Abstract
Background
Homelessness is uncommon but is frequently a characteristic in adults with invasive pneumococcal disease (IPD). In Calgary, homeless persons comprise approximately 0.2% of the population. We evaluated the relationship of homelessness and IPD in Calgary.
Methods
Demographic, clinical, and microbiologic data were collected by the Calgary Streptococcus pneumoniae Epidemiology Research (CASPER) team through prospective, population-based surveillance of all IPD cases. Here, we report on cases in adults (≥18 years) from 2000 to 2016.
Results
Of 1729 IPD cases, 321 (18.8%) occurred in homeless persons. Compared with nonhomeless persons, homeless persons were younger, more often male, smokers, alcohol abusers, illegal drug users, and had a primary diagnosis of pneumonia. In multivariable models of outcomes, homeless persons had lower odds of being admitted to the ICU (odds ratio [OR], 0.7; P = .02) and lower odds of death (OR, 0.6; P = .146). IPD caused by serotypes 4, 5, or 8, which have caused outbreaks in Calgary, was more common in homeless persons (54.4% vs 21.0%; P < .001). In addition, regardless of homeless status, persons with IPD caused by serotypes 4, 5, or 8 had lower odds of ICU admission and mortality (OR, 0.7; P = .017; and OR, 0.4; P = .004; respectively).
Conclusions
Homelessness is overrepresented in IPD cases in Calgary, despite most homeless persons having fewer risk factors than the overall population of persons with IPD. Most cases are caused by serotypes in both the 23-valent polysaccharide vaccine and the 13-valent conjugate vaccine. Thus, enhanced efforts are needed to deliver both vaccines to this vulnerable population.
Keywords: homelessness, pneumococcal vaccine, Streptococcus pneumoniae
Streptococcus pneumoniae commonly colonizes the upper airway asymptomatically, primarily in healthy children. It can cause invasive or noninvasive infections at all ages, with peaks in young children and older adults, and is a leading cause of infectious disease morbidity and mortality worldwide [1, 2].
Polyvalent protein–polysaccharide pneumococcal conjugate vaccines for routine use in infants and children have led to the near elimination of vaccine serotype invasive pneumococcal disease (IPD) in vaccinated children [3]. They have also resulted in a decline of vaccine serotype disease in unvaccinated adults through an indirect herd effect [3–5]. In Alberta, the 7-valent pneumococcal conjugate (PCV7) vaccine was introduced for routine use in infants in Alberta in 2002 and was replaced by the 13-valent pneumococcal protein–polysaccharide conjugate vaccine (PCV13) in 2010. Vaccination with PPV23, a 23-valent pneumococcal polysaccharide vaccine, is recommended and publicly funded for all adults aged 65 years and older, as well as younger persons with high-risk conditions [6]. Similar to PPV23, PCV13 is recommended, but not publicly funded, for older adults and is recommended and publicly funded for persons age 2–65 years with high-risk conditions [7]. Those with high-risk conditions are recommended to receive both vaccines, preferably PCV13 followed by PPV23 [6, 7].
Vaccine uptake is low in disadvantaged adult populations, including homeless persons. In Canada, homelessness is described as a continuum of living situations, ranging from individuals without any type of shelter to individuals with insecure housing [8, 9]. It is estimated that homeless persons currently comprise approximately 0.2% of the adult Calgary population (2911 persons in 2018) [10]. PPV23 has been recommended for use in homeless persons in Canada since 2008 [11], but PCV13 has not yet been recommended for routine use in homeless persons in Canada. The purpose of this study was to explore IPD trends and outcomes in the homeless adult population in Calgary, Alberta.
METHODS
Population
The study population included all adults aged 18 years and older diagnosed with IPD at a health care facility in the Calgary Zone of Alberta Health Services (Calgary Health Region before 2009) between January 1, 2000, and December 31, 2016. If an adult presented with more than 1 episode of IPD within the study time period, all episodes were included.
