor agonist–dependent activation and β-arrestin recruitment
3.1.1. 5-HT2C receptor agonist effect on IP production after 20 h incubation of cells with vehicle only
The potencies (pEC50) and efficacies (maximal efficacy [Emax]) of agonist ligands at the 5-HT2C receptor IP signaling pathway following 20 h of incubation of cells with vehicle only are shown in Table 1. Post–hoc tests revealed that mCPP and aripiprazole were less potent than 5-HT, DOI, Ro 60–0175, WAY–161503, and lorcaserin (P < 0.05). The pEC50 values of the other 5-HT2C agonists in Table 1 were not statistically different from each other. Regarding efficacy, Ro 60–0175, WAY–161503, lorcaserin, mCPP, and DOI were full agonists, while MBP, PAT, and aripiprazole were partial agonists (P < 0.05), relative to 5-HT (Table 1).
3.1.2. 5-HT2C receptor–β-arrestin recruitment
The pEC50 and Emax values of 5-HT, Ro 60–0175, lorcaserin, MBP, and aripiprazole to recruit β-arrestin to the 5-HT2C receptor are shown in Table 2. Lorcaserin showed increased Emax relative to all other agonists tested (P < 0.05). Both MBP and aripiprazole had negligible effects on 5-HT2C receptor β-arrestin recruitment. The effect of Ro 60–0175 was not different from the endogenous ligand, 5-HT.
Table 2.
A5-HT2C agonists pEC50 and Emax values, relative to 5-HT: β-arrestin recruitment.
| Agonist | pEC50 | Emax (% 5-HT) |
|---|---|---|
| lorcaserin | 6.93 ± 0.04a | 118 ± 5a |
| 5-HT | 8.21 ± 0.08 | 100 ± 6 |
| Ro 60–0175 | 7.86 ± 0.11 | 92 ± 4 |
| MBP | 6.84 ± 0.10a | 7 ± 4a |
| aripiprazole | < 6a | 8 ± 1a |
n = 3.
= P < 0.05 different from 5-HT via one-way ANOVA.
3.1.3. Agonist-dependent desensitization of 5-HT2C receptor IP production
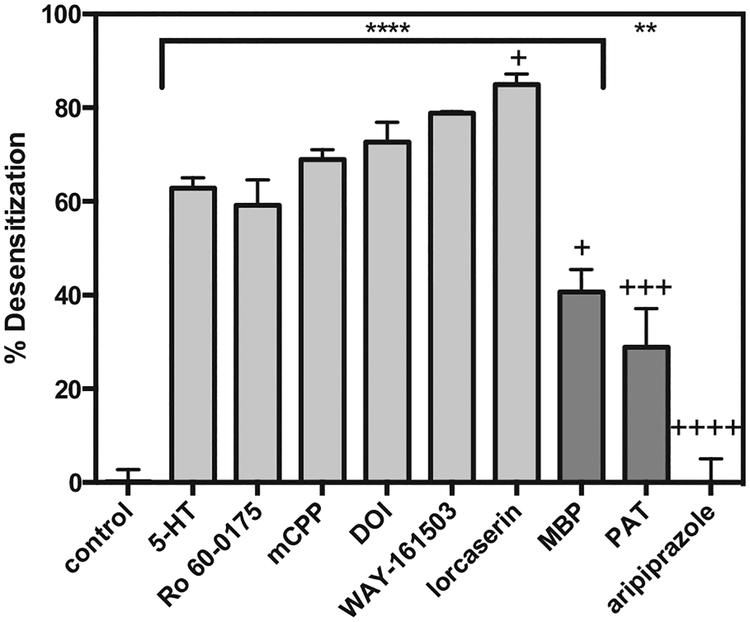
Pretreatment of 5-HT2C-expressing CHO-K1 cells for 20 h with 1 μM of test agonists (5-HT, Ro 60–0175, WAY–161503, lorcaserin, mCPP, DOI, MBP, PAT, and aripirazole) did not alter the pEC50 to stimulate IP production, (P < 0.05), data not shown. In contrast, with the exception of aripiprazole, pretreatment with test agonists resulted in desensitization as measured by a significant reduction in agonist ligand efficacy (Emax) (F9,36, = 63.5; P < 0.0001) (Fig. 3). The 5-HT2C full agonist lorcaserin produced the largest degree of desensitization, with an 85 ± 2.6% reduction in Emax after desensitization compared to control conditions. 5-HT preincubation resulted in a 63 ± 3.2% reduction in subsequent Emax. The other 5-HT2C full agonists tested (Ro 60–0175, DOI, mCPP, and WAY–161503) desensitized 5-HT2C-mediated IP signaling with a magnitude similar to 5-HT, as evidenced by reductions in Emax values from the desensitized condition that were 59 ± 5.4%, 73 ± 4.1%, 69 ± 1.8%, and 79 ± 0.5% (respectively) of the corresponding Emax values obtained from control conditions. Preincubation with the partial agonists PAT and MBP resulted in 29 ± 5.0% and 38 ± 4.9% reductions in subsequent Emax values, respectively. The rank order for agonist–induced desensitization of 5-HT2C receptor IP signaling was: lorcaserin > WAY-161503 > (±)–DOI > mCPP > 5-HT > Ro 60–0175 > MBP > PAT > aripiprazole.
Fig. 3. Agonist–induced desensitization of the 5-HT2C receptor IP pathway.
Cells expressing the 5-HT2C receptor were treated with 1 μM of agonist ligand for 20 h, washed, then re–stimulated with the same agonist (1–10,000 nM). The Emax for each agonist at the desensitized 5-HT2C receptor was compared to the Emax of the agonist at the non–desensitized 5-HT2C receptor (control condition), and reported as percent desensitization. n = 3 or 4; ** = P < 0.01 compared to control; **** = P < 0.0001 compared to control; + = P < 0.05 compared to 5-HT–induced desensitization; +++ = P < 0.001 compared to 5-HT–induced desensitization; ++++ = P < 0.0001 compared to 5-HT–induced desensitization, via one-way ANOVA.
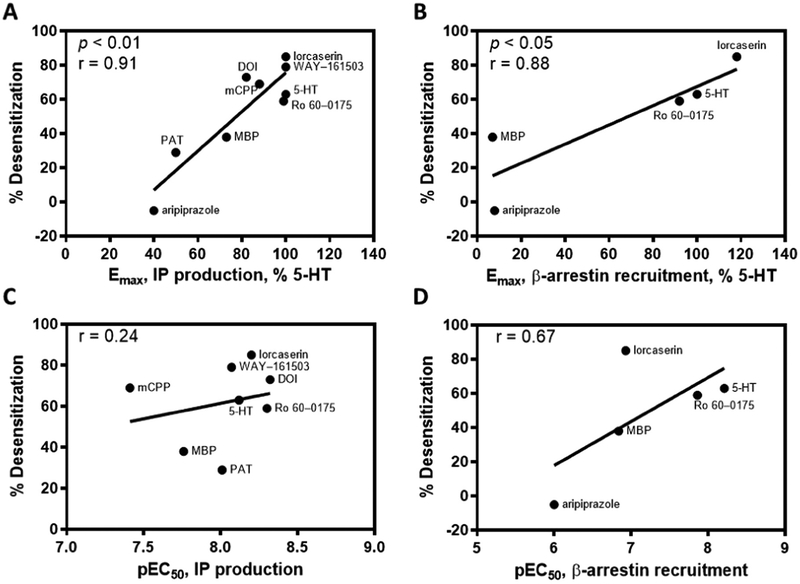
Pearson correlation analyses assessed the relationship between desensitization and ligand pharmacology at 5-HT2C receptor IP and β-arrestin signaling pathways (Fig. 4). As shown in Fig. 4A and B, there were significant positive correlations between ligand Emax values for stimulating 5-HT2C receptor IP (Fig. 4A) or β-arrestin (Fig. 4B) signaling and desensitization of IP signaling (r = 0.91, P < 0.01 and r = 0.88, P < 0.05, respectively). In contrast, ligand potency (pEC50) at either pathway did not correlate with desensitization (Fig. 4C and D).
Fig. 4.
5-HT2C receptor agonist efficacy—but not potency—at either IP or β-arrestin pathways significantly correlates with 5-HT2C agonist-induced receptor desensitization of the IP pathway. A. IP production Emax; B. β-arrestin recruitment Emax; C. IP production pEC50; D. β-arrestin recruitment pEC50.
3.2. Inverse agonist–dependent inhibition and sensitization of 5-HT2C receptor IP production
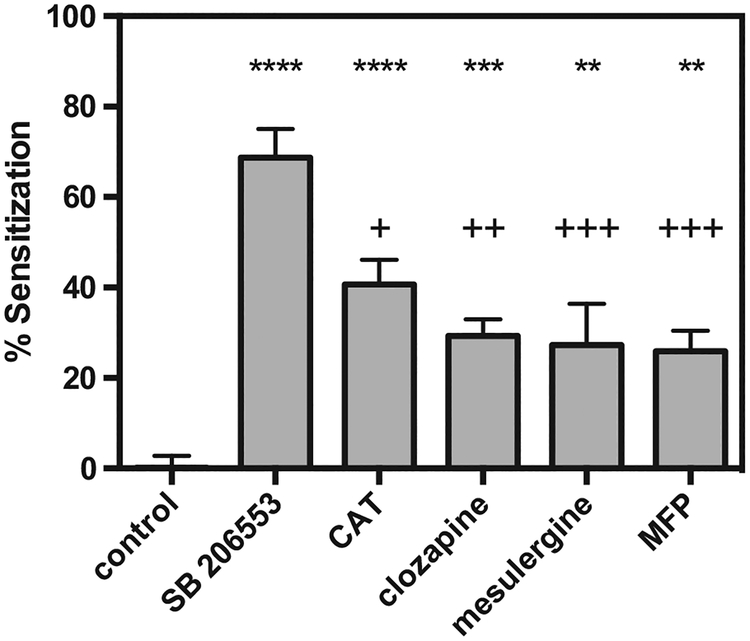
The potency and efficacy of the 5-HT2C receptor inverse agonists following 20 h of vehicle administration are shown in Table 3. Clozapine was the most efficacious, and SB 206553 was the most potent, 5-HT2C inverse agonist, respectively, at reducing basal 5-HT2C-mediated basal IP production. To assess 5-HT2C receptor sensitization, CHO-K1 cells expressing the 5-HT2C receptor were incubated with 1 μM inverse agonists for 20 h. After washing the cells, the efficacy of 5-HT to stimulate 5-HT2C–mediated IP production was assessed. Sensitization was defined as an elevated Emax value of 5-HT in the sensitization condition compared to cells without pretreatment of inverse agonists. As shown in Fig. 5, each of the 5-HT2C inverse agonists caused sensitization of the 5-HT2C receptor (F5, 25 = 27.84; P < 0.0001). The efficacy of 5-HT was increased by 69 ± 6.3%, 41 ± 4.7%, 29 ± 3.2%, 27 ± 9.1%, 26 ± 4.0% in cells pretreated with the inverse agonists SB 206553, CAT, clozapine, mesulergine, and MFP, respectively.
Table 3.
5-HT2C inverse agonists pIC50 and Imax values: Inositol phosphate reduction.
| Inverse Agonist | pIC50 | Imax (% decrease from basal) |
|---|---|---|
| clozapine | 6.89 ± 0.10 | 68 ± 4 |
| SB 206553 | 7.77 ± 0.02 | 32 ± 5 |
| CAT | 7.17 ± 0.21 | 26 ± 4 |
| MFP | 7.62 ± 0.10 | 25 ± 3 |
| mesulergine | 7.62 ± 0.13 | 17 ± 2 |
n = 3.
Fig. 5. Inverse agonist sensitization of the 5-HT2C receptor.
Cells expressing the 5-HT2C receptor were treated with 1 μM inverse agonist for 20 h, washed, then re–stimulated with 5-HT (1–10,000 nM). n = 3; ** = P < 0.01 compared to control; *** = P < 0.001 compared to control; ****= P < 0.0001 compared to control; + = P < 0.05 compared to SB 206553–induced sensitization; ++ = P < 0.01 compared to SB 206553–induced sensitization; +++ = P < 0.001 compared to SB 206553–induced sensitization, via one-way ANOVA.
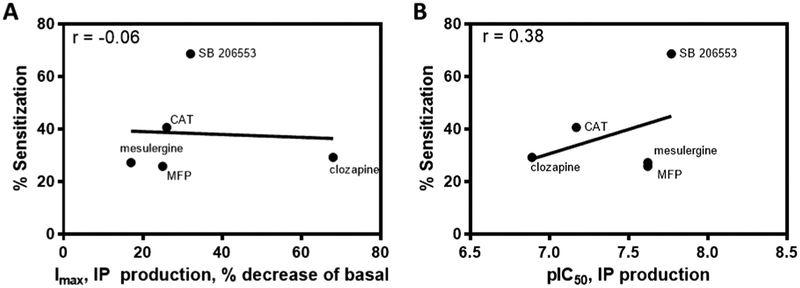
Pearson correlation analyses assessed the relationship between sensitization and inverse agonist pharmacology at inhibiting basal 5-HT2C–induced IP production (Fig. 6). As shown in Fig. 6A and B, there were no significant positive correlations between inverse agonist efficacy or potency values for inhibiting 5-HT2C IP production sensitization of IP signaling (r = −0.06 and r = 0.38, respectively).
Fig. 6.
Neither 5-HT2C receptor inverse agonist efficacy nor potency at the IP pathway correlates with 5-HT2C receptor sensitization of the IP pathway. A. IP reduction, Imax (decrease from basal); B. IP production, pIC50.
3.3. Agonist–dependent desensitization of S407A 5-HT2C receptor IP production
The 5-HT2C receptor serine residue S407, at the distal end of the intracellular C terminus, is in a position homologous to 5-HT2A receptor residue S421 that is involved in agonist–induced desensitization, putatively, via a phosphorylation event (Gray et al., 2003). To test for a functional role of S407 in 5-HT2C–IP desensitization, residue S407 was mutated to alanine (S407A), precluding its phosphorylation. 5-HT2C–IP functional assays then were performed, as above. Agonist ligands assessed were 5-HT, mCPP, RO 60–0175, DOI, lorcaserin, and MBP. The potency (but not efficacy—see below) of only mCPP at the S407A 5-HT2C receptor was significantly reduced compared to WT 5-HT2C receptor; pEC50 = 8.15 ± 0.11 and 7.41 ± 0.21 M, respectively (P < 0.05).
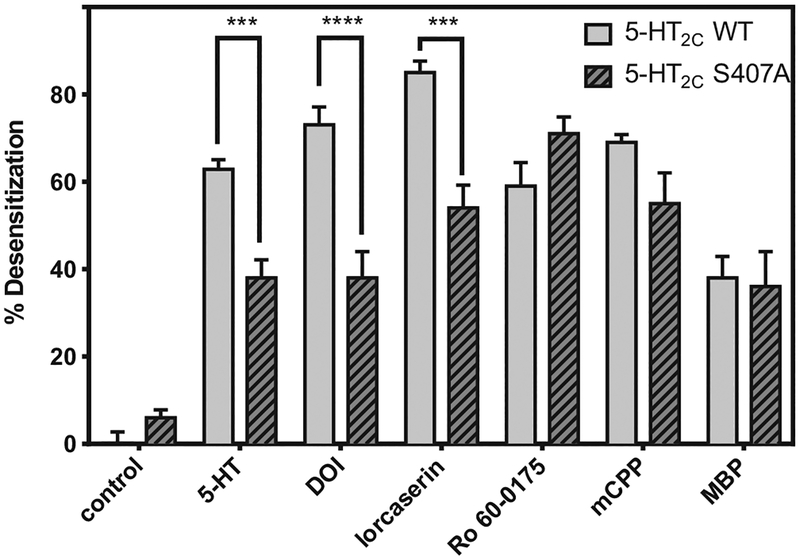
Similar to results for the WT 5-HT2C receptor, all agonists caused desensitization (lowered Emax) of S407A 5-HT2C–IP signaling, however, desensitization caused by 5-HT, DOI, and lorcaserin was blunted at S407A relative to the WT receptor (Fig. 7). The Emax of 5-HT, DOI, and lorcaserin at S407A was reduced by 38 ± 4.1%, 38 ± 6.0%, and 54 ± 5.2%, respectively, in the desensitization conditions, magnitudes significantly lower compared to desensitization observed at WT 5-HT2C (P < 0.001). The Emax for Ro 60–0175, mCPP, and MBP at S407A was reduced by 71 ± 3.8%, 55 ± 7.0%, and 36 ± 8.0%, respectively, in the desensitization conditions—magnitudes not different from desensitization observed at WT 5-HT2C.
Fig. 7. Agonist–induced desensitization of the S407A 5-HT2C receptor.
Cells expressing the WT 5-HT2C (solid bars) or S407A 5-HT2C (striped bars) receptor were treated with 1 μM of agonist ligand for 20 h, washed, then re–stimulated with the same agonist (1–10,000 nM). The Emax for each agonist at the desensitized 5-HT2C receptor was compared to the Emax of the agonist at the non–desensitized 5-HT2C receptor (control condition), and reported as percent desensitization. n = 3 or 4; *** = P < 0.001 compared to 5-HT2C WT desensitization, **** = P < 0.0001 compared to 5-HT2C WT desensitization, via two-way ANOVA.
3.4. Effect of PKC inhibition on 5-HT-induced 5-HT2C receptor desensitization
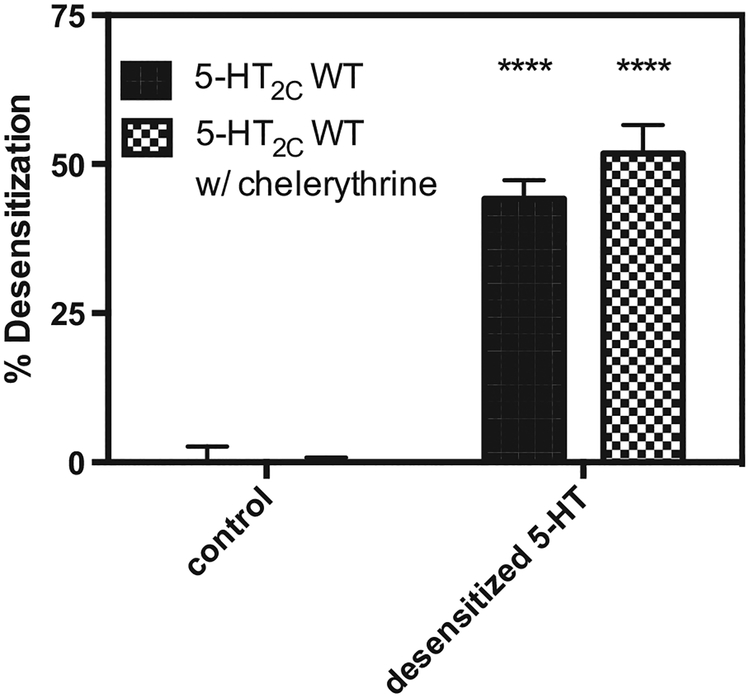
PKC is a downstream effector of the 5-HT2C receptor. We hypothesized that PKC phosphorylates activated 5-HT2C receptors contributing to desensitization via a negative feedback mechanism. To assess this hypothesis, 5-HT2C receptor–expressing cells were treated with 10 μM chelerythrine, a selective, cell–permeable PKC inhibitor, 10 min prior to performing the receptor desensitization protocol. In the absence of chelerythrine, 5-HT-induced desensitization resulted in the reduction of 5-HT efficacy to stimulate IP production by 45 ± 3.0% compared to 5-HT2C receptor–expressing cells that were not pretreated with 5-HT. When cells were treated with 10 μM chelerythrine (which has no effect on 5-HT-induced IP production in non-desensitized cells, data not shown), 10 min prior to 5-HT-induced desensitization, the efficacy of 5-HT at desensitized receptors was reduced by 52 ± 4.8%, an effect not statistically different than control conditions (n = 3) (Fig. 8).
Fig. 8. The PKC inhibitor chelerythrine did not affect 5-HT–induced 5-HT2C receptor desensitization.
Cells expressing the 5-HT2C receptor were treated with 10 μM of 5-HT and vehicle (solid bars) or 10 μM chelerythrine (checkered bars) for 1 h, washed, then re–stimulated with 5-HT (1–10,000 nM). n = 3; **** = P < 0.0001 different than 5-HT2C WT control, via two-way ANOVA.
4. Discussion
We assessed several structural classes of 5-HT2C receptor ligands and observed that desensitization and sensitization of 5-HT2C receptor canonical signaling is dependent on ligand pharmacology and structure. For example, ligands with partial agonism, including, the arylpiperazine aripiprazole and the phenylaminotetralins PAT and MBP cause nil or low desensitization, while full agonists such as indole 5-HT and benzazepine lorcaserin cause substantially more 5-HT2C receptor desensitization. We observed a significant positive correlation between agonist efficacy and desensitization magnitude at both canonical 5-HT2C–IP signaling as well as 5-HT2C–mediated β-arrestin signaling pathways, suggesting agonist efficacy impacts 5-HT2C receptor desensitization. In contrast, agonist potency did not correlate with desensitization. DOI and MBP, for example, showed similar potencies as 5-HT2C agonists, but DOI caused more robust desensitization than MBP. Regarding 5-HT2C receptor sensitization, neither inverse agonist efficacy nor potency correlated with the magnitude of sensitization.
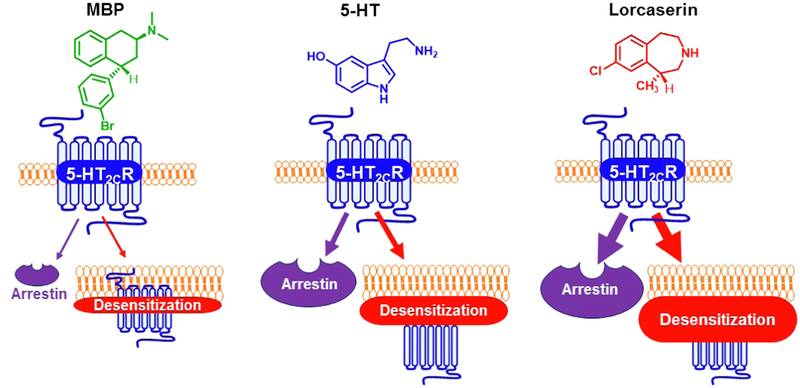
The magnitude of 5-HT2C receptor signaling desensitization was different for agonists of different structural classes. Desensitization was greatest after prolonged stimulation with the benzazepine lorcaserin. Surprisingly, lorcaserin desensitized the 5-HT2C receptor more robustly than the endogenous indole 5-HT, despite each having similar maximal efficacies (Emax) and potencies (pEC50) at the 5-HT2C IP signaling pathway. Lorcaserin also desensitized the 5-HT2C receptor more than the indole Ro 60–0175, the phenethylamine DOI, the aminotetralins MBP and PAT, the piperazines mCPP, WAY-161503, and the arylpiperazine aripiprazole, as shown in Fig. 9.
Fig. 9.
Schematic representation of 5-HT2C receptor desensitization by MBP, 5-HT, and Lorcaserin.
Lorcaserin was a “super agonist” at recruiting β-arrestin to the 5-HT2C receptor, with an efficacy significantly greater than the 5-HT2C full agonists 5-HT and Ro 60–0175. The extremely high efficacy of lorcaserin to recruit β-arrestin may account for it’s remarkable desensitization of 5-HT2C IP signaling that is more robust than any other agonist tested (Roth et al., 2017; Wacker et al., 2017; Rajagopal and Shenoy, 2018). In contrast, MBP and aripiprazole showed very low to nil efficacy to recruit β–arrestin, and they produced low to nil 5-HT2C receptor desensitization effects. These data suggest MBP and aripiprazole are biased toward PLC/IP signaling, or that a certain degree of canonical Gαq activation is required to recruit β-arrestin to 5-HT2C, which subsequently causes receptor desensitization. MBP and aripiprazole are partial agonists of the 5-HT2C–Gαq–IP pathway, and a recent report concludes that G protein activation is required for β-arrestin recruitment (Grundmann et al., 2018). MBP- and aripiprazole-mediated partial activation of 5-HT2C and Gαq may be insufficient to recruit β-arrestin, therefore preventing 5-HT2C–Gαq desensitization.
Inverse agonist ligand structure also appears to impact 5-HT2C receptor sensitization. Preincubation of the 5-HT2C receptor with the benzo-dipyrrole inverse agonist SB 206553 sensitized the 5-HT2C receptor to activation by 5-HT (i.e., increased Emax of 5-HT) to an extent that was larger than all other inverse agonists, including the aminotetralins MFP and CAT, the ergoline mesulergine, and the benzazepine clozapine. Interestingly, SB 206553 and MFP have about equal 5-HT2C receptor inverse agonist potency and efficacy, however, SB 206553 sensitized the 5-HT2C receptor to a greater extent than MFP.
The 5-HT2C receptor sensitization we observed after 20 h of inverse agonist exposure likely is due to an increase of functional receptor at the cell surface, which has been shown in 5-HT2C expressing cells treated for 18 h with 1 μM SB 206553 (Chanrion et al., 2008). During optimization, we observed that pretreatment with SB 206553 for 8 h or less does not sensitize the 5-HT2C receptor (data not shown). Similarly, others have shown that a 30 min SB 206553 pretreatment is insufficient to sensitize 5-HT2C receptors (Schlag et al., 2004). These data suggest that new mRNA translation, post-translational modifications, trafficking and insertion into the membrane may underlie 5-HT2C sensitization after inverse agonist treatment.
Like other GPCRs, ligand-induced 5-HT2C receptor desensitization and sensitization is likely a consequence of unique interactions between ligand and receptor that impact G-protein coupling, receptor phosphorylation, and β-arrestin binding (Liggett, 2011; Nobles et al., 2011; Rajagopal and Shenoy, 2018). We observed that the structure of the agonist ligand also impacts involvement of 5-HT2C receptor residue S407 (homologue of 5-HT2A receptor S421) in the desensitization process. For example, desensitization of the S407A point-mutated 5-HT2C receptor by lorcaserin, 5-HT, and DOI was less than for the WT 5-HT2C receptor. Meanwhile, desensitization by MBP, mCPP, and Ro 60–0175 was the about the same for the WT and S407A 5-HT2C receptor. These results suggest that structurally-different agonists may stabilize different active receptor conformations, altering spatial orientation of 5-HT2C receptor serine residues and perhaps impacting access of serine/threonine kinases.
Molecular modeling and mutagenesis studies reported in the literature provide support that structurally-different agonists interact with the same GPCR differently and result in different pharmacological potency and efficacy. For example, 5-HT, DOI, lorcaserin, mCPP, Ro 60–0175 and WAY-161503 (all shown here to be high efficacy agonists at the canonical 5-HT2C receptor signaling pathway) contain one or more chemical moieties capable of forming hydrogen or halogen bonding interactions. The 5-OH moiety of 5-HT is proposed to hydrogen bond with 5-HT2C receptor residues D3.32, S3.36, and Y7.43 (Bray and Goddard, 2008; Canal et al., 2011; Liu et al., 2017), putatively, stabilizing a 5-HT2C receptor conformation that leads to robust activation. Experimental results supporting this proposal include that tryptamine (same structure as 5-HT except without the 5-OH moiety) is a 5-HT2C agonist with reduced affinity and lower functional potency and efficacy than serotonin (Porter et al., 1999; Canal et al., 2011). Analogously, the aminotetralins PAT and MBP lack a hydrogen bond donor in a position that is analogous to the 5-OH moiety of 5-HT, perhaps, explaining why these compounds are partial agonists at the 5-HT2C receptor (Table 1). Thus, it appears that agonist chemical structure impacts efficacy to, in turn, impact 5-HT2C receptor desensitization.
Our data are consistent with previous observations that PKC is not involved in short-term (< 1 h) 5-HT-induced desensitization of the 5-HT2C receptor (Berg et al., 2001). It was previously shown that PKC plays a significant role only in long-term (> 24 h of agonist pretreatment) 5-HT2C receptor desensitization processes, whereas, GRK, may mediate short-term 5-HT2C receptor desensitization (< 10 min). In contrast, for the 5-HT2A receptor, there is a significant role for PKC in short–term 5-HT-induced desensitization (Anji et al., 2001; Bhattacharyya et al., 2002), indicating unique signaling regulatory mechanisms for the highly homologous 5-HT2A and 5-HT2C receptors.
5. Conclusions
In summary, our studies suggest that ligand chemical structure impacts 5-HT2C receptor desensitization and sensitization. There is a robust, positive correlation between the efficacy of 5-HT2C receptor agonist–elicited IP production and β-arrestin recruitment and the magnitude of 5-HT2C receptor desensitization. Accordingly, agonist structure, efficacy to activate G proteins, and efficacy to recruit β-arrestin should be considered when designing 5-HT2C receptor agonists to possess low desensitization liability, i.e. to avoid tolerance to clinical effects. Conversely, drug design involving 5-HT2C receptor inverse agonism should take into account the phenomenon of 5-HT2C receptor sensitization that can result in elevated endogenous 5-HT2C receptor signaling after discontinuation of the inverse agonist treatment. Results here indicated that the magnitude of 5-HT2C receptor signaling desensitization or resensitization was different for agonists or inverse agonists of different structural classes, suggesting, structure—activity relationships to inform drug design targeting the 5-HT2C (and closely-related) GPCRs could be unique for each medicinal chemical scaffold.
Acknowledgements
Drs. Myong Sang Kim, Rajeev Sakhuja, and Zhuming Sun synthesized the PAT compounds, Dr. John Allen determined expression level of 5-HT2C receptors in U2OS cells, and Dr. David Janero, Charles Perry, Caitlin Schlagal helped edit the manuscript. These studies were funded by grants from the National Institute of Mental Health and National Institute on Drug Abuse (RO1MH081193, RO1DA030989, R21DA040907), and the Department of Defense (CDMRP PR141869, AR160095). The NIMH, NIDA, and DOD did not have a role in study design, data collection, analysis, and interpretation, writing the manuscript, or in the decision to submit the paper for publication.
Footnotes
Conflict of interest
The authors declare they have no financial conflicts of interest.
References
- Absolinova H, Jancar L, Jancarova I, Vicar J, Kuban V, 2010. Spectrophotometric study of time stability and acid-base characteristics of chelerythrine and dihydrochelerythrine. Cent. Eur. J. Chem 8, 626–632. [Google Scholar]
- Anji A, Hanley N, Kumari M, Hensler J, 2001. The role of protein kinase C in the regulation of serotonin-2A receptor expression. J. Neurochem 77, 589–597. [DOI] [PubMed] [Google Scholar]
- Arena., Pharmaceuticals, 2012. FDA Briefing Document, NDA 22529 Lorcaserin hydochloride tablets, 10 mg approval letter Endocrinologic and Metabolic Drugs Advisory Committee Meeting -May 10, 2012, <http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM303198.pdf>.
- Benovic J, Pike L, Cerione R, Stanieszewski C, Yoshimasa T, Codina J, Caron M, Lefkowitz R, 1985. Phosphorylation of the mammalian beta-adrenergic receptor by cyclic amp-dependent protein kinase. J. Biol. Chem 260, 7094–7101. [PubMed] [Google Scholar]
- Berg K, Stout B, Maayani S, Clarke W, 2001. Differences in rapid desensitization of 5-hydroxytryptamine2A and 5-hydroxytryptamine2C receptor-mediated phospholipase C activation. J. Pharmacol. Exp. Ther 299, 593–602. [PubMed] [Google Scholar]
- Bhattacharyya S, Puri S, Miledi R, Panicker M, 2002. Internalization and recycling of 5-HT2A receptors activated by serotonin and protein kinase C-mediated mechanisms. Proc. Natl. Acad. Sci. USA 99, 14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R, Fang L, Huang Y, Wilczynski A, Sivendran S, 2009. 1R, 3S)-(−)-Trans-PAT: a novel full-efficacy serotonin 5-HT2C receptor agonist with 5-HT2A and 5-HT2B receptor inverse agonist/antagonist activity. Eur. J. Pharmacol 615, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray J, Goddard WI, 2008. The structure of human serotonin 2c G-protein-coupled receptor bound to agonists and antagonists. J. Mol. Graph. Model 27, 66–81. [DOI] [PubMed] [Google Scholar]
- Canal C, Booth R, Morgan D, 2013a. Support for 5-HT2C receptor functional selectivity in vivo utilizing structurally diverse, selective 5-HT2C receptor ligands and the 2,5-dimethoxy-4-iodoamphetamine elicited head-twitch response model. Neuropharmacology 70, 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal C, Cordova-Sintjago T, Liu Y, Kim M, Morgan D, Booth R, 2013b. Molecular pharmacology and ligand docking studies reveal a single amino acid difference between mouse and human serotonin 5-HT2A receptors that impacts behavioral translation of novel 4-phenyl-2-dimethylaminotetralin ligands. J. Pharmacol. Exp. Ther 347, 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal C, Cordova-Sintjago T, Villa N, Fang L, Booth R, 2011. Drug discovery targeting human 5-HT2C receptors: residues S3.36 and Y7.43 impact ligand—binding pocket structure via hydrogen bond formation. Eur. J. Pharmacol 673, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal C, Morgan D, Felsing D, Kondabolu K, Rowland N, Robertson K, Sakhuja R, Booth R, 2014. A novel aminotetralin-type serotonin (5-HT) 2C receptor-specific agonist and 5-HT2A competitive antagonist/5-HT2B inverse agonist with preclinical efficacy for psychoses. J. Pharmacol. Exp. Ther 349, 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanrion B, Mannoury la Cour C, Gavarini S, Seimandi M, Vincent L, Pujol J, Bockaert J, Marin P, Maillan M, 2008. Inverse agonist and neutral antagonist actions of antidepressants at recombinant and native 5-Hydroxytryptamine2C receptors: differential modulation of cell surface expression and signal transduction. Mol. Pharmacol 73, 748–757. [DOI] [PubMed] [Google Scholar]
- Collins GT, Gerak LR, France CP, 2017. The behavioral pharmacology and therapeutic potential of lorcaserin for substance use disorders. Neuropharmacology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J, Compton-Toth B, Roth B, 2003. Identification of two serine residues essential for agonist-induced 5-HT2A receptor desensitization. Biochemistry 42, 10853–10862. [DOI] [PubMed] [Google Scholar]
- Gray J, Roth B, 2001. Paradoxical trafficking and regulation of 5-HT2A receptors by agonists and antagonists. Brain Res. Bull 56, 441–451. [DOI] [PubMed] [Google Scholar]
- Grundmann M, Merten N, Malfacini D, Inoue A, Preis P, Simon K, Rüttiger N, Ziegler N, Benkel T, Schmitt NK, Ishida S, Müller I, Reher R, Kawakami K, Inoue A, Rick U, Kühl T, Imhof D, Aoki J, König GM, Hoffmann C, Gomeza J, Wess J, Kostenis E, 2018. Lack of beta-arrestin signaling in the absence of active G proteins. Nat. Commun 9, 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey-Lewis C, Li Z, Higgins G, Fletcher P, 2016. The 5-HT2C receptor agonist lorcaserin reduces cocaine self-administration, reinstatement of cocaine-seeking and cocaine induced locomotor activity. Neuropharmacology 101, 237–245. [DOI] [PubMed] [Google Scholar]
- Herbert J, Augereau J, Gleye J, Maggrand J, 1990. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun 172, 993–999. [DOI] [PubMed] [Google Scholar]
- Higgins G, Sellers E, Fletcher P, 2013. From obesity to substance abuse: therapeutic opportunities for 5-HT2C receptor agonists. Trends Pharmacol. Sci 34, 560–570. [DOI] [PubMed] [Google Scholar]
- Julius D, Huang K, Livelli T, Axel R, Jessell T, 1990. The 5HT2 receptor defines a family of structurally distinct but functionally conserved serotonin receptors. Neurobiology 87, 928–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagaya A, Mikuni M, Kusumi I, Yamamoto H, Takahashi K, 1990. Serotonin-induced acute resensitization receptors in human platelets via a mechanism involving protein kinase C. J. Pharmacol. Exp. Ther 255, 305–311. [PubMed] [Google Scholar]
- Kasper J, Tikamdas R, Kim M, Macfadyen K, Aramini R, Ladd J, Bisceglia S, Booth R, Peris J, 2013. The serotonin-2 receptor modulator, (−)-trans-PAT, decreases voluntary ethanol consumption in rats. Eur. J. Pharmacol 15, 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E, Bailey C, Henderson G, 2008. Agonist-selective mechanisms of GPCR desensitization. Br. J. Pharmacol 153, 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggett SB, 2011. Phosphorylation barcoding as a mechanism of directing GPCR signaling. Sci. Signal 4, pe36. [DOI] [PubMed] [Google Scholar]
- Liu Y, Canal CE, Cordova-Sintjago TC, Zhu W, Booth RG, 2017. Mutagenesis analysis reveals distinct amino acids of the human serotonin 5-HT2C receptor underlying the pharmacology of distinct ligands. ACS Chem. Neurosci 8, 28–39. [DOI] [PubMed] [Google Scholar]
- Morgan D, Canal C, Kondabolu K, Sakhuja R, Robertson K, Rowland N, Booth R, 2012. A novel serotonin-2 (5-HT2) modulator as a candidate drug to treat impulsive behavioral disorders and psychoses without weight gain as a side effect. Neuropsychopharmacology 38, S104–S105. [Google Scholar]
- Morgan D, Kondabolu K, Kuipers A, Sakhuja R, Robertson K, Rowland N, Booth R, 2013. Molecular and behavioral pharmacology of two novel orally-active 5HT2 modulators: potential utility as antipsychotic medications. Neuropharmacology 70, 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobles KN, Xiao K, Ahn S, Shukla AK, Lam CM, Rajagopal S, Strachan RT, Huang TY, Bressler EA, Hara MR, Shenoy SK, Gygi SP, Lefkowitz RJ, 2011. Distinct phosphorylation sites on the beta(2)-adrenergic receptor establish a barcode that encodes differential functions of beta-arrestin. Sci. Signal 4, ra51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil PM, Smith SR, Weissman NJ, Fidler MC, Sanchez M, Zhang J, Raether B, Anderson CM, Shanahan WR, 2012. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity(Silver Spring) 20, 1426–1436. [DOI] [PubMed] [Google Scholar]
- Pitcher J, Freedman N, Lefkowitz R, 1998. G protein-coupled receptor kinases. Annu. Rev. Biochem 67, 653–692. [DOI] [PubMed] [Google Scholar]
- Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ, 1999. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br. J. Pharmacol 128, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Neuman R, 1993. Multiple mechanisms of serotonin 5-HT2 receptor desensitization. Eur. J. Pharmacol 238, 173–180. [DOI] [PubMed] [Google Scholar]
- Rajagopal S, Shenoy SK, 2018. GPCR desensitization: acute and prolonged phases. CellSignal 41, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Irwin JJ, Shoichet BK, 2017. Discovery of new GPCR ligands to illuminate new biology. Nat. Chem. Biol 13, 1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhuja R, Kondabolu K, Cordova-Sintjago T, Travers S, Vincek A, Kim M,Abboud K, Fang L, Sun Z, Canal C, Booth R, 2015. Novel 4-substituted-N,N-dimethyltetrahydronaphthalen-2-amines: synthesis, affinity, and in silico docking studies at serotonin 5-HT2-type and histamine H1 G protein-coupled receptors. Bioorg. Med. Chem 23, 1588–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlag B, Lou Z, Fennell M, Dunlop J, 2004. Ligand dependency of 5-hydro-xytryptamine 2C receptor internalization. J. Pharmacol. Exp. Ther 310, 865–870. [DOI] [PubMed] [Google Scholar]
- Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR, 2010. Multicenter, placebo-controlled trial of lorcaserin for weight management. N. Engl. J. Med 363, 245–256. [DOI] [PubMed] [Google Scholar]
- Stout B, Clarke W, Berg K, 2002. Rapid desensitization of the serotonin2C receptor system: effector pathway and agonist dependence. J. Pharmacol. Exp. Ther 302, 957–962. [DOI] [PubMed] [Google Scholar]
- Van Oekelen D, Luyten W, Leysen J, 2003. 5-HT2A and 5-HT2C receptors and their atypical regulation properties. Life Sci 72, 2429–2449. [DOI] [PubMed] [Google Scholar]
- Wacker D, Wang S, McCorvy JD, Betz RM, Venkatakrishnan AJ, Levit A, Lansu K, Schools ZL, Che T, Nichols DE, Shoichet BK, Dror RO, Roth BL, 2017. Crystal structure of an LSD-bound human serotonin receptor. Cell 168, 377–389 (e312). [DOI] [PMC free article] [PubMed] [Google Scholar]