Abstract
Background
This study aimed to determine the diagnostic role of serum levels of complement C1q, Bb, and H in nonpregnant women, women with normal pregnancy, and women with severe pre-eclampsia.
Material/Methods
Healthy nonpregnant women (n=30), women with early, middle, and late normal pregnancy (n=30, respectively), and women with severe pre-eclampsia (n=73) were studied. The pre-eclampsia study group included early-onset cases (n=43) and late-onset cases (n=30). Serum levels of Bb were determined by enzyme-linked immunosorbent assay (ELISA), and C1q and H were tested by a turbidimetric immunoassay method.
Results
In the pre-eclampsia study group, compared with women with normal pregnancy, serum levels of C1q remained stable throughout pregnancy, and Bb levels declined from mid-pregnancy (p=0.250). Serum levels of factor H increased in the middle and late stages of pregnancy, and C1q and H were lower in early-onset severe pre-eclampsia (p<0.001, p=0.009, respectively) and late-onset severe pre-eclampsia (p<0.001, p=0.031, respectively) compared with the early-onset control and late-onset control groups. Serum levels of Bb increased in early-onset severe pre-eclampsia (p=0.001) and late-onset severe pre-eclampsia (p=0.003) compared with early-onset control and late-onset control groups. The area under the receiver operator curve (ROC) for serum C1q, Bb, and H for the diagnosis of early-onset severe pre-eclampsia were 0.814 (95% CI, 0.712–0.917), 0.743 (95% CI, 0.638–0.859), and 0.681(95% CI, 0.556–0.806), and late-onset severe pre-eclampsia were 0.805 (95% CI, 0.694–0.913), 0.796 (95% CI, 0.680–0.911), and 0.662 (95% CI, 0.524–0.800).
Conclusions
The classical and alternative pathways of complement were activated in patients with severe pre-eclampsia. Serum levels of C1q, Bb, and H should be studied further as potential diagnostic markers for severe pre-eclampsia.
MeSH Keywords: Complement C1q, Complement Factor B, Complement Factor H, Pre-Eclampsia
Background
Pre-eclampsia is associated with pregnancy and is a leading cause of maternal and perinatal morbidity and mortality. Worldwide, the incidence of pre-eclampsia is between 2–8% [1]. Pre-eclampsia is defined as new hypertension (blood pressure of ≥140/90 mmHg) with proteinuria (≥300 mg/24 h) at or after 20 gestational weeks of pregnancy [2]. The etiology and pathogenesis of pre-eclampsia may be related to an imbalance of the immune system and disturbed placental function, but remains unclear [1–4].
Complement activation plays a crucial role in sustaining a healthy pregnancy, whereas the excessive activation or inappropriate regulation of the complement system could contribute to the development of pre-eclampsia [3,5]. A recent study of human pre-eclamptic placenta showed that lack of complement function is linked to an inability to clear trophoblast from the placenta. As a consequence, fibrinoid material accumulates that can result in vascular abnormalities in the maternal circulation [5].
C1q is a recognition molecule of the classical complement pathway. Animal studies have shown that pregnant C1q-deficient (C1q−/−) mice showed some of the key features of human pre-eclampsia, including hypertension, albuminuria, and endotheliosis [6,7]. C1q has an important role in the maintenance of immune tolerance by clearing apoptotic cells and antigens, and deficiency in C1q is associated with reduced clearance of apoptotic cells [5]. Activation of the classical pathway has been shown in the kidneys of women with pre-eclampsia [8]. The alternative pathway begins with the activation of C3, which is initiated by complement factors B, D, and properdin. The complement activation product Bb is derived from factor B. Lynch and colleagues reported that circulating levels of Bb in early pregnancy were associated with the subsequent development of pre-eclampsia [9]. Factor H is an abundant glycoprotein that acts as the main regulator of the activation of C3 complement, and deficiency of factor H results in excessive C3 activation [10,11].
Therefore, this study aimed to determine the diagnostic role of serum levels of complement C1q, Bb, and H in nonpregnant women, women with normal pregnancy, and women with severe pre-eclampsia.
Material and Methods
Ethical statement
This study was approved by Peking University Third Hospital Medical Research Ethics Committee (project no. IRB00006761-M2016122, item 221-002), and written informed consent was provided by all study participants.
Study participants
Pregnant women with pre-eclampsia and healthy pregnant women were selected from the Obstetrics and Gynecology Department of the Peking University Third Hospital (Beijing, China) between January 2017 to March 2018. All participants were from the Chinese Han population.
Normal pregnant women were recruited (n=50) at the early, middle, and late stage of pregnancy when they attended hospital for routine prenatal visits. Women with multiple pregnancy, chronic hypertension, diabetes mellitus, renal disease, and autoimmune disease were excluded from the study. Blood samples were collected for routine prenatal examination, and serum samples were stored at −80°C. After delivery, their medical records were reviewed, and only women without complications (gestational hypertension, preeclampsia, gestational diabetes, pregnancy with nephropathy) throughout the pregnancy were selected. The controls were matched by age and body mass index (BMI), and included 30 normal pregnant women at early, middle, and late pregnancy.
There were 30 healthy nonpregnant women who were selected with similar age and BMI of the study group. Women with chronic hypertension, diabetes mellitus, renal disease, and autoimmune disease were excluded. The characteristic data of the study participants are shown in Table 1.
Table 1.
Clinical characteristics and complement factors levels of normal nonpregnant women and normal pregnant women.
| variable | Nonpregnancy | Early pregnancy | Middle pregnancy | Late pregnancy | P value |
|---|---|---|---|---|---|
| Age, y | 30±4 | 32±4 | 31±3 | 31±4*** | 0.514 |
| Nulliparous, n, % | – | 18 (60) | 21 (70) | 21 (70)*** | 0.796 |
| Gravidity | – | 2 (2–3) | 2 (2–3) | 2 (1–3) | 0.436 |
| Parity | – | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.524 |
| Gestational age, weeks | – | 11.0±2.1 | 24.2±2.2 | 30.1±2.8 | – |
| BMI, kg/m2 | 22.7 (19.8–26.4) | 23.6 (20.1–26.7) | 22.3 (19.1–25.4) | 20.9 (19.5–22.6)*** | 0.260 |
| Systolic BP, mmHg | 114 (93–106) | 115 (108–120) | 109 (98–115) | 117 (111–127)*** | 0.38 |
| Diastolic BP, mmHg | 75 (71–82) | 72 (65–78) | 68 (63–71) | 71 (61–80)*** | 0.42 |
| C1q, μg/mL | 196 (180–225) | 174 (163–204) | 190 (172–208) | 187 (174–223)*** | 0.060 |
| Bb, ng/mL | 408 (302–480) | 430 (371–468) | 410 (327–478) | 403 (350–452)*** | 0.250 |
| H, μg/mL | 314 (279–339) | 337 (296–374) | 385 (343–412)*,** | 370 (350–410)*,**,*** | <0.001 |
| C3, μg/mL | 1016 (905–1125) | 1103 (1010–1184)* | 1402 (1275–1528)** | 1438 (1285–1599)*** | <0.001 |
| C4, μg/mL | 187 (159–213) | 221 (201–251)* | 229 (172–289) | 223 (197–267)*** | 0.006 |
Non-normally distributed data are listed as median (IQR), normally distributed data are listed as mean ±SD.
Compared with NP, p<0.05;
compared with EP, p<0.05;
compared with MP, p>0.05;
‘−‘ – not applicable.
Forty-three patients with early-onset severe pre-eclampsia and 30 patients with late-onset severe pre-eclampsia were included in the study. Blood samples were collected after admission to hospital. One hundred healthy pregnant women were followed up throughout pregnancy. Thirty normal pregnant women were selected as the early-onset control group, and 30 normal pregnant women were included in the late-onset control group, according to the number of weeks of gestation. The patient characteristic data are shown in Table 2. Women with multiple pregnancy, chronic hypertension, diabetes mellitus, renal disease, and autoimmune disease were excluded.
Table 2.
Clinical characteristics and complememt factors levels of EOSPE, E-control, LOSPE, L-control.
| Variable | EC (n=30) | Early-onset SPE (n=43) | LC (n=30) | Late-onset SPE (n=30) | P value* | P value** | P value# | P value## |
|---|---|---|---|---|---|---|---|---|
| Age, y | 31.2±4.0 | 32.8±4.7 | 30.5±4.2 | 33.4±4.5 | 0.954 | 0.080 | 0.586 | 0.164 |
| Nulliparous, n, % | 21(70) | 25 (58.1) | 20 (67.7) | 22 (73.3) | 0.302 | 0.573 | 0.182 | 0.781 |
| Gestational age, weeks | 29.1±2.5 | 29.7+3.1 | 35.3±3.8 | 36.2±2.2 | 0.122 | 0.739 | <0.001 | <0.001 |
| BMI, kg/m2 | 22.9 (19.5–28.6) | 24.6 (22.1–28.2) | 22.0 (20–27.5) | 22.7 (20.5–28.1) | 0.073 | 0.249 | 0.146 | 0.122 |
| Systolic BP, mmHg | 116 (111–129) | 158 (149–170) | 115 (94–127) | 157 (144–162) | <0.001 | −5.435 | 0.224 | 0.307 |
| Diastolic BP, mmHg | 72 (63–80) | 100 (95–110) | 74 (71–82) | 97 (89–103) | <0.001 | <0.001 | 0.015 | 0.477 |
| Proteinuria,g/24 h | <0.3 | 1.63 (0.75–4.90) | <0.3 | 0.86 (0.40–1.84) | <0.001 | <0.001 | 0.015 | - |
| C3, μg/mL | 1438 (1264–1603) | 1120 (1019–1311) | 1434 (1271–1602) | 1196 (1012–1340) | <0.001 | <0.001 | 0.480 | 0.906 |
| C4, μg/mL | 223 (197–269) | 139 (107–178) | 235 (188–280) | 136 (103–180) | <0.001 | <0.001 | 0.426 | 0.408 |
| C1q, μg/mL | 187 (148–247) | 159 (142–176) | 194 (179–211) | 155 (129–177) | <0.001 | <0.001 | 0.071 | 0.325 |
| Bb, ng/mL | 389 (312–470) | 491 (423–669) | 374 (323–418) | 503 (367–773) | 0.001 | 0.003 | 0.695 | 0.487 |
| H, μg/mL | 370 (339–398) | 351 (319–374) | 366 (348–408) | 354 (321–372) | 0.009 | 0.031 | 0.964 | 0.779 |
Non-normally distributed data are listed as median (IQR), normally distributed data are listed as mean ±SD. SPE – severe pre-eclampsia; EC – controls for early-onset SPE; LC – controls for late-onset SPE.
Early-onset SPE vs. EC;
late-onset SPE vs. LC;
early-onset SPE vs. late-onset SPE;
EC vs. LC; ‘−‘ not applicable.
Early-onset pre-eclampsia was defined as pre-eclampsia that occurred before 34 weeks of gestation, whereas late-onset pre-eclampsia was defined as pre-eclampsia that developed after 34 weeks of gestation [12]. Severe pre-eclampsia was defined according to guidelines provided by American College of Obstetricians and Gynecologists (ACOG) [13], and included systolic blood pressure ≥160 mmHg or diastolic blood pressure ≥110 mmHg, onset of hypertension after 20 weeks of gestation, thrombocytopenia, impaired liver function, progressive renal insufficiency, pulmonary edema, and new-onset cerebral or visual disturbances. Patients with pre-eclampsia with any of these features were diagnosed with severe pre-eclampsia.
Serum sample preparation
Fasting blood samples were collected. Serum samples were obtained by centrifugation at 3000×g for 10 min at 4°C. Samples were centrifuged and stored within 4 hours. The serum samples were frozen at −80°C until further investigation.
Biochemical analysis of complement factors
Complement factors C1q, C3, C4, and factor H were measured using an immune transmission turbidity method on an automated AU 5800 biochemical analyzer (Beckman Coulter, Brea, CA, USA). The reagents used for the detection of C1q and factor H were from Shanghai Beijia Biochemical Reagent Co. Ltd. (No. 20170922 and No. 20183020). The reagents used for the detection of C3 and C4 were from Diasys Diagnostic System (Shanghai) Co., Ltd (Shanghai) (No. 23889 and No. 23539). All reagents were applied with matching calibrators and quality control material. Bb was measured using an enzyme-linked immunosorbent assay (ELISA) kits from Quidel (San Diego, CA, USA).
Statistical analysis
Data were analyzed using SPSS version 22.0 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 5 (GraphPad Inc., San Diego, CA, USA). The Shapiro-Wilk test was used to assess the normality of continuous variables. Data with normal distribution were presented as the mean and standard deviation (SD). Data with skewed distribution were presented as the median and interquartile range (IQR). To compare continuous variables among multiple groups, we applied the analysis of variance (ANOVA) to compare the mean between groups, if normally distributed, or the Kruskal-Wallis test, if not normally distributed. To compare means between two groups, the independent t-test was used if normally distributed, or the non-parametric test if not normally distributed. Categorical variables were presented as frequency and percentage. To compare categorical variables between multiple groups, the chi-squared (χ2) or Fisher’s exact test were applied. Receiver operating characteristic (ROC) area under the curve (AUC) was analyzed to determine the diagnostic role of complement factors. A P-value <0.05 was considered to be statistically significant.
Results
Serum levels of C1q, Bb and H in normal nonpregnant women, and normal pregnant women
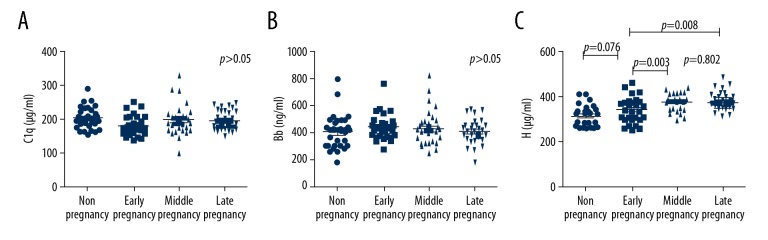
Clinical characteristics of study groups and serum levels of complement factors are shown in Table 1. The differences in serum levels of C1q and Bb among the groups of normal nonpregnant women, early-stage, middle-stage, and late-stage pregnant women did not show statistical significance (p=0.061, p=0.250). Serum levels of factor H increased from middle pregnancy. Serum H levels showed significant differences between early and middle pregnancy (p=0.003), early and late pregnancy (p=0.008) (Table1, Figure 1).
Figure 1.
Complement factor levels of normal nonpregnant women and normal pregnant women. (A) Serum levels of C1q. (B) Serum levels of Bb. (C) Serum levels of complement factor H. Serum levels of (A) C1q, (B) Bb, and (C) H were analyzed in healthy nonpregnant women (n=30), normal pregnant women at early pregnancy (n=30), middle pregnancy (n=30), and late pregnancy (n=30).
Serum C1q, Bb and H levels in early-onset and late-onset severe pre-eclampsia and early-onset control and late-onset control groups
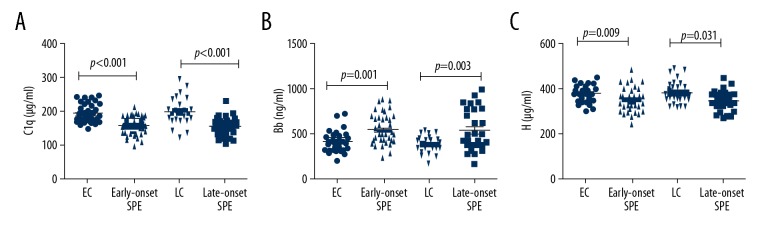
Demographic data and biochemical characteristics of the four groups are shown in Table 2. The serum levels of C1q, H, C3 and C4 of early-onset severe pre-eclampsia group were significantly decreased compared with the early-onset control group (p <0.001, p=0.009, p<0.001 and p<0.001, respectively). The serum levels of factor Bb were increased in the early-onset severe pre-eclampsia group compared with the early-onset control group (p=0.001) (Figure 2).
Figure 2.
Complement factor levels of early-onset and late-onset severe pre-eclampsia (SPE) groups and the control groups for early-onset (EC) and late-onset (LC) group SPE. (A) Serum levels of C1q. (B) Serum levels of Bb. (C) Serum levels of complement factor H. Serum levels of (A) C1q, (B) Bb, and (C) H were analyzed in patients with early-onset severe pre-eclampsia (n=43) and the early-onset control group (n=30), patients with late-onset severe pre-eclampsia patients (n=30) and the late-onset control group (n=30). SPE – severe pre-eclampsia; EC – control group for early-onset SPE; LC – control group for late-onset severe pre-eclampsia.
Serum levels of C1q, H, C3 and C4 of late-onset severe pre-eclampsia group were also significantly decreased compared with late-onset control group (p<0.001, p=0.031, p<0.001 and p<0.001, respectively). The serum levels of Bb were increased in the late-onset severe pre-eclampsia group compared with the late-onset control group (p=0.003) (Figure 2). There were no significant differences of the serum levels of C1q, Bb, H, C3 and C4 between early-onset severe pre-eclampsia and late-onset severe pre-eclampsia groups or between the early-onset control and late-onset control groups (Table 2).
Receiver operating characteristic (ROC) area under the curve (AUC) analysis
As shown in Table 3, the ROC AUC for serum C1q, Bb, H diagnosed early-onset severe pre-eclampsia were 0.814 (95% CI, 0.712–0.917), 0.743 (95% CI, 0.638–0.859), 0.681 (95% CI, 0.556–0.806); for diagnosed late-onset severe pre-eclampsia were 0.805 (95% CI, 0.694–0.913), 0.796 (95% CI, 0.680–0.911), 0.662 (95% CI, 0.524–0.800). The AUC ROC for combined C1q, Bb, H, C3 and C4 diagnosed early-onset severe pre-eclampsia were 0.916 (95% CI, 0.879–0.964); for diagnosed late-onset severe pre-eclampsia were 0.939 (95% CI, 0.901–0.977).
Table 3.
ROC analyses of serum complement factors in pregnant women with SPE.
| Variable | AUC | P value | 95% CI |
|---|---|---|---|
| Early-onset SPE | |||
| C1q | 0.814 | <0.001 | 0.712–0.917 |
| H | 0.681 | <0.001 | 0.556–0.806 |
| Bb | 0.743 | <0.001 | 0.638–0.859 |
| C3 | 0.803 | <0.001 | 0.768–0.939 |
| C4 | 0.812 | <0.001 | 0.713–0.912 |
| Combined* | 0.916 | <0.001 | 0.879–0.964 |
| Late-onset SPE | |||
| C1q | 0.805 | <0.001 | 0.697–0.913 |
| H | 0.662 | 0.031 | 0.524–0.800 |
| Bb | 0.796 | <0.001 | 0.680–0.911 |
| C3 | 0.805 | <0.001 | 0.761–0.906 |
| C4 | 0.821 | <0.001 | 0.699–0.943 |
| Combined* | 0.939 | <0.001 | 0.901–0.977 |
SPE – severe pre-eclampsia;
ROC analysis with all the 5 complement factors: C1q, H, Bb, C3 and C4.
Discussion
Pre-eclampsia is a heterogeneous disorder and is subdivided into early-onset and late-onset disease [12]. Previous studies have shown that early-onset and late-onset pre-eclampsia may have different pathogenesis [14,15]. In this study, we divided the severe pre-eclampsia into two groups: early-onset severe pre-eclampsia and late-onset severe pre-eclampsia, and we investigated the complement activation factors C1q and Bb, the regulatory complement factor H, and complement C3 and C4 in the maternal circulation.
In the present study, the findings showed that in normal pregnancy, serum levels of C3, C4, and factor H increased significantly in middle and late pregnancy, which may be due to the activation of the complement pathways in a healthy pregnancy, as increased factor H helps to regulate the alternative pathway. The classical pathway can be activated by apoptotic cells formed in trophoblast during normal pregnancy [3]. Overactivation of factor H occurs in the placenta of patients with pre-eclampsia associated with oxidative stress [3,7].
The serum levels of C1q remains relatively stable throughout normal pregnancy, which may help to maintain moderate activation of the classical pathway in normal pregnancy. In this study, the median serum Bb levels in early pregnancy were higher than those in middle and late pregnancy, but this difference did not reach statistical significance. It has previously been suggested that the activation of the alternative pathway in early pregnancy is to protect the fetus and placenta against infection by microorganisms [16].
The findings from the present study showed that serum levels of complement C1q and H decreased in women with early-onset severe pre-eclampsia and late-onset severe pre-eclampsia when compared with the control groups. This finding is supported by the findings of Agostinis et al. [17] and Derzsy et al. [3]. Reduced levels of C1q is associated with a reduced level of C4, which may be due to increased consumption of C4 and indicates the abnormal activation of the classical pathway of the complement system [18]. The reduction in levels of factor H may be caused by deficiency or consumption of factor H, which results in dysregulation of complement activation [3,19]. Although it has previously been reported that serum levels of C1q decreased in early-onset severe pre-eclampsia but increased in late-onset severe pre-eclampsia [20], we found no significant difference of C1q levels between women with early-onset severe pre-eclampsia and late-onset severe pre-eclampsia. However, further studies are needed to support the findings of the present study, which indicated that the classical pathway is activated in both early-onset severe pre-eclampsia and late-onset severe pre-eclampsia.
In this study, we found increased serum levels of complement Bb in both the early-onset and late-onset severe pre-eclampsia groups. A previous study by He et al. [20] had also shown similar results and suggested that increased plasma levels of Bb during early pregnancy were associated with the pathogenesis of pre-eclampsia [16]. The findings of these studies indicated that the alternative pathway was activated in severe pre-eclampsia, and Bb could be one of the initial factors of pre-eclampsia.
We also evaluated the diagnostic role of serum levels of complement factors C1q, Bb, and H for early-onset and late-onset severe pre-eclampsia. As shown in Table 3, among these three factors, C1q showed a better area under the curve (AUC) of 0.814 for early-onset and 0.805 for late-onset severe pre-eclampsia. The best AUC result was when the five complement factors were combined and were 0.916 for early-onset and 0.939 for late-onset severe pre-eclampsia. These complement factors may have potential as diagnostic markers for severe pre-eclampsia.
This study had several limitations. This study had a small sample size and was a case-control study, which may have resulted in selection bias. Also, a few selected complement factors only were investigated, and other complement activation factors, such as C3a, C5a, and C4d, were not studied. Also, we only investigated severe pre-eclampsia and did study women with mild pre-eclampsia.
Conclusions
This study aimed to determine the diagnostic role of serum levels of complement C1q, Bb, and H in nonpregnant women, women with normal pregnancy, and women with severe pre-eclampsia. The classical and alternative pathways of complement were activated in women with severe pre-eclampsia, and serum levels of C1q, Bb, and H should be studied further as potential diagnostic markers for severe pre-eclampsia.
Footnotes
Source of support: Peking University Third Hospital Clinical Key Project Youth Project (Y76439-01)
Conflict of interest
None.
References
- 1.Steegers EA, von Dadelszen P, Duvekot JJ, et al. Pre-eclampsia. Lancet. 2010;376(9741):631–44. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 2.Milne F, Redman C, Walker J, et al. The pre-eclampsia community guideline (PRECOG): how to screen for and detect onset of pre-eclampsia in the community. BMJ. 2005;330(7491):576–80. doi: 10.1136/bmj.330.7491.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derzsy Z, Prohaszka Z, Rigo J, Jr, et al. Activation of the complement system in normal pregnancy and pre-eclampsia. Mol Immunol. 2010;47(7–8):1500–6. doi: 10.1016/j.molimm.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed A, Singh J, Khan Y, et al. A new mouse model to explore therapies for pre-eclampsia. PLoS One. 2010;5(10):e13663. doi: 10.1371/journal.pone.0013663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lokki A, Heikkinen-Eloranta J, Jarva H, et al. Complement activation and regulation in preeclamptic placenta. Front Immunol. 2014;5:312. doi: 10.3389/fimmu.2014.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh J, Ahmed A, Girardi G. Role of complement component C1q in the onset of pre-eclampsia in mice. Hypertension. 2011;58(4):716–24. doi: 10.1161/HYPERTENSIONAHA.111.175919. [DOI] [PubMed] [Google Scholar]
- 7.Agostinis C, Tedesco F, Bulla R. Alternative functions of the complement protein C1q at embryo implantation site. J Reprod Immunol. 2017;119(2):74–80. doi: 10.1016/j.jri.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Penning M, Chua JS, van Kooten C, et al. Classical complement pathway activation in the kidneys of women with preeclampsia. Hypertension. 2015;66(1):117–25. doi: 10.1161/HYPERTENSIONAHA.115.05484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch AM, Murphy JR, Byers T, et al. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of pre-eclampsia. Am J Obstet Gynecol. 2008;198(4):385e1–e9. doi: 10.1016/j.ajog.2007.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Józsi M, Zipfel PF. Factor H family proteins and human diseases. Trends Immunol. 2008;29(8):380–87. doi: 10.1016/j.it.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Parente R, Clark SJ, Inforzato A, Day AJ. Complement factor H in host defense and immune evasion. Cell Mol Life Sci. 2017;74(9):1605–24. doi: 10.1007/s00018-016-2418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22(2):143–48. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 13.Roberts JM, August PA, Bakris G, et al. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 14.Nelson DB, Ziadie MS, McIntire DD, et al. Placental pathology suggesting that pre-eclampsia is more than one disease. Am J Obstet Gynecol. 2014;210(1):66.e1–7. doi: 10.1016/j.ajog.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Raymond D, Peterson E. A critical review of early-onset and late-onset pre-eclampsia. Obstet Gynecol Surv. 2011;66(8):497–506. doi: 10.1097/OGX.0b013e3182331028. [DOI] [PubMed] [Google Scholar]
- 16.Lynch AM, Wagner BD, Giclas PC, et al. The relationship of longitudinal Levels of complement Bb during pregnancy with pre-eclampsia. Am J Reprod Immunol. 2016;75(2):104–11. doi: 10.1111/aji.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agostinis C, Stampalija T, Tannetta D, et al. Complement component C1q as a potential diagnostic but not predictive marker of pre-eclampsia. Am J Reprod Immunol. 2016;76(6):475–81. doi: 10.1111/aji.12586. [DOI] [PubMed] [Google Scholar]
- 18.Kouser L, Madhukaran SP, Shastri A, et al. Emerging and novel functions of complement Protein C1q. Front Immunol. 2015;6:317. doi: 10.3389/fimmu.2015.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zipfel PF. Complement factor H: Physiology and pathophysiology. Semin Thromb Hemost. 2001;27(3):191–99. doi: 10.1055/s-2001-15248. [DOI] [PubMed] [Google Scholar]
- 20.He Y, Xu B, Song D, et al. Expression of the complement system’s activation factors in plasma of patients with early/late-onset severe pre-eclampsia. Am J Reprod Immunol. 2016;76(3):205–11. doi: 10.1111/aji.12541. [DOI] [PubMed] [Google Scholar]