Data Collection
The Calgary Area Streptococcus pneumoniae Epidemiology Research (CASPER) team has conducted prospective, active population-based surveillance of all IPD cases in the Calgary area since 1998. This study reports on adult (≥18 years of age) cases identified from 2000 to 2016. The CASPER team was notified by the Calgary central laboratory system whenever a case of IPD was identified from a patient who presented to a health care facility within the Calgary Zone. As a part of the province-wide Alberta Health Services publicly funded health system, the Calgary Zone includes the city of Calgary and several neighboring communities (population ~1.62 million in 2016, with 1.31 million aged 16 and older) in the province of Alberta, Canada [12]. Cases of IPD are defined as an individual presenting with an acute infection and 1 or more cultures yielding S. pneumoniae from normally sterile body fluids. After case identification, members of the CASPER team conducted a detailed chart review and interview for patients who consented to participation. Demographic information, pertinent medical history, and current health status were collected. Pneumococcal vaccination history for each case was extracted from the chart review, patient interview, and, for cases since 2010, from an electronic public health database (Meditech).
Definitions
Based on the continuum of homelessness in Canada, the Canadian Observatory on Homelessness (COH) classified homelessness into 3 major categories: homeless not in a shelter (living outside or in places not intended for human habitation), homeless in a shelter (staying in overnight shelters), and provisionally accommodated (temporary living arrangements such as jail, hotel, or with others) [8]. The COH also includes a fourth category, at risk of homelessness, describing individuals who are not currently homeless but are at risk of losing their homes or live in substandard housing. The CASPER study collected data to categorize cases in the first 3 categories, but not the fourth.
Measured comorbidities include conditions known to increase the risk of pneumococcal disease in adults, as defined in the Canadian Immunization Guide [13]. These comorbidities were defined as immunocompetent (chronic cerebrospinal fluid [CSF] leak, chronic neurologic condition that may impair clearance of oral secretions, chronic heart disease, diabetes mellitus, chronic kidney disease, chronic liver disease including hepatic cirrhosis due to any cause, and chronic lung disease including asthma requiring medical care in the preceding 12 months) or immunocompromising (sickle cell disease, congenital or acquired asplenia or splenic dysfunction, congenital immunodeficiencies involving any part of the immune system, immunocompromising therapy including use of long-term corticosteroids, chemotherapy, radiation therapy, post–organ transplant therapy, people with HIV infection, hematopoietic stem cell transplant [recipient], malignant neoplasms including leukemia and lymphoma, nephrotic syndrome, and solid organ or islet transplant [candidate or recipient]).
Smoking status was defined as current smoker, past smoker, and never smoker. Alcohol abuse was captured in the chart review if it was documented in the patient’s hospital chart as such. Any details regarding the amount of alcohol consumed were recorded from the chart, along with any previous symptoms of alcohol withdrawal, such as withdrawal tremors or seizures. For most admitted patients with a history of alcoholism, the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) is conducted while the patient is admitted and can help support the diagnosis [14]. For this study, alcohol abuse was categorized as yes or no.
The primary IPD diagnosis was assigned based on severity because some patients had multiple diagnoses. Meningitis was considered the most severe, followed by pneumonia +/- empyema, other focal invasive disease, and bacteremia without focus. Other focal invasive disease included peritonitis, osteomyelitis, septic arthritis, and endocarditis.
Data Analysis
Statistical analysis was performed using Stata/IC 14.1 (StataCorp, College Station, TX). Comparison of age between homeless and nonhomeless persons was done through 2-sample t test with equal variance. Due to previously reported serotype-specific outbreaks of serotypes 4, 5, and 8 in homeless persons [1, 15–17], the total number of cases and case fatality rates due to these 3 serotypes were compared between homeless persons and nonhomeless persons. Univariable tests of proportions were used to compare different demographic, clinical, and microbiological features between homeless and nonhomeless persons with IPD. The total population size varied between each risk factor and outcome due to missing information. Multivariable logistic regression models were used to explore the relationship between ICU admission and risk factors, as well as the relationship between mortality and various risk factors.
This study was approved by the Conjoint Health Research Ethics Board (CHREB) at the University of Calgary.
RESULTS
A total of 1729 adult IPD cases were identified in the Calgary Zone area between 2000 and 2016. The cases occurred in 1652 separate patients; therefore, 77 cases (4.6%) occurred in persons who had presented at least once before with IPD. Full information was available for 89.5% of cases. The highest rate of missing information was for smoking status (11.5% missing).
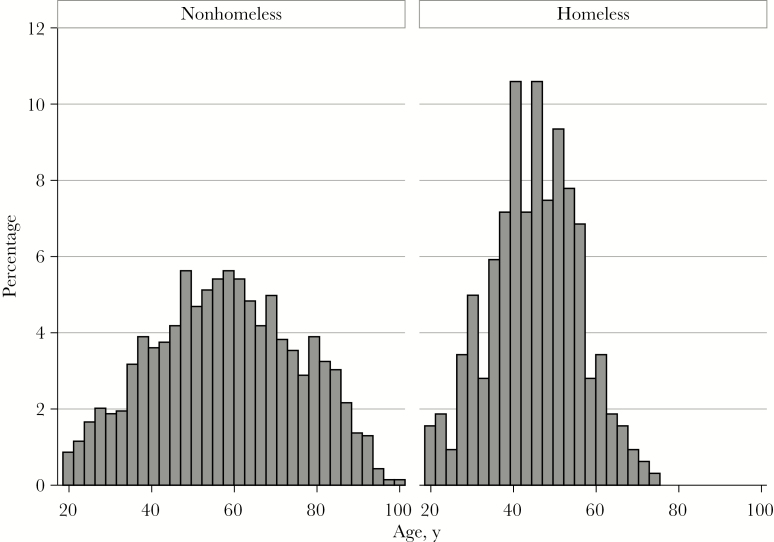
Of the 1729 cases, 321 cases (18.8%) occurred in homeless persons. Compared with the estimated prevalence of homelessness in the adult population (0.2%), homelessness was over-represented in IPD cases by a factor of 94 times. The mean age in homeless persons was 45.0 years (median [range], 45.0 [18.6–72.9] years), compared with a mean age of 57.9 years (median [range], 60.6 [18.5–101.3] years) in nonhomeless persons (P < .001) (Figure 1). In addition, 310 IPD cases (96.6%) in homeless persons occurred in persons under 65 years of age, compared with 896 IPD cases (64.6%) in nonhomeless persons occurring in persons under 65 years. In the descriptive analysis of age, gender, and primary diagnosis among the different levels of homelessness (Table 1), homeless persons were more commonly male, and the majority presented with a primary diagnosis of pneumonia and empyema.
Figure 1.
Histogram of age distribution of invasive pneumococcal disease cases in adults who were homeless or nonhomeless, 2000–2016.
Table 1.
Age, Gender, and IPD Clinical Diagnosis With Different Levels of Homelessness
| Homeless Status (Total = 321)a | Mean Age, y | Gender | IPD Clinical Diagnosis | ||
|---|---|---|---|---|---|
| Homeless in a shelter (total = 235) | 46.1 | Male | 81.7% | Pneumonia/empyema | 92.3% |
| Female | 18.3% | Meningitis | 2.1% | ||
| Bacteremia/other | 5.1% | ||||
| Homeless not in a shelter (total = 64) | 41.5 | Male | 70.3% | Pneumonia/empyema | 96.9% |
| Female | 29.7% | Meningitis | 1.6% | ||
| Bacteremia/other | 1.6% | ||||
| Provisionally accommodated (total = 18) | 40.7 | Male | 72.2% | Pneumonia/empyema | 88.9% |
| Female | 27.8% | Meningitis | 0.0% | ||
| Bacteremia/other | 11.1% | ||||
Abbreviation: IPD, invasive pneumococcal disease.
aStatus known for 317/321 cases.
Pneumococcal vaccination status was determined for 956 IPD cases (55.3%). Although it was assumed that the great majority of the 773 IPD cases (44.7%) with missing data had never been vaccinated, we did not impute results for these cases to avoid erroneous misclassification. Further, we did not include vaccination status in any of the multivariate models to avoid having to exclude the large number of cases with missing data.
For the 956 IPD cases with known pneumococcal vaccination status, 283 (29.6%) had previously received at least 1 dose of PPV23 vaccine. In those under 65 years of age, 102/624 (16.4%) had received a dose, compared with 181/332 (54.5%) of those aged 65 and older. When considering all cases with missing data as unvaccinated, the proportion of 1729 overall IPD cases with at least 1 known dose of PPV23 vaccine was 16.4% (283/1729). The PPV23 vaccination status by age group was 8.4% in those under 65 years (102/1221) and 34.7% in those aged 65 and older (176/508).
Among the 321 IPD cases in homeless persons, pneumococcal vaccination status was known for 134 cases (41.8%) and 24/134 (17.9%) had received at least 1 dose of PPV23 vaccine. When considering all IPD cases in homeless persons with missing data as unvaccinated, 24/321 (7.5%) had received at least 1 dose of PPV23 vaccine. As noted previously, 96.6% of IPD cases in homeless persons occurred in persons under 65, and further, 95.8% (23/24) of homeless persons who had been vaccinated were under 65 years of age.
Just 0.6% (n = 11) of all IPD cases had previously received at least 1 dose of PCV7 or PCV13, and these small numbers were not considered further in this analysis.
Since 2011 (1 year after the introduction of routine childhood PCV13 vaccination in Calgary), PCV13 serotypes accounted for 52.1% of IPD cases in homeless persons compared with 32.2% of cases in nonhomeless persons (P < .001), whereas PPV23 accounted for 90.6% of IPD cases in homeless persons compared with 75.6% in nonhomeless persons (P < .001). Serotypes 4, 5, and 8 (outbreak-associated serotypes) [1, 15–17] were identified in 54.4% of cases in homeless persons, compared with 21.0% of cases in nonhomeless persons (P < .001). The case fatality rate among patients with serotypes 4, 5, or 8 was 5.2%, compared with 14.9% among patients with other serotypes (P < .001), regardless of homeless status.
With univariable analysis (Table 2), the proportions of cases who were male, smokers, alcohol abusers, and illegal drug users were significantly higher in homeless persons than nonhomeless persons. There was no significant difference in proportion of homeless and nonhomeless people with a comorbidity that increased their risk of IPD. Homeless persons had significantly higher proportions of infection by PCV13 serotypes, PCV13-only serotypes, and PPV23 serotypes compared with nonhomeless persons.
Table 2.
Comparison of Demographic, Clinical, and Microbiological Characteristics of Homeless and Nonhomeless Adults With IPD, 2000–2016
| Characteristic | Nonhomeless n = 1398,a % of Total | Homeless n = 321,a % of Total | Difference if Homeless, % | 95% CI of Difference | P Value |
|---|---|---|---|---|---|
| Demographic & clinical | |||||
| Male gender | 768 (55.4) | 252 (78.8) | +23.4 | 18.2 to 28.6 | <.001 |
| Smoking statusb | 591 (47.6) | 252 (88.7) | +41.1 | 36.6 to 45.8 | <.001 |
| Alcohol abuse | 263 (19.3) | 230 (72.6) | +53.3 | 47.9 to 58.6 | <.001 |
| Illegal drug use | 169 (12.4) | 156 (49.2) | +36.8 | 31.1 to 42.6 | <.001 |
| Increased risk of IPDc | 870 (63.7) | 184 (58.0) | –5.7 | –11.7 to 0.3 | .059 |
| Primary diagnosis pneumonia +/- empyema +/- meningitisd | 1198 (86.4) | 304 (94.7) | +8.3 | 5.3 to 11.4 | <.001 |
| Outcome | |||||
| Case fatality | 185 (13.5) | 22 (6.9) | –6.6 | –3.3 to –9.9 | .001 |
| ICU admission | 342 (25.1) | 86 (27.1) | +2.1 | –3.4 to 7.5 | .449 |
| Microbiological | |||||
| PCV7 serotype | 299 (21.9) | 57 (17.9) | –3.9 | –0.8 to 8.7 | .121 |
| PCV13 serotype | 681 (49.6) | 182 (57.2) | +7.6 | 1.6 to 13.6 | .015 |
| PCV13-only serotype | 380 (27.8) | 125 (39.3) | +11.5 | 5.7 to 17.4 | <.001 |
| PPV23 serotype | 1115 (81.3) | 286 (89.9) | +8.6 | 4.7 to 12.5 | <.001 |
| Outbreak serotypes 4, 5, & 8 | 288 (21.0) | 173 (54.4) | +33.3 | 27.5 to 39.3 | <.001 |
Abbreviations: CI, confidence interval; ICU, intensive care unit; IPD, invasive pneumococcal disease; PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; PPV23, 23-valent pneumococcal polysaccharide vaccine.
aTotal number varied in some factors due to missing information.
bNonsmoker/former smoker vs current smoker.
cProportion of cases with 1 or more immunocompetent or immunocompromising comorbidities [13].
dPneumonia +/- empyema +/- meningitis vs all other IPD.
The first multivariable model examined the relationship between ICU admission and risk factors (Table 3). Factors that increased the odds of ICU admission included being a current smoker, alcohol abuse, immunocompetent comorbidity, and having a primary diagnosis of pneumonia/empyema or meningitis. Factors that decreased the odds of ICU admission included homelessness, age ≥85 years, and IPD caused by serotypes 4, 5, or 8. All other factors were not significant.
Table 3.
Multivariable Logistic Regression of Clinical and Microbiological Factors Associated With ICU Admission in Adults With IPD, 2000–2016
| Factor | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Homeless | 0.65 | 0.45 to 0.93 | .020 |
| Male | 0.87 | 0.67 to 1.12 | .283 |
| Alcohol abuse | 2.23 | 1.64 to 3.03 | <.001 |
| Illegal drug use | 1.20 | 0.85 to 1.68 | .298 |
| Serotypes 4, 5, or 8 | 0.69 | 0.51 to 0.94 | .017 |
| Age group | |||
| 18–64 y | Reference group | ||
| 65–84 y | 0.80 | 0.57 to 1.11 | .179 |
| 85+ y | 0.14 | 0.04 to 0.47 | .001 |
| Smoking | |||
| Nonsmoker | Reference group | ||
| Current smoker | 1.46 | 1.01 to 2.12 | .046 |
| Former smoker | 1.29 | 0.85 to 1.96 | .231 |
| Primary diagnosis | |||
| Bacteremia/other | Reference group | ||
| Pneumonia/empyema | 1.65 | 1.05 to 2.60 | .031 |
| Meningitis | 19.64 | 9.76 to 39.49 | <.001 |
| Comorbiditya | |||
| No comorbidity | Reference group | ||
| Immunocompetent comorbidity | 1.38 | 1.03 to 1.85 | .031 |
| Immunocompromising comorbidity | 1.15 | 0.81 to 1.63 | .440 |
Abbreviations: CI, confidence interval; ICU, intensive care unit; IPD, invasive pneumococcal disease.
aProportion of cases with 1 or more immunocompetent or immunocompromising comorbidities [13].
The second multivariable model examined the relationship between mortality and risk factors (Table 4). Factors that increased the odds of mortality included older age, immunocompromising or immunocompetent comorbidities, and alcohol abuse. IPD caused by serotypes 4, 5, or 8 had lower odds of mortality. All other factors, including homelessness, were not significant.
Table 4.
Multivariable Logistic Regression of Clinical and Microbiological Factors Associated with 30-Day Mortality in Adults with IPD, 2000–2016
| Risk Factor | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Homeless | 0.62 | 0.33 to 1.18 | .146 |
| Male | 0.91 | 0.64 to 1.31 | .619 |
| Alcohol abuse | 1.91 | 1.19 to 3.05 | .007 |
| Illegal drug use | 0.62 | 0.33 to 1.14 | .123 |
| Serotypes 4, 5, or 8 | 0.45 | 0.26 to 0.77 | .004 |
| Age group | |||
| 18–64 y | Reference group | ||
| 65–84 y | 2.05 | 1.35 to 3.11 | .001 |
| 85+ y | 3.88 | 1.95 to 7.73 | <.001 |
| Smoking | |||
| Nonsmoker | Reference group | ||
| Current smoker | 1.13 | 0.70 to 1.81 | .623 |
| Former smoker | 0.67 | 0.40 to 1.11 | .116 |
| Primary diagnosis | |||
| Bacteremia/other | Reference group | ||
| Pneumonia/empyema | 0.95 | 0.56 to 1.63 | .863 |
| Meningitis | 2.07 | 0.94 to 4.52 | .069 |
| Comorbiditya | |||
| No comorbidity | Reference group | ||
| Immunocompetent comorbidity | 2.60 | 1.61 to 4.21 | <.001 |
| Immunocompromising comorbidity | 3.05 | 1.82 to 5.12 | <.001 |
Abbreviations: CI, confidence interval; ICU, intensive care unit; IPD, invasive pneumococcal disease.
aProportion of cases with 1 or more immunocompetent or immunocompromising comorbidities [13].
DISCUSSION
Homelessness was present in nearly 1 in 5 cases of IPD in adults in Calgary and so was significantly overrepresented, by a factor of 94 times, compared with the estimated population prevalence of homelessness. Compared with adults who had IPD and were not homeless, those who were homeless had a lower mean age, and there was a higher proportion of males and a higher proportion of smokers, alcohol abusers, and illegal drug users. Homeless persons with IPD had underlying comorbid health conditions as commonly as did nonhomeless persons with IPD. Similar to nonhomeless persons, the IPD clinical diagnosis in those who were homeless was most often pneumonia. However, homeless persons had lower odds of ICU admission than nonhomeless persons, and they had the same odds of mortality from IPD.
Homelessness associated with IPD has been reported as an important risk factor in other Canadian studies, which reported higher rates of IPD in homeless persons compared with the general population [18–21]. Plevneshi et al. observed a higher incidence of IPD among homeless persons compared with the general population in Toronto, Canada, and also found that these homeless persons were younger and more likely to be smokers, alcohol abusers, and intravenous drug users [18]. Outbreaks of IPD in homeless shelters have also been reported in the United States and Europe [22–24].
Despite a specific recommendation in Canada to provide PPV23 vaccine to all homeless persons, the proportion vaccinated in Calgary (nearly all of whom were <65 years of age) was very low and much lower than in all persons 65 years and older, which is another group for whom it is recommended that all receive PPV23 vaccine. From discussions with staff at homeless shelters in Calgary, it is known that homeless persons are a particularly difficult population to deliver vaccinations to (personal communication, J.A. Lemay).
The large burden of health issues in homeless persons including chronic medical conditions, infectious diseases (eg, tuberculosis, HIV), and substance abuse [9, 25], along with crowded living conditions, has been discussed as these are factors that may be associated with the observed increased incidence of IPD in homeless persons [18, 21]. As in our study, other studies found that the average age of homeless persons presenting with IPD was younger compared with nonhomeless persons with IPD [17, 18].
After accounting for the independent influence of younger age, behavioral factors, comorbidity, and clinical diagnosis, as well as pneumococcal serotype, homelessness was not independently associated with IPD mortality but was associated with a lower odds of ICU admission.
The influence of older age, underlying comorbidity, and clinical severity on increasing IPD mortality in adults is well established [26]. In the current study, we additionally observed the influence of serotypes 4, 5, and 8 as a result of outbreaks of serotypes 5 and 8 in 2005–2007 [1, 16, 27] and serotype 4 in 2015–2017 [15, 17], all of which predominated in homeless persons compared with nonhomeless persons. These serotypes may be associated with less severe disease. Both outbreaks were also seen in other areas of western Canada, particularly in the province of British Columbia [16, 17, 27].
When grouped together in the current study, IPD cases caused by 1 of these 3 serotypes had lower odds of admission to the ICU and lower mortality. There is some evidence to support this observation. Zurawska et al. observed lower incidence of necrotizing pneumonia and lower hospital mortality in adults with IPD due to serotype 5 compared with adults with non–serotype 5 IPD; however, no difference in rate of ICU admission was found between both groups [2]. A meta-analysis conducted by Weinberger et al. investigated serotype-specific disease outcomes in patients with IPD, using serotype 14 as the reference. Serotype 8 was found to have a decreased risk of death, and serotypes 4 and 5 were also observed to have a decreased, but not statistically significant, risk of death [28]. In contrast, it has also been reported that host factors are more associated with death than isolate serotype [29] and that single IPD serotypes would most likely not have a large confounding effect on outcome [26].
Strengths of this study include the long-term, large population–based surveillance, a sufficiently large sample of both homeless and nonhomeless persons with IPD, and extensive and complete demographic, clinical, and microbiological information on each case. This enabled descriptive analysis of multiple factors and evaluation of the relationship between homelessness and important outcomes (ICU admission and mortality).
Our study does have some limitations. We do not have a complete understanding of homeless persons’ interactions with our health care system to understand what potential barriers to accessing preventive or emergency health care may have influenced the likelihood of developing IPD in this group. We also do not have accurate information on the duration of acute illness symptoms before presentation to a health care center. Thus, despite having extensive information for each case, there may be unmeasured factors that influenced the likelihood of homelessness or IPD risk or both. Pneumococcal vaccination status was missing in a large proportion of all cases and in a higher proportion of homeless persons than nonhomeless persons, which made it difficult to evaluate the current impact of vaccination with PPV23. Finally, our study population included only persons who had IPD.
In summary, homelessness is highly overrepresented in adults with IPD in Calgary. Serotypes found in both PPV23 and PCV13 remain common causes of IPD in homeless persons. The most effective public health intervention to specifically prevent IPD is vaccination. Enhanced efforts are needed to deliver both PPV23 and PCV13 vaccinations to this vulnerable population.
Acknowledgments
The authors acknowledge the contributions of the other CASPER investigators (Drs. Jia Hu and Otto Vanderkooi) and research staff (Shannon Pyra, Joslyn Gray, Nicole McMillan).
Financial support. Dr. Kellner received funding to support the CASPER study through an unrestricted investigator-initiated grant-in-aid from Pfizer Canada.
Potential conflicts of interest. Dr. Kellner received funding to support the CASPER study through an unrestricted investigator-initiated grant-in-aid from Pfizer Canada. All other authors reported no conflicts of interest.
References
- 1. Vanderkooi OG, Church DL, MacDonald J, et al. Community-based outbreaks in vulnerable populations of invasive infections caused by Streptococcus pneumoniae serotypes 5 and 8 in Calgary, Canada. PLoS One 2011; 6:e28547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zurawska JH, Romney MG, Wong H, et al. Outcomes of critically ill patients who have serotype 5 invasive pneumococcal disease [published online ahead of print January 1, 2017]. J Intensive Care Med. doi: 10.1177/0885066617728895. [DOI] [PubMed] [Google Scholar]
- 3. Kellner JD, Scheifele D, Vanderkooi OG, et al. Effects of routine infant vaccination with the 7-valent pneumococcal conjugate vaccine on nasopharyngeal colonization with Streptococcus pneumoniae in children in Calgary, Canada. Pediatr Infect Dis J 2008; 27:526–32. [DOI] [PubMed] [Google Scholar]
- 4. Ricketson LJ, Wood ML, Vanderkooi OG, et al. ; Calgary Streptococcus pneumoniae Epidemiology Research (CASPER) investigators Trends in asymptomatic nasopharyngeal colonization with Streptococcus pneumoniae after introduction of the 13-valent pneumococcal conjugate vaccine in Calgary, Canada. Pediatr Infect Dis J 2014; 33:724–30. [DOI] [PubMed] [Google Scholar]
- 5. Cabaj JL, Nettel-Aguirre A, MacDonald J, et al. Influence of childhood pneumococcal conjugate vaccines on invasive pneumococcal disease in adults with underlying comorbidities in Calgary, Alberta (2000–2013). Clin Infect Dis 2016; 62:1521–6. [DOI] [PubMed] [Google Scholar]
- 6. Alberta Health Services. Pneumococcal polysaccharide vaccine biological page. 2018. Available at: https://www.albertahealthservices.ca/assets/info/hp/cdc/if-hp-cdc-pnumo-polysac-vac-bio-pg-07-290.pdf. Accessed 8 March 2019. [Google Scholar]
- 7. Alberta Health Services. Pneumococcal 13-valent conjugate vaccine biological page. 2018. Available at: https://www.albertahealthservices.ca/assets/info/hp/cdc/if-hp-cdc-pneu-13-conjate-vac-bio-pg-07-291.pdf. Accessed 8 March 2019. [Google Scholar]
- 8. Gaetz S, Barr C, Friesen A, et al. Canadian definition of homelessness. 2012. Available at: https://www.homelesshub.ca/sites/default/files/COHhomelessdefinition.pdf. Accessed 8 March 2019. [Google Scholar]
- 9. Frankish CJ, Hwang SW, Quantz D. Homelessness and health in Canada: research lessons and priorities. Can J Public Health 2005; 96(Suppl 2):S23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Turner Strategies. 2018 Alberta point-in-time homeless count. 2018. Available at: http://calgaryhomeless.com/content/uploads/2018-Alberta-Point-in-Time-Count-Technical-Report.pdf. Accessed 8 March 2019. [Google Scholar]
- 11.National Advisory Committee on Immunization. Statement on the recommended use of pneumococcal 23-valent polysaccharide vaccine in homeless persons and injection drug users. An Advisory Committee Statement (ACS). Can Commun Dis Rep Wkly 2008; 34(ACS-5):1–12. [PubMed] [Google Scholar]
- 12. Government of Alberta. Interactive health data application. Available at: http://www.ahw.gov.ab.ca/IHDA_Retrieval/. Accessed 7 April 2019. [Google Scholar]
- 13. Public Health Agency of Canada. Canadian immunization guide. Available at: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-16-pneumococcal-vaccine.html#a3. Accessed 8 March 2019. [Google Scholar]
- 14. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict 1989; 84:1353–7. [DOI] [PubMed] [Google Scholar]
- 15. Kellner J, Ricketson L, Vanderkooi O, et al. Whole genome phylogenetic analysis of Streptococcus pneumoniae causing an outbreak of serotype 4 invasive pneumococcal disease (IPD) in Alberta, Canada. Paper presented at: 11th International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD); April 2018; Melbourne, Australia. [Google Scholar]
- 16. Tyrrell GJ, Lovgren M, Ibrahim Q, et al. Epidemic of invasive pneumococcal disease, western Canada, 2005-2009. Emerg Infect Dis 2012; 18:733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McKee G, Choi A, Madill C, et al. Outbreak of invasive Streptococcus pneumoniae among an inner-city population in Victoria, British Columbia, 2016–2017. Can Commun Dis Rep Wkly 2018; 44:317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Plevneshi A, Svoboda T, Armstrong I, et al. ; Toronto Invasive Bacterial Diseases Network Population-based surveillance for invasive pneumococcal disease in homeless adults in Toronto. PLoS One 2009; 4:e7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shariatzadeh MR, Huang JQ, Tyrrell GJ, et al. Bacteremic pneumococcal pneumonia: a prospective study in Edmonton and neighboring municipalities. Medicine (Baltimore) 2005; 84:147–61. [DOI] [PubMed] [Google Scholar]
- 20. Marrie TJ, Tyrrell GJ, Majumdar SR, Eurich DT. Invasive pneumococcal disease: still lots to learn and a need for standardized data collection instruments. Can Respir J 2017; 2017:2397429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schillberg E, Isaac M, Deng X, et al. Outbreak of invasive Streptococcus pneumoniae serotype 12F among a marginalized inner-city population in Winnipeg, Canada, 2009-2011. Clin Infect Dis 2014; 59:651–7. [DOI] [PubMed] [Google Scholar]
- 22. Birtles A, McCarthy N, Sheppard CL, et al. Multilocus sequence typing directly on DNA from clinical samples and a cultured isolate to investigate linked fatal pneumococcal disease in residents of a shelter for homeless men. J Clin Microbiol 2005; 43:2004–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mercat A, Nguyen J, Dautzenberg B. An outbreak of pneumococcal pneumonia in two men’s shelters. Chest 1991; 99:147–51. [DOI] [PubMed] [Google Scholar]
- 24. DeMaria A Jr, Browne K, Berk SL, et al. An outbreak of type 1 pneumococcal pneumonia in a men’s shelter. JAMA 1980; 244:1446–9. [PubMed] [Google Scholar]
- 25. Turnbull J, Muckle W, Masters C. Homelessness and health. CMAJ 2007; 177:1065–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ricketson LJ, Nettel-Aguirre A, Vanderkooi OG, et al. Factors influencing early and late mortality in adults with invasive pneumococcal disease in Calgary, Canada: a prospective surveillance study. PLoS One 2013; 8:e71924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Romney MG, Hull MW, Gustafson R, et al. Large community outbreak of Streptococcus pneumoniae serotype 5 invasive infection in an impoverished, urban population. Clin Infect Dis 2008; 47:768–74. [DOI] [PubMed] [Google Scholar]
- 28. Weinberger DM, Harboe ZB, Sanders EA, et al. Association of serotype with risk of death due to pneumococcal pneumonia: a meta-analysis. Clin Infect Dis 2010; 51:692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alanee SR, McGee L, Jackson D, et al. ; International Pneumococcal Study Group Association of serotypes of Streptococcus pneumoniae with disease severity and outcome in adults: an international study. Clin Infect Dis 2007; 45:46–51. [DOI] [PubMed] [Google Scholar]