Abstract
Familial hypocalciuric hypercalcemia (FHH) causes hypercalcemia by three genetic mechanisms: inactivating mutations in the calcium-sensing receptor, the G-protein subunit α11, or adaptor-related protein complex 2, sigma 1 subunit. While hypercalcemia in other conditions causes significant morbidity and mortality, FHH generally follows a benign course. Failure to diagnose FHH can result in unwarranted treatment or surgery for the mistaken diagnosis of primary hyperparathyroidism (PHPT), given the significant overlap of biochemical features. Determinations of urinary calcium excretion greatly aid in distinguishing PHPT from FHH, but overlap still exists in certain cases. It is important that 24-h urine calcium and creatinine be included in the initial workup of hypercalcemia. FHH should be considered if low or even low normal urinary calcium levels are found in what is typically an asymptomatic hypercalcemic patient. The calcimimetic cinacalcet has been used to treat hypercalcemia in certain symptomatic causes of FHH.
Keywords: familial hypocalciuric hypercalcemia, Ca-sensing receptor, Hyperparathyroidism
Background
Introduction
Hypercalcemia is a frequent biochemical abnormality in adults. In most cases, if left untreated, the underlying etiology or the hypercalcemia itself will often lead to significant morbidity over time. Primary hyperparathyroidism (PHPT) is a common etiology for hypercalcemia in adults that often triggers surgical intervention, if end-organ damage is documented or if the age of the patient is young enough that potential risks of long-term observation are sufficiently uncertain to prompt definitive treatment [1]. The majority of patients with PHPT in the developed world today are diagnosed, not because their symptoms suggest PHPT, but because serum calcium, now included in many serum basic metabolic panels, is checked as part of routine clinical practice [2]. Likewise, patients may undergo testing for PHPT as part of an evaluation for secondary causes of low bone mineral density (BMD), detected by screening postmenopausal women for osteoporosis [3]. Thus, patients diagnosed in these ways lack most, if not all, of the classic symptoms of PHPT [4]. Therefore, a relatively uncommon but key diagnosis to consider in the asymptomatic hypercalcemic outpatient is familial hypocalciuric hypercalcemia (FHH). This condition has important implications for management of such a patient and, indeed, his or her entire family.
When the clinical syndrome came to attention in the early 1970’s, a term coined to describe this condition was familial benign hypercalcemia. One of the first well-described kindreds involved a proband whose affected family members all had mild hypercalcemia associated with low urinary calcium excretion [5]. The proband, a 7-year-old boy, was incidentally found to have mild hypercalcemia upon workup for headaches. He was treated with prednisone with no decrease in serum calcium. Additional evaluation included surgical exploration that revealed normal parathyroid glands and a calcium infusion during which he demonstrated an inability to suppress parathyroid hormone (PTH) levels. Increased dietary calcium intake did not increase the urine calcium excretion, and there was only a slight increase in urine calcium excretion after several days of furosemide, a loop diuretic predicted to stimulate calcium excretion. Review of the family pedigree revealed that the frequency of biochemical abnormalities was compatible with an autosomal dominant pattern of inheritance [5]. This phenotype of asymptomatic hypercalcemia and hypocalciuria appeared to be “benign” over time, with the oldest living affected relative being 76 years old. As such, the term familial benign hypercalcemia came into common use to describe this increasingly recognized disorder [5]. Over time, however, it became clear that not all patients with this form of hypercalcemia follow an entirely benign course and the term familial hypocalciuric hypercalcemia has become the preferred designation for the disorder.
Since those early reports, multiple developments in understanding the calcium-sensing receptor (CaSR), including the cloning of the CASR cDNA and genetic investigations of numerous families, have led to the elucidation of the genetic bases for three different forms of FHH. These disorders have been further characterized biochemically and clinically, and the molecular and cell biologic aspects of the many mutations have been studied in some detail. Different genotypephenotype relationships have been described, and some of the subtleties that the different disorder subtypes can produce in humans have now become increasingly evident. Additionally, the exploration of the scientific underpinnings for FHH has brought new insights into CaSR signal transduction pathways not only in the parathyroid gland and kidney, but also in tissues outside of calcium-sensing and mineral metabolism. These studies have opened up whole new fields of study and may someday yield novel therapeutics for disorders like asthma, pulmonary hypertension, cancer, Alzheimer’s disease, and osteoporosis [6].
Disorders of both CaSR inactivation and activation have also been explored as well as the downstream proteins responsible for mediating receptor activity. Disorders of CaSR constitutive activation have led to an appreciation for the causes of two inherited forms of hypoparathyroidism, namely autosomal dominant hypocalcemia (ADH) type 1 and 2. Thus, through concerted efforts of clinical and bench investigators in a dynamic bench-to-bedside translational effort, rapid understanding of previously poorly understood disorders has ensued.
Genetics and subtypes
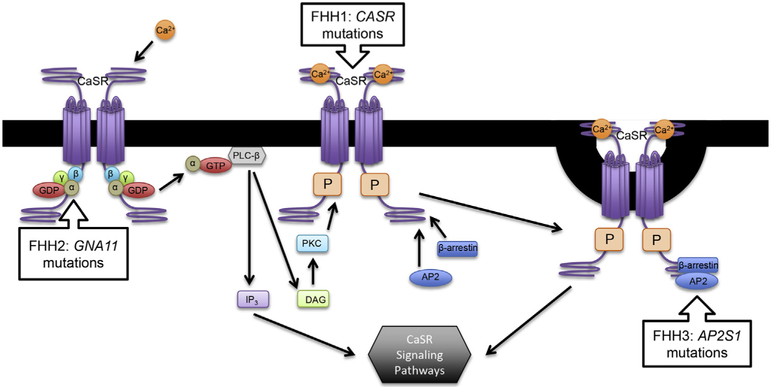
FHH1 has almost complete penetrance and follows an autosomal dominant pattern of disease inheritance. Sporadically occurring new mutations are not uncommon either occurring in 15–30% of new index cases. Three subtypes or variants have been described: FHH1, FHH2, and FHH3 (Fig. 1). The majority of cases are FHH1, due to heterozygous inactivating mutations in CASR on chromosome 3. No strong genotype-phenotype correlations have been described in terms of specific CASR mutations, and over 200 mutations in CASR have been reported (www.CASRdb.mcgill.ca). Mutations involving a region on chromosome 19p13.3 are categorized either as FHH2, if due to inactivating mutations in the GNA11 gene encoding the G-protein subunit α11 [7], or FHH3, if due to inactivating mutations in the AP2S1 gene encoding the adaptor-related protein complex 2, sigma 1 (AP2σ) subunit [8]. The phenotypes of these different subtypes of FHH are generally similar, aside from some reports of more symptomatic disease with certain cases of FHH3 with AP2S1 mutations [9,10]. As many as 13–22% of CASR mutation-negative FHH cases have been subsequently classified as FHH3 with documented heterozygous AP2S1 p.R15 mutations [11]. Studies have demonstrated that G-protein subunit α11 is a key mediator of downstream CaSR signal transduction, whereas AP2σ is involved in clathrin-mediated endocytosis of the CaSR, which has an effect on CaSR signaling and trafficking as studied in heterologous cell expression systems [9].
Fig. 1.
Schematic model of calcium-sensing receptor and selected downstream pathways.
AP2: Adaptor Protein 2, β-arrestin, Ca2+: Calcium ion, CaSR: Calcium-Sensing Receptor, DAG: Diacylglycerol, GDPαβγ: G-protein, PLC-β: Phospholipase C β, PKC: Protein kinase C, P: Phosphothreonine, IP3: Inositol triphosphate.
FHH: Familial Hypocalciuric Hypercalcemia, CASR: Calcium-sensing receptor gene, GNA11: G Protein Subunit Alpha 11 gene, AP2S1: Adaptor Related Protein Complex 2 Sigma 1 Subunit gene.
Additionally, acquired auto-antibodies that block the interaction of extracellular calcium with CaSR have been found in patients presenting with the FHH phenotype without mutations in CASR. Similarly, antibodies that activate the CaSR have been identified in rare patients with acquired hypoparathyroidism [12–14]. For the remainder of this paper, the term FHH will refer to any type of FHH in patients with phenotype of high serum calcium, low urinary calcium excretion, and non-suppressed PTH. When genetics are known, FHH1, FHH2, or FHH3 will be noted accordingly.
At the time that FHH was being described as a clinical disorder, an extremely rare but highly morbid condition of moderate to very severe hypercalcemia, often accompanied by hypercalciuria, was being reported in infants and children. This disorder, designated neonatal severe primary hyperparathyroidism (NSHPT), is characterized by severe symptomatic hypercalcemia (e.g., failure to thrive, dehydration, demineralized skeleton, rib cage deformities, fractures, hypotonia, constipation, impaired cognitive development) in the first week of life. Mortality is high, and surgical management with parathyroidectomy is generally necessary for survival. Hyperplastic parathyroid glands are typically found. However, some of the milder cases have been managed conservatively and, eventually, some of these individuals became asymptomatic.
Initially, studies of the extended kindreds of these dramatically affected infants with NSHPT linked the cases to FHH in consanguineous parents. This observation ultimately led to the conclusion that some cases of NSHPT resulted from homozygous CASR mutations from two parents carrying heterozygous CASR mutations [15–18]. Other cases, those without known parents with hypercalcemia, have been linked to sporadic heterozygous CASR mutations [19,20] that have a strong inactivating effect on parathyroid CaSR function in vivo (even in the presence of one wild-type CaSR allele). It is thought that such sporadic heterozygous mutations exert a dominant negative effect on the function of the wild-type CaSR, or that the maternal serum calcium plays a role in the regulation of serum calcium in the fetus such that the normal extracellular calcium to which the developing fetus is exposed triggers fetal parathyroid hyperplasia and PTH hypersecretion which gradually lessens postnatally but does not disappear completely.
Other CaSR-related conditions (noted above) include gain-of-function CASR mutations resulting in ADH with hypercalciuria (ADHH), or ADH1 and Bartter’s syndrome type V [21,22]. Likewise, heterozygous activating mutations in GNA11 result in ADH2 [9], withADH1 less symptomatic than ADH2 [23]. Select mutations and associated phenotypes are summarized in Table 1.
Table 1.
Calcium-sensing receptor mutations and associated phenotypes.
| Gene | Protein | Type of Mutation | Resulting Phenotype |
|---|---|---|---|
| CASR | CaSR | Inactivating Activating |
FHH1, NSHPT ADHH, ADH1, Bartter’s syndrome type V |
| GNA11 | G-protein subunit α11 | Inactivating Activating |
FHH2 ADH2 |
| AP2S1 | Adaptor-related protein complex 2, sigma 1 subunit | Inactivating | FHH3 |
Physiology of calcium-sensing and its dysregulation in FHH
The bovine parathyroid CASR cDNA was identified by an expression cloning approach in 1993 by Brown, Hebert, and colleagues [24]. Their seminal work demonstrated that calcium as well as other divalent and trivalent cations could act as extracellular first-messengers to trigger G-protein dependent signal transduction and that signaling responsiveness was conferred to heterologous cells that were transfected with CASR cDNA. The bovine parathyroid CaSR is comprised of 1085 amino acids with a very large predominantly hydrophilic N-terminal extracellular domain (613 amino acids), seven transmembrane domains characteristic of the G protein-coupled receptor superfamily (250 amino acids), and a large hydrophilic intracellular C-terminal tail (222 amino acids) [24].
The closely related human CASR was shown to be located on the long arm of chromosome 3 (3q21-q24) by linkage analysis of four unrelated kindreds with FHH [25]. The human CASR cDNA encodes 1078 amino acids with 93% sequence similarity to the bovine CaSR, also with a 612 amino acid N-terminal extracellular domain, a 250 amino acid central membrane spanning domain, and a relatively long 216 amino acid C-terminal intracellular domain [26]. While CaSRs are broadly expressed in many tissues, their effects on systemic calcium homeostasis are largely exerted by actions in the parathyroid glands, kidneys, gut, and skeleton.
The wild-type CaSR is thought to function as a homodimer (or a higher order multimer). In cases of FHH, heterodimerization between one wild-type CaSR and the other mutant CaSR appears to alter calcium-sensing function in the parathyroid glands and kidneys, producing the phenotype. The heterodimer is expected to lead to altered sensing of the ambient serum calcium concentration in FHH. It is hypothesized that mild hypercalcemia is produced because the receptor complexes (containing mutant CaSRs) fail to shut off PTH secretion efficiently, despite a mildly elevated serum calcium, and additionally, fail to excrete calcium in the urine, despite the signal of hypercalcemia [27]. In examples of other mutations in the CaSRs, receptors are synthesized within the cells but fail to be expressed on the cell surface in sufficient numbers compared to the wild-type CaSR. These hypothetical explanations result from extensive studies of transfected CaSRs in mammalian cells but are not based on CaSR expression and trafficking in parathyroid or renal cells, which would be even more informative.
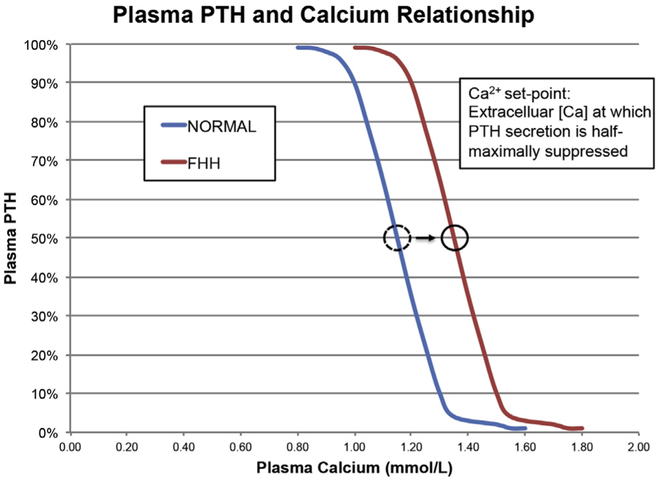
In describing the behavior of parathyroid cells in response to extracellular calcium, the concept of the calcium-PTH secretory “set point” developed. This is the extracellular calcium concentration at which PTH secretion is half-maximally suppressed. Parathyroid glands, from which cells have been made and studied from individuals with NSHPT, have shown markedly reduced sensitivity to the suppressive effects of extracellular calcium on PTH secretion [19,28]. In FHH as well, this set point is thought to be increased such that the typical inverse sigmoidal relationship between ionized calcium and plasma PTH is shifted rightward (Fig. 2). In normal humans, this set point is 1.0‒1.2 mmol/L (4.41‒4.81 mg/dL) [29]. In FHH, this abnormality results in a higher level of plasma PTH at any plasma calcium level and a higher threshold for calcium to suppress PTH [27].
Fig. 2.
Relationship between plasma PTH (expressed asa%of maximal secretion) and plasma ionized calcium (mmol/L) showing the extremely steep relationship between PTH levels in vivo and plasma calcium concentrations (blue curve). The set-point is defined as the plasma or extracellular calcium concentration at which PTH secretion is 50% suppressed versus maximal secretion. Patients with FHH typically have a modest shift to the right in the set-point for secretion as shown (red curve).
In the parathyroid glands, the Gq/11 family of G proteins represent the major signaling partners for the CaSR [7], leading to dissociation of the Gα subunits to activate phospholipase C-beta (PLC-β) enzyme which, in turn, hydrolyzes membrane constituent polyphosphoinositides to produce inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 stimulates rapid release of intracellular calcium, while DAG activates protein kinase C (PKC) and eventually the mitogen-activated protein kinase (MAPK) cascade. Together, these pathways decrease PTH secretion, preproparathyroid hormone mRNA stability and gene transcription, parathyroid cell proliferation, and renal tubular calcium absorption [30,31]. The β-arrestin protein and adaptor-related protein complex 2 (AP2) are thought to facilitate CaSR internalization via clathrin-mediated endocytosis as likely determinants of CaSR expression on the cell surface [8] (Fig. 1).
In the kidney, sensing of serum calcium and downstream effects beyond the CaSR is also abnormal in FHH. Clinically, this results in the characteristic failure to produce a hypercalciuric response to hypercalcemia, resulting in hypocalciuria or potentially inappropriately normal urinary calcium levels [32]. The CaSR is expressed throughout the kidney with the primary site of renal calcium transport determined to be the thick ascending limb (TAL) of the loop of Henle, which is responsible for the reabsorption of ~25% of filtered calcium [33]. Additional sites with purported sensitivity to extracellular calcium include the proximal tubule, collecting duct, and juxtaglomerular apparatus with varying effects of CaSR in those segments, including decrease in renin release, phosphate excretion, and calcium reabsorption via antagonizing influences on PTH and cAMP [34,35]. The renal CaSR is stimulated by high serum calcium concentration to reduce the urinary concentrating capacity via decreased expression of aquaporin-2 [36]. This leads to increased renal calcium excretion in the TAL, which is impaired in FHH due to inactivating CASR mutations. In FHH, the tubular reabsorption of calcium is higher, such that renal threshold for calcium is increased, and may additionally be subject to synergistic effects of higher PTH levels.
CaSR is widely expressed in bone, including in both osteoblasts and osteoclasts [37–40]. Stimulation of CaSRs by high (supraphysiologic) extracellular calcium levels in vitro can result in inhibition of osteoclast-mediated resorptive activities of the osteoclast [41,42]. The role of osteoblast CaSRs in vivo appears to be stimulation of bone formation, which has been demonstrated in mouse early bone development [43], although the role in adult animals and in humans is less clear. Skeletal effects of mutant CaSRs as those in individuals with FHH1 are uncertain. These individuals sometimes have frankly elevated or high normal PTH levels, which may offset the effects of inactivating mutations in their osteoblast and osteoclast CaSRs. Individuals with FHH have generally been described to have normal bone mineral density without significant perturbations in bone turnover makers [44].
Epidemiology
The prevalence of FHH is difficult to estimate since many of those affected remain asymptomatic and therefore go undetected. The majority (~65%) of cases studied are identified as FHH1 with CASR mutations, with more cases of FHH2 and FHH3 being clearly identified when sequencing studies are done looking for GNA11 and AP2S1 mutations [9]. Previous population-based studies have suggested prevalence of FHH approximately in the range of 1:10,000 to 1:100,000 [27,45]. If considered an atypical form of PHPT, FHH makes up approximately 2% of PHPT cases [46]. Of those patients who underwent failed neck exploration for PHPT, 9%‒23% of them have been reported to ultimately have FHH [27,47]. Thus, it is likely that because FHH is asymptomatic and sometimes not diagnosed, or has such a varied spectrum of clinical presentations, it is actually more prevalent than reported.
Diagnosis
Clinical presentation
While typically asymptomatic, FHH has been associated with at least a few clinically significant manifestations, particularly in those who come to medical attention. In the earlier analyses of index cases and their kindreds, those who had FHH (genotype unknown at the time) with mild hypercalcemia reported more symptoms of muscle weakness, fatigue, arthralgias, and increased thirst than their normocalcemic relatives [48]. These symptoms were seen primarily in the index cases when stratified analyses were performed. Other case reports have described acute pancreatitis [47,49], chondrocalcinosis [50], and even nephrolithiasis [51], but a separate group has challenged the link between FHH and pancreatitis [52]. Additionally, with the developments in characterizing the genetic basis of FHH, certain FHH variants (i.e., FHH3, in particular, which is discussed in Genetics and subtypessection) have been thought to lead to more symptomatic hypercalcemia [9,10] and perhaps in some individuals reduced bone mass and even osteomalacia in setting of hypophosphatemia [53]. Phenotypic distinctions may become more apparent as greater numbers of individuals undergo gene sequencing and careful clinical observations are made.
Even parathyroid adenomas have rarely been described in FHH [54], and parathyroid gland enlargement has been found in some cases of FHH, although the enlargement is not as significant as in PHPT [55]. Prior to the availability of genetic studies, clinical characteristics suggestive of FHH included family history of hypercalcemia following an autosomal dominant pattern and recurrent or persistent hypercalcemia after parathyroidectomy.
Diagnostic evaluation
Standard biochemical evaluation of hypercalcemia should be undertaken, with added emphasis on collection and interpretation of 24-h urine calcium and creatinine. Individuals with FHH have findings of lifelong hypercalcemia, typically below 3.0 mmol/L(12 mg/dL), with an inappropriately low urinary calcium excretion. Serum phosphate levels are often reduced, intact PTH levels are typically inappropriately normal in 80% of patients and mildly elevated in the remainder, 25(OH) vitamin D levels are normal, calcitriol levels are normal or elevated, renal function is preserved, and mild hypermagnesemia may be present [7,16]. Because of the significant overlap between FHH and PHPT, serum biochemical studies may not be helpful in differentiating between these two conditions.
From the 24-h urine studies, several indices have been investigated to assist with discrimination between FHH and PHPT. These include the 24-h renal calcium excretion measured directly, the 24-h renal calcium/creatinine excretion ratio (calculated as 24-h urine calcium excretion/24-h urine creatinine excretion), and the renal calcium/creatinine clearance ratio (CCCR) [calculated as (24-h urine calcium/total plasma calcium)/(24-h urine creatinine/plasma creatinine)]. Of these indices, the CCCR is the most favorable in assisting with the diagnosis of FHH [56]. Different cut-points have been described for the CCCR, where <0.01 may serve as a good separation point [47], but 20–35% of patients FHH have ratios above this point [16,56]. Other groups suggest testing for mutations in the CASR gene for all patients with CCCR of0.020 of less, which yields a 98% diagnostic sensitivity [27]. There is no consensus on this issue, and clinicians must use all the biochemical and patient and family level data to guide accurate diagnosis.
Imaging of the parathyroid glands with ultrasound is typically unrevealing. Surgically excised parathyroid glands of FHH patients have been described as slightly heavier than normal with histology showing normal or hyperplastic parathyroid tissue [57]. Additionally, investigation of hypercalcemia in younger patients should include consideration of genetic testing [58]. Ultimately, genetic testing should be considered for cases of suspected FHH given the substantial clinical and biochemical overlap with PHPT cases, especially if surgery is being considered.
Prognosis and treatment
The majority of cases of FHH do not require any treatment, and morbidity is often a result of inappropriate surgical intervention. While there are reports of symptoms and morbidity related to hypercalcemia in patients with FHH, these make up the minority of cases. However, there have been recent developments in the approach to FHH as well as potential for medical therapies in this heterogeneous population. While the convention has largely been to observe individuals with FHH due to the usual asymptomatic nature of the condition, recent literature has suggested that calcimimetic drugs could play a role in treatment of FHH when treatment is needed [59–61]. In particular, a review of case reports showed success of calcimimetic treatment in 14 of 16 cases (88%) of FHH, including FHH1, FHH3, NSHPT, and FHH cases associated with recurrent pancreatitis [62]. Treatment with cinacalcet has also been reported in a case of FHH2 [63] and 3 cases of FHH3 [60], with successful normalization of serum calcium concentrations. Caution should be exercised, however, as cinacalcet was reported to induce symptoms of hypocalcemia in a case of FHH3 in an adolescent patient with concurrent 22q11.2 deletion syndrome [64].
Subtotal parathyroidectomy is generally ineffective and therefore not recommended. Total parathyroidecomy is recommended only for the most severe cases, such as those with NSHPT, due to the obligatory resultant permanent hypoparathyroidism [59].
Research
While our knowledge of the calcium-sensing and signaling pathway has greatly expanded over the last 25 years since the cloning of the CaSR cDNA, further research on the basic calcium-signaling pathway in tissues beyond the parathyroid gland and kidney is needed. Additionally, the locations and roles of CaSR in the kidney have not been completely delineated. Future studies should investigate the molecular basis for significant overlap in CCCR between patients with FHH and PHPT. While animal studies have been able to elucidate many important mechanisms, manifestations in mice have not always correlated with findings in humans. Furthermore, the role of CaSR in the skeleton is likewise still under investigation, as targeting the CaSR in that tissue and in specific bone cell populations may have therapeutic potential [65].
Finally, in addition to the utility of using CaSR-directed therapies in the treatment of PHPT [66–70], uremic secondary HPT [71–73] and parathyroid carcinoma [74], treatment with calcimimetics has been reported to be effective in patients with FHH [59–64] and NSHPT [75] with varying degrees of success. Future controlled trials of these medications in the context of specific genetic mutations would be difficult to conduct due to the rarity of FHH and NSHPT, but small case series and reports on long-term outcomes would be beneficial to those with clinically significant symptomatic FHH.
Summary
In summary, FHH is a genetically heterogeneous condition that merits consideration in the differential diagnosis of usually mild and asymptomatic hypercalcemia. There is significant biochemical and clinical overlap with PHPT that makes discerning the diagnosis challenging, as evidenced by those who are diagnosed only after surgical intervention is unsuccessful. Inappropriately low urinary calcium excretion is a clue that FHH may be the explanation, and subsequent genetic testing for CASR mutations should be considered, particularly in cases with suggestive family history. Consideration of GNA11 or AP2S1 mutations should be entertained if FHH is highly suspected and CASR sequencing is unrevealing. Morbidity of FHH is generally low, although select cases may be amenable to calcimimetic treatment, such as those associated with significant symptoms or recurrent pancreatitis. Subtotal parathyroidectomy should be avoided, but in rare cases, total parathyroidectomy may be indicated and, in NSHPT, lifesaving.
Practice points.
FHH should be considered on the differential diagnosis of asymptomatic hypercalcemia
Calculation of the 24-h urine calcium/creatinine clearance ratio may guide the diagnostic evaluation of hypercalcemia, particularly in distinguishing FHH from PHPT
Genetic studies should be pursued when urine calcium excretion is low or inappropriately normal for serum calcium levels, particularly in younger patients
Morbidity is generally low in FHH, although mild symptoms of hypercalcemia and pancreatitis have been reported, and chondrocalcinosis has been reported with higher frequency in FHH
CASR mutations comprise the majority of FHH cases, but GNA11 and AP2S1 mutations should be considered in CASR-negative cases of suspected FHH
Calcimimetics can be considered in patients with symptomatic FHH, but surgery should generally be avoided
Research agenda.
Systematic or randomized controlled trials of calcimimetic therapy in FHH management to discern whether mutant CaSRs vary in their sensitivity to allosteric modulation by the calcimimetic cinacalcet
Further research on calcium sensing and signaling pathways in tissues where the CaSR is strongly expressed but where the physiologic functions in vivo are not well understood
Identification of the molecular pathways activated by the CaSR in tissues and other molecules with which the CaSR interacts in bringing about changes in PTH secretion, parathyroid cell proliferation, and renal effects on calcium, water and electrolyte handling
References
- [1].Wilhelm SM, Wang TS, Ruan DT, et al. The American association of endocrine surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg 2016;151:959–68. [DOI] [PubMed] [Google Scholar]
- [2].Heath H 3rd, Hodgson SF, Kennedy MA. Primary hyperparathyroidism. Incidence, morbidity, and potential economic impact in a community. N Engl J Med 1980;302:189–93. [DOI] [PubMed] [Google Scholar]
- [3].Griebeler ML, Kearns Ae, Ryu E, et al. Secular trends in the incidence of primary hyperparathyroidism over five decades (1965–2010). Bone 2015;73:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[4].Walker MD, Silverberg SJ. Primary hyperparathyroidism. Nat Rev Endocrinol 2018;14:115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[5].Foley TP Jr, Harrison HC, Arnaud CD, et al. Familial benign hypercalcemia. J Pediatr 1972;81:1060–7. [DOI] [PubMed] [Google Scholar]
- [6].Diaz-Soto G, Rocher A, Garcia-Rodriguez C, et al. The calcium-sensing receptor in health and disease. Int Rev Cell Mol Biol 2016;327:321–69. [DOI] [PubMed] [Google Scholar]
- *[7].Nesbit MA, Hannan FM, Howles SA, et al. Mutations affecting G-protein subunit alpha11 in hypercalcemia and hypocalcemia. N Engl J Med 2013;368:2476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[8].Nesbit MA, Hannan FM, Howles SA, et al. Mutations in AP2S1 cause familial hypocalciuric hypercalcemia type 3. Nat Genet 2013;45:93–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hannan FM, Babinsky VN, Thakker RV. Disorders of the calcium-sensing receptor and partner proteins: insights into the molecular basis of calcium homeostasis. J Mol Endocrinol 2016;57:R127–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hannan FM, Howles SA, Rogers A, et al. Adaptor protein-2 sigma subunit mutations causing familial hypocalciuric hypercalcaemia type 3 (FHH3) demonstrate genotype-phenotype correlations, codon bias and dominant-negative effects. Hum Mol Genet 2015;24:5079–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hendy GN, Canaff L, Newfield RS, et al. Codon Arg15 mutations of the AP2S1 gene: common occurrence in familial hypocalciuric hypercalcemia cases negative for calcium-sensing receptor (CASR) mutations.J Clin Endocrinol Metab 2014;99:E1311–5. [DOI] [PubMed] [Google Scholar]
- [12].Kifor O, Moore FD Jr, Delaney M, et al. A syndrome of hypocalciuric hypercalcemia caused by autoantibodies directed at the calcium-sensing receptor. J Clin Endocrinol Metab 2003;88:60–72. [DOI] [PubMed] [Google Scholar]
- [13].Pallais JC, Kemp EH, Bergwitz C, et al. Autoimmune hypocalciuric hypercalcemia unresponsive to glucocorticoid therapy in a patient with blocking autoantibodies against the calcium-sensing receptor. J Clin Endocrinol Metab 2011;96:672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Makita N, Sato J, Manaka K, et al. An acquired hypocalciuric hypercalcemia autoantibody induces allosteric transition among active human Ca-sensing receptor conformations. Proc Natl Acad Sci U S A 2007;104:5443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Marx SJ, Attie MF, Levine MA, et al. The hypocalciuric or benignvariantof familial hypercalcemia: clinical and biochemical features in fifteen kindreds. Medicine (Baltimore) 1981;60:397–412. [DOI] [PubMed] [Google Scholar]
- [16].Heath DA. Familial hypocalciuric hypercalcemia. Rev Endocr Metab Disord 2000;1:291–6. [DOI] [PubMed] [Google Scholar]
- [17].Pollak MR, Chou YH, Marx SJ, et al. Familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Effects of mutant gene dosage on phenotype. J Clin Invest 1994;93:1108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[18].Marx SJ, Attie MF, Spiegel AM, et al. An association between neonatal severe primary hyperparathyroidism and familial hypocalciuric hypercalcemia in three kindreds. N Engl J Med 1982;306:257–64. [DOI] [PubMed] [Google Scholar]
- [19].Bai M, Pearce SH, Kifor O, et al. In vivo and in vitro characterization of neonatal hyperparathyroidism resulting from a de novo, heterozygous mutation in the Ca2+-sensing receptor gene: normal maternal calcium homeostasis as a cause of secondary hyperparathyroidism in familial benign hypocalciuric hypercalcemia. J Clin Invest 1997;99:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pearce sH, Trump D, Wooding C, et al. Calcium-sensing receptor mutations in familial benign hypercalcemia and neonatal hyperparathyroidism. J Clin Invest 1995;96:2683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Thakker RV. Diseases associated with the extracellular calcium-sensing receptor. Cell Calcium 2004;35:275–82. [DOI] [PubMed] [Google Scholar]
- [22].Pearce SH, Williamson C, Kifor O, et al. A familial syndrome of hypocalcemia with hypercalciuria due to mutations in the calcium-sensing receptor. N Engl J Med 1996;335:1115–22. [DOI] [PubMed] [Google Scholar]
- [23].Roszko KL, Bi RD, Mannstadt M. Autosomal dominant hypocalcemia (hypoparathyroidism) types 1 and 2. Front Physiol 2016;7:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[24].Brown EM, Gamba G, Riccardi D, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 1993;366:575–80. [DOI] [PubMed] [Google Scholar]
- *[25].Chou YH, Brown EM, Levi T, et al. The gene responsible for familial hypocalciuric hypercalcemia maps to chromosome 3q in four unrelated families. Nat Genet 1992;1:295–300. [DOI] [PubMed] [Google Scholar]
- [26].Garrett JE, Capuano IV, Hammerland LG, et al. Molecular cloning and functional expression of human parathyroid calcium receptor cDNAs. J Biol Chem 1995;270:12919–25. [DOI] [PubMed] [Google Scholar]
- [27].Christensen SE, Nissen PH, Vestergaard P, et al. Familial hypocalciuric hypercalcaemia: a review. Curr Opin Endocrinol Diabetes Obes 2011;18:359–70. [DOI] [PubMed] [Google Scholar]
- [28].Marx SJ, Lasker RD, Brown EM, et al. Secretory dysfunction in parathyroid cells from a neonate with severe primary hyperparathyroidism. J Clin Endocrinol Metab 1986;62:445–9. [DOI] [PubMed] [Google Scholar]
- [29].Brent GA, LeBoff MS, Seely EW, et al. Relationship between the concentration and rate of change of calcium and serum intact parathyroid hormone levels in normal humans. J Clin Endocrinol Metab 1988;67:944–50. [DOI] [PubMed] [Google Scholar]
- [30].Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol 2003;4:530–8. [DOI] [PubMed] [Google Scholar]
- [31].Conigrave AD, Ward DT. Calcium-sensing receptor (CaSR): pharmacological properties and signaling pathways. Best Pract Res Clin Endocrinol Metab 2013;27:315–31. [DOI] [PubMed] [Google Scholar]
- [32].Toka HR, Al-Romaih K, Koshy JM, et al. Deficiency of the calcium-sensing receptor in the kidney causes parathyroid hormone-independent hypocalciuria. J Am Soc Nephrol 2012;23:1879–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Attie MF, Gill JR Jr, Stock JL, et al. Urinarycalcium excretion in familial hypocalciuric hypercalcemia. Persistence of relative hypocalciuria after induction of hypoparathyroidism. J Clin Invest 1983;72:667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Riccardi D, Brown EM. Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am J Physiol Renal Physiol 2010;298:F485–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[35].Riccardi D, Valenti G. Localization and function of the renal calcium-sensing receptor. Nat Rev Nephrol 2016;12:414–25. [DOI] [PubMed] [Google Scholar]
- [36].Sands JM, Naruse M, Baum M, et al. Apical extracellular calcium/polyvalent cation-sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct. J Clin Invest 1997;99:1399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].House MG, Kohlmeier L, Chattopadhyay N, et al. Expression of an extracellular calcium-sensing receptor in human and mouse bone marrow cells. J Bone Miner Res 1997;12:1959–70. [DOI] [PubMed] [Google Scholar]
- [38].Yamaguchi T, Kifor O, Chattopadhyay N, et al. Expression of extracellular calcium (Ca2 + o)-sensing receptor in the clonal osteoblast-like cell lines, UMR-106 and SAOS-2. Biochem Biophys Res Commun 1998;243:753–7. [DOI] [PubMed] [Google Scholar]
- [39].Chang W, Tu C, Chen TH, et al. Expression and signal transduction of calcium-sensing receptors in cartilage and bone. Endocrinology 1999;140:5883–93. [DOI] [PubMed] [Google Scholar]
- [40].Dvorak MM, Chen TH, Orwoll B, et al. Constitutive activity of the osteoblast Ca2+-sensing receptor promotes loss of cancellous bone. Endocrinology 2007;148:3156–63. [DOI] [PubMed] [Google Scholar]
- [41].Kanatani M, Sugimoto T, Kanzawa M, et al. High extracellular calcium inhibits osteoclast-like cell formation by directly acting on the calcium-sensing receptor existing in osteoclast precursor cells. Biochem Biophys Res Commun 1999;261: 144–8. [DOI] [PubMed] [Google Scholar]
- [42].Kameda T, Mano H, Yamada Y, et al. Calcium-sensing receptor in mature osteoclasts, which are bone resorbing cells. Biochem Biophys Res Commun 1998;245:419–22. [DOI] [PubMed] [Google Scholar]
- [43].Chang W, Tu C, Chen TH, et al. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci Signal 2008;1:ra1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Christensen SE, Nissen PH, Vestergaard P, et al. Skeletal consequences of familial hypocalciuric hypercalcaemia vs. primary hyperparathyroidism. Clin Endocrinol (Oxf) 2009;71:798–807. [DOI] [PubMed] [Google Scholar]
- [45].Hinnie J, Bell E, McKillop E, et al. The prevalence of familial hypocalciuric hypercalcemia. Calcif Tissue Int 2001;68:216–8. [DOI] [PubMed] [Google Scholar]
- *[46].Marx SJ. Familial hypocalciuric hypercalcemia as an atypical form of primary hyperparathyroidism. J Bone Miner Res 2018;33:27–31. [DOI] [PubMed] [Google Scholar]
- [47].Marx SJ. Familial hypocalciuric hypercalcemia: recognition among patients referred after unsuccessful parathyroid exploration. Ann Intern Med 1980;92:351. [DOI] [PubMed] [Google Scholar]
- [48].Law WM Jr, Heath H 3rd. Familial benign hypercalcemia (hypocalciuric hypercalcemia). Clinical and pathogenetic studies in 21 families. Ann Intern Med 1985;102:511–9. [DOI] [PubMed] [Google Scholar]
- [49].Davies M, Klimiuk PS, Adams PH, et al. Familial hypocalciuric hypercalcaemia and acute pancreatitis. Br Med J (Clin Res Ed) 1981;282:1023–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Volpe A, Guerriero A, Marchetta A, et al. Familial hypocalciuric hypercalcemia revealed by chondrocalcinosis. Jt Bone Spine 2009;76:708–10. [DOI] [PubMed] [Google Scholar]
- [51].Marx SJ. Familial hypocalciuric hypercalcemia. N Engl J Med 1980;303:810–1. [DOI] [PubMed] [Google Scholar]
- [52].Stuckey BG, Gutteridge DH, Kent GN, et al. Familial hypocalciuric hypercalcaemia and pancreatitis: no causal link proven. Aust N Z J Med 1990;20:718–9. 725. [DOI] [PubMed] [Google Scholar]
- [53].McMurtry CT, Schranck FW, Walkenhorst DA, et al. Significant developmental elevation in serum parathyroid hormone levels in a large kindred with familial benign (hypocalciuric) hypercalcemia. Am J Med 1992;93:247–58. [DOI] [PubMed] [Google Scholar]
- [54].Forde HE, Hill AD, Smith D. Parathyroid adenoma in a patient with familial hypocalciuric hypercalcaemia. BMJ Case Rep 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Thorgeirsson U, Costa J, Marx SJ. The parathyroid glands in familial hypocalciuric hypercalcemia. Hum Pathol 1981;12: 229–37. [DOI] [PubMed] [Google Scholar]
- [56].Christensen SE, Nissen PH, Vestergaard P, et al. Discriminative power of three indices of renal calcium excretion for the distinction between familial hypocalciuric hypercalcaemia and primary hyperparathyroidism: a follow-up study on methods. Clin Endocrinol (Oxf) 2008;69:713–20. [DOI] [PubMed] [Google Scholar]
- [57].Law WM Jr, Carney JA, Heath H 3rd. Parathyroid glands in familial benign hypercalcemia (familial hypocalciuric hypercalcemia). Am J Med 1984;76:1021–6. [DOI] [PubMed] [Google Scholar]
- [58].Starker LF, Akerstrom T, Long WD, et al. Frequent germ-line mutations of the MEN1, CASR, and HRPT2/CDC73 genes in young patients with clinically non-familial primary hyperparathyroidism. Horm Cancer 2012;3:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[59].Marx SJ. Calcimimetic use in familial hypocalciuric hypercalcemia-a perspective in endocrinology. J Clin Endocrinol Metab 2017;102:3933–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Howles SA, Hannan FM, Babinsky VN, et al. Cinacalcet for symptomatic hypercalcemia caused byAP2S1 mutations. N Engl J Med 2016;374:1396–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Brown EM. Clinical utility of calcimimetics targeting the extracellular calcium-sensing receptor (CaSR). Biochem Pharmacol 2010;80:297–307. [DOI] [PubMed] [Google Scholar]
- [62].Mayr B, Schnabel D, Dorr HG, et al. Genetics in endocrinology: gain and loss of function mutations of the calcium-sensing receptor and associated proteins: current treatment concepts. Eur J Endocrinol 2016;174:R189–208. [DOI] [PubMed] [Google Scholar]
- [63].Gorvin CM, Hannan FM, Cranston T, et al. Cinacalcet rectifies hypercalcemia in a patient with familial hypocalciuric hypercalcemia type 2 (FHH2) caused by a germline loss-of-function Galpha11 mutation. J Bone Miner Res 2018;33: 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tenhola S, Hendy GN, Valta H, et al. Cinacalcet treatment in an adolescent with concurrent 22q11.2 deletion syndrome and familial hypocalciuric hypercalcemia type 3 caused by AP2S1 mutation. J Clin Endocrinol Metab 2015;100:2515–8. [DOI] [PubMed] [Google Scholar]
- [65].Santa Maria C, Cheng Z, Li A, et al. Interplay between CaSR and PTH1R signaling in skeletal development and osteoa-nabolism. Semin Cell Dev Biol 2016;49:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Peacock M, Bilezikian JP, Klassen PS, et al. Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J Clin Endocrinol Metab 2005;90:135–41. [DOI] [PubMed] [Google Scholar]
- [67].Marcocci C, Chanson P, Shoback D, et al. Cinacalcet reduces serum calcium concentrations in patients with intractable primary hyperparathyroidism. J Clin Endocrinol Metab 2009;94:2766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Peacock M, Bolognese MA, Borofsky M, et al. Cinacalcet treatment of primary hyperparathyroidism: biochemical and bone densitometric outcomes in a five-year study. J Clin Endocrinol Metab 2009;94:4860–7. [DOI] [PubMed] [Google Scholar]
- [69].Peacock M, Bilezikian JP, Bolognese MA, et al. Cinacalcet HCl reduces hypercalcemia in primary hyperparathyroidism across a wide spectrum of disease severity. J Clin Endocrinol Metab 2011;96:E9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Khan A, Bilezikian J, Bone H, et al. Cinacalcet normalizes serum calcium in a double-blind randomized, placebo-controlled study in patients with primary hyperparathyroidism with contraindications to surgery. Eur J Endocrinol 2015;172: 527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Block GA, Bushinsky DA, Cheng S, et al. Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA 2017;317:156–64. [DOI] [PubMed] [Google Scholar]
- [72].Block GA, Bushinsky DA, Cunningham J, et al. Effectof etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA 2017;317:146–55. [DOI] [PubMed] [Google Scholar]
- [73].Investigators ET, Chertow GM, Block GA, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 2012;367:2482–94. [DOI] [PubMed] [Google Scholar]
- [74].Silverberg SJ, Rubin MR, Faiman C, et al. Cinacalcet hydrochloride reduces the serum calcium concentration in inoperable parathyroid carcinoma. J Clin Endocrinol Metab 2007;92:3803–8. [DOI] [PubMed] [Google Scholar]
- [75].Fisher MM, Cabrera SM, Imel EA. Successful treatment of neonatal severe hyperparathyroidism with cinacalcet in two patients. Endocrinol Diabetes Metab Case Rep 2015;2015:150040. [DOI] [PMC free article] [PubMed] [Google Scholar